Abstract
BACKGROUND AND PURPOSE
GABAA receptors mediate both synaptic and extrasynaptic actions of GABA. In several neuronal populations, α4 and δ subunits are key components of extrasynaptic GABAA receptors that strongly influence neuronal excitability and could mediate the effects of neuroactive agents including neurosteroids and ethanol. However, these receptors can be difficult to study in native cells and recombinant δ subunits can be difficult to express in heterologous systems.
EXPERIMENTAL APPROACH
We engineered concatemeric (fused) subunits to ensure δ and α4 subunit expression. We tested the pharmacology of the concatemeric receptors, compared with a common synaptic-like receptor subunit combination (α1 +β2 +γ2L), and with free-subunit α4/δ receptors, expressed in Xenopus oocytes.
KEY RESULTS
δ-β2 −α4 +β2-α4 cRNA co-injected into Xenopus oocytes resulted in GABA-gated currents with the expected pharmacological properties of α4/δ-containing receptors. Criteria included sensitivity to agonists of different efficacy, sensitivity to the allosteric activator pentobarbital, and modulation of agonist responses by DS2 (4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridine-3-yl benzamide; a δ-selective positive modulator), furosemide, and Zn2+. We used the concatemers to examine neurosteroid sensitivity of extrasynaptic-like, δ-containing receptors. We found no qualitative differences between extrasynaptic-like receptors and synaptic-like receptors in the actions of either negative or positive neurosteroid modulators of receptor function. Quantitative differences were explained by the partial agonist effects of the natural agonist GABA and by a mildly increased sensitivity to low steroid concentrations.
CONCLUSIONS AND IMPLICATIONS
The neurosteroid structure-activity profile for α4/δ-containing extrasynaptic receptors is unlikely to differ from that of synaptic-like receptors such as α1/β2/γ2-containing receptors.
Keywords: neuropharmacology, molecular pharmacology, acetylcholine, GABA, ligand-gated channels, steroids/neurosteroids
Introduction
GABAA receptors containing the δ subunit are thought to mediate extrasynaptic effects on the excitability of neurones that express these subunits and δ subunit-containing receptors may be major targets of neuroactive compounds, including neurotherapeutics (Farrant and Nusser, 2005; Belelli et al., 2009; receptor nomenclature follows Alexander et al., 2011). Receptors containing the δ subunit are difficult to study in native cells, because of the presence of other types (e.g. synaptic) of GABAA receptors. They can also be difficult to study in heterologous systems because of poor δ subunit expression (Borghese and Harris, 2007). Absence of δ subunits can be difficult to detect functionally because it is not an obligatory subunit, and the properties of α/β and α/β/δ receptors can be similar. Recently, concatemeric (tandem, fused) receptors were explored in the context of α1/δ subunits (Baur et al., 2010), a subunit combination thought to be relevant for some neurones (Glykys et al., 2007). Here we explore a tool to ensure α4/δ-subunit expression, a subunit combination thought to occur natively in many principal cell types. We find that concatemeric δ-β2-α4 +β2-α4 combinations assemble and exhibit the properties expected of α4/δ receptors. We anticipate that this tool will aid investigators interested in developing α4/δ-selective ligands.
Receptors containing δ subunits mediate small-standing tonic currents in dentate granule neurones of the hippocampal formation, in cerebellar granule neurones, and in thalamocortical neurones, among other neuronal classes (Brickley et al., 1996; Nusser and Mody, 2002; Cope et al., 2005; Chandra et al., 2006; Wei et al., 2003). Although small in amplitude, the sustained nature of these conductances has a profound effect on neuronal excitability, and the conductances are therapeutic targets for anticonvulsants, anxiolytics, hypnotics and anaesthetics, among others (Caraiscos et al., 2004; Hemmings et al., 2005; Coulter and Carlson, 2007; Belelli et al., 2009). In cell types expressing the δ subunit, the α4 subunit is often preferentially expressed with δ subunits in extrasynaptic somatodendritic regions of cells (Wei et al., 2003). Therefore, α4/δ-containing receptors represent a good system for studying drug effects on extrasynaptic GABAA receptors.
δ-Containing GABAA receptors may also be a primary target for ethanol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003). However, there is controversy over these results, stemming from potential difficulties in δ expression in heterologous systems (Borghese et al., 2006; Borghese and Harris, 2007). We sought to obviate these issues by forcing δ expression using tandem expression with other subunits of the pentameric GABAA receptor. We synthesized δ-β2-α4 and β2-α4 concatemeric subunits and co-expressed the tandem subunits to promote pentameric receptor expression. We tested the characteristics expected of bona fideα4/δ subunits and found that the concatemer behaves as expected. We also tested modulation by a series of neurosteroid analogues, including negative modulators and positive modulators. Negative modulators exhibited characteristic uncompetitive antagonism at concatemeric α4/δ-containing receptors, similar to synaptic-like receptors. For neurosteroids that positively modulate, we found that, although δ subunit-containing receptors are especially sensitive to neurosteroids at high agonist concentration, the structure-activity relationship (SAR) for neurosteroids acting at δ-containing receptors is essentially similar to that at a typical α1 +β2 +γ2L synaptic receptor subunit combination. Thus, we conclude that neurosteroids may not represent a suitable framework for the development of δ subunit-selective ligands.
Methods
Concatemeric subunits were created using human α4, rat β2 and rat δ subunits as described previously (Akk et al., 2009; Bracamontes and Steinbach, 2009). In brief, we first generated the β2-α4 concatemer (referred to as β2-α4 construct), which had a linker with 23 amino acid residues: Q5A3PAQ2AGP2A2Q5, with a FLAG tag on the N terminus of the β2 subunit between residues 4 and 5 of the mature peptide. The α4 subunit was generated by PCR, with a partial linker sequence containing an FseI restriction site at the 5′ end and was subsequently subcloned into an existing β2-α1 construct, generating β2-α4, joined together with the linker. The δ-β2-α4 tandem was also generated by subcloning the α4 PCR fragment into an existing δ-β2-α1 clone, generating the δ-β2-α4 construct with the 26-amino acid residue sequence Q5A3PTGQ(QA)2A2PA2Q5 between the δ and β subunits. The human α4 subunit (kindly provided by Dr Paul Whiting, Merck, Harlow, Essex, UK), rat β2 subunit (kindly provided by Dr D. Weiss, University of Texas Health Science Center, San Antonio, TX, USA) and rat δ subunit (kindly provided by Dr Robert Macdonald, Vanderbilt, Nashville, TN, USA) were used. Concatemers were generated in pcDNA3 (Invitrogen, Carlsbad, CA, USA), and the full length of the insert was sequenced.
Oocyte expression
Stage V–VI oocytes were harvested from sexually mature female Xenopus laevis (Xenopus One, Northland, MI, USA) under 0.1% tricaine (3-aminobenzoic acid ethyl ester) anaesthesia, according to protocols approved by the Washington University Animal Studies Committee. Oocytes were defolliculated by shaking for 20 min at 37°C in collagenase (2 mg mL−1) dissolved in calcium-free solution containing (in mM): NaCl (96), KCl (2), MgCl2 (1) and HEPES (5) at pH 7.4. Capped mRNA, encoding rat GABAA receptor α1, β2, γ2L subunits and the concatemers were transcribed in vitro using the mMESSAGE mMachine Kit (Ambion, Austin, TX, USA) from linearized pcDNA3 vectors containing receptor-coding regions. Subunit transcripts were injected in equal parts (3–13 ng RNA for each of the free α1, α4, β2, γ2L, and δ subunits, up to 39 ng total, and 46 ng total RNA for the concatemers) 16–24 h following defolliculation. Oocytes were incubated up to 5 days at 18°C in ND96 medium containing (in mM): 96 NaCl, 1 KCl, 1 MgCl2, 2 CaCl2 and 10 HEPES at pH 7.4, supplemented with pyruvate (5 mM), penicillin (100 U mL−1), streptomycin (100 µg mL−1) and gentamycin (50 µg mL−1).
One possible caveat is that concatemeric constructs may be subject to proteolysis, yielding free-subunit receptors. Although we cannot completely exclude this possibility, we found it difficult to express free α4 and δ subunits (Supporting Information Figure S1). Thus, we view this possibility as unlikely. Also, linkers in the tandem receptors have no known protease sites, and previous studies of similar concatemers found no evidence for significant degradation by Western blot analysis (Bracamontes et al., 2011).
Oocyte electrophysiology
Two-electrode voltage-clamp experiments were performed with an OC725 amplifier (Warner Instruments, Hamden, CT, USA), 2–5 days following RNA injection. The extracellular recording solution was ND96 medium with no supplements. Intracellular recording pipettes were filled with 3 M KCl and had open tip resistances of ∼1 MΩ. GABA and the modulators were applied from a common tip via a gravity-driven multibarrel delivery system. Unless indicated otherwise, drugs were co-applied with no pre-application period. Cells were voltage clamped at −70 mV for all experiments, and the peak current (for potentiated responses) or the current at the end of 30 s drug applications (for inhibition of responses) was measured for quantification of current amplitudes.
Experimental design and analysis
Design of individual experiments is described in the Results and Figure legends. Wherever possible, each cell served as its own control, and experimental conditions and drug applications were interleaved to negate any time-dependent changes in cell responsiveness.
Data acquisition and analysis were performed with pCLAMP 9.0 software (Molecular Devices, Union City, CA, USA).
Tadpole anaesthesia screen
Identification of some neurosteroid analogues for testing on concatemeric, α4/δ-containing subunits relied on biological activity in a tadpole anaesthesia screen. Details of the screen have been published elsewhere (Wittmer et al., 1996). A reversible loss of righting reflex achieved at concentrations below 10 µM was taken to indicate positive biological activity in the tested compounds.
