Abstract
BACKGROUND AND PURPOSE
Peripheral blockade of cannabinoid CB1 receptors has been proposed as a safe and effective therapy against obesity, putatively devoid of the adverse psychiatric side effects of centrally acting CB1 receptor antagonists. In this study we analysed the effects of LH-21, a peripherally acting neutral cannabinoid receptor antagonist with poor brain penetration, in an animal model of diet-induced obesity.
EXPERIMENTAL APPROACH
To induce obesity, male Wistar rats were fed a high-fat diet (HFD; 60 kcal% fat) whereas controls received a standard diet (SD; 10 kcal% fat). Following 10 weeks of feeding, animals received a daily i.p. injection of vehicle or 3 mg·kg−1 LH-21 for 10 days. Plasma and liver samples were used for biochemical analyses whereas visceral fat-pad samples were analysed for lipid metabolism gene expression using real-time RT-PCR. In addition, the potential of LH-21 to interact with hepatic cytochrome P450 isoforms and cardiac human Ether-à-go-go Related Gene (hERG) channels was evaluated.
KEY RESULTS
LH-21 reduced feeding and body weight gain in HFD-fed animals compared with the control group fed SD. In adipose tissue, this effect was associated with decreased gene expression of: (i) leptin; (ii) lipogenic enzymes, including SCD-1; (iii) CB1 receptors; and (iv) both PPARα and PPARγ. Although there were no significant differences in plasma parameters between HFD- and SD-fed rats, LH-21 did not seem to induce hepatic, cardiac or renal toxicity.
CONCLUSIONS AND IMPLICATIONS
These results support the hypothesis that treatment with the peripherally neutral acting CB1 receptor antagonist, LH-21, may promote weight loss through modulation of visceral adipose tissue.
Keywords: cannabinoids, diet-induced obesity, rat, adipose tissue, appetite, PPARs, adiponectin, leptin, SCD-1
Introduction
Although the incidence of obesity and related metabolic disorders continues to increase in modern society, there are no pharmacological therapies for its treatment. Thus, development of safe and effective therapies against obesity represents a priority for both researchers and health systems.
Among the new targets for pharmaceutical development of anti-obesity drugs, the endocannabinoid system remains a focus of attention. A growing body of evidence has clearly demonstrated that endocannabinoids and their receptors (mainly cannabinoid CB1 receptor) play a fundamental role in appetite regulation and energy homeostasis. Obesity and its complications appear to be associated with a dysregulated and hyperactive endocannabinoid system in humans and rodents displaying an increase in circulating endocannabinoid levels and altered expression of the components of this system in several organs such as adipose tissue, liver and pancreas. In fact, increased concentrations of endocannabinoids might overstimulate CB1 receptors in a pathophysiological manner contributing to obesity (Engeli et al., 2005; Bluher et al., 2006; Di Marzo, 2008; Engeli, 2008; Matias et al., 2008; Starowicz et al., 2008).
Consequently several cannabinoid CB1 receptor antagonists have been developed as anti-obesity drugs. Synthetic and plant-derived cannabinoid CB1 receptor blockers have been reported to suppress food intake, whereas chronic treatments lead to weight loss and improved cardiometabolic risk profile in rodents including both genetic and dietary models of obesity (Hildebrandt et al., 2003; Ravinet Trillou et al., 2003; Vickers et al., 2003; Thornton-Jones et al., 2006; Gary-Bobo et al., 2007; Riedel et al., 2009; Tam et al., 2010). One of these antagonists, rimonabant (also referred as SR141716A), reached the market after successful clinical trials revealing both, metabolic benefits and body weight reduction in overweight and obese subjects (Van Gaal et al., 2005; Pi-Sunyer et al., 2006). However, due to the appearance of psychiatric adverse effects, derived from central cannabinoid CB1 receptor blockade and its inverse agonistic activity, the drug was withdrawn from the market (Christensen et al., 2007; Christopoulou and Kiortsis, 2011).
A growing number of new CB1 blockers with limited brain penetration and/or neutral antagonism activity are being synthesized and characterized to prevent adverse effects observed with rimonabant while maintaining anti-obesity properties.
Molecules acting as neutral CB1 antagonists include AM4113 and VCHSR1, and both have been evaluated in food intake behaviour. For example, VCHSR1 is a rimonabant-derived compound with lower affinity for CB1 that inhibits milk ingestion and growth in newborn mice (Fride et al., 2007). AM4113 has been more extensively studied; it is a pyrazole congener of AM251 that also acts as a neutral CB1 antagonist and reduces feeding in rats on high fat, high carbohydrate and lab chow diets (Chambers et al., 2007; Sink et al., 2008). Another recent study has reported that rats treated with AM4113, i.p. or p.o., display a transient reduction in food intake, which results in a long-term reduction in body weight gain (Cluny et al., 2011).
Because it was demonstrated that peripheral cannabinoid CB1 receptor blockade is sufficient to suppress appetite (Gomez et al., 2002), increase energy expenditure and reduce lipogenesis on both liver and adipose tissue (Cota et al., 2003; Osei-Hyiaman et al., 2005), the use of cannabinoid receptor antagonists with limited penetration into the brain is a matter of active research. Thus, several CB1 receptor antagonists with restricted brain penetrability have been developed and evaluated in obesity. For example, non-brain-penetrant CB1 antagonists JD-2114 and JD-5006 have been shown to reduce body weight gain and improve metabolic parameters in obese mice maintained on a high-fat diet (HFD; McElroy et al., 2008). URB447, a mixed CB1 antagonist/CB2 agonist with reduced brain penetration reduces feeding and body weight gain in mice (LoVerme et al., 2009). Another interesting compound is AM6545, a neutral CB1 antagonist with relatively poor penetrability into the brain, as shown in Table 1 (Tam et al., 2010). This drug reduces food intake in animals fed high-fat and high-carbohydrate diets, but it is less efficacious at reducing lab chow intake (Randall et al., 2010). AM6545 improves the metabolic profile of mice with genetic or diet-induced obesity in a food intake-independent manner (Tam et al., 2010). Recently, it has been reported that AM6545 reduces food intake and body weight gain in rodents including CB1 gene-deficient mice, but not in CB1/CB2 double knockout mice (Cluny et al., 2010).
Table 1.
Structures and pharmacological characteristics of LH-21 and AM6545
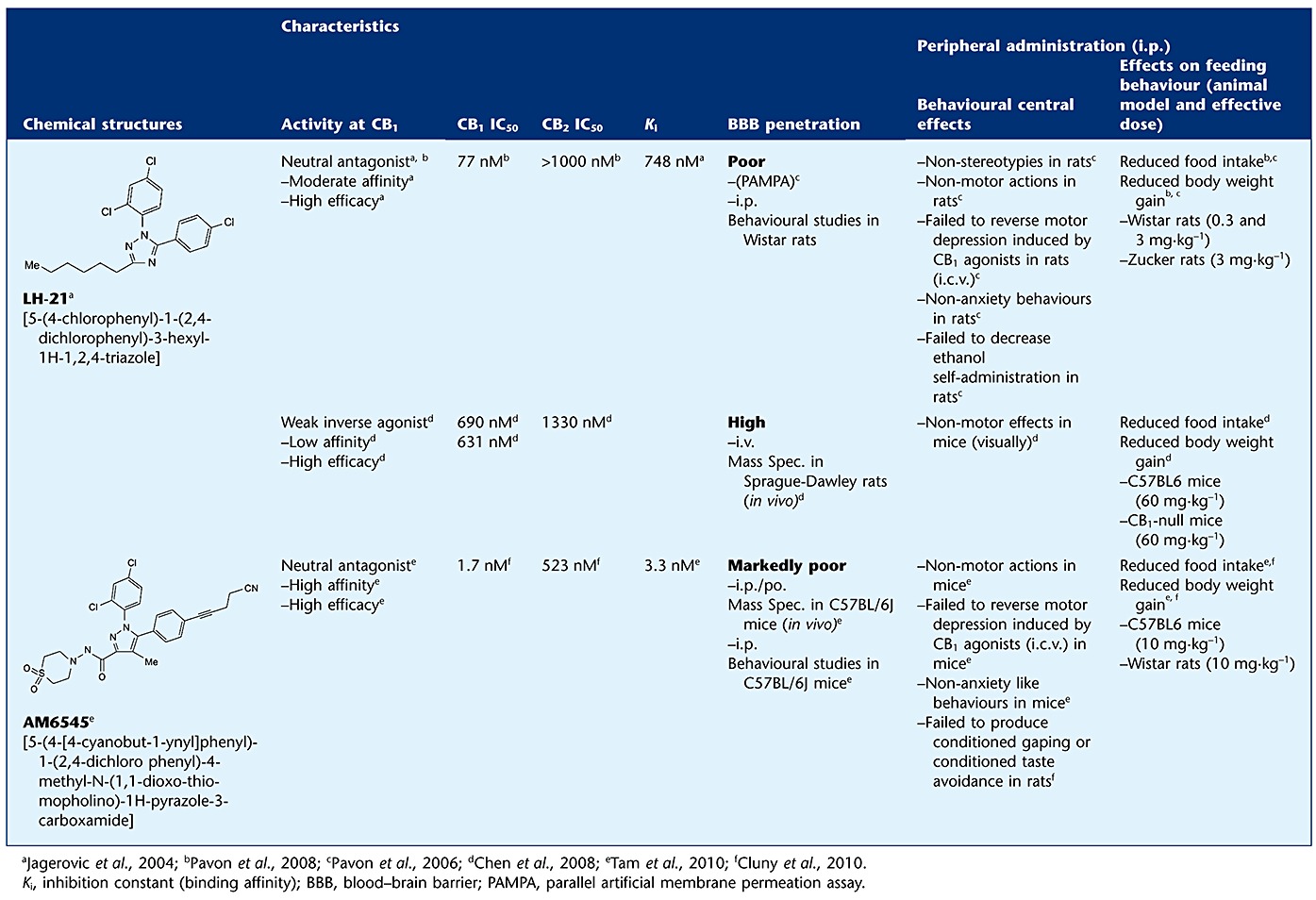 |
Following the research of this new range of compounds based on peripheral CB1 blockade, the present study has focused on LH-21, a triazole-derived compound with anorectic properties, which was synthesized and identified initially as a neutral CB1 receptor antagonist with a paradoxical low affinity for CB1 receptors (Jagerovic et al., 2004) (Table 1), although recently it has been considered a weak CB1 inverse agonist by Chen and colleagues (Chen et al., 2008). Diverse studies in rodents have demonstrated that this anorectic drug shows a different pharmacological profile in comparison with rimonabant (Pavon et al., 2006; 2008). These studies reported that acute administration of LH-21 reduces feeding behaviour in rats, whereas subchronic administration reduces food intake and body weight gain in genetically obese Zucker rats (fa/fa, missense mutation in the leptin receptor), albeit with no metabolic benefits (Pavon et al., 2008). Both, in vivo and in vitro assays have demonstrated that LH-21 has low permeability through the blood-brain barrier. The poor penetration of LH-21 predicted by in vitro permeability assay into the brain was confirmed in vivo by the absence of effects on anxiety-like behaviours, motor stereotypies and ethanol self-administration (Pavon et al., 2006). Indeed, systemic administration (i.p.) of LH-21 was unable to antagonize the motor inhibition induced by central administration of the CB1 receptor agonist CP55940 in Wistar rats (Pavon et al., 2006). These studies suggest that its effects on feeding behaviour and body weight are mainly mediated by peripheral targets, i.e. cannabinoid CB1 receptors located in metabolically relevant organs, such as adipose tissue or the liver. Although a recent report (Chen et al., 2008) suggested that LH-21 penetrates freely into the brain of Sprague-Dawley rats, this was not sufficiently demonstrated as: (i) the study lacks positive and negative control drugs for brain penetration in their analysis; (ii) the study used an i.v. administration route instead of p.o. or i.p. route, skipping the liver influence; and (iii) they do not report in vivo behavioural data. Moreover, their data on efficacy on feeding inhibition showed effective doses of 60 mg·kg−1, whereas we found that the drug was effective at doses three times lower that this in mice and 20 times lower in rats.
