Abstract
BACKGROUND AND PURPOSE
The rostral ventrolateral medulla (RVLM) maintains sympathetic nerve activity (SNA), and integrates adaptive reflexes. Orexin A-immunoreactive neurones in the lateral hypothalamus project to the RVLM. Microinjection of orexin A into RVLM increases blood pressure and heart rate. However, the expression of orexin receptors, and effects of orexin A in the RVLM on splanchnic SNA (sSNA), respiration and adaptive reflexes are unknown.
EXPERIMENTAL APPROACH
The effect of orexin A on baseline cardio-respiratory variables as well as the somato-sympathetic, baroreceptor and chemoreceptor reflexes in RVLM were investigated in urethane-anaesthetized, vagotomized and artificially ventilated male Sprague-Dawley rats (n= 50). orexin A and its receptors were detected with fluorescence immunohistochemistry.
KEY RESULTS
Tyrosine hydroxylase-immunoreactive neurones in the RVLM were frequently co-localized with orexin 1 (OX1) and orexin 2 (OX2) receptors and closely apposed to orexin A-immunoreactive terminals. Orexin A injected into the RVLM was pressor and sympatho-excitatory. Peak effects were observed at 50 pmol with increased mean arterial pressure (42 mmHg) and SNA (45%). Responses to orexin A (50 pmol) were attenuated by the OX1 receptor antagonist, SB334867, and reproduced by the OX2 receptor agonist, [Ala11, D-Leu15]orexin B. Orexin A attenuated the somato-sympathetic reflex but increased baroreflex sensitivity. Orexin A increased or reduced sympatho-excitation following hypoxia or hypercapnia respectively.
CONCLUSIONS AND IMPLICATIONS
Although central cardio-respiratory control mechanisms at rest do not rely on orexin, responses to adaptive stimuli are dramatically affected by the functional state of orexin receptors.
Keywords: orexin A, sympathetic vasomotor tone, somato-sympathetic reflex, baroreflex, chemoreflex
Introduction
Tonically active sympathetic premotor neurones in the rostral ventrolateral medulla (RVLM) project to the intermediolateral cell column of the spinal cord and provide an excitatory input to sympathetic preganglionic neurones so as to maintain the resting level of sympathetic vasomotor tone (Pilowsky and Goodchild, 2002; Pilowsky et al., 2009). The RVLM is the key site for cardiovascular homoeostasis. Neurones in the RVLM integrate information from the centre and periphery, including: respiration, and baro-, chemo- and somato-sympathetic reflex afferent neurones. Although synaptic input modulates the excitability of RVLM neurones (Pilowsky et al., 2009), the sources of this input and the transmitters involved are not thoroughly characterized. Glutamate and GABA are the key contributors for the short-term control of RVLM neurones. Regulation of RVLM neurones by metabotropic transmitters, including neuropeptides that have long-term effects on cell function, may affect adaptive reflexes, and encode distinct patterns of autonomic output (Pilowsky et al., 2009).
Orexin A and orexin B (also known as hypocretin 1 and hypocretin 2) have well-established roles in feeding and sleep-wakefulness. Recent evidence suggests a role for orexins as neuromodulators in central cardio-respiratory regulation. Orexin A increases BP and heart rate (HR) following intracisternal injection, or microinjection into the RVLM, in conscious or anaesthetized rats (Chen et al., 2000; Machado et al., 2002). However, the effects of activating orexin receptors in the RVLM on sympathetic nerve activity, or autonomic reflexes, are unknown.