Data analysis
Data plotting and curve fitting were done with Sigma Plot 10.0 software (SPSS Science, Chicago, IL, USA). Fits to concentration response relationships were achieved using a least squares minimization to the Hill equation: y=a[xb/(cb+xb)], where a is the maximum potentiation, b is the Hill coefficient and c is the EC50 concentration of drug. Data are presented in the text and figures as mean ± SEM. Statistical differences were determined using a two-tailed Student's t-test.
Materials
Most drugs were from Sigma (St. Louis MO, USA) except DS2 (4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridine-3-yl benzamide; a δ-selective positive modulator) (Tocris Bioscience, Ellisville, MO, USA). The steroids XJ-18, B-260, ent-androsterone and ent-etiocholanolone (see Fig 8) were prepared as described previously (Han et al., 1996; Katona et al., 2008; Covey and Jiang, 2010). Steroids ECN, B-384, B-249, AKB-2 and KK-95 (Fig 8) were prepared using multi-step synthetic procedures and had spectroscopic properties (infrared spectroscopy, and both proton and carbon nuclear magnetic resonance spectroscopy) consistent with the structures shown. These compounds were determined to be pure by TLC and by either elemental analysis or high resolution mass spectrometry. Full synthetic details will be reported elsewhere. Steroids were prepared as stock solutions in DMSO. Final DMSO concentration was always below 0.13% and solutions were matched for DMSO concentration.
Figure 8.
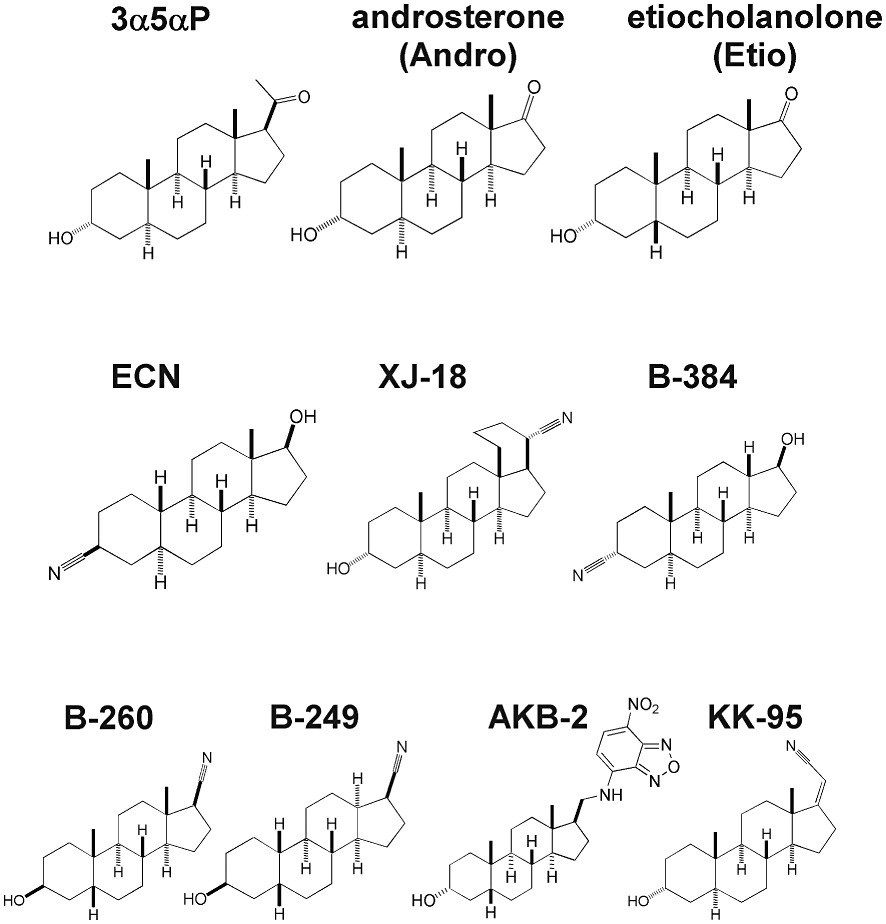
Structures of steroid analogues used to survey SAR at concatemeric receptors. The analogues 3α5αP, androsterone and etiocholanolone were used in studies of enantiomeric pairs; not shown are the unnatural enantiomers in which the stereochemistry of each chiral center was reversed. Analogues ECN, XJ-18, B-384, B-260 and B-249 were chosen for weak activity at α1 +β2 +γ2L receptors but strong biological activity in a tadpole anaesthesia screen. AKB-2 and KK-95 were chosen as representative weak potency and weak efficacy modulators respectively.
Results
Agonist pharmacology
To ensure δ subunit incorporation into heterologous receptors that represent an endogenous extrasynaptic combination for several classes of CNS neurones, we engineered concatemeric receptors composed of δ-β2-α4 and β2-α4 fused subunits. cRNA transcribed from plasmids encoding the fused subunits was injected into Xenopus oocytes for functional studies. We reliably observed GABA-evoked currents from the concatemeric subunits when δ-β2-α4 and β2-α4 subunits were co-injected. In batches of oocytes in which co-injection of δ-β2-α4 and β2-α4 resulted in robust currents to 30 µM GABA, neither δ-β2-α4 alone (n= 7 oocytes from three batches) nor β2-α4 alone (n= 15 oocytes from seven batches) formed functional channels, suggesting that functional receptors formed only with the intended subunit stoichiometry. Maximum current amplitudes from δ-β2-α4 +β2-α4 receptors were ∼200 nA at −70 mV to saturating GABA concentrations, substantially smaller than maximum currents from oocytes expressing individual, non-concatenated subunits of a primary synaptic receptor, α1 +β2 +γ2L (∼5 µA, n= 6 oocytes).
We performed several pharmacological assays to test α4/δ-containing receptor function and to ensure that the concatemeric receptors served as good models of endogenous, wild-type receptors. In Figure 1, we evaluated the response of δ-β2-α4 +β2-α4 receptors to agonists. One feature of endogenous and recombinant δ-containing receptors is their high sensitivity (low EC50) for GABA, typical of receptors sensitive to the low concentrations of GABA found extrasynaptically (Stell and Mody, 2002). We found the EC50 for peak GABA responses was 1.4 ± 0.3 µM in eight oocytes, consistent with previous reports for α4/δ receptors (Figure 1A,C) (Brown et al., 2002; Storustovu and Ebert, 2006; Mortensen et al., 2010). By contrast, synaptic-like α1 +β2 +γ2L receptors demonstrated an EC50 for peak GABA responses of 19 ± 2.4 µM (Figure 1B,C, n= 6; P < 0.05 compared with α4/δ concatemer EC50). As expected, responses to saturating GABA concentrations were weakly desensitizing for δ-β2-α4 +β2-α4 but were strongly desensitizing for α1 +β2 +γ2L receptors. The Hill coefficient was 1.2 ± 0.1 for the concatemeric receptors, considerably lower than that for α1 +β2 +γ2L receptors (2.4 ± 0.4, n= 6, P < 0.05). Hill coefficients of near 1 have been reported for agonists at δ-containing receptors previously (Storustovu and Ebert, 2006; Mortensen et al., 2010).
Figure 1.
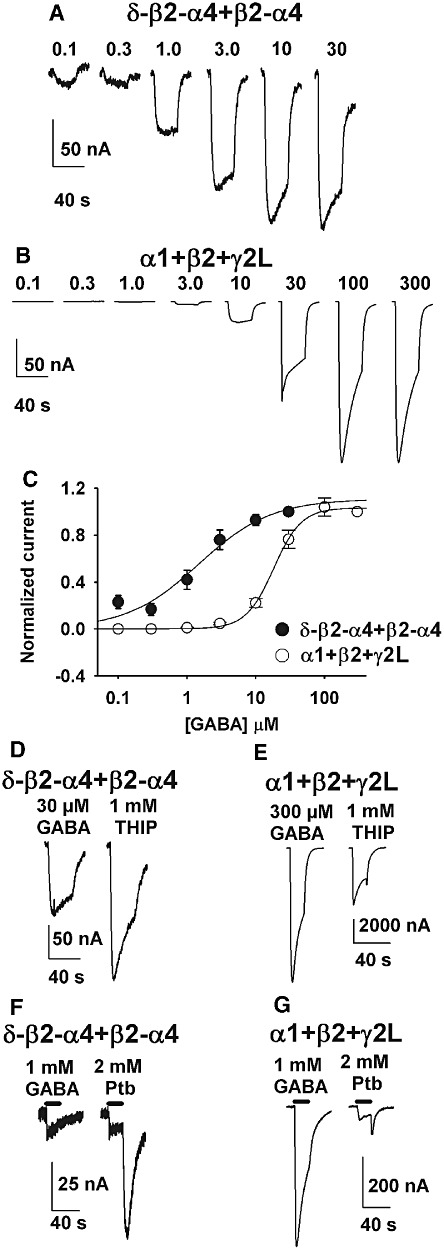
Sensitivity of extrasynaptic-like δ-β2-α4 +β2-α4 receptors to agonists. (A) Responses of a representative oocyte expressing concatemeric δ-β2-α4 +β2-α4 receptors to increasing concentrations of GABA. (B) Responses of a representative oocyte expressing α1 +β2 +γ2L to the same series of GABA concentrations. (C) Summary concentration-response curves for eight oocytes expressing concatemeric receptors and six oocytes expressing α1 +β2 +γ2L subunits. The solid lines represent fits to the Hill equation as described in the Methods. The fits yielded an EC50 of 1.2 µM for concatemeric δ-β2-α4 +β2-α4 receptors and 19 µM for α1 +β2 +γ2L receptors. Hill slopes were also significantly different, as described in the Results. (D) and (E) Representative responses of oocytes expressing the indicated subunit combinations to saturating GABA and THIP. THIP was a full agonist at concatemeric α4/δ-containing receptors but a partial agonist at synaptic-like receptors. (F) and (G) Pentobarbital (Ptb) tail currents exceed responses to GABA in α4/δ-containing concatemeric receptors. The traces are representative responses under the indicated conditions. The duration of agonist presentation is indicated by the horizontal bars.