In the present study we investigated the toxicity and effects of a 10 day treatment with LH-21 in a diet-induced obesity model, and compared its effects in rats fed a HFD compared with a standard/low-fat diet (SD). We have focused this study on visceral white adipose tissue and liver because both organs have a critical role in energy homeostasis and obesity/overweight leads to a dysfunction caused by excessive fat accumulation. Our results indicate that: (i) LH-21 reduces feeding and body weight gain in HFD-induced obese rats by modulating lipogenic pathways in the adipose tissue; and (ii) this drug has a safe profile, confirming the therapeutic utility of peripheral blockade of cannabinoid receptors in obesity.
Methods
Animals
Feeding studies and experiments related to diet-induced obesity were performed on 10–12 week-old male Wistar rats (Charles Rivers, Barcelona, Spain) weighing 200–250 g. Animals were housed in pairs under a 12 h light/dark cycle (lights off 20 h 00 min) in a room with temperature and humidity control. Unless otherwise indicated, water and rat chow pellets were available ad libitum throughout the course of these studies. Additional feeding studies were performed on adult male mice weighing 25–30 g. Both wild-type (129S1/SvImJ, stock #002448) and PPARα-null (129S4/SvJae-Pparatm1Gonz/J, stock #003580) mice were originally obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained as an inbred colony of mice (Suardiaz et al., 2007). All animal care and experimental procedures were conducted in accordance with the European Community Directive 86/609 regulating animal research.
Drugs
LH-21 was synthesized in our laboratory as previously described (Hernandez-Folgado et al., 2008). LH-21 was dissolved in vehicle (10% Tocrisolve™ 100, Tocris Bioscience, Bristol, UK, diluted in saline). The following doses of LH-21 were used: (i) 1, 3, 10 and 30 mg·kg−1 for acute studies in rats deprived of food (fasted rats); (ii) 3 mg·kg−1 for a 10 day administration in diet-induced obese rats; and (iii) 20 mg·kg−1 for acute studies using mice deprived of food (fasted mice). Drugs were injected i.p. at the beginning of light cycle (09 h–10 h) at a dose volume of 1 mL·kg−1 body weight in rats or 10 mL·kg−1 body weight in mice.
The drug/molecular target nomenclature used in this study conforms with the BJP's Guide to Receptors and Channels (Alexander et al., 2011).
Cytochrome P450 (CYP) fluorometric inhibition assay
CYP inhibition assay was used to study if LH-21 could produce adverse drug reactions or toxicity, and performed in collaboration with the Fundación Medina (Centro de Excelencia en Investigación de Medicamentos Innovadores en Andalucía, Granada, Spain). The fluorometric assays represent the most common approach used to test compounds, as CYP inhibitors, in early drug discovery. They are high-throughput systems based on metabolism of substrates into highly fluorescent products. LH-21 and control inhibitors (ketoconazole, sulphafenazole and quinidine) were serially diluted by using a dilution factor of 2:1 to provide different concentrations. Dimethyl sulphoxide (DMSO; 0.35%) and acetonitrile (0.65%) were used as organic solvents with a maximum dose in the assay established as 105 µM. Both fluorescence interference and quenching interference were determined for each compound in triplicate. Therefore, IC50 values were obtained in fluorometric CYP inhibition assays with three different CYPs: CYP3A4; CYP2C9; and CYP2D6. LH-21 was categorized as moderate or weak inhibitor when compared with its respective control.
Human Ether-à-go-go Related Gene (hERG) channel assay using a cell fluorescence functional assay
Testing the interaction of drugs with the hERG potassium channels in heterologous expression systems is recommended in order to identify drugs, such as LH-21, that may have the potential to cause cardiotoxicity. This study was also performed in collaboration with the above mentioned institution.
The FluxOR™ Potassium Ion Channel Assay is based on the activation of a fluorescent dye using thallium influx as a surrogate indicator of K+ channel activity. HEK-293 (human embryonic kidney) cells expressing hERG K+ channels were seeded into poly-D-lysine-coated 96-wells plates. The FluxOR™ potassium channel assay was performed as outlined in the product information sheet (available from Invitrogen Co., Carlsbad, CA, USA), and measured at room temperature via FLIPR Tetra System (Molecular Devices Inc., Sunnyvale, CA, USA). After 24 h of incubation, the plates were washed with assay buffer of the following composition (mM): 165 NaCl, 4.5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES and 10 glucose, pH 7.4. Next, loading buffer containing the FluxOR™ dye mix was added into each well and the cells were incubated for 1 h at room temperature (23–25°C). They were washed once in assay buffer and then incubated with the same buffer containing LH-21 and control inhibitors (amiodarone, bepridil, haloperidol and terfenadin) at a dilution of 1:200, on the EP3 pipetting platform. Subsequently, compounds and LH-21 were measured in triplicate in each of three independent plates seeded with hERG-expressing HEK-293 cells, using 12 point curves (1:2 dilutions, and 150 µM as maximum concentration). Astemizol (1 µM) was added to each well as positive control whereas 0.5% DMSO was used as negative control. Next, plates containing both control inhibitors and LH-21 were allowed to equilibrate for 30 min at room temperature. Finally, stimulation buffer (Tl2SO4+ K2SO4) was added to the plates via FLIPR Tetra System for kinetic analysis during 120 s, and IC50 values were obtained.
Acute feeding studies in LH-21-treated rodents
The acute effects of LH-21 on feeding behaviour were analysed in adult male Wistar rats (n= 6–8 per dose group), and both wild-type and PPARα-null adult male mice (n= 10–11 per group).
For acute feeding studies, animals were deprived of food for 24 h but had free access to water (Gomez et al., 2002; Pavon et al., 2006). Before the beginning of this study, LH-21 at different doses was administered i.p. for 15 min before access to food and water. Animals were then returned to their individual home cages without any bedding material. Subsequently, a measured amount of food and water were placed into the cages (t = 0 h). For Wistar rats, food pellets and food spillage were then weighed at time intervals of 30, 60, 120 and 240 min, and 24 h. Although a similar protocol was used for wild-type and PPARα-null mice, pellets were weighed at 15, 30, 60, 120 and 240 min. The cumulative food intake (g·kg−1 body weight) was calculated from these data.
Diet-induced obesity models
To induce obesity, Wistar rats (n= 16 per diet group) were fed an HFD of 5.24 kcal·g−1 energy density (D12492; 60 kcal% fat, 20 kcal% protein, 20 kcal% carbohydrate), whereas controls received a standard diet (SD) containing 3.85 kcal·g−1 (D12450B; 10 kcal% fat, 20 kcal% protein, 70 kcal% carbohydrate) (Research Diets, Inc., New Brunswick, NJ, USA) for 10 weeks. HFD contained fat constituted by soybean oil (9.26 kcal% of total fat content) and lard (90.74 kcal% of total fat content; cholesterol 300.8 mg·kg−1 lard) (formulated by E.A. Ulman, PhD, Research Diets, Inc., 26 August 1998 and 11 March 1999). Food intake (kcal·kg−1 body weight) and body weight (g) were measured daily for 10 weeks.
A 10 day study of feeding behaviour in rats treated with LH-21
Following 10 weeks of feeding, animals received a daily i.p. injection of vehicle or LH-21 (3 mg·kg−1) for 10 consecutive days. Eight rats per group were used. Food intake (kcal·kg−1 body weight) and body weight (g) were measured daily.
Sample collection
The animals were killed 2 h after the last dose. LH-21-treated and control animals were anaesthetized (sodium pentobarbital, 50 mg·kg−1, i.p.) and blood samples were collected from the retro-orbital plexus. Blood was centrifuged (2100× g C9 for 8 min, 4°C) and the plasma kept for further analysis. Visceral white adipose tissue and liver samples were then removed, snap-frozen in liquid nitrogen and stored at −80°C until analyses.
RNA isolation and RT-PCR analysis
Total RNA was extracted from 100 mg frozen visceral adipose tissue and liver samples using Trizol Reagent (Gibco BRL Technologies, Baltimore, MD, USA), and reverse-transcribed with the Transcriptor Reverse Transcriptase kit (Transcriptor RT; Roche Diagnostics GmbH, Mannheim, Germany). Primer sequences were designed by our laboratory and synthesized by Sigma-Proligo (Paris, France). PCR amplification was performed using primer sets outlined in Table 2. All primer sequences were determined through established GenBank sequences. The cDNA obtained from each sample was used as a template for qPCR using both the QuantiTec SYBR Green PCR Kit (Qiagen, Hilden, Germany) and the iCycler iQReal-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). Samples omitting reverse transcriptase were included as negative controls in each set of reactions. The rat SP-1 and Rpl19 genes were amplified as controls and their expression was used to normalize gene expression levels.
Table 2.