Although orexin-containing cell bodies are restricted to the lateral hypothalamus, perifornical area and dorsomedial hypothalamus, orexin-immunoreactive fibres are widely distributed in the brain, including many brainstem areas that influence cardio-respiratory function, including the nucleus tractus solitarius (NTS), RVLM, rostral ventromedial medulla (RVMM), pre-Bötzinger complex, retrotrapezoid nucleus (RTN), dorsal motor nucleus of the vagus and the raphe (de Lecea et al., 1998; Peyron et al., 1998; Date et al., 1999; Nambu et al., 1999; Machado et al., 2002; Ciriello et al., 2003; Young et al., 2005). Orexin binds to two GPCRs (coupled to Gαq/11 and/or Gαi), orexin 1 (OX1) and orexin 2 (OX2) receptors (nomenclature follows Alexander et al., 2011). OX1 receptors have preferential affinity for orexin A, whereas OX2 receptors exhibit similar affinities for orexin A and orexin B in vitro (Sakurai et al., 1998). Orexin receptors are also widespread throughout the brain including thalamus, hypothalamus, brainstem areas like the nucleus ambiguus, RVMM, pre-Bötzinger complex, NTS and spinal cord (Trivedi et al., 1998; Marcus et al., 2001; Sunter et al., 2001; Cluderay et al., 2002; Ciriello and de Oliveira, 2003). The distribution of OX1 and OX2 receptors in relation to putative sympatho-excitatory neurones in the RVLM is unknown.
The aims of this study were (i) to determine whether OX1 and OX2 receptors were expressed within neurones in the RVLM, and to determine if they were co-localized with putative sympatho-excitatory catecholamine-containing neurones of the rostral C1 cell group; (ii) to evaluate the actions of orexin A in the RVLM on splanchnic sympathetic (sSNA) and phrenic nerve activity (PNA), and on the baro-, chemo- and somato-sympathetic reflexes; and (iii) to determine which type of orexin receptor mediated the central effects of orexin.
Our principal findings are that OX1 and OX2 receptors were expressed abundantly in the RVLM neurones. Bilateral injection of orexin A in the RVLM increased mean arterial pressure (MAP), HR, sSNA and phrenic nerve amplitude (PNamp). Orexin A in the RVLM attenuated the somato-sympathetic reflex but increased barosensitivity. Following orexin A injection into the RVLM, sympatho-excitatory responses to hypoxia or hypercapnia were increased or reduced respectively. The findings demonstrate a role for orexin A in the RVLM in both the tonic and reflex regulation of central cardio-respiratory control.
Methods
All animal care and experimental procedures complied with the guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (http://www.nhmrc.gov.au/guidelines/publications/ea16) and were approved by the Animal Ethics Committee of Macquarie University, Sydney, Australia. We used male Sprague-Dawley rats (n= 50, 350–500 g), housed under controlled conditions with a standard 12 h light/dark cycle. Standard laboratory rat chow and tap water were available ad libitum. They were allowed to acclimatize for at least 7 days before experimental manipulations. Food and water were withdrawn 30 min before anaesthesia.
Immunohistochemical experiments
Rats (n= 3) were anaesthetized (pentobarbitone sodium, 70 mg·kg−1, i.p.), transcardially perfused and brainstems were processed for fluorescence immunohistochemistry (IHC) as described previously (Li et al., 2005; Padley et al., 2007). In brief, 40 µm sections were incubated with species-specific primary antibodies to detect tyrosine hydroxylase (TH; mouse, 1:2500), orexin A (rabbit, 1:2000), OX1 receptors (rabbit, 1:50) and OX2 receptors (goat, 1:50). Sections were then washed (3 × 30 min) in TPBS buffer (10 mM Tris HCl, 10 mM sodium phosphate buffer, 0.9% NaCl, pH 7.4) and incubated with fluorophore-conjugated secondary antibodies (Alexa Fluor 488 donkey anti-mouse IgG, and Cy3 donkey anti-rabbit IgG or Cy3 donkey anti-sheep IgG; 1:500) to reveal TH, orexin A, OX1 and OX2 receptors respectively. Finally, sections were mounted on glass slides, coverslipped with Vectashield (Vector Laboratories, QLD, Australia) and sealed with nail polish.
Brainstem sections, including the RVLM region, extending from 11.6 to 12.8 mm caudal to Bregma, were examined using an epifluorescence microscope (Axio-Imager Z1; Zeiss, Göttingen, Lower Saxony, Germany). The RVLM was defined as a triangular area ventral to the nucleus ambiguus, medial to the spinal trigeminal tract, and lateral to the pyramidal tract or the inferior olive. Labelled neurones in the RVLM were counted from six to seven sections, 200 µm apart. Counts were made for OX1, OX2 receptors and TH-immunoreactive neurones, as well as all double-labelling combinations. Results were expressed as the mean ± SEM at 200 µm intervals. Images were captured in grey scale with an Axiocam MR3 digital camera (Göttingen, Lower Saxony, Germany) and pseudocolouring was applied. Staining for all antigens was observed throughout the depth of the section indicating adequate antibody penetration.