Higher efficacy agonism by 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP [gaboxadol]) in contrast to partial agonism by GABA also characterizes α4/δ-containing receptors. At typical synaptic subunit combinations THIP acts as a partial agonist compared with GABA (Ebert et al., 1994; Adkins et al., 2001; Brown et al., 2002; Mortensen et al., 2010; Meera et al., 2011). This profile predicts that THIP should generate larger currents than a saturating GABA concentration in concatemeric δ-β2-α4 +β2-α4 receptors. Indeed, THIP-gated (1 mM) currents were 1.9 ± 0.3 times those produced by 300 µM GABA (Figure 1D). By contrast, at synaptic α1 +β2 +γ2L receptors, currents to 1 mM THIP were 0.4 ± 0.2 of those to 300 µM GABA (Figure 1E, n= 6 oocytes). THIP at 1 mM was saturating because increasing the THIP concentration to 10 mM did not alter the result (n= 4 oocytes, data not shown). Thus, these results confirm that THIP agonism is as expected at concatemeric δ-β2-α4 +β2-α4 receptors.
The presence of the δ subunit in receptor-complexes can also be ascertained from the prominent tail currents observed when the channels are directly gated by high concentrations of pentobarbital. The tail currents result from rapid relief of channel block during the removal of free pentobarbital allowing a transient increase in pentobarbital-elicited activity, unhindered by block. The magnitude of tail current can be thus used as a measure of channel gating efficacy. Receptors consisting of free α4 +β2 +δ subunits are strongly activated by pentobarbital whereas GABA is a weak agonist (Akk et al., 2004a). In single-channel recordings the channel open probability was significantly greater in the presence of pentobarbital compared with GABA. In whole-cell recordings, this manifested in pentobarbital tail currents that were several-fold larger than the peak current elicited by a saturating concentration of GABA. In the present work, concatemeric δ-β2-α4 +β2-α4 receptors yielded tail currents to 2 mM pentobarbital that were 4.0 ± 0.6-fold (n= 7 cells) larger than the peak response to 1 mM GABA (Figure 1F). By comparison, α1 +β2 +γ2L receptors exhibited tail currents that were 0.5 ± 0.3 (n= 5 cells) of the peak response to 1 mM GABA (Figure 1G).
Antagonist pharmacology
Responses to inhibitors also help define bona fideα4/δ-containing receptors. For instance the presence of an auxiliary δ or γ subunit lowers the sensitivity to the non-competitive antagonist Zn2+ (Smart et al., 1991; Nagaya and Macdonald, 2001; Storustovu and Ebert, 2006). We found that δ-β2-α4 +β2-α4 receptors had weak sensitivity to inhibition by 1 µM Zn2+ (Figure 2A,D). Inhibition was also weak at synaptic α1 +β2 +γ2L (Figure 2B,D). As expected, α1 +β2 receptors (no auxiliary subunit) were strongly inhibited by 1 µM Zn2+ (Figure 2C,D).
Figure 2.
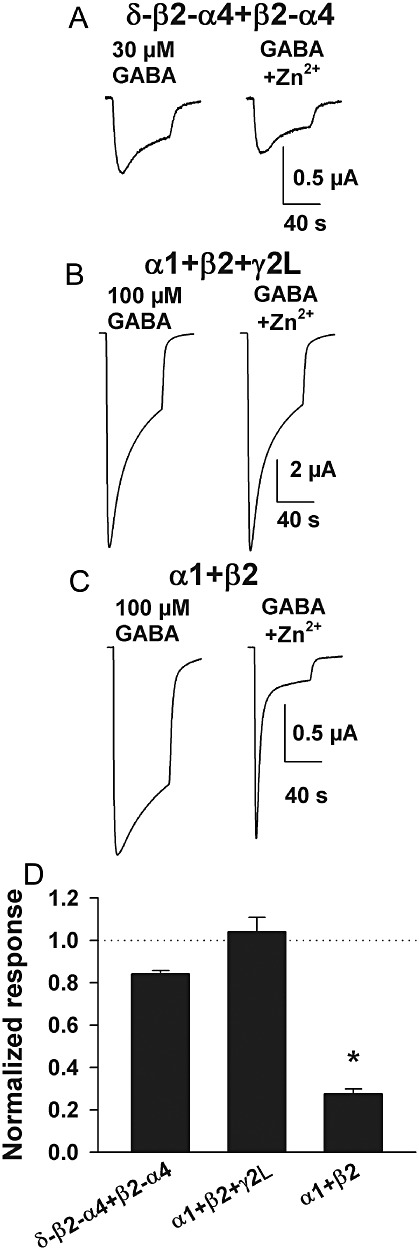
The antagonist Zn2+ exhibits appropriate selectivity at concatemeric receptors. (A–C) Representative responses of the indicated subunit combinations to a high GABA concentration in the absence and presence of Zn2+ (1 µM). (D) Summary data (n= 4–8 oocytes per subunit combination) for Zn2+ sensitivity under the conditions depicted in A–C. As expected Zn2+ sensitivity was highest in receptors with no auxiliary subunit and was intermediate at α4/δ-containing concatemers. *P < 0.05, significantly different from each of the other two values; two-tailed unpaired t-test.
Furosemide is an antagonist with relative selectivity for α4- and α6-containing receptors over other α subunit-containing receptors (Wafford et al., 1996). Furosemide (300 µM) depressed the response of δ-β2-α4 +β2-α4 receptors to 1 µM GABA to 0.64 ± 0.04 (n= 4; Figure 3A,C). This is consistent with the degree of block observed on α4 receptors in previous work (Wafford et al., 1996). By contrast, 300 µM furosemide depressed responses of α1 +β2 +γ2L receptors to 0.92 ± 0.02 (n= 4; Figure 3B,C).
Figure 3.
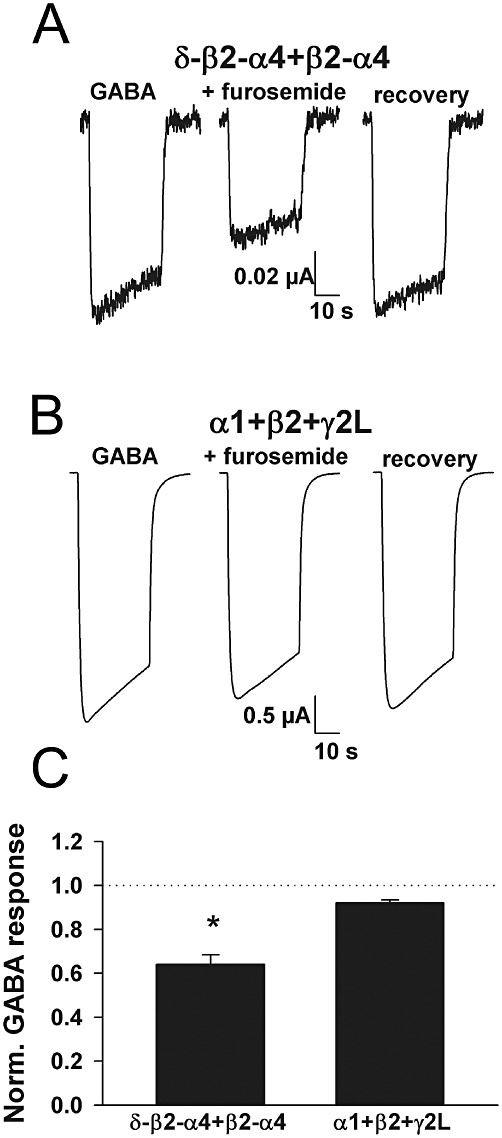
The antagonist furosemide exhibits appropriate selectivity at concatemeric receptors. (A and B) Representative responses of the indicated subunit combinations to ∼EC50 GABA concentration in the absence and presence of furosemide (300 µM). (C) Summary data (n= 4 oocytes per subunit combination). As expected furosemide sensitivity was higher in α4/δ- containing receptors than in synaptic-like α1 +β2 +γ2L receptors. *P < 0.05, significantly different from α1 +β2 +γ2L; two-tailed unpaired t-test.
Allosteric positive modulators
Figure 4 shows responses of concatemeric α4/δ receptors to positive allosteric modulators. Receptors containing α4/δ subunits do not exhibit sensitivity to benzodiazepines. In agreement with these results with free subunits, we found that 1 µM lorazepam had no effect on responses to 0.3 µM GABA at concatemeric δ-β2-α4 +β2-α4 receptors (Figure 4A). By contrast, 1 µM lorazepam reliably potentiated responses to low GABA concentrations at α1 +β2 +γ2L synaptic-like receptors (Figure 4B and 2.9 ± 0.6 times that of control, n= 4 oocytes).
Figure 4.