Primers sequences used for RT-PCR
| Gene description | GenBank® accession | Oligosense (5′→ 3′) | Product size (bp) |
|---|---|---|---|
| Oligoantisense (5′→ 3′) | |||
| Ppara (PPARα) | NM_013196.1 | 5′TGCTGTCCTCCTTGATGAAC | 270 |
| 5′GCTTGAGCACGTGCACAATC | |||
| Acox1 (acyl-CoA oxidase 1) | NM_017340.1 | 5′CACCTTCGAGGGAGAGAACA | 75 |
| 5′CGCACCTGGTCGTAGATTTT | |||
| Pparg (PPARγ) | NM_013124.1 | 5′GACCACTCCCATTCCTTTGA | 153 |
| 5′CGCACTTTGGTATTCTTGGAG | |||
| Fasn (fatty acid synthase) | NM_017332.1 | 5′AGTTTCCGTGAGTCCATCCT | 182 |
| 5′TCAGGTTTCAGCCCCATAGA | |||
| Scd1 (stearoyl-CoA desaturase 1) | NM_139192.1 | 5′GAAGCGAGCAACCGACAG | 71 |
| 5′GGTGGTCGTGTAGGAACTGG | |||
| Cnr1 (CB1) | NM_012784.3 | 5′ACAGCCAGCATGCACAGGGC | 94 |
| 5′CGGCGGACGTGTCTGTGGAC | |||
| Cnr2 (CB2) | NM_020543.4 | 5′AGGATAAGCAGGAGTTGGGAGGAGA | 80 |
| 5′TGAATCTGCCAGAGACAGCATGGG | |||
| Gpr55 (GPCR 55) | XM_576605.2 | 5′AAAACCTTTGGGATCTGCTG | 62 |
| 5′TAGATGGGGATGCTTCCAAC | |||
| Adipoq (adiponectin hormone) | NM_144744.2 | 5′AGGGATTACTGCAACCGAAG | 211 |
| 5′TCCTGTCATTCCAGCATCTC | |||
| Lep (leptin hormone) | NM_013076.1 | 5′AGGAAAATGTGCTGGAGACC | 160 |
| 5′ATACCGACTGCGTGTGTGAA | |||
| Sp1 (Sp1 transcription factor) | NM_012655.2 | 5′GCTATAGCAAACACCCCAGGT | 115 |
| 5′GATCAGGGCTGTTCTCTCCTT | |||
| Rpl19 (ribosomal protein L19) | NM_031103.1 | 5′TGCCGGAAGAACACCTTG | 121 |
| 5′GCAGGATCCTCATCCTTCG |
Liver triglyceride extraction and fatty acid analyses
Total lipids were extracted from frozen liver samples as described previously (Bligh and Dyer, 1959), and the liver fat content was expressed as a percentage of tissue weight. The lipid extracts were separated by TLC using hexane-diethylether-acetic acid (80:20:1, v/v/v) as the solvent system. After separation, the lipid spots corresponding to triglycerides were scraped from silica gel plates (Merck KGaA, Darmstadt, Germany), and fatty acid methyl esters (FAME) extracted with methanol benzene (4:1, v/v) for 1 h at 90°C. FAME were then analysed by gas-liquid chromatography, and individual fatty acids were identified with the following synthetic standards purchased from Supelco Analytical (Sigma Aldrich Co, St. Louis, MO, USA): lauric (12:0); myristic (14:0); palmitic (16:0); stearic (18:0); arachidic (20:0); palmitoleic (16:1); oleic (18:1); vaccenic (18:1); eicosenoic (20:1); linoleic (18:2); γ-linolenic (18:3); α-linolenic (18:3); eicosadienoic (20:2); dihomo-γ-linolenic (20:3); arachidonic (20:4); eicosapentaenoic (20:5); adrenic (22:4); docosapentaenoic (22:5); and docosahexaenoic acid (22:6). Fatty acids were quantified by area normalization and each fatty acid was expressed as a percentage of total fatty acids. They were finally grouped into saturated (SFA), mono-unsaturated (MUFA) or polyunsaturated (PUFA) fatty acids.
Biochemical assays
The following plasma metabolites were measured in plasma obtained from the retro-orbital plexus of LH-21-treated and control animals: urea, creatinine, uric acid, cholesterol, HDL-cholesterol, glutamate-pyruvate transaminase (GPT), glutamate-oxaloacetate transaminase (GOT), and γ-glutamyl transpeptidase (GGt). They were analysed in the Hematology Service at the Carlos Haya Hospital (Málaga, Spain) using commercial kits according to manufacturer's instructions and a Hitachi 737 Automatic Analyser (Hitachi Ltd, Tokyo, Japan).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.04 (GraphPad Software Inc., San Diego, CA, USA) by one-way or two-way anova followed by Bonferroni's post-hoc test as appropriate. The results are expressed as the mean ± SEM of 6–8 rats and 10–11 mice in each group. A P-value of less than 0.05 was considered significant.
Results
LH-21 does not inhibit P450 activity
The CYP family of enzymes is primarily responsible for drug metabolism. Drug-induced changes in CYP activity are a major source of adverse drug reactions, because CYP inhibition may affect the metabolism and clearance of many therapeutic drugs. Indeed, the inhibition of CYP enzymes may produce elevated blood concentrations of drugs resulting in adverse drug effects and toxicity.
As shown in Table 3, LH-21 did not display fluorescent or quenching interference. LH-21 exhibited much higher IC50 values than the control inhibitors of CYP isoenzymes (CYP3A4, CYP2C9 and CYP2D6). LH-21 can thus be considered to be a moderate/weak CYP inhibitor. Consequently, LH-21 is not expected to induce drug–drug interactions that could lead to adverse reactions or toxicity.
Table 3.
Interaction of LH21 with hepatic cytochrome P450 isoforms
| Cytochrome P450 fluorometric inhibition assay | |||||||
|---|---|---|---|---|---|---|---|
| Control Inhibitors* | LH-21 | ||||||
| CYP subtype | Inhibition IC50 (µM) | Lower 95% CL | Upper 95% CL | Inhibition IC50 (µM) | Lower 95% CL | Upper 95% CL | Inhibitor category |
| CYP3A4 | 0.052A | 0.041A | 0.066A | 1.62 | 1.46 | 1.79 | Moderate |
| CYP2C9 | 0.23B | 0.18B | 0.29B | 8.14 | 6.84 | 9.70 | Moderate |
| CYP2D6 | 0.018C | 0.015C | 0.021C | >105 | – | – | Weak |
Control Inhibitors in each CYP inhibition assay: (A) Ketoconazole; (B) Sulphafenazole; (C) Quinidine. CL, confidence level.
LH-21 produces no cardiotoxic effects
Drug-induced sudden cardiac death is associated with a prolongation of the QT interval in the electrocardiogram. When the QT interval is prolonged, there is an increased risk of ventricular tachyarrhythmia due to an excessive time between depolarization and repolarization. Inhibition of the delayed rectifier K+ current (IKr) is the most common mechanism responsible for drug-induced prolongation of QT interval in humans and such inhibition is conducted by hERG potassium channels.
In our studies with HEK-293 cells expressing hERG, LH-21 displayed no activity in this assay. Thus, it exhibits a high IC50 value (>150 µM) as compared with standard control inhibitors (IC50 values for amiodarone, 1.7 µM; bepridil, 2.2 µM; haloperidol, 1.9 µM; terfenadine, 1.0 µM). Thus, LH-21 is a drug with no inhibitory activity on hERG and can be considered a safe drug devoid of cardiotoxicity.
LH-21 causes a dose-dependent decrease in food intake in fasted Wistar rats
In order to select an effective anorectic dose of LH-21 for subsequent sub-chronic studies, we performed an acute study on feeding behaviour in 24 h-fasted Wistar rats. We evaluated the effect of different doses of LH-21 on feeding at different times during a period of 240 min (Figure 1A), and 24 h after the injection of the drug (Figure 1B). Two-way anova analysis of acute effects revealed the different doses of LH-21 that produced a significant effect on cumulative food intake (F4,108= 39.86; P < 0.0001) with no interaction with time (F12,108= 0.57; n.s.). As expected, the time factor reflected the increase in food intake (F3,108= 78.13; P < 0.0001). Bonferroni's post-hoc test indicated that LH-21 produced a dose-dependent inhibitory effect on feeding, resulting in a significant decrease with all doses tested: 1 mg·kg−1 (P < 0.001 at 60 and 120 min; P < 0.05 at 240 min), 3 mg·kg−1 (P < 0.001 at 60 min; P < 0.01 at 120; P < 0.05 at 240 min), 10 mg·kg−1 (P < 0.001 at 30, 60, 120 and 240 min) and 30 mg·kg−1 of LH-21 (P < 0.001 at 30, 60, 120 and 240 min), when compared with the vehicle-treated group (Figure 1A). At 240 min all tested doses (1, 3, 10 and 30 mg·kg−1, i.p.) exhibited a significant reduction of cumulative food intake (relative percentages over vehicle-treated rats of 77, 78, 68 and 59%, respectively).
Figure 1.
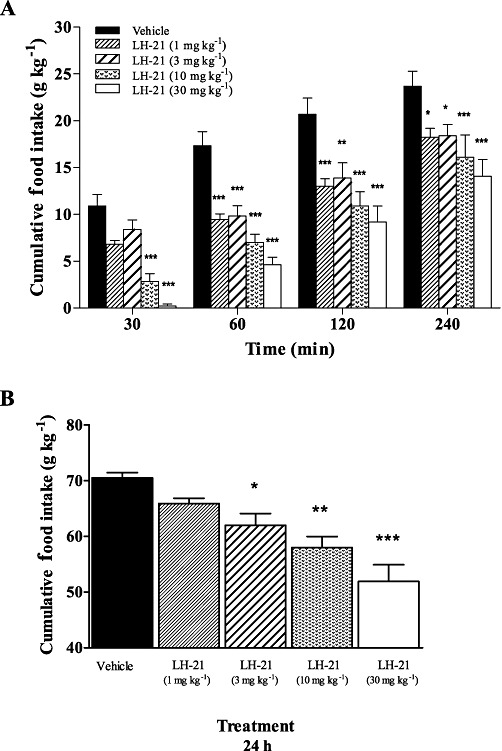
Effects of acute administration of LH-21 on cumulative food intake in male Wistar rats. Time course of the dose-response effect of LH-21 (1, 3, 10 and 30 mg·kg−1, i.p.) at 30, 60, 120 and 240 min (A), and 24 h (B) on cumulative food intake (g·kg−1 body weight). Columns are means ± SEM (n= 8 animals per group). Data were analysed by one- or two-way anova (treatment and time) and Bonferroni's post-hoc test. *P < 0.05, **P < 0.01 and ***P < 0.001 denote significant differences compared with the vehicle-treated group.
Twenty-four hours after the injection of LH-21, the cumulative food intake was evaluated again and a one-way anova showed that the different doses had a marked effect on feeding (F4,27= 12.33; P < 0.0001). Significant dose-dependent reductions in feeding were observed with LH-21 at doses of 3 mg·kg−1 (P < 0.05), 10 mg·kg−1 (P < 0.001) and 30 mg·kg−1 (P < 0.001) when compared with the vehicle-treated group (Figure 1B). Relative percentages of cumulative food intake were approximately 93, 88, 82 and 74%, with respect to vehicle-treated rats after administration of LH-21, 1, 3, 10 and 30 mg·kg−1 i.p., respectively.
Overall, the results indicate that LH-21 is a potent hypophagic drug capable of maintaining its anorectic properties for at least 24 h. In accord with previous studies in rats (Pavon et al., 2006; 2008 2008), 3 mg·kg−1 of LH-21 was an effective dose to perform a 10 day treatment in diet-induced obese rats.