Electrophysiological experiments
Experimental design
Electrophysiological experiments were conducted as described previously (Shahid et al., 2011). Briefly, rats (n= 47) were anaesthetized with urethane (1.2–1.4 g·kg−1, i.p.). Supplemental doses of urethane (30–40 mg, i.v.) were given when necessary if nociceptive stimuli (tested every half an hour) caused a change in MAP of more than 10 mmHg. Rectal temperature was maintained at ∼36.5°C with a thermostatically controlled heating pad (Harvard Apparatus, Holliston, MA, USA) and infrared heat lamp.
The left jugular vein and right carotid artery were cannulated with polyethylene tubing (internal diameter = 0.58 mm; outer diameter = 0.96 mm) for administration of drugs and fluids and for the measurement of BP. In some experiments, both femoral veins were cannulated to enable administration of sodium nitroprusside (SNP) or phenylephrine hydrochloride. The trachea was cannulated to enable artificial ventilation and a 3-lead electrocardiogram was fitted. HR was derived from the BP. The left greater splanchnic nerve and phrenic nerve were isolated, tied with silk thread, and cut distally to permit recording of efferent sSNA and PNA respectively. In an additional subset of animals, the sciatic nerve was isolated, tied and cut distally for electrical stimulation to allow activation of somato-sympathetic reflexes. Rats were secured in a stereotaxic frame, vagotomized, paralyzed (pancuronium bromide; 0.8 mg initially, then 0.4 mg·h−1) and artificially ventilated with oxygen-enriched room air. End-tidal CO2 was monitored and maintained between 4.0% and 4.5% (Capstar 100, CWE Inc., Ardmore, PA, USA). Arterial blood gas was analysed to maintain pH at 7.35–7.45 (pH 7.4 ± 0.03; PaCO2 40.4 ± 0.9) by electrolyte and blood gas analyzer (IDEXX Vetstat, West brook, ME, USA). Animals were infused with 5% glucose in water (1.0–2.0 mL·h−1) to ensure hydration. Nerve recordings were made with bipolar silver wire electrodes. The neurograms were amplified (×10 000, CWE Inc.), bandpass filtered (0.1–2 kHz), sampled at 3 kHz (1401 plus, CED Ltd., Cambridge, UK) and recorded on computer using Spike2 software (v7, CED Ltd.).
The dorsal medulla was exposed after an occipital craniotomy. Bilateral microinjections of L-glutamate (100 mM, 5 nmol in 50 nL), orexin A (250 µM, 500 µM, 1 mM, 2 mM equivalent to 12.5, 25, 50 and 100 pmol; 50 nL per side) and vehicle (PBS) into the RVLM were made, using single- or multi-barrel glass pipettes, as described previously (Miyawaki et al., 2001; Rahman et al., 2011a). The RVLM was functionally identified by an increase of >30 mmHg in MAP following microinjection of L-glutamate. All variables were allowed to return to baseline (>30 min) before microinjection of PBS. After a further 30 min, orexin A was microinjected into the same site and observation was continued for a further 60 min. For all experiments, microinjections (50 nL) were performed over 5 s. Injection sites were marked with 2% rhodamine beads added to the PBS (50 nL) which was also microinjected as a volume and vehicle control.