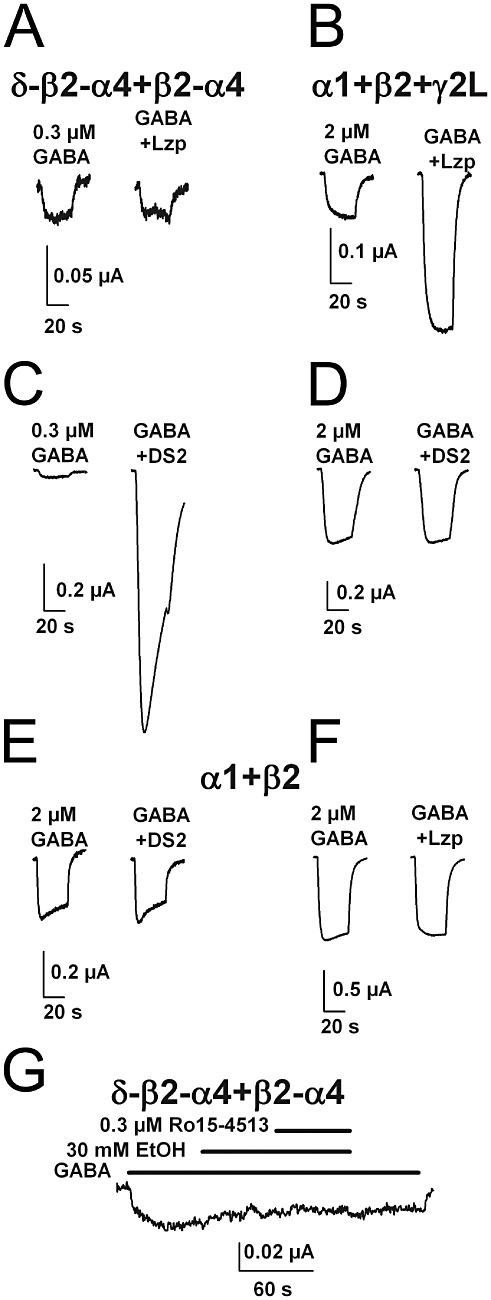
Allosteric positive modulators exhibit appropriate selectivity at concatemeric α4/δ-containing receptors. (A and B) Representative responses of oocytes expressing the indicated subunits to the indicated GABA concentrations and co-applied 1 µM lorazepam (Lzp). (C and D) Representative oocyte responses to the indicated GABA concentration and to co-applied DS2 (1 µM). (E and F) Oocytes lacking an auxiliary subunit failed to respond to either lorazepam or to DS2. (G) Lack of effect of ethanol (30 mM) and Ro15-4513 (0.3 µM) on GABA (0.5 µM)-activated concatemer currents.
Recently, δ subunit-selective benzamide allosteric potentiators have been described (Wafford et al., 2009). We found that the δ-selective benzamide DS2 (1 µM) dramatically potentiated responses to low GABA concentrations in concatemeric δ-β2-α4 +β2-α4 receptors but not in synaptic-like α1 +β2 +γ2L receptors (Figure 4C,D and 12.9± 0.7 times the baseline GABA response on concatemers, n= 4; 1.1 ± 0.05 times the baseline GABA response at α1 +β2 +γ2L receptors). As expected, α1 +β2 receptors were insensitive to both lorazepam and DS2, because of the lack of γ2 subunit and δ subunit, respectively (Figure 4E,F).
Some studies have suggested that α4/δ-containing receptors may underlie the neuroactive effects of low concentrations of ethanol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003). Other studies have failed to find a preferential role of ethanol on these receptors (Borghese et al., 2006), and a recent study of concatemeric α1-β3-δ receptors failed to find potentiating ethanol effects, regardless of subunit arrangement (Baur et al., 2010). Our δ-β2-α4 +β2-α4 concatemeric receptors also failed to exhibit a detectable effect of ethanol up to 100 mM (tested with 0.3–0.5 µM GABA, Figure 4G; average ethanol effect (1.16 ± 0.18 relative to GABA alone, n= 7). Further, we found no effect of the putative ethanol antagonist compound Ro15-4513 on the responses (0.99 ± 0.07 relative to GABA alone; Figure 4G). Possibly the lack of ethanol effect in our receptors is related to the β2 subunit. A β3 subunit may be quantitatively important for low-concentration ethanol effects (Wallner et al., 2003). We also cannot exclude the possibility of posttranslational changes not recapitulated in our system that may underlie effects of ethanol on extrasynaptic receptors.
Comparison with individual α4 +β2 +δ responses
We attempted to express individual subunits for pharmacological comparison with concatemer responses. Our efforts were fraught with the difficulties that our concatemer approach was intended to avoid. In addition to δ subunit expression, we also had problems with α4 subunit expression. Oocytes injected with two separate batches of α4 RNA with β2 subunit RNA exhibited Zn2+ (1 µM)-sensitive standing conductances, small GABA currents (Supporting Information Figure S1), but very large pentobarbital currents (data not shown). We attributed these aberrant responses to β2 homomer expression because injection of β2 RNA alone yielded similar responses (Supporting Information Figure S1). Using Zn2+-sensitive standing conductances as an indication of contaminating homomer expression, we identified three batches of oocytes injected with a 5:1 and 5:1:5 ratios of α4:β2 and α4:β2:δ that yielded responses indicative of mostly multimeric receptors.
Data from these oocytes yielded a GABA EC50 for α4 +β2 +δ RNA of 0.8 ± 0.1 µM with a Hill coefficient of 0.8 ± 0.1 (n= 6 oocytes), consistent with concatemer data. Oocytes injected with α4 +β2 (without δ) yielded an EC50 of 0.6 ± 0.1 µM and a Hill coefficient of 1.3 ± 0.1. We hypothesized that DS2 sensitivity may be the single most reliable indicator of δ subunit incorporation. Indeed, oocytes injected with free α4 +β2 +δ subunits (5:1:5) exhibited strong DS2 sensitivity, while oocytes from the same batches injected with α4 +β2 subunits failed to exhibit DS2 sensitivity (Figure 5A–C). Other pharmacological tests also qualitatively validated the expected agonist/antagonist pharmacology (Figure 5D–F). Maximum THIP responses exceeded maximum GABA responses, and δ did not alter THIP efficacy (Figure 5D), consistent with a recent report (Meera et al., 2011). Zn2+ sensitivity was stronger in the absence of a δ subunit, as expected; however, overall sensitivity was somewhat higher with free subunits than with concatemeric receptors (Figure 5E). Furosemide sensitivity of both α4 +β2 and of α4 +β2 +δ injected oocytes was stronger than that observed for synaptic-like receptors not containing α4 (Figure 5F); however, with free δ subunits present, we observed higher sensitivity of responses to furosemide than in concatemeric receptors (cf. Figure 3). The reason for the quantitative differences in Zn2+ and furosemide sensitivity between free-subunit and concatemeric receptors is unclear, but we speculate that differences in subunit stoichiometry, including the presence of homomeric receptors (Supporting Information Figure S1) may contribute. It is also possible that different positioning of δ subunits in the receptor pentamer affects responses.
Figure 5.
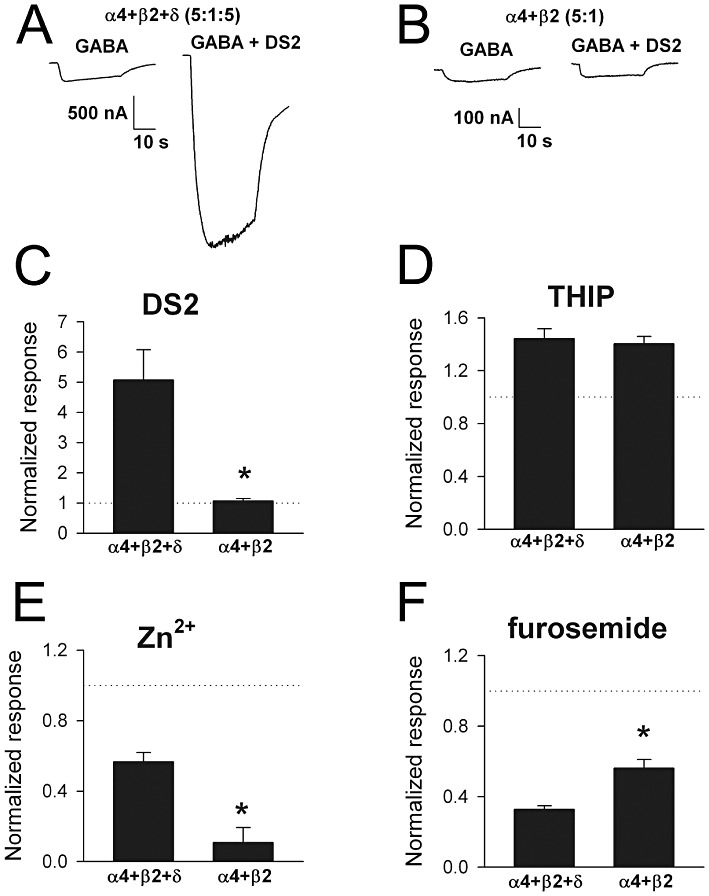
Properties of free-subunit receptors. (A and B) Representative examples of DS2 potentiation in oocytes injected with α4 +β2 +δ or with α4 +β2 free subunits. The ratios of RNA amounts were 5:1:5 and 5:1, respectively (see Supporting InformationFigure 1). GABA (0.3 µM) responses were potentiated by DS2 (1 µM) in oocytes injected with δ subunit RNA but not in oocytes injected with α4 +β2 alone. (C) Summary of oocytes challenged as in A and B (n= 8 and 10 respectively). *P < 0.05, significantly different from α4 +β2 +δ; two-tailed unpaired t-test. (D) Demonstration of stronger efficacy of THIP (1 mM) compared with 30 µM GABA on both classes of receptor (n= 8 and 10 respectively). (E) Zn2+ sensitivity of δ-lacking receptors was higher (n= 8 and 10 respectively, 1 µM GABA, 1 µM Zn2+). *P < 0.05, significantly different from α4 +β2 +δ; two-tailed unpaired t-test (F) Furosemide sensitivity at both classes of receptor was higher than observed on synaptic-like receptors (cf. Figure 3), but sensitivity to furosemide was higher on δ subunit-containing receptors (n= 7 and 10). *P < 0.05, significantly different from α4 +β2 +δ; two-tailed unpaired t-test.