LH-21 inhibits body weight gain and reduces the food intake in diet-induced obese rats
To further explore the anti-obesity properties of LH-21 previously studied on naïve rats and genetically obese rats (fa/fa, missense mutation in the leptin receptor), we generated another animal model of obesity, induced by exposure to HFD for 10 weeks. As shown in Figure 2A, both diets had a significant effect on cumulative body weight gain of Wistar rats (two-way anova, F1,330= 43.77; P < 0.0001). This analysis also revealed a significant interaction between diet and time (F10,330= 2.553; P= 0.0056), because the differences in weights due to diet were greater with time. The HFD produced a significant enhancement in weight gain when compared with the SD, that started on the 6th week of exposure (P < 0.05, 6th and 7th week; P < 0.01, 8th and 9th week; P < 0.001, 10th week). At the beginning of the treatment with LH-21 (10th week), HFD-fed rats displayed an increase of 10% in cumulative body weight gain compared with SD-fed rats. With respect to food intake, the statistical analysis revealed differences in feeding between both diets (diet effect, F1,300= 151.1; P < 0.0001) and a significant interaction between diet and time of exposure (F9,300= 43.77; P= 0.0007) (Figure 2B). HFD-exposure resulted in a marked and significant increase in cumulative food intake that started on the 4th week (P < 0.05, 4th week; P < 0.01, 5th week; P < 0.001, 6th to 10th week) as compared with SD-fed rats. Significant time effects were detected in weight gain and feeding using anova (data not shown).
Figure 2.
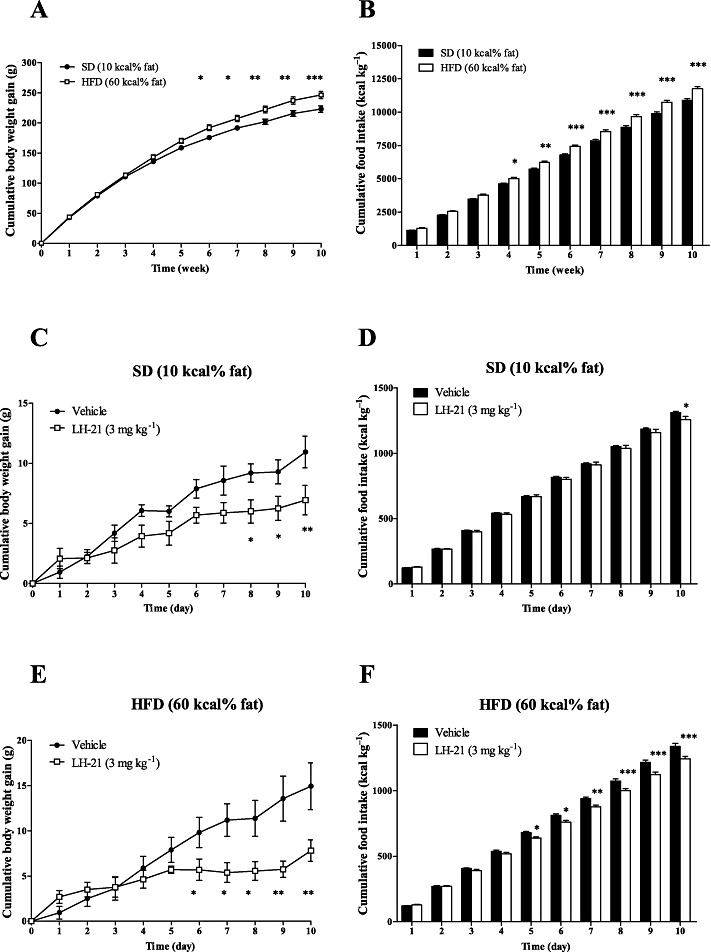
Effects on body weight and food intake in male Wistar rats fed SD or HFD, and following a 10 day treatment with LH-21. Cumulative body weight gain (A) and cumulative food intake (B) were evaluated weekly for 10 week exposure to SD or HFD. Points and columns are means ± SEM (n= 16 animals per group). Data were analysed by two-way anova (diet and time) and Bonferroni's post-hoc test. *P < 0.05, **P < 0.01 and ***P < 0.001 denote significant differences compared with the SD-fed group. Cumulative body weight gain (C, E) and cumulative food intake (D, F) in SD and HFD-fed animals, respectively, were evaluated after a 10 day exposure to vehicle or LH-21 (3 mg·kg−1, daily, i.p.). Points and columns are means ± SEM (n= 8 animals per group). Data were analysed by two-way anova (diet and treatment) and Bonferroni's post-hoc. *P < 0.05, **P < 0.01 and ***P < 0.001 denote significant differences compared with the vehicle-treated group.
After a significant segregation of both diet groups were observed, we examined the effect of prolonged LH-21 treatment on body weight and feeding for 10 days (3 mg·kg−1, once daily, i.p.). In rats fed SD, LH-21 reduced the body weight gain (two-way anova analysis, F1,154= 24.82; P < 0.0001) and only produced a modest effect on cumulative food intake (F1,140= 5.185; P < 0.05) when compared with the vehicle-treated group (Figure 2C and D). However, LH-21 produced a significant inhibition of body weight gain (F1,154= 25.06; P < 0.0001) and a marked reduction of feeding (F1,140= 61.43; P < 0.0001) at all time points analysed in the HFD-fed rats that started on the 5th day of treatment (Figure 2E and F). This higher efficacy detected with LH-21 on reducing food intake in HFD was statistically confirmed when we analysed the differences on feeding in each diet the last day (10th day) after a continuous treatment. Therefore, an additional two-way anova showed significant effects caused by treatment (F1,28= 12.80; P= 0.0013) as expected, but the post-test revealed a significant reduction of cumulative food intake only in HFD-fed rats (P < 0.01) with no effects on SD when compared with vehicle-treated rats.
These data indicate that LH-21 is able to maintain its anti-obesity properties in diet-induced obesity at doses that do not target brain CB1 receptors, as was reported in leptin signalling-deficient animals (Pavon et al., 2006; 2008).
LH-21 does not affect fat composition and content in liver
As the liver is an essential organ involved in lipid metabolism, we investigated the lipid content and its fatty acid composition to identify any effects of the diet and/or LH-21 treatment. As shown in Table 4, two-way anova revealed that LH-21 had no effect on the lipid variables analysed (total fat, triglycerides and fatty acid composition) in both diet-groups. However, important changes were found between SD and HFD groups regardless of whether animals received LH-21 or not. HFD induced a twofold increase in total fat content in liver as compared with SD rats (F1,28= 32.40; P < 0.0001); a marked increase in triglycerides (threefold) was observed in HFD rats (F1,28= 52.75; P < 0.0001). Additionally, liver fatty acids of triglycerides were identified and quantified by chromatography. The distribution of the most abundant fatty acids in HFD rats were different from those found in vehicle-treated SD group, with the exception of oleic acid. LH-21 did not appear to have any effect on the fatty acid distribution. The relative percentage of palmitic, oleic and linoleic acid represents about 85% of total for each group, and were as follows: [palmitic acid (16:0): vehicle-treated SD 36.88 ± 2.51%, LH-21-treated SD 39.86 ± 1.70%, vehicle-treated HFD 28.86 ± 1.58% (P < 0.05), LH-21-treated HFD 26.20 ± 1.03% (P < 0.001); oleic acid (18:1): vehicle-treated SD 30.43 ± 0.66%, LH-21-treated SD 32.89 ± 1.02%, vehicle-treated HFD 29.84 ± 0.83%, LH-21-treated HFD 31.34 ± 0.96%; linoleic acid (18:2): vehicle-treated SD 16.64 ± 1.68%, LH-21-treated SD 12.03 ± 1.38%, vehicle-treated HFD 25.34 ± 0.81% (P < 0.001), LH-21-treated HFD 26.62 ± 0.82% (P < 0.001)] (see Supporting Information Table S1).
Table 4.
Hepatic lipid content of diet-induced obese male Wistar rats treated with LH-21
| Treatment and diet | ||||
|---|---|---|---|---|
| SD | HFD | |||
| Hepatic lipid levels | Vehicle | LH-21 (3 mg·kg−1) | Vehicle | LH-21 (3 mg·kg−1) |
| Total fat content (% tissue) | 4.08 ± 0.34 | 4.39 ± 0.36 | 8.21 ± 1.03*** | 7.91 ± 0.71** |
| Triglycerides (% tissue) | 0.86 ± 0.09 | 0.88 ± 0.10 | 2.913 ± 0.42*** | 2.74 ± 0.31*** |
| SFA (% total fatty acids) | 41.96 ± 2.89 | 45.02 ± 1.84 | 33.56 ± 1.86* | 30.78 ± 1.07** |
| MUFA (% total fatty acids) | 38.68 ± 1.11 | 41.45 ± 1.54 | 32.96 ± 0.96* | 34.67 ± 1.20 |
| PUFA (% total fatty acids) | 19.36 ± 2.21 | 13.53 ± 1.47 | 33.48 ± 1.26*** | 34.55 ± 1.18*** |
| SFA/(MUFA + PUFA) | 0.75 ± 0.09 | 0.83 ± 0.07 | 0.51 ± 0.04* | 0.45 ± 0.02** |
Wistar rats fed standard (SD: 10 kcal% fat) or high fat diet (HFD: 60 kcal% fat) for 10 weeks, then treated with vehicle or LH-21 (3 mg·kg−1, i.p.) daily for 10 days. Lipid levels in liver were analysed 2 h after the last injection as described in the Methods section. Data were analysed by two-way anova (diet and treatment) for each parameter (see details in the Results section).
P < 0.05,
P < 0.01 and
P < 0.001 denote significant differences compared with vehicle-treated SD group (Bonferroni's post-hoc test). Values are expressed as mean ± SEM (n= 8 animals per group).
Data were also analysed by a two-way anova (diet and treatment) after being grouped as SFA, MUFA and PUFA. The type of diet had a significant effect on each type of fatty acid analysed in both treatment-subgroups (vehicle and LH-21) [SFA: (F1,28= 31.41; P < 0.0001); MUFA (F1,28= 26.22; P < 0.0001); and PUFA (F1,28= 123.13; P < 0.0001)], and only PUFA showed an interaction between treatment and diet (F1,28= 4.77; P= 0.0379). To calculate the SFAs/unsaturated fatty acids ratio we used the formula SFA/(MUFA + PUFA). Two-way anova revealed a significant effect of the HFD on this lipid ratio (F1,28= 27.25; P < 0.0001). Within-group differences were obtained by Bonferroni's post hoc test (see Table 4).
Effects of LH-21 on blood biochemical parameters
In order to investigate the effect of LH-21 on blood biochemistry indices of toxicity, we analysed the following plasma values: urea, creatinine and uric acid, primary nitrogenous waste products of protein, which are excreted by the kidneys; GPT, GOT and GGt, transaminases whose activities are related to liver toxicity; and finally cholesterol and HDL-C.