Cardiorespiratory reflexes were evoked as described previously (Abbott and Pilowsky, 2009; Shahid et al., 2011; Rahman et al., 2011b), with sequential i.v. injection of SNP and phenylephrine (10 µg·kg−1) or electrical stimulation (10–25 V, 50, 0.2 ms pulses at 1 Hz) of the sciatic nerve. Chemoreceptors were activated by ventilating animals with either 100% N2 (12–14 s, brief hypoxia; peripheral; BOC gas and gear, NSW, Australia) or 5% CO2:95%O2 (3 min, hypercapnia; central; BOC gas and gear). Reflexes were activated before and after bilateral microinjection of PBS or orexin A (50 pmol, per side, 50 nL). Following death (3 M KCl, 0.5 mL, i.v.), brainstems were removed and injection sites were verified. Preliminary experiments revealed that tachyphylaxis occurs following orexin A injection; therefore, each animal received only one injection of orexin A. Different reflexes and antagonist studies were conducted on different sets of animals. Cardio-respiratory coupling was analysed from the same group used to determine the cardio-respiratory responses.
Data acquisition
Neurograms were rectified and smoothed (sSNA, 1 s time constant; PNA, 50 ms). Minimum background activity after death was taken as zero sSNA, and this value was subtracted from sSNA before analysis with off-line software (Spike 2 version 7). Baseline values were obtained by averaging 60 s of data 5 min prior to orexin A or PBS injection and maximum changes were expressed as absolute or percentage changes from baseline values. Phrenic-triggered ensemble averages of sSNA were generated from 60 s portions of data to evaluate cardio-respiratory coupling. The area under the curve (AUC), less baseline, of sSNA activity during the inspiratory and post-inspiratory phases was determined. sSNA was rectified and smoothed at 1 s and 5 ms time constants to analyse baroreceptor reflex and somato-sympathetic reflex respectively. To analyse reflexes, sSNA was normalized between the activity of sSNA before PBS injection (100%) and the sSNA after death (0%). The sSNA response to sciatic nerve stimulation was analysed using peristimulus waveform averaging. The AUC of the sympatho-excitatory peaks was analysed. The response to hypoxia (100% N2 inhalation for 12–14 s) was quantified by comparing the average maximum sSNA during hypoxia compared with a control period during normal hyperoxic ventilation. The maximum response to stimulation was then expressed as a percentage change from the baseline (control) considering maximum reflex response in control as 100%.
Data analysis
Grouped data are expressed as mean ± SEM. Statistical analysis was conducted with GraphPad Prism (version 5.0) (GraphPad, La Jolla, CA, USA).. A two-way, repeated measures, anova with Bonferroni's correction was used to compare values after orexin A (50 pmol) administration on sSNA and PNA with the PBS value. Paired t-test was used to analyse peak effects and reflexes. P < 0.05 was considered significant.
Materials
Orexin A (MW = 3561.16; Cat. No: H-4172; Lot No: 1013059) was obtained from, Bachem AG (Bubendorf, Switzerland); the OX1 receptor antagonist, SB334867 (N-(2-methyl-6-benzoxazolyl)-N′-1,5-naphthyridin-4-yl urea) (Cat. No: 1960; Batch No: 5) and the OX2 receptor agonist, [Ala11, D-Leu15]orexin B (Cat. No: 2142; Batch No: 10), were from Tocris Bioscience (Bristol, UK). Urethane, L-glutamate, glucose, phenylephrine and SNP were purchased from Sigma-Aldrich (Sydney, Australia); pancuronium bromide was from AstraZeneca Pty Ltd (Sydney, Australia); pentobarbitone sodium was from Merial Australia Pty Ltd (NSW, Australia); rhodamine microbeads were from Molecular Probes (Sydney, Australia); and PBS (10 mM in 0.9% NaCl) tablets were from AMRESCO Inc. (Solon, OH, USA). Primary antibodies for orexin A (Cat No: PC362), OX1 receptors (Cat. No: AB3092; Lot No: LV1387169), OX2 receptors (Cat. No: sc-8074) and TH were from Merck (Darmstadt, Germany), Millipore (Billerica, MA, USA), Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and Sigma-Aldrich respectively. Fluorophore-conjugated secondary antibodies such as Alexa Fluor 488 donkey anti-mouse IgG (Cat. No: A21202; Lot No: 811493), Cy3 donkey anti-rabbit IgG (Cat. No: 711–166-152; Lot No: 92082) or Cy3 donkey anti-sheep IgG (Cat. No: 713–166-147; Lot No: 89763) were from Invitrogen (Victoria, Australia) and Jackson ImmunoResearch Laboratories (West Grove, PA, USA), and vectashield was from Vector Laboratories. orexin A, [Ala11, D-Leu15]orexin B and rhodamine (2% v/v) were dissolved and further diluted in PBS (10 mM; pH 7.4). SB334867 was dissolved and further diluted in 10% dimethyl sulphoxide. PBS, phenylephrine and SNP were prepared in de-ionized water. Urethane was dissolved in 0.9% NaCl and L-glutamate in PBS.