Neurosteroid effects-inhibitory steroids
Having established that concatemeric receptors retain many properties expected of α4/δ-containing receptors, we explored the effects of different classes of neurosteroids on these receptors. Neurosteroids are of interest because they are endogenous positive and negative regulators of GABAergic transmission. Further, it has been proposed that positive neurosteroids preferentially modulate δ subunit-containing receptors, with implications for anxiety, epilepsy and alcohol intoxication (Wohlfarth et al., 2002; Stell et al., 2003). The effects of some endogenous potentiating neurosteroids have been studied at α4/δ receptors (Brown et al., 2002; Meera et al., 2009), but the effects of non-competitive antagonist neurosteroids have been studied less extensively (Brown et al., 2002). We investigated three sulphated neurosteroids. At synaptic-like α1 +β2 +γ2L receptors, sulphated steroids such as pregnenolone sulphate (PS) exhibit characteristic use-dependent antagonism; antagonism is weaker against low GABA concentrations and against partial agonists (Eisenman et al., 2003). When tested on the concatemers, PS (1 µM) antagonism was relatively weak against a saturating concentration (30 µM) of the partial agonist GABA but was stronger against the higher-efficacy agonist THIP (Figure 6A). In eight oocytes in which PS was tested against both 30 µM GABA and 1 mM THIP at steady-state, PS reduced responses to 0.40 ± 0.05 for responses elicited by GABA and to 0.30 ± 0.02 for responses to THIP (P < 0.05, paired t-test). With both GABA and THIP, PS exhibited stronger effects on steady-state currents than on peak currents, consistent with activation-dependent enhancement of antagonism previously observed with α1 +β2 +γ2L receptors (Figure 6B) (Shen et al., 2000; Eisenman et al., 2003).
Figure 6.
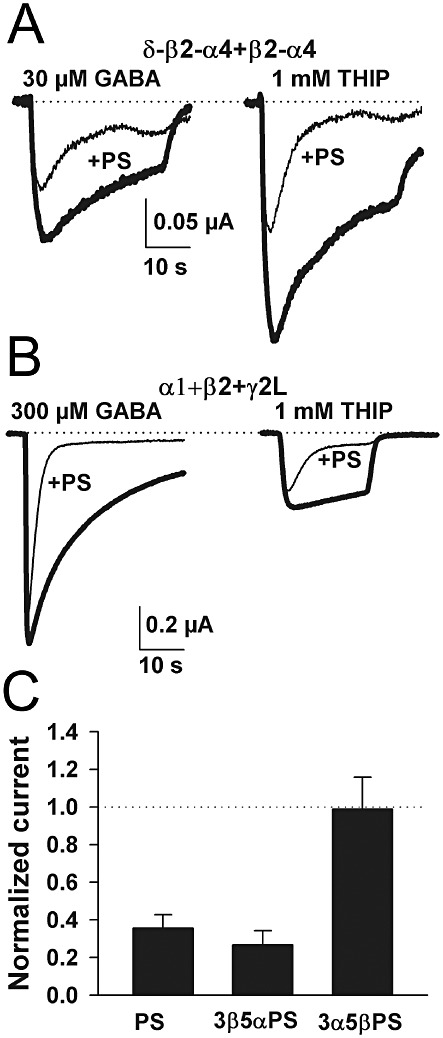
α4/δ-containing receptors exhibit sensitivity to antagonist sulphated steroids similar to that previously reported for synaptic-like receptors. (A) Responses of representative oocytes to the partial agonist GABA and the full agonist THIP in the absence and presence of PS. Responses in the presence of PS exhibited stronger apparent desensitization, and PS inhibition increased when the full agonist was used to activate receptors. Dotted line indicates zero-current level. (B) Similar experiment using α1 +β2 +γ2L receptors and the indicated agonists. Note the reverse pattern of agonist and antagonist effects. Antagonism is strongest with the full agonist. (C) SAR for sulphated steroid antagonism against GABA (30 µM) responses at concatemeric δ−β2 −α4 +β2 −α4 receptors was similar to that previously reported for α1 +β2 +γ2L receptors (Wang et al., 2002), with pregnane sulphated steroids exhibiting diastereoselectivity.
At α1 +β2 +γ2L receptors, PS and 3β5αPS exhibit stronger inhibition than 3α5βPS (Wang et al., 2002). In the present studies, 1 µM PS depressed α1 +β2 +γ2L responses to saturating GABA (300 µM) to 0.21 ± 0.4 (Figure 6B), while 1 µM 3α5βPS depressed currents to only 0.76 ± 0.03 (n= 4 oocytes). At concatemeric receptors, we observed a similar pattern of antagonism of GABA responses (Figure 6C), suggesting a similar SAR at α4/δ receptors to that at α1 +β2 +γ2L receptors. In fact 3α5βPS at 1 µM showed very little inhibition against GABA responses at concatemeric α4/δ receptors (Figure 6C). However, responses to the higher efficacy agonist THIP (1 mM) were characterized by stronger antagonism to 1 µM 3α5βPS (0.69 ± 0.01; n= 4). Thus 3α5βPS antagonism is not qualitatively absent at concatemers. Rather, a confluence of factors (diastereoselectivity, the partial agonism of GABA, and the uncompetitive nature of antagonism) gives rise to the weak inhibition shown in Figure 6C. In summary, mechanistic features of sulphated steroid antagonism are essentially similar at extrasynaptic α4/δ receptors and synaptic-like α1 +β2 +γ2L receptors.
Neurosteroid effects – potentiating steroids
The natural 3α-hydroxy neurosteroids 3α5αP and 3α5αTHDOC potentiated concatemeric receptors over a slightly lower concentration range compared with synaptic-like receptors. For 3α5αP there was about a twofold lower EC50 (Figure 7A–C) (0.26 ± 0.08 µM for concatemer and 0.53 ± 0.05 µM for α1 +β2 +γ2L, n= 8 and 4, respectively, P < 0.05). The EC50 for α1 +β2 +γ2L receptors was nearly identical to the value we have previously obtained in independent studies (0.6 µM, Chisari et al., 2009). The EC50 for 3α5αTHDOC differed by about fivefold between concatemeric α4/δ receptors and α1 +β2 +γ2L receptors (0.39 ± 0.09 µM for concatemers and 1.9 ± 0.4 µM for α1 +β2 +γ2L receptors, n= 8 oocytes each, P < 0.05). On the other hand, maximum potentiation was actually larger for THDOC actions at synaptic-like receptors (normalized values of 25 ± 4.6-fold vs. 13 ± 1.6-fold at concatemeric receptors, P < 0.05). Robust potentiation was observed at both receptor types at the lowest concentrations of 3α5αTHDOC tested. For instance, 100 nM 3α5αP potentiated responses to 4.7 ± 0.9 times baseline GABA current for concatemers and 5.6 ± 0.8 times baseline for α1 +β2 +γ2L receptors. Responses were also detectably potentiated by 30 nM 3α5αP at both receptor types (Figure 7D,E and 2.0± 0.2 times GABA response for α1 +β2 +γ2L and 2.4 ± 0.2 for concatemers, n= 4 each). Therefore, despite the EC50 difference, we caution that neurosteroid selectivity may be difficult to achieve at δ subunit-containing receptors even at low concentrations.
Figure 7.
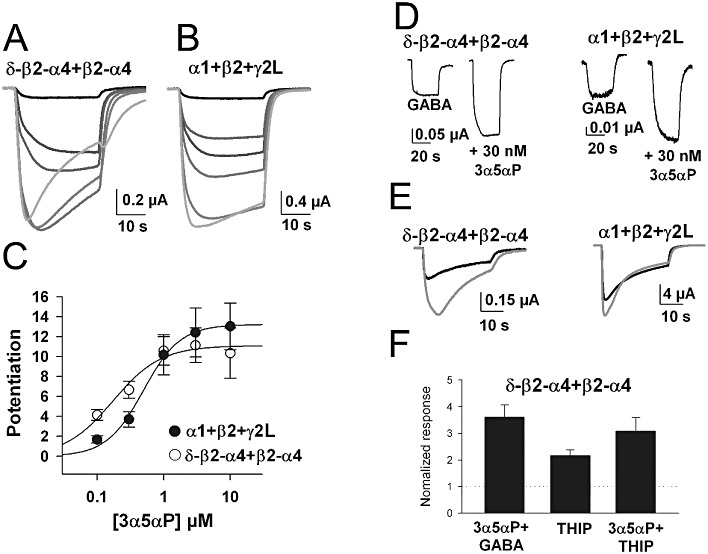
Responses of concatemeric receptors to the positive modulator neurosteroid 3α5αP. (A) Responses to 0.3 µM GABA alone (black, smallest trace) and increasing concentrations of 3α5αP, represented by increasingly lighter shades of grey. (B) Sample responses of an oocyte expressing α1 +β2 +γ2L to 2 µM GABA and increasing 3α5αP concentrations. (C) 3α5αP concentration-response relationship for eight oocytes expressing concatemeric α4/δ-containing receptors and four oocytes expressing α1 +β2 +γ2L receptors. Solid lines represent fits to the Hill equation. Parameters for the fits are given in the Results. (D) Representative responses to low steroid concentration, showing that both synaptic-like and extrasynaptic-like receptors respond detectably. (E) Representative responses to saturating GABA concentration (30 µM for left traces and 100 µM for right traces) in the absence and presence of 1 µM 3α5αP. Weak GABA efficacy was correlated with stronger potentiation at a high GABA concentration. (F) Responses to the higher efficacy agonist THIP are potentiated to the same maximum level as responses to saturating GABA. Oocytes expressing concatemeric receptors were challenged with 30 µM GABA, 30 µM GABA plus 1 µM 3α5αP, 1 mM THIP and 1 mM THIP plus 1 µM 3α5αP. All responses are normalized to the response to GABA alone in the same oocyte.