Two-way anova revealed that LH-21 had minimal effects on biochemical metabolic parameters related to toxicity in plasma (Table 5). Indeed, LH-21 induced only a significant decrease in the creatinine level (F1,28= 42.67; P < 0.0001), which showed no interaction with diet. However, diet produced significant effects on both urea and uric acid levels in plasma. Thus, HFD reduced urea (F1,28= 13.76; P= 0.0009) and uric acid levels (F1,28= 4.26; P= 0.0484) as compared with the SD-fed group. Neither diet nor LH-21 treatment-dependent effects were observed on the other parameters (cholesterol, HDL-C, GPT, GOT and GGt).
Table 5.
Metabolic biochemical parameters in plasma of diet-induced obese male Wistar rats treated with LH-21
| Treatment and Diet | ||||
|---|---|---|---|---|
| SD | HFD | |||
| Plasma biochemical parameter | Vehicle | LH-21 (3 mg kg−1) | Vehicle | LH-21 (3 mg kg−1) |
| Urea (mg L−1) | 3.95 ± 0.18 | 3.68 ± 0.14 | 3.24 ± 0.18 | 3.28 ± 0.09 |
| Creatinine (mg mL−1) | 82.4 ± 2.5 | 64.9 ± 3.9 | 86.3 ± 2.1 | 59.8 ± 4.5 |
| Uric acid (mg mL−1) | 57.3 ± 17.1 | 90.0 ± 28.5 | 42.5 ± 7.1 | 34.3 ± 3.3 |
| Cholesterol (mg L−1) | 9.20 ± 0.74 | 7.36 ± 0.84 | 8.16 ± 0.87 | 7.19 ± 0.58 |
| HDL-C (mg L−1) | 2.74 ± 0.12 | 2.34 ± 0.16 | 2.49 ± 0.27 | 2.36 ± 0.11 |
| GPT (U L−1) | 41.13 ± 3.89 | 52.25 ± 8.38 | 48.13 ± 4.75 | 50.25 ± 6.93 |
| GOT (U L−1) | 149.88 ± 41.66 | 119.50 ± 14.78 | 112.38 ± 20.62 | 114.25 ± 10.79 |
| GGt (U L−1) | 6.13 ± 0.74 | 4.50 ± 0.76 | 5.13 ± 0.87 | 6.25 ± 0.56 |
Wistar rats fed standard (SD: 10 kcal% fat) or high-fat diet (HFD: 60 kcal% fat) for 10 weeks, then treated with vehicle or LH-21 (3 mg·kg−1, i.p.) daily for 10 days. Plasma parameters were analysed 2 h after the last injection as described in the Methods section. Data were analysed by two-way anova (diet and treatment) for each parameter (see details in the Results section).
Values are expressed as mean ± SEM (n= 8 animals per group).
GGt, γ-glutamyl transpeptidase; GOT, glutamate-oxaloacetate transaminase; GPT, glutamate-pyruvate transaminase; HDL-C, high-density lipoprotein cholesterol.
Effects of LH-21 on lipid metabolism-related gene expression in adipose tissue
The expression of the mRNA of both, enzymes and receptors related to lipid metabolism, was analysed by RT-PCR in adipose tissue of treated animals. We selected genes implicated in pathways for lipolysis [PPARα and acyl-CoA oxidase (ACOX)] or lipogenesis [PPARγ, fatty acid synthase (FAS) and SCD-1]. Overall, LH-21 modulated both pathways as can be seen in Figure 3.
Figure 3.
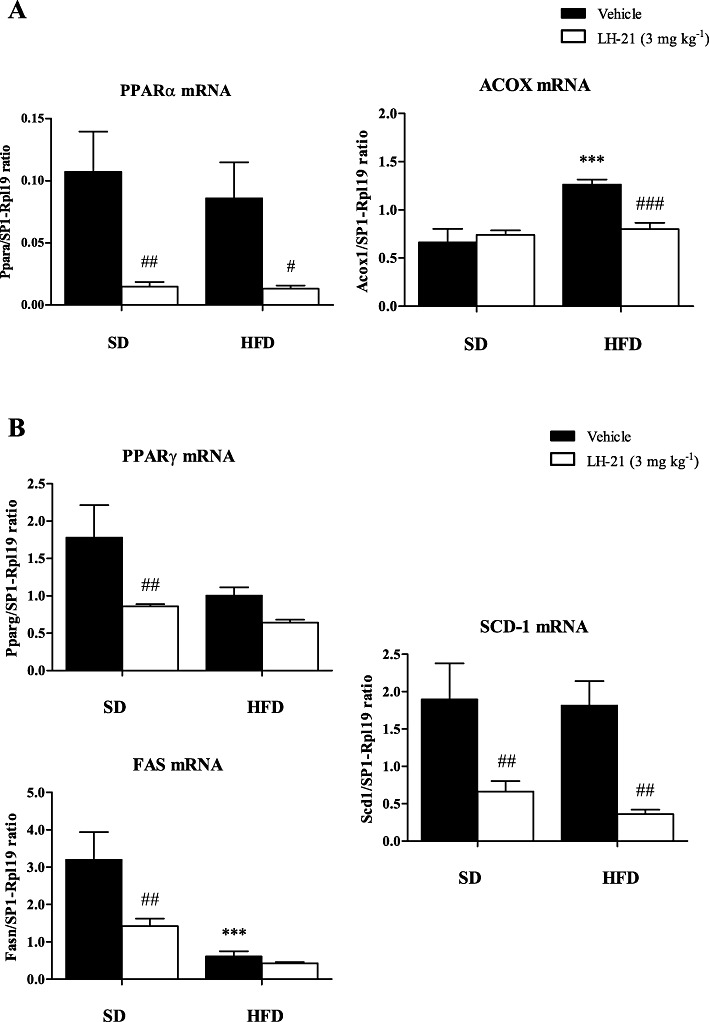
Effect of 10 day treatment with LH-21 on the gene expression of lipid metabolism-related proteins in visceral adipose tissue. Gene expression of PPARα and ACOX (Acox1) were determined as lipolytic molecules (A); and gene expression of PPARγ, FAS (Fasn) and SCD-1 (Scd1) were determined as lipogenic molecules (B) in SD and HFD-fed animals after 10 day exposure to vehicle or LH-21 (3 mg·kg−1, i.p.). Columns are means ± SEM (n= 8 animals per group). Data were normalized with a ratio of housekeeping genes (Sp1 and Rpl19) and analysed by two-way anova (diet and treatment) using Bonferroni's post-hoc. ***P < 0.001 denotes significant differences compared with the vehicle-treated group with SD; #P < 0.05, ##P < 0.01 and ###P < 0.001 denote significant differences compared with the corresponding vehicle-treated group.
Although diet did not affect the mRNA expression of PPARα (Ppara) (Figure 3A) LH-21 treatment had a strong effect on this nuclear receptor (F1,27= 15.08; P= 0.0006) inhibiting significantly its expression in both SD-fed (P < 0.01) and HFD-fed (P < 0.05) rats, when compared with vehicle groups. In contrast, ACOX expression (Acox1) was affected by both, diet and treatment. Thus, HFD induced a significant increase in the gene expression of ACOX (P < 0.001) in vehicle-treated animals when compared with SD-fed animals. However, LH-21 prevented selectively this increase only in the HFD group (P < 0.01). A two-way anova showed that both treatment (F1,27= 5.73; P= 0.0238) and diet (F1,27= 16.76; P= 0.0003) had significant effects on this oxidative enzyme. Additionally, a significant interaction between diet and treatment was also detected (F1,27= 11.31; P= 0.0023) confirming a selective effect of LH-21 on ACOX mRNA in HFD-fed rats.
Regarding lipogenic genes, effects due to diet and treatment are depicted in Figure 3B. Gene expression of PPARγ (Pparg) was significantly affected by both diet and treatment in an independent way. In particular, diet induced significant effects on this transcriptional factor (F1,26= 5.62; P= 0.0254), where HFD reduced its expression in the adipose tissue. LH-21 reduced PPARγ expression in both diet groups (F1,26= 9.38; P= 0.0051) with a higher effect in SD-fed rats (P < 0.01).
Fatty acid synthase (FAS) is an enzyme that plays a key role in fatty acid synthesis. A two-way anova revealed that diet and treatment induced significant changes in the expression of FAS (Fasn) mRNA, and both factors displayed a significant interaction (F1,26= 4.72; P= 0.0391). Thus, diet had a very strong effect on FAS (F1,26= 24.00; P < 0.0001); HFD-fed rats had a significant decrease in FAS when compared with vehicle-treated animals (P < 0.001). LH-21 also decreased significantly FAS expression (F1,26= 7.08; P= 0.0132), but this effect was only observed in the SD-fed group because of the strong inhibition of HFD on FAS expression (P < 0.01). The lipogenic enzyme SCD-1 is associated with increased fat accumulation and mono-unsaturation of SFAs. THe gene expression of SCD-1 (Scd1) was only affected by LH-21 (F1,26= 22.46; P < 0.0001) with no interaction between diet and treatment; LH-21 produced a strong reduction in SCD-1 in both SD-fed (P < 0.01) and HFD-fed (P < 0.01) animals.
Effects of LH-21 on gene expression of cannabinoid receptors and GPR55 in adipose tissue
Classical CB1 and CB2 cannabinoid receptors and the GPR55 orphan receptor were also analysed in adipose tissue. GPR55 was included as it was originally reported to be a putative cannabinoid receptor (Baker et al., 2006), although conflicting pharmacological data do not further support this classification because this receptor displays great affinity for cannabinoid antagonists but not for endocannabinoids. These receptors were determined by RT-PCR in adipose tissue (Figure 4). Gene expression of CB1 receptor (Cnr1) was significantly modified by diet and treatment as revealed by two-way anova with no interaction between these factors. Diet had a significant influence on CB1 mRNA (F1,26= 7.28; P= 0.0121) and the post-hoc test confirmed this effect. Thus, vehicle-treated HFD-fed group showed a significant increase (∼2-fold) of CB1 as compared with vehicle-treated SD group (P < 0.05). Interestingly, LH-21 also exerted a significant effect on CB1 (F1,26= 17.57; P= 0.0003). In fact, LH-21 markedly decreased the gene expression of CB1 in both diet groups, suppressing the diet-induced increase by 50% (P < 0.01) in HFD-fed rats (Figure 4A). CB2 receptor expression (Cnr2) was found to be elevated in rats fed a HFD (F1,27= 18.72; P= 0.0002) when compared with the respective SD-fed groups. However, LH-21 had no effect on CB2 receptor expression, independently of diet, as shown in Figure 4B.
Figure 4.