Results
Expression of orexin A terminals and its receptors in the RVLM
The expression of orexin A, OX1 and OX2 receptors in the RVLM was examined using fluorescence IHC. Orexin A-immunoreactive fibres and terminals were found commonly throughout the RVLM (Figure 1A). Orexin A-immunoreactive terminals were also closely apposed to TH-immunoreactive cell bodies as well as dendrites (n= 3, Figure 1A).
Figure 1.
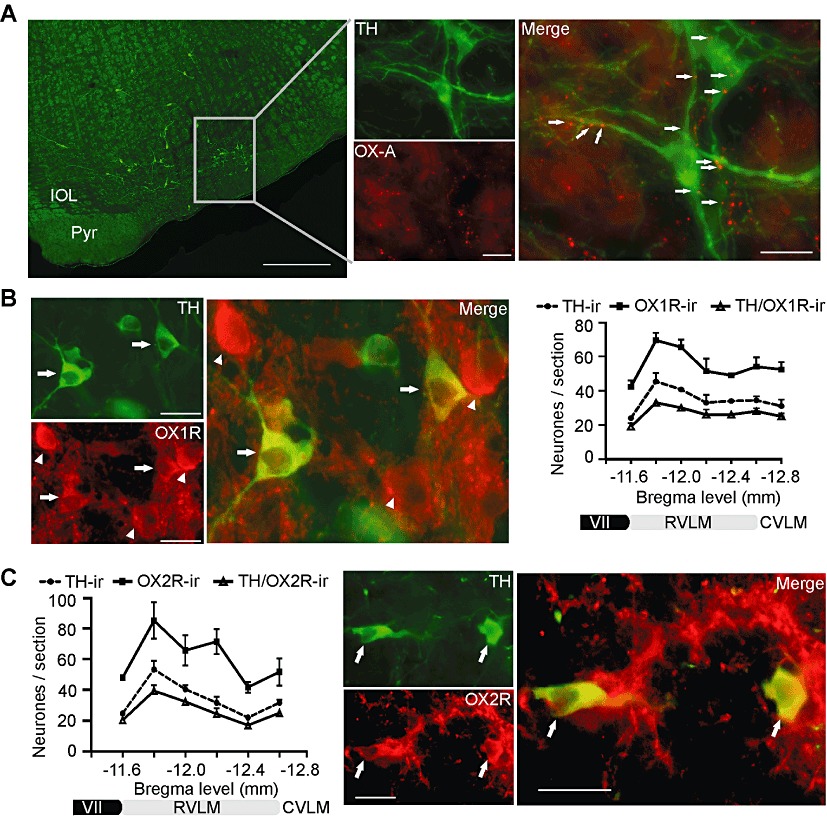
Immunohistochemical location of orexin A (OX-A) terminals, OX1 and OX2 receptors in the RVLM. (A) TH-positive (TH-ir) C1 neurones (green) in the RVLM are surrounded by orexin A-immunoreactive (OX-A-ir) varicosities (red) that form close appositions with TH-positive cell bodies and dendrites (arrows). (B) Immunostaining for OX1 receptors in the RVLM. OX1 receptors (OX1R-ir; red) were expressed in TH-immunoreactive neurones (green) (arrows) as well as in some non-TH neurones (arrowheads). XY plot represents rostrocaudal distribution of neurones staining for TH (TH-ir), those staining for OX1 receptors (OX1R-ir) and those staining for both TH and OX1- receptors(TH/OX1R-ir). (C) XY plot represents rostrocaudal distribution of neurones staining for TH (TH-ir), those staining for OX2 receptors (OX2R-ir)and those staining for both TH and OX2- receptors (TH/OX2-ir). OX2 receptors (red) are co-localized with TH-immunoreactive neurones (green; arrows). VII, facial nucleus; CVLM, caudal ventrolateral medulla. Scale bars: 500 µm (A, left); 20 µm (A, right); 25 µm (B and C).