A major difference between neurosteroid actions at concatemers and synaptic-like receptors was revealed at high GABA concentrations. Concatemers responded to saturating 30 µM GABA, which exhibits partial efficacy, with significant neurosteroid potentiation (Figure 7E). In contrast, because GABA is a higher efficacy agonist at synaptic-like α1 +β2 +γ2L receptors, neurosteroids did not strongly potentiate responses to 100 µM GABA (Figure 7E). These patterns of potentiation were expected from previously published results on δ-containing receptors (Brown et al., 2002; Wohlfarth et al., 2002). We also examined the effect of 3α5αP on THIP responses in concatemeric receptors (Figure 7F). As expected, THIP responses were larger than saturating GABA concentrations, and the effect of 3α5αP was to increase response amplitude to the same level as that achieved in the presence of GABA plus 3α5αP (Figure 7F).
It is unclear from previous work whether α4/δ extrasynaptic receptors share a similar SAR with synaptic-like receptors. Differences in the SAR could suggest an ability to selectively manipulate extrasynaptic receptors for therapeutic or experimental purposes. We approached the question using our database of neurosteroid analogues (Akk et al., 2007). Using the rationale described below, we tested the neurosteroid analogues whose structures are shown in Figure 8.
One of the most curious aspects of neurosteroid SAR at synaptic-like receptors is that different neurosteroid families exhibit different enantioselectivity profiles. 5α-Pregnane-based neurosteroids exhibit enantioselectivity such that the activity of natural enantiomers is much greater than that of unnatural enantiomers (Wittmer et al., 1996; Hu et al., 1997), while androstane-based neurosteroids exhibit reverse enantioselectivity (activity of unnatural enantiomers greater than that of natural enantiomers)(Li et al., 2007; Katona et al., 2008). We reasoned that this peculiar pattern of enantioselectivity might reveal differences in SAR at synaptic-like versus extrasynaptic-like α4/δ-containing receptors. However, we found that the pattern of enantioselectivity at δ-β2-α4 +β2-α4 was indistinguishable from that observed on α1 +β2 +γ2L receptors, suggesting no enantiomeric SAR differences (Figure 9A). Furthermore, the 5β-reduced pair (etiocholanolone) exhibited reduced activity at both synaptic-like and extrasynaptic-like receptors compared with the 5α-reduced pair (androsterone). Thus, neither patterns of diastereoselectivity nor of enantioselectivity appear to differ between synaptic-like and extrasynaptic-like α4/δ receptors.
Figure 9.
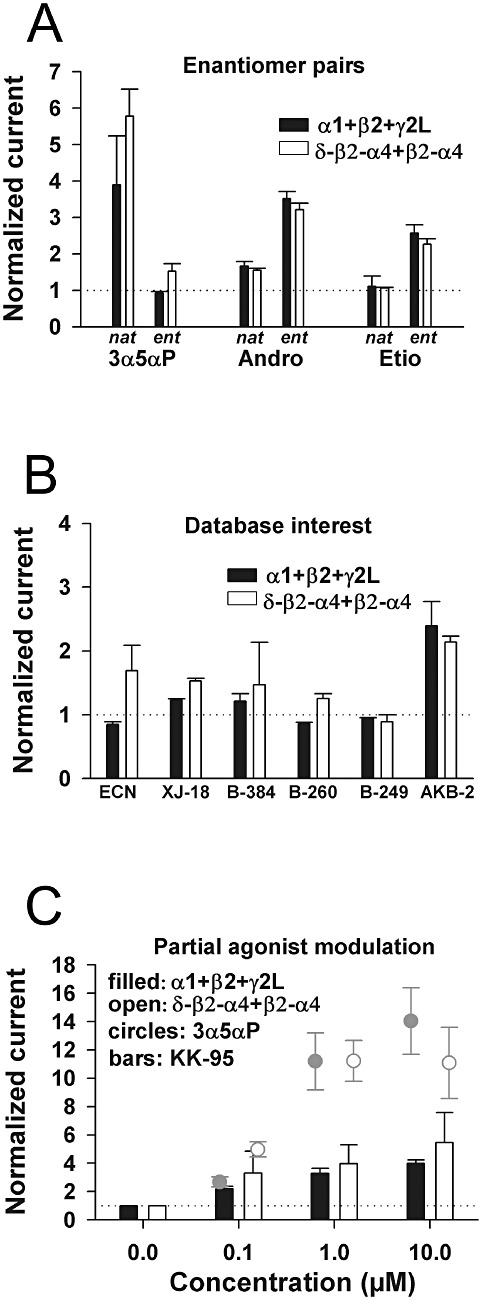
SARs for positively modulating steroid analogues are indistinguishable at extrasynaptic-like and synaptic-like subunit combinations. (A) Potentiation by enantiomer pairs (natural (nat) and unnatural (ent); 1 µM) using 0.3 µM GABA as the agonist at concatemeric α4/δ-containing receptors and an equiactive GABA concentration (2 µM) at α1 +β2 +γ2L receptors.. (B) We examined our database of structural analogues for modulators with no detectable action at α1 +β2 +γ2L receptors but with biological activity in a tadpole assay of anaesthesia. AKB-2 is a fluorescent neurosteroid analogue with weak activity at α1 +β2 +γ2L receptors in the absence of light and serves as a positive control for detection of weak effects. (C) KK-95 was tested as an unusual partial agonist analogue, with potent actions (easily detectable potentiation at 0.1 µM, similar to 3α5αP), but with weak maximum potentiation. The data for 3α5αP at the indicated concentrations are repeated from Figure 7 for reference. In all cases, analogue actions at concatemeric receptors were similar to those on α1 +β2 +γ2L synaptic-like receptors. For all experiments in panels A–C, n= 3–11 oocytes.
We also examined our database of more than 300 compounds previously screened for activity at α1 +β2 +γ2L receptors and in a tadpole anaesthesia assay (Wittmer et al., 1996). Typically, functional effects on α1 +β2 +γ2L correlates with activity in a tadpole behaviour screen (Akk et al., 2007). We reasoned that δ subunit-selective compounds may be represented by the small subset of compounds that have behavioural effects in animals but no effect on recombinant α1 +β2 +γ2L receptors. We identified five compounds from varying structural classes that met this functional profile for testing on our concatemeric δ-β2-α4 +β2-α4 receptors. As with α1 +β2 +γ2L receptors, compounds exhibited poor activity on GABA responses when tested at 1 µM at concatemeric receptors (Figure 9B). Increasing the concentration of each of the analogues 10-fold to 10 µM produced no increase in potentiation of GABA responses to four of the five compounds (normalized responses in four oocytes of 1.1 ± 0.7, 0.9 ± 0.4, 1.0 ± 0.2, and 1.3 ± 0.1 for ECN, B-384, B-260, and B-249, respectively; XJ-18 potentiation increased modestly to 2.1 ± 0.7-fold over control, n= 4). We conclude that the biological effects in the tadpole behavioural screen of steroid analogues with no detectable activity on α1 +β2 +γ2L receptors probably does not relate to actions at extrasynaptic α4/δ GABAA receptors and biological activity more likely reflects alternative targets, such as voltage-gated Ca2+ channels (Nakashima et al., 1999; Todorovic et al., 2004) or others. Although XJ-18 appeared inert up to 10 µM on our initial screen of α1 +β2 +γ2L (hence, its examination on concatemers here), results at concatemers prompted a re-evaluation of high XJ-18 concentrations. We found that XJ-18 exhibited detectable activity at 10 µM on α1 +β2 +γ2L receptors (2.6 ± 0.9-fold over control GABA response, n= 3), akin to XJ-18 actions at α4/δ concatemers. Thus, in no case did we find evidence for a different SAR at α4/δ-containing receptors than at synaptic-like receptors.
At α1 +β2 +γ2L receptors, KK-95 represents a neurosteroid analogue with unusual actions. It exhibits potentiation at quite low concentrations (∼tripling of current amplitudes at 0.1 µM), similar to the natural neurosteroid 3α5αP, but KK-95 fails to exhibit maximum potentiation equivalent to that of 3α5αP (Figure 9C). Therefore, KK-95 might be considered a high-potency (active at low concentration), low-efficacy (weak maximum potentiation) allosteric modulator. This same pattern of modulation was evident at δ-β2-α4 +β2-α4 concatemers (Figure 9C), suggesting that partial-agonist modulation is also preserved at α4/δ receptors.