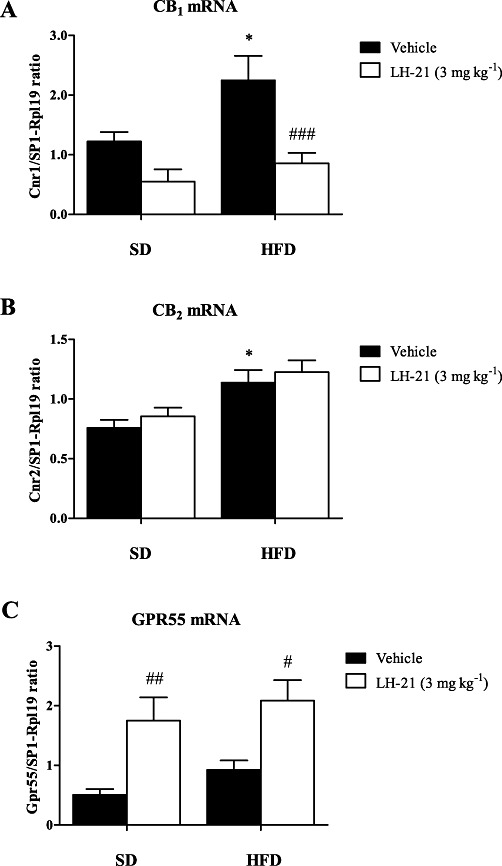
Effect of 10 day treatment with LH-21 on the gene expression of cannabinoid receptors in visceral adipose tissue. Gene expression of CB1 (Cnr1) (A), CB2 (Cnr2) (B), and GPR55 (C) were determined in SD and HFD-fed animals after 10 day exposure to vehicle or LH-21 (3 mg·kg−1, i.p.). Columns are means ± SEM (n= 8 animals per group). Data were normalized with a ratio of housekeeping genes (Sp1 and Rpl19) and analysed by two-way anova (diet and treatment) using Bonferroni's post-hoc. *P < 0.05 denotes significant differences compared with the vehicle-treated group with SD; #P < 0.05, ##P < 0.01 and ###P < 0.001 denote significant differences compared with the corresponding vehicle-treated group.
The mRNA expression of GPR55 (Gpr55) receptor was only affected by LH-21(F1,27= 17.80; P= 0.0002), which exerted a robust and significant increase in GPR55 mRNA in both the SD and HFD groups (∼three- and ∼twofold, respectively) when compared with vehicle-treated rats (P < 0.01 and P < 0.05) (Figure 4C).
Effects of LH-21 on gene expression of adipose-derived hormones
Both, leptin and adiponectin (Adipoq) are peptide hormones released from adipose tissue that play a key role in regulating energy intake and energy expenditure. With regard to leptin, we observed a clear diet × treatment interaction (F1,24= 7.04; P= 0.0139). Thus, HFD increased leptin (Lep) expression in the adipose tissue (F1,24= 23.09; P < 0.0001) and LH-21 significantly decreased leptin expression (P < 0.001) in HFD-fed animals. In the SD group, LH-21 showed a similar profile but this decrease in leptin expression was not significant (Figure 5A).
Figure 5.
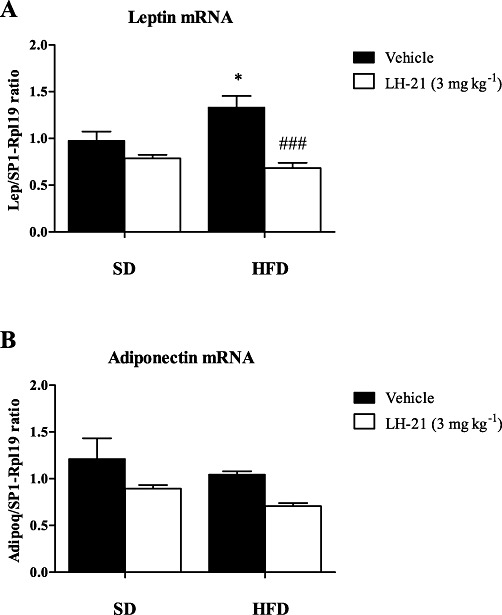
Effect of 10 day treatment with LH-21 on the gene expression of adipocyte-derived hormones in visceral adipose tissue. Gene expression of leptin (Lep) (A) and adiponectin (Adipoq) (B) were determined in SD and HFD-fed animals after 10 day exposure to vehicle or LH-21 (3 mg·kg−1, i.p.). Columns are means ± SEM (n= 8 animals per group). Data were normalized with a ratio of housekeeping genes (Sp1 and Rpl19) and analysed by one- and two-way anova (diet and treatment) using Bonferroni's post-hoc. *P < 0.05 denotes significant differences compared with the vehicle-treated group with SD; ###P < 0.001 denotes significant differences compared with the corresponding vehicle-treated group.
On the other hand, diet had no effects on Adipoq mRNA, although LH-21 significantly decreased its expression, as revealed by two-way anova (F1,26= 8.96; P < 0.0060) (Figure 5B).
LH-21 causes a decrease in food intake in both PPARα-null and wild-type mice
As LH-21 was able to suppress PPARα mRNA expression (Ppara) dramatically in adipose tissue of both diet groups (Figure 3A), we investigated if it could influence feeding through the well-described actions of PPARα on feeding behaviour (Fu et al., 2003; Rodriguez de Fonseca et al., 2001). To this end, the actions of LH-21 were evaluated in 24 h-fasted PPARα-null mice and wild-type control mice. We tested a single effective dose of LH-21 (20 mg·kg−1, i.p.) on feeding for 240 min, measuring cumulative food intake at different times (Figure 6). We detected no differences between both genotypes. Thus, statistical analysis revealed that neither LH-21 treatment (F3,195= 75.05; P < 0.0001) nor time intervals (F4,195= 114.41; P < 0.0001) were different between the genotypes, on which we observed a clear reduction of feeding induced by LH-21 treatment that was sustained over time. LH-21-treated mice displayed a significant decrease in cumulative food intake at 15 min (P < 0.05 in PPARα-null mice), 30 min (P < 0.05 in wild-type mice; P < 0.01 in PPARα-null mice), 60 min (P < 0.01 in wild-type mice; P < 0.001 in PPARα-null mice), 120 min (P < 0.001 in wild-type mice; P < 0.001 in PPARα-null mice) and 240 min (P < 0.001 in wild-type mice; P < 0.001 in PPARα-null mice) when compared with their respective vehicle-treated mice (Figure 6). At 120 min the anorectic effect on feeding behaviour between mice exposed to LH-21 and vehicle reached the highest difference as indicated by the relative percentages of cumulative food intake for each genotype compared to vehicle-treated mice: in wild-type was approximately 27% whereas in PPARα-null mice was 12%. Food intake after 120 min was practically the same as that during the first 15 min in both genotypes. However, at 240 min these feeding values were increased to 61% and 35% in wild-type and PPARα-null mice, respectively. Therefore, a single dose of 20 mg·kg−1 i.p. of LH-21 in mice was enough to induce a significant and sustained decrease in food intake for at least 4 h and the absence of PPARα had no effect on this anorectic activity.
Figure 6.
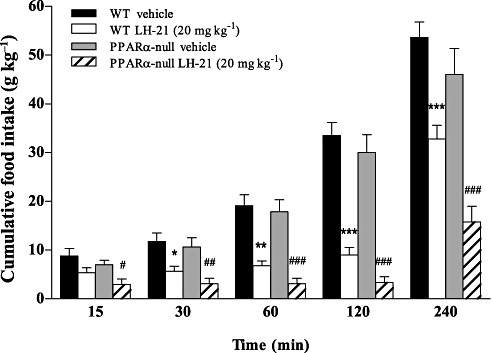
Effects of acute administration of LH-21 on cumulative food intake in wild-type and PPARα-null mice. Time course of the effect of LH-21 (20 mg·kg−1, i.p.) on cumulative food intake (g·kg−1 body weight). Columns are means ± SEM (n= 9 animals per group). Data were analysed by two-way anova (treatment and time) and Bonferroni's post-hoc. *P < 0.05, **P < 0.01 and ***P < 0.001 denote significant differences compared with the wild-type vehicle-treated group; #P < 0.05, ##P < 0.01 and ###P < 0.001 denote significant differences compared with the PPARα-null vehicle-treated group.
Discussion and conclusions
Three main important findings on the pharmacological properties of LH-21 arose from the present study. Firstly, this cannabinoid CB1 receptor antagonist displays a safe pharmacological profile, as: (i) it does not interact with the hERG cardiac potassium channels; (ii) it has no or moderate effects on the activity of different isoforms of hepatic cytochrome P450; and (iii) it does not induce liver or kidney toxicity after short-term repeated administration. Secondly, LH-21 maintains its anorectic activity in a model of diet-induced obesity, as previously reported in either food-deprived Wistar rats or obese Zucker rats (Pavon et al., 2006; 2008), and is more effective at reducing food intake and body weight gain in animals fed a HFD as compared with an SD. Thirdly, these effects were associated with changes in mRNA expression of genes related to lipogenesis and leptin in the adipose tissue, whereas we detected no changes in metabolic abnormalities in liver or plasma due to diet in contrast to other CB1 blockers.
In accordance with previous observations, a 10 week exposure to HFD caused a higher increase in body weight gain and food intake in comparison with SD-fed animals (Crespillo et al., 2010). A 10 day treatment with LH-21 produced a significant reduction in cumulative food intake and body weight gain in both diets. These results show that the changes observed on body weight in LH-21-treated rats were more evident than changes in food intake. Indeed, several studies have reported that body weight reductions observed in diet-induced obese mice treated with CB1 antagonists, such as rimonabant and AM6545, have been related to food intake-independent of effects on energy expenditure (Kunos and Tam, 2011; Ravinet Trillou et al., 2003; Tam et al., 2010). LH-21 appeared to be more efficacious as an anti-obesity agent in rats fed HFD than SD. This increased sensitivity of HFD-obese animals has been described previously (Matias et al., 2008; Starowicz et al., 2008; Izzo et al., 2009; 2010) and can be explained by a higher sensitivity to the effects of CB1 antagonists due to the dysregulation and overactivation of the endocannabinoid system. Increased concentrations of endocannabinoids in peripheral tissues, for example, increased 2-AG levels in adipose tissue (Matias et al., 2006) and increased anandamide levels in liver (Osei-Hyiaman et al., 2005), have been reported after exposure to HFDs in obese rodents. Additionally, studies with AM251 have demonstrated that its anorectic action is enhanced in animals fed a HFD (Judge et al., 2009). Also, AM6545, a non-brain penetrant neutral CB1 antagonist that reduces caloric intake of high fat and high carbohydrate diets, is less effective at reducing lab chow intake (Randall et al., 2010). Further, classical studies with rimonabant show that it is more effective in obese rats than in lean controls (Colombo et al., 1998; Bensaid et al., 2003; Ravinet Trillou et al., 2003; Vickers et al., 2003).