Discussion and conclusions
Our study introduces a tool to examine α4/δ-containing, extrasynaptic-like receptors in heterologous systems with assurance of known subunit stoichiometry. Because α/β-only receptors can exhibit functional expression (presumably by incorporation of an extra α or β subunit), δ subunit expression is not assured with standard heterologous expression strategies even when δ is used in excess. Furthermore, our own experience with free subunits yielded the unexpected observation that α4 expression can be problematic. We confirmed that the functional receptors produced with our concatemeric constructs have properties expected of α4/δ-containing receptors, including modulation by α4- and δ-specific ligands. Finally, we used the concatemers to demonstrate that negative and positive neurosteroid modulation obeys a similar SAR at this type of α4/δ-containing receptors as at synaptic-like α1 +β2 +γ2L receptors.
Difficulties with the expression of the δ subunit have caused others to adopt alternative approaches to confirm incorporation of δ subunits into functional receptors. These include the development of permanently transfected cell lines (Brown et al., 2002; Borghese et al., 2006), study of native cells' tonic GABA currents (Glykys et al., 2008), and the introduction of a benzodiazepine-sensitive functional ‘tag’ into the δ subunit (Meera et al., 2010). Others have also used the tandem subunit approach to incorporate δ along with α1 and β3 subunits (Kaur et al., 2009; Baur et al., 2010). However, it has been noted that there can be some difficulties in ensuring that all subunits in a concatemer are successfully incorporated into the functional receptor (see White, 2006; Barrera and Edwardson, 2008; Sack et al., 2008; Sigel et al., 2009). Accordingly, a major aspect of our work was to confirm the presence of the α4 and δ subunits in the functional receptors by pharmacological tests. We cannot rule out the possibility that a fraction of the functional receptors lack a δ subunit.
We chose to utilize concatemers which would be expected to place the δ subunit in the position of a γ subunit in the pentamer (Baumann et al., 2002). Recent work has reported that the δ subunit occupies other positions when the α1 or α6 (Baur et al., 2010) subunits, combined with β3 subunit, are used to generate concatemers. This also appears to occur with the ε subunit (Bollan et al., 2008), whereas the γ2S subunit may be able to assemble as a modulatory subunit outside the pentamer (Boileau et al., 2010). These data indicate that future work will require the generation and characterization of additional concatemers utilizing the α4 and δ subunits to allow better understanding of putative extrasynaptic GABAA receptors and comparisons to responses in native cells.
We chose the β2 subunit as our extrasynaptic-like β subunit. Other studies have used β3 subunits in combination with δ subunits (Baur et al., 2010; Meera et al., 2010). Although β2 and β3 subunits are often considered together as molecularly and functionally similar, the β subunit may be important for some modulatory aspects of receptor function, notably ethanol sensitivity and phosphorylation (Wallner et al., 2003; Houston et al., 2008). We chose β2 subunits for our concatemeric receptors mainly because recent evidence suggests that these subunits are expressed in extrasynaptic receptors, in at least some neurones expressing δ and α4 subunits (Herd et al., 2008).
One disadvantage of our present concatemers is that maximum currents to saturating concentrations of agonists (even the full agonist THIP) were smaller than maximum currents generated by α1 +β2 +γ2L receptors in the same batches of oocytes. It is likely that expression of both individual subunits and concatemers could be improved by attention to expression vectors tailored to oocytes or possibly to intracellular retention sequences. Subunit arrangement has also been shown to affect functional expression levels (Baur et al., 2010).
In some cases, engineered tandem subunits change the agonist EC50 relative to receptors formed from the individual subunits, although other key aspects of pharmacology and physiology were retained (Baumann et al., 2002; 2003; Baur and Sigel, 2005; Akk et al., 2009; Bracamontes et al., 2011). Similarly, we found that δ-β2-α4 +β2-α4 concatemers exhibited key features (including agonist EC50) expected of α4/δ-containing receptors. These include low GABA EC50, stronger efficacy by THIP, intermediate Zn2+ sensitivity, high furosemide sensitivity and strong modulation by DS2, a δ subunit-selective ligand (Wafford et al., 2009).
DS2 appears to be a particularly useful compound for detecting the presence of δ subunits. Our experiments with free subunits extend previous work (Wafford et al., 2009) by demonstrating that α4 +β2 free-subunit receptors, which share a number of properties with α4/δ-containing receptors, fail to respond to DS2 (Figure 5). Interestingly, we observed stronger potentiation with DS2 in both free-subunit receptors and in concatemeric receptors than observed by Wafford et al. (2009). It is possible that stoichiometry considerations, species differences or expression system differences explain these quantitative discrepancies.
We failed to observe detectable ethanol modulation of our concatemers, in agreement with some studies of δ subunit-containing receptors (Borghese et al., 2006; Baur et al., 2010), but in contrast to others that have suggested strong ethanol modulation (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003). It seems likely that species differences, post-translational modifications or subunit copy number (Wagoner and Czajkowski, 2010) might contribute to discrepant ethanol results. Notwithstanding the results with ethanol results and some minor quantitative differences between the pharmacology of free subunits (Figure 5) and concatemeric receptors, the aggregate of our results suggests that this concatemeric construct represents a good model for studying modulation of the extrasynaptic receptors expressed by certain neuronal cell classes.
Neurosteroids have two distinct effects at GABAA receptors (Eisenman et al., 2004; Reddy, 2010). Sulphated neurosteroids uncompetitively antagonize receptor function (Barker et al., 1987; Eisenman et al., 2003), likely through an allosteric effect on gating/desensitization rather than through uncompetitive open channel block (Akk et al., 2001). Certain 3α-hydroxysteroids potentiate receptor function through several distinct kinetic effects on channel function (Akk et al., 2004b).
To our knowledge, the sulphated steroid SAR has not previously been investigated at δ subunit-containing receptors. Qualitatively, we find that sulphated neurosteroids antagonize δ-containing receptors and synaptic-like receptors similarly. Quantitative reductions in antagonism at δ-containing receptors appear to be explained by the low efficacy of GABA. The full agonist THIP promoted stronger antagonism, consistent with the uncompetitive and use-dependent nature of sulphated steroid antagonism. Conversely, use of partial agonists at synaptic-like receptors reduces antagonism (Eisenman et al., 2003).
Potentiating 3α-hydroxy neurosteroids have been proposed to selectively modulate δ subunit-containing extrasynaptic receptors at low concentrations (Mihalek et al., 1999; Wohlfarth et al., 2002; Stell et al., 2003). This might suggest that neurosteroid analogues with selective actions at δ-containing receptors could be developed and exploited to modulate neuronal excitability experimentally or therapeutically. We employed structural analogues spanning a range of activities at α1 +β2 +γ2L receptors to determine whether the SAR at δ-containing receptors was likely to differ from that at synaptic-like receptors. We found that both classes of receptor are modulated in parallel across a broad range of neurosteroid structural analogues. These analogues include enantiomer pairs of pregnane and androstane steroids, 5α and 5β reduced steroids, and steroids with little activity at synaptic-like receptors but with strong biological activity (measured in a tadpole behaviour screen). Furthermore, the natural neurosteroids 3α5αP and 3α5αTHDOC modulated δ subunit-containing receptors and synaptic-like receptors at similar threshold concentrations. As with sulphated steroids, the main difference between receptor types appears to emerge as a result of the partial agonist, low-efficacy agonist activity of GABA, which affords neurosteroid potentiation even at high agonist concentrations (Brown et al., 2002; Wohlfarth et al., 2002; Meera et al., 2009). Our conclusions are broadly consistent with the finding that residues responsible for steroid modulation of α4/δ-containing receptors are analogous to those mediating modulation of α1/γ-containing receptors (Hosie et al., 2009), with critical interaction sites resident on the α subunit.
In summary we report a concatemeric GABAA receptor that should aid investigators interested in the function and modulation of extrasynaptic GABAA receptors. This engineered receptor appeared to express with the intended subunit stoichiometry and exhibited major pharmacological and functional features expected of α4/δ-containing receptors. In addition, we explored the actions of sulphated and 3α-hydroxy neurosteroids at these concatemeric receptors. Our exploration of a variety of negative and positive neurosteroid modulators uncovered no evidence for differences in neurosteroid SAR at δ subunit-containing receptors. Although we cannot exclude the possibility that examination of additional structural features of neurosteroids might reveal selective modulation, our multi-pronged approach failed to reveal evidence for SAR differences. We conclude that neurosteroids may not be a productive framework for the development of δ subunit-selective ligands.
Acknowledgments
We thank other members of our research groups for advice, discussion and technical support. This work was supported by GM47969, NS54174, AA017413 and the Bantly Foundation. KW was supported by an HHMI SURF fellowship from Washington University. JHS is the Russell and Mary Shelden Professor of Anesthesiology.
Glossary
- 3α5αP
(3α,5α)-3-hydroxypregnan-20-one
- 3α5αTHDOC
(3α,5α)-3,21-dihydroxypregnan-20-one
- 3α5βPS
(3α,5β)-3-hydroxypregnan-20-one sulphate
- 3β5αPS
(3β,5α)-3-hydroxypregnan-20-one sulphate
- DS2
4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridine-3-yl benzamide
- PS
pregnenolone sulphate
- SAR
structure-activity relationship
- THIP
(gaboxadol), 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol
Conflict of interest
DFC holds a patent on XJ-18. DFC and CFZ have a financial interest in Sage Therapeutics. Sage Therapeutics did not support this work.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Free-subunit injections requireexcess α4 RNA. A. Representative example of a response to 100μM GABA and GABA plus 1 μM Zn2+ from an oocyte injected with equal quantities of α4 and β2 cRNA. Zn2+ caused an overshooting blockade of the GABA current. B. In a different oocyte injected with α4 + β2 RNA, Zn2+ gated a large outward current in the absence of GABA. C. GABA responses from an oocyte injected with a five-fold excess of α4 RNA. D. The odd responses in panels A and Blikely resulted from homomeric β2 receptors, as small GABA responses and Zn2+-sensitive standing conductances also characterized oocytes injected with β2 subunit alone (26 ngcRNA injected). Although we did not test other antagonists of GABAA receptors, Zn2+-sensitive standing conductances were never observed in uninjected oocytes or in oocytes injected with equal parts α1 + β2 RNA.
Please note: Wiley–Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, et al. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH. Pregnenolone sulfate block of GABAA receptors: mechanism and involvement of a residue in the M2 region of the α subunit. J Physiol (Lond) 2001;532:673–684. doi: 10.1111/j.1469-7793.2001.0673e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH. Activation of GABAA receptors containing the α4 subunit by GABA and pentobarbital. J Physiol (Lond) 2004a;556:387–399. doi: 10.1113/jphysiol.2003.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol (Lond) 2004b;558:59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABAA receptors. Pharmacol Ther. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Steinbach JH. Activation and modulation of concatemeric GABAA receptors expressed in human embryonic kidney cells. Mol Pharmacol. 2009;75:1400–1411. doi: 10.1124/mol.108.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JL, Harrison NL, Lange GD, Owen DG. Potentiation of γ-aminobutyric-acid-activated chloride conductance by a steroid anaesthetic in cultured rat spinal neurones. J Physiol (Lond) 1987;386:485–501. doi: 10.1113/jphysiol.1987.sp016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera NP, Edwardson JM. The subunit arrangement and assembly of ionotropic receptors. Trends Neurosci. 2008;31:569–576. doi: 10.1016/j.tins.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Individual properties of the two functional agonist sites in GABA(A) receptors. J Neurosci. 2003;23:11158–11166. doi: 10.1523/JNEUROSCI.23-35-11158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R, Sigel E. Benzodiazepines affect channel opening of GABAA receptors induced by either agonist binding site. Mol Pharmacol. 2005;67:1005–1008. doi: 10.1124/mol.104.008151. [DOI] [PubMed] [Google Scholar]
- Baur R, Kaur KH, Sigel E. Diversity of structure and function of α1α6β3δ GABAA receptors: comparison with α1β3δ and α6β3δ receptors. J Biol Chem. 2010;285:17398–17405. doi: 10.1074/jbc.M110.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau AJ, Pearce RA, Czajkowski C. The short splice variant of the γ2 subunit acts as an external modulator of GABAA receptor function. J Neurosci. 2010;30:4895–4903. doi: 10.1523/JNEUROSCI.5039-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollan KA, Baur R, Hales TG, Sigel E, Connolly CN. The promiscuous role of the epsilon subunit in GABAA receptor biogenesis. Mol Cell Neurosci. 2008;37:610–621. doi: 10.1016/j.mcn.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant δ-containing γ-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, et al. The δ subunit of γ-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Bracamontes JR, Steinbach JH. Steroid interaction with a single potentiating site is sufficient to modulate GABAA receptor function. Mol Pharmacol. 2009;75:973–981. doi: 10.1124/mol.108.053629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracamontes J, McCollum M, Esch C, Li P, Ann J, Steinbach JH, et al. Occupation of either site for the neurosteroid allopregnanolone potentiates the opening of the GABAA receptor induced from either transmitter binding site. Mol Pharmacol. 2011;80:79–86. doi: 10.1124/mol.111.071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497(Pt 3):753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Newell JG, You-Ten KE, Elliott EM, Rosahl TW, Wafford KA, et al. Selective enhancement of tonic GABAergic iInhibition in murine hippocampal neurons by low concentrations of the volatile anesthetic isoflurane. J Neurosci. 2004;24:8454–8458. doi: 10.1523/JNEUROSCI.2063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, et al. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, et al. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low affinity interaction. J Neurophysiol. 2009;102:1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res. 2007;163:235–243. doi: 10.1016/S0079-6123(07)63014-3. [DOI] [PubMed] [Google Scholar]
- Covey DF, Jiang X. Neuroactive 13,24-cyclo-18,21-dinorcholanes and structurally related pentacyclic steroids. 2010. United States Patent, 7,781,421 B2, issued Aug. 24, 2010., Patent US (ed)
- Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Molecular pharmacology of γ-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different α, β, and γ receptor subunit combinations. Mol Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- Eisenman LN, He Y, Fields C, Zorumski CF, Mennerick S. Activation-dependent properties of pregnenolone sulfate inhibition of GABAA receptor-mediated current. J Physiol (Lond) 2003;550(Pt 3):679–691. doi: 10.1113/jphysiol.2003.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman LN, He Y, Covey DF, Zorumski CF, Mennerick S. Potentiation and inhibition of GABAA receptor function by neuroactive steroids. In: Smith SS, editor. Neurosteroid Effects in the Central Nervous System. Boca Raton: CRC Press; 2004. pp. 95–117. [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Zorumski CF, Covey DF. Neurosteroid analogues. 4. The effect of methyl substitution at the C-5 and C-10 positions of neurosteroids on electrophysiological activity at GABAA receptors. J Med Chem. 1996;39:4218–4232. doi: 10.1021/jm960304p. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, et al. The expression of GABAAβ subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol (Lond) 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Houston CM, Hosie AM, Smart TG. Distinct regulation of β2 and β3 subunit-containing cerebellar synaptic GABAA receptors by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2008;28:7574–7584. doi: 10.1523/JNEUROSCI.5531-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Wittmer LL, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Neurosteroid analogues. Part 5. Enantiomers of neuroactive steroids and benz[e]indenes: total synthesis, electrophysiological effects on GABAA receptor function and anesthetic actions in tadpoles. J Chem Soc Perkin Trans. 1997;1:3665–3671. [Google Scholar]
- Katona BW, Krishnan K, Cai ZY, Manion BD, Benz A, Taylor A, et al. Neurosteroid analogues. 12. Potent enhancement of GABA-mediated chloride currents at GABAA receptors by ent-androgens. Eur J Med Chem. 2008;43:107–113. doi: 10.1016/j.ejmech.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Kaur KH, Baur R, Sigel E. Unanticipated structural and functional properties of δ-subunit-containing GABAA receptors. J Biol Chem. 2009;284:7889–7896. doi: 10.1074/jbc.M806484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, Akk G. Natural and enantiomeric etiocholanolone interact with distinct sites on the rat α1β2γ2L GABAA receptor. Mol Pharmacol. 2007;71:1582–1590. doi: 10.1124/mol.106.033407. [DOI] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant α4β3δ and α4β3 GABAA receptors expressed in HEK cells. Neuropharmacology. 2009;56:155–160. doi: 10.1016/j.neuropharm.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. Alcohol- and alcohol antagonist-sensitive human GABAA receptors: tracking δ subunit incorporation into functional receptors. Mol Pharmacol. 2010;78:918–924. doi: 10.1124/mol.109.062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. J Neurophysiol. 2011;106:2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, et al. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor δ subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol (Lond) 2010;588(Pt 8):1251–1268. doi: 10.1113/jphysiol.2009.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Macdonald RL. Two γ2L subunit domains confer low Zn2+ sensitivity to ternary GABAA receptors. J Physiol (Lond) 2001;532(Pt 1):17–30. doi: 10.1111/j.1469-7793.2001.0017g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima YM, Pereverzev A, Schneider T, Covey DF, Lingle CJ. Blockade of Ba2+ current through human alpha1E channels by two steroid analogs, (+)-ACN and (+)-ECN. Neuropharmacology. 1999;38:843–855. doi: 10.1016/s0028-3908(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack JT, Shamotienko O, Dolly JO. How to validate a heteromeric ion channel drug target: assessing proper expression of concatenated subunits. J Gen Physiol. 2008;131:415–420. doi: 10.1085/jgp.200709939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Mennerick S, Covey DF, Zorumski CF. Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABAA receptor desensitization. J Neurosci. 2000;20:3571–3579. doi: 10.1523/JNEUROSCI.20-10-03571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Kaur KH, Luscher BP, Baur R. Use of concatamers to study GABAA receptor architecture and function: application to delta-subunit-containing receptors and possible pitfalls. Biochem Soc Trans. 2009;37:1338–1342. doi: 10.1042/BST0371338. [DOI] [PubMed] [Google Scholar]
- Smart TG, Moss SJ, Xie X, Huganir RL. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br J Pharmacol. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic SM, Pathirathna S, Brimelow BC, Jagodic MM, Ko SH, Jiang X, et al. 5β-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol Pharmacol. 2004;66:1223–1235. doi: 10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human gamma-aminobutyric acidA receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, et al. Novel compounds selectively enhance δ subunit containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56:182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Wagoner KR, Czajkowski C. Stoichiometry of expressed α4β2δγ-aminobutyric acid type A receptors depends on the ratio of subunit cDNA transfected. J Biol Chem. 2010;285:14187–14194. doi: 10.1074/jbc.M110.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4 β3 δ and α6β3δγ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng CM, Mathews J, et al. 3β -hydroxypregnane steroids are pregnenolone sulfate-like GABAA receptor antagonists. J Neurosci. 2002;22:3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MM. Pretty subunits all in a row: using concatenated subunit constructs to force the expression of receptors with defined subunit stoichiometry and spatial arrangement. Mol Pharmacol. 2006;69:407–410. doi: 10.1124/mol.105.020727. [DOI] [PubMed] [Google Scholar]
- Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Enantioselectivity of steroid-induced γ-aminobutyric acidA receptor modulation and anesthesia. Mol Pharmacol. 1996;50:1581–1586. [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