Overall, these observations support the idea that CB1 blockers such as LH-21 display a strong ability to reduce weight gain via CB1 receptor blockade with a greater potency in obese animals exposed to highly caloric diets. The effects of global cannabinoid CB1 receptor blockade on weight reduction are the combination of central and peripheral mechanisms. Although central actions include a decrease in caloric intake via interference with the hypothalamic and limbic mechanisms controlling appetite, peripheral mechanisms include an increase in energy expenditure involving tissues such as adipose tissue and muscle. The fact that LH-21 has poor penetration into the CNS indicates the importance of peripheral CB1 receptor blockade to produce weight reduction without interfering with neurobehavioural control of appetite, as has been observed with other non-brain penetrant CB1 antagonists (Tam et al., 2010). Thus, our effects of LH-21 on appetite and weight gain were probably mediated through its interaction with CB1 receptors located peripherally in gut sensory terminals that control satiety (Gomez et al., 2002), and also with cannabinoid receptors located in metabolically relevant tissues (Cota et al., 2003; Tam et al., 2010).
Obesity can lead to some forms of hepatic steatosis, mainly due to excessive fat accumulation in the liver. Here, we showed diet-induced alterations in the level and composition of lipids in rat liver; HFD-fed animals displayed an increase in liver fat content and triglycerides compared with SD-fed rats. Significant changes in fatty acid composition of hepatic triglycerides were also observed. The reduction in SFA observed in HFD-fed rats was basically produced by a decrease in palmitic acid levels. This observation might be related to inhibition of lipogenesis detected in these animals, as palmitic acid is the first product of fatty acid synthesis. HFD-fed rats also exhibited an elevation in PUFA, mainly due to the increase in essential fatty acids (linoleic and arachidonic acids); these changes could be caused by the specific composition of the fat-enriched diet, which contained soya oil. Systemic administration of LH-21 did not modify the total percentage of liver fat content or fatty acid composition in any of the groups, it did not reduce the elevated fat content produced by HFD. Again, these results accord with previous observations demonstrating that LH-21 does not reduce liver fat stores in leptin signalling-deficient obese rats (Pavon et al., 2008). This absence of metablic benefits of LH-21 contrasts with the hepatic fat reduction induced by CB1 receptor inverse agonists, rimonabant and AM251 (Osei-Hyiaman et al., 2005), and peripheral CB1 receptor neutral antagonists such as AM6545 (Tam et al., 2010). The fact that these compounds can improve or not the obesity-associated hepatic steatosis might suggest a different profile due to several factors. They include: (i) the inverse agonism/antagonism activity at cannabinoid CB1 receptors; (ii) penetrability into the brain; (iii) bioavailability to act at CB1 receptors according to their chemical properties and vehicle used; and (iv) the existence of other non-cannabinoid targets that could counteract or interfere in the metabolic effects promoted with CB1 blockers in liver. Indeed, pyrazole-derived compounds structurally related to rimonabant have been found to be potent activators of nuclear receptors in the nanomolar range, specifically PPARα (Alvarado et al., 2008).
In addition to the liver, adipose tissue has a key role in the peripheral energy homeostasis, as obesity is also associated with dysfunctional adipose tissue. Weight reduction improves the function of adipose tissue, probably by reducing fat mass. In our study, we observed that chronic exposure to a fat-enriched diet induced important changes at the transcriptional level of several enzymes and hormones involved in lipid metabolism and energy in adipocytes. We grouped our data into lipolytic or lipogenic genes to evaluate the net effects caused by diet and/or treatment. A 10 day treatment with LH-21 had an important effect on the gene expression of receptors (PPARs) and enzymes involved in lipid metabolism. LH-21 inhibited mRNA levels of PPARα in both diet groups in a similar way, as well as abolishing the elevation in ACOX mRNA expression observed in the HFD-fed animals. It is important to note that ACOX expression is PPARα-dependent. Interestingly, LH-21 exerted an important inhibitory effect on the pro-lipogenic PPARγ receptor and on each lipogenic enzyme analysed, as we observed a reduced mRNA expression of FAS and SCD-1. These data accord with results from previous studies conducted by Matias and colleagues, who showed that the cannabinoid CB1 receptor agonist HU-210 increases PPARγ expression in mouse adipocytes (Matias et al., 2006). Similar effects on lipogenic enzymes have been described after treatment with rimonabant and AM6545 in fat enriched diet-induced obese mice with a decrease in SCD-1 and FAS expression in visceral adipose tissue (Jourdan et al., 2010; Tam et al., 2010). Gene expression of SCD-1 was similarly reduced in both diet groups, which might implicate a decrease in fat accumulation and mono-unsaturation of SFAs in adipose tissue in LH-21-treated animals. The suppressed transcription of the lipogenic genes may be related to the enhancement of PUFA observed in these animals (Mater et al., 1998). Additionally, this strong inhibitory effect of LH-21 on lipogenesis signalling could be due to compensatory actions of the suppressor effects on lipolytic genes described previously, for example PPARα. Adipose tissue could be improving its functionality in order to recover an energy balance status by potentiating a down-regulation of the lipogenic pathway, reducing lipid accumulation in the adipocyte, and consequently decreasing lipolysis. Similar observations have been described in obese humans with a decreased expression of lipogenic gene in adipose tissue (Diraison et al., 2002).
As LH-21 is a CB1 receptor antagonist, we evaluated the expression of both CB1 and CB2 receptors in adipose tissue, which controls adipogenesis and lipogenesis in adipocytes (Bellocchio et al., 2008; Vettor and Pagano, 2009). Our data support the presence of cannabinoid CB1 and CB2 receptors in the visceral adipose tissue (Roche et al., 2006). Indeed, HFD produced significant alterations in the expression of both CB1 and CB2 receptors; an increase in their mRNA levels was associated with the diet-induced lipogenesis. As mentioned previously, the endocannabinoid system is dysregulated in obesity (Engeli, 2008) and genetically obese rats show an overexpression of CB1 receptors in adipose tissue compared with lean rats (Bensaid et al., 2003). LH-21 had selective and important effects on these cannabinoid receptors; it reduced the gene expression of CB1 in the HFD group to values of SD-fed animals, preventing the increase in CB1 induced by the HFD. However, LH-21 decreased the expression of CB1 receptors in both diet groups. Hence, the CB1 blockade under a 10 day regimen may induce a down-regulation of such receptor signalling in adipose tissue. As some authors have suggested a direct adipogenic role of CB1 activation (Matias et al., 2006), the CB1 receptor blockade may be a mechanism to promote oxidative pathways or/and to block synthetic pathways, which is consistent with effects on gene expression of proteins related to lipid metabolism in adipose tissue. In contrast, the expression of CB2 receptors was unchanged by LH-21 and LH-21 did not affect the increased expression of CB2 mRNA produced by the HFD. Our results confirm a selective effect of LH-21 on CB1 versus CB2 receptors as described previously (Jagerovic et al., 2004; Chen et al., 2008; Pavon et al., 2008). However, recently very high doses of LH-21 have been shown to have effects in CB1 knockout mice, suggesting alternative targets for this compound (Chen et al., 2008). In this regard, AM6545, a potent neutral CB1 antagonist with reduced brain penetration, inhibits food intake in CB1 receptor gene-deficient mice, but not in CB1/CB2 receptor double knockout mice, also indicating the existence of other alternative pathways to inhibit appetite. Two of these pathways may be either the PPARα receptors or the orphan receptor GPR55. We observed that the mRNA expression of PPARα in adipose tissue was reduced after 10 day exposure with LH-21, suggesting a down-regulation of this nuclear receptor. As other pyrazole-derived drugs structurally related to LH-21 and other CB1 antagonists were found to activate PPARα receptors (Alvarado et al., 2008), we tested whether the hypophagia induced by LH-21 in vivo was through a PPARα-mediated pathway by using PPARα-null mice. LH-21 (20 mg·kg−1, i.p.) potently reduced food intake in both the PPARα-null mice and wild-type animals, which suggests that the suppressive effect on feeding produced by LH-21 was via PPARα-independent mechanism. However, we cannot exclude long-term metabolic effects of LH-21 though interactions with PPARα receptors. LH-21 treatment clearly increased the expression of the orphan receptor GPR55, suggesting a potential interaction of this drug with GPR55-regulatory mechanisms. This receptor is strongly activated by rimonabant and AM251 (Henstridge et al., 2009; 2010; Kapur et al., 2009), therefore, the interaction of LH-21 with GPR55 will be addressed in future studies to elucidate its role.
Adipose tissue is also an endocrine organ. Leptin and Adipoq are the major adipocyte-derived hormones and both are involved in the regulation of energy homeostasis, glucose and lipid metabolism (Zhang et al., 1994; Scherer et al., 1995; Hu et al., 1996). Leptin is known to regulate appetite and body weight by decreasing caloric intake and increase peripheral energy expenditure (Friedman, 1998; Elmquist et al., 1999), although its functionality is lost in obese rodents that display leptin resistance as a consequence of the constantly elevated circulating levels of the hormone. In our study, leptin was the main adipocyte-derived hormone that was affected by both diet and LH-21 treatment. Leptin expression was up-regulated in HFD-fed rats, perhaps indicating the onset of HFD-induced leptin resistance, and LH-21 significantly reduced leptin transcription in both the HFD and SD groups. A decrease in leptin is related to weight reduction and it may indicate a recovery of leptin function. LH-21, however, failed to up-regulate Adipoq, producing only a moderate decrease in its expression. Although it has been reported that Adipoq mRNA levels in adipose tissue are lower in obese than in lean humans and rodents (Arita et al., 1999; Weyer et al., 2001; Milan et al., 2002), we observed no changes in Adipoq mRNA levels with diet. Nonetheless, the effects of CB1 antagonists on Adipoq levels are contradictory. Some studies have reported an increase in the expression of Adipoq in the circulation and in adipose tissue of obese Zucker rats and diet-induced obese rats treated with rimonabant (Bensaid et al., 2003; Thornton-Jones et al., 2006). Also, rimonabant has been shown to stimulate Adipoq expression and secretion in vitro by murine adipocytes (Matias et al., 2006; Perwitz et al., 2006), and AM6545 was reported to increase serum Adipoq levels in obese mice (Tam et al., 2010). In contrast, other studies have found no differences on Adipoq expression after CB1 activation in mature human adipocytes (Pagano et al., 2007).
In summary, our results demonstrate the anti-obesity effect of the neutral CB1 receptor antagonist LH-21 in a non-genetic model of obesity. LH-21 produced hypophagia and inhibited weight gain in diet-induced obese rats. In contrast, continuous treatment with this antagonist was unable to counteract some conditions associated with obesity, such as hepatic steatosis. LH-21 affected lipid metabolism through a general inhibitory effect on gene expression in the adipocyte, mainly lipogenic proteins and leptin. These observations suggest that visceral adipose tissue is the main peripheral target of LH-21 responsible for its weight reduction effect.
Acknowledgments
This study was supported by grants to F.R.F. from the European Union's 7th Framework Programme (Health-F2-2008-223713, Reprobesity); the following grants from the Spanish Ministry of Science and Innovation: Grants SAF 2010-20521; SAF 2009-12422-C02-02; National Institute of Health ‘Carlos III’ (PI07/0880), UE-ERDF, Red de Trastornos Adictivos (RD06/0001 and RETICS RD06/001/0014), and CIBER OBN; grants from the Consejería de Economía, Innovación y Ciencia de la Junta de Andalucía, UE/ERDF(CTS433, CVI1038 and 740486); and a grant from the Consejería de Salud de la Junta de Andalucía (PI0232/2008), Spain. L.H.F. is recipient of a postdoctoral contract (JAEDoc_07_00204) from CSIC. MMG is supported by the Research Stabilization Program of the Instituto de Salud Carlos III (CES 10/004). FJBS holds Miguel Servet research contracts CD07/00283 and JS holds a Sara Borrell postdoctoral contract CD08/00203, all from the National Institute of Health ‘Carlos III’, Madrid, Spain.
Glossary
- CYP
cytochrome P450
- FAME
fatty acid methyl esters
- hERG
Ether-à-go-go Related Gene
- HFD
high fat diet
- LH-21
5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1H-1,2,4-triazole
- SD
standard diet
Conflict of interest
The authors state no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1 Fatty acid composition (%) of hepatic triglycerides of diet-induced obese male Wistar rats treated with LH-21
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado M, Goya P, Macias-Gonzalez M, Pavon FJ, Serrano A, Jagerovic N, et al. Antiobesity designed multiple ligands: synthesis of pyrazole fatty acid amides and evaluation as hypophagic agents. Bioorg Med Chem. 2008;16:10098–10105. doi: 10.1016/j.bmc.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Bellocchio L, Cervino C, Vicennati V, Pasquali R, Pagotto U. Cannabinoid type 1 receptor: another arrow in the adipocytes' bow. J Neuroendocrinol. 2008;20(Suppl. 1):130–138. doi: 10.1111/j.1365-2826.2008.01682.x. [DOI] [PubMed] [Google Scholar]
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bluher M, Engeli S, Kloting N, Berndt J, Fasshauer M, Batkai S, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, et al. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–R2193. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Frassetto A, Lao JZ, Huang RR, Xiao JC, Clements MJ, et al. Pharmacological evaluation of LH-21, a newly discovered molecule that binds to cannabinoid CB1 receptor. Eur J Pharmacol. 2008;584:338–342. doi: 10.1016/j.ejphar.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Christopoulou FD, Kiortsis DN. An overview of the metabolic effects of rimonabant in randomized controlled trials: potential for other cannabinoid 1 receptor blockers in obesity. J Clin Pharm Ther. 2011;36:10–18. doi: 10.1111/j.1365-2710.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol. 2010;161:629–642. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluny NL, Chambers AP, Vemuri VK, Wood JT, Eller LK, Freni C, et al. The neutral cannabinoid CB receptor antagonist AM4113 regulates body weight through changes in energy intake in the rat. Pharmacol Biochem Behav. 2011;97:537–543. doi: 10.1016/j.pbb.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespillo A, Suarez J, Bermudez-Silva FJ, Rivera P, Vida M, Alonso M, et al. Expression of the cannabinoid system in muscle: effects of a high-fat diet and CB1 receptor blockade. Biochem J. 2010;433:175–185. doi: 10.1042/BJ20100751. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–1367. doi: 10.1007/s00125-008-1048-2. [DOI] [PubMed] [Google Scholar]
- Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2002;282:E46–E51. doi: 10.1152/ajpendo.2002.282.1.E46. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Engeli S. Dysregulation of the endocannabinoid system in obesity. J Neuroendocrinol. 2008;20(Suppl. 1):110–115. doi: 10.1111/j.1365-2826.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E, Braun H, Matan H, Steinberg S, Reggio PH, Seltzman HH. Inhibition of milk ingestion and growth after administration of a neutral cannabinoid CB1 receptor antagonist on the first postnatal day in the mouse. Pediatr Res. 2007;62:533–536. doi: 10.1203/PDR.0b013e3181559d42. [DOI] [PubMed] [Google Scholar]
- Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutr Rev. 1998;56(Pt 2):s38–s46. doi: 10.1111/j.1753-4887.1998.tb01685.x. discussion s54–75. [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, et al. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology. 2007;46:122–129. doi: 10.1002/hep.21641. [DOI] [PubMed] [Google Scholar]
- Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009;23:183–193. doi: 10.1096/fj.08-108670. [DOI] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Schroder R, Kargl JK, Platzer W, Martini L, et al. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br J Pharmacol. 2010;160:604–614. doi: 10.1111/j.1476-5381.2009.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Folgado L, Schmuck C, Tomic S, Piantanida I. A novel pyrene-guanidiniocarbonyl-pyrrole cation efficiently differentiates between ds-DNA and ds-RNA by two independent, sensitive spectroscopic methods. Bioorg Med Chem Lett. 2008;18:2977–2981. doi: 10.1016/j.bmcl.2008.03.060. [DOI] [PubMed] [Google Scholar]
- Hildebrandt AL, Kelly-Sullivan DM, Black SC. Antiobesity effects of chronic cannabinoid CB1 receptor antagonist treatment in diet-induced obese mice. Eur J Pharmacol. 2003;462:125–132. doi: 10.1016/s0014-2999(03)01343-8. [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, et al. Peripheral endocannabinoid dysregulation in obesity: relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br J Pharmacol. 2009;158:451–461. doi: 10.1111/j.1476-5381.2009.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Piscitelli F, Capasso R, Marini P, Cristino L, Petrosino S, et al. Basal and fasting/refeeding-regulated tissue levels of endogenous PPAR-alpha ligands in Zucker rats. Obesity (Silver Spring) 2010;18:55–62. doi: 10.1038/oby.2009.186. [DOI] [PubMed] [Google Scholar]
- Jagerovic N, Hernandez-Folgado L, Alkorta I, Goya P, Navarro M, Serrano A, et al. Discovery of 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1h-1,2,4-triazole, a novel in vivo cannabinoid antagonist containing a 1,2,4-triazole motif. J Med Chem. 2004;47:2939–2942. doi: 10.1021/jm031099y. [DOI] [PubMed] [Google Scholar]
- Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes. 2010;59:926–934. doi: 10.2337/db09-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge MK, Zhang Y, Scarpace PJ. Responses to the cannabinoid receptor-1 antagonist, AM251, are more robust with age and with high-fat feeding. J Endocrinol. 2009;203:281–290. doi: 10.1677/JOE-09-0210. [DOI] [PubMed] [Google Scholar]
- Kapur A, Zhao P, Sharir H, Bai Y, Caron MG, Barak LS, et al. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J Biol Chem. 2009;284:29817–29827. doi: 10.1074/jbc.M109.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunos G, Tam J. The case for peripheral CB(1) receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol. 2011;163:1423–1431. doi: 10.1111/j.1476-5381.2011.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVerme J, Duranti A, Tontini A, Spadoni G, Mor M, Rivara S, et al. Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorg Med Chem Lett. 2009;19:639–643. doi: 10.1016/j.bmcl.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mater MK, Pan D, Bergen WG, Jump DB. Arachidonic acid inhibits lipogenic gene expression in 3T3-L1 adipocytes through a prostanoid pathway. J Lipid Res. 1998;39:1327–1334. [PubMed] [Google Scholar]
- Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA, Di Marzo V. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: effect of high fat diets. Mol Cell Endocrinol. 2008;286(Suppl. 1):S66–S78. doi: 10.1016/j.mce.2008.01.026. [DOI] [PubMed] [Google Scholar]
- McElroy J, Sieracki K, Chorvat R. Non-brain-penetrant CB1 receptor antagonists as a novel treatment of obesity and related metabolic disorders. Obesity (Silver Spring) 2008;16:S47. [Google Scholar]
- Milan G, Granzotto M, Scarda A, Calcagno A, Pagano C, Federspil G, et al. Resistin and adiponectin expression in visceral fat of obese rats: effect of weight loss. Obes Res. 2002;10:1095–1103. doi: 10.1038/oby.2002.149. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano C, Pilon C, Calcagno A, Urbanet R, Rossato M, Milan G, et al. The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab. 2007;92:4810–4819. doi: 10.1210/jc.2007-0768. [DOI] [PubMed] [Google Scholar]
- Pavon FJ, Bilbao A, Hernandez-Folgado L, Cippitelli A, Jagerovic N, Abellan G, et al. Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1H-1,2,4-triazole–LH 21. Neuropharmacology. 2006;51:358–366. doi: 10.1016/j.neuropharm.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Pavon FJ, Serrano A, Perez-Valero V, Jagerovic N, Hernandez-Folgado L, Bermudez-Silva FJ, et al. Central versus peripheral antagonism of cannabinoid CB1 receptor in obesity: effects of LH-21, a peripherally acting neutral cannabinoid receptor antagonist, in Zucker rats. J Neuroendocrinol. 2008;20(Suppl. 1):116–123. doi: 10.1111/j.1365-2826.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- Perwitz N, Fasshauer M, Klein J. Cannabinoid receptor signaling directly inhibits thermogenesis and alters expression of adiponectin and visfatin. Horm Metab Res. 2006;38:356–358. doi: 10.1055/s-2006-925401. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Randall PA, Vemuri VK, Segovia KN, Torres EF, Hosmer S, Nunes EJ, et al. The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behaviour. Pharmacol Biochem Behav. 2010;97:179–184. doi: 10.1016/j.pbb.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- Riedel G, Fadda P, McKillop-Smith S, Pertwee RG, Platt B, Robinson L. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br J Pharmacol. 2009;156:1154–1166. doi: 10.1111/j.1476-5381.2008.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche R, Hoareau L, Bes-Houtmann S, Gonthier MP, Laborde C, Baron JF, et al. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem Cell Biol. 2006;126:177–187. doi: 10.1007/s00418-005-0127-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, et al. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:946–955. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz KM, Cristino L, Matias I, Capasso R, Racioppi A, Izzo AA, et al. Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring) 2008;16:553–565. doi: 10.1038/oby.2007.106. [DOI] [PubMed] [Google Scholar]
- Suardiaz M, Estivill-Torrus G, Goicoechea C, Bilbao A, Rodriguez de Fonseca F. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain. 2007;133:99–110. doi: 10.1016/j.pain.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Kennett GA, Benwell KR, Revell DF, Misra A, Sellwood DM, et al. The cannabinoid CB1 receptor inverse agonist, rimonabant, modifies body weight and adiponectin function in diet-induced obese rats as a consequence of reduced food intake. Pharmacol Biochem Behav. 2006;84:353–359. doi: 10.1016/j.pbb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Vettor R, Pagano C. The role of the endocannabinoid system in lipogenesis and fatty acid metabolism. Best Pract Res Clin Endocrinol Metab. 2009;23:51–63. doi: 10.1016/j.beem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