Both OX1 and OX2 receptors were expressed in the RVLM. Intense immunoreactivity for both receptors was observed on cell bodies as well as on dendrites, fibres and terminals throughout the RVLM (Figure 1B,C). Immunoreactivity for OX1 receptors and TH were frequently co-localized in the RVLM neurones (Figure 1B). OX1 receptor immunoreactivity was found in 78 ± 2% (561 of 727 neurones across the region counted from −11.6 to −12.8; n= 3) of TH-immunoreactive neurones in the RVLM (Figure 1B); OX2 receptor immunoreactivity was found in 77 ± 3% (475 of 611 neurones) of TH-immunoreactive neurones in the RVLM (Figure 1C). Within the RVLM, about 51% of OX1 receptors (590 of 1151 neurones across the entire region counted from −11.6 to −12.8, n= 3) and 56% of OX2 receptors (615 of 1090 neurones across the entire region counted from −11.6 to −12.6, n= 3) were expressed in non-TH-immunoreactive or non-C1 neurones, suggesting that both C1 and non-C1 neurones in the RVLM contain both orexin receptors (Figure 1B,C). Immunoreactivities to OX1 and OX2 receptors and to TH showed similar patterns of distribution in the RVLM, being expressed most frequently in the rostral pole (Figure 1B,C).
Effects of orexin A in the RVLM on cardio-respiratory parameters
Bilateral microinjection of orexin A at four different doses (12.5, 25, 50 and 100 pmol, per side) evoked a pressor response, tachycardia and sympatho-excitation in a dose-dependent manner (Figures 2A, 2B) with maximum effects attained following injection of 50 pmol orexin A (Figure 2B). Bilateral injection of PBS (vehicle) in the RVLM was without effect on MAP, HR or sSNA (Figure 2B).
Figure 2.
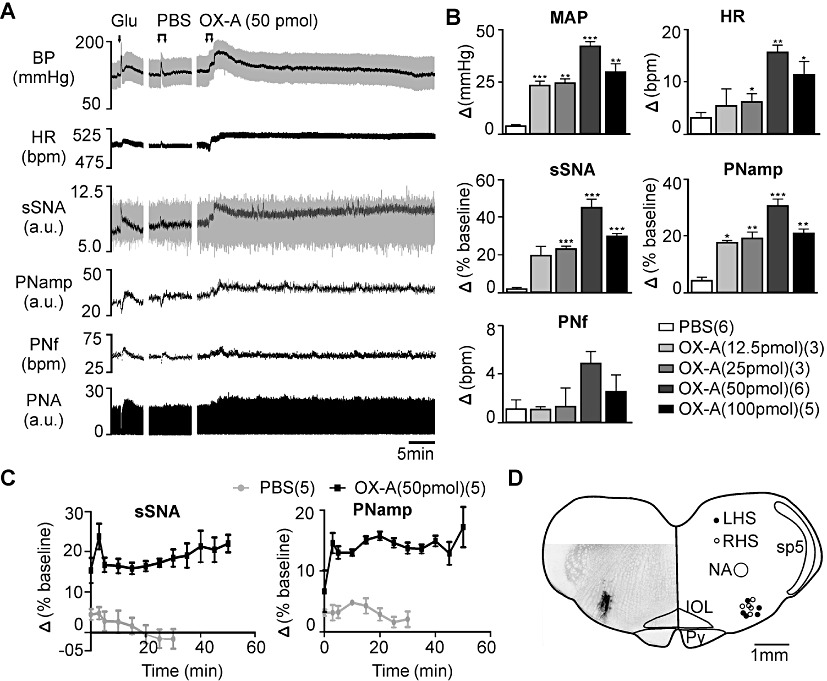
Effect of bilateral microinjection of orexin A (OX-A) in the RVLM. (A) Continuous recording of BP (grey – pulsatile and black – mean), HR, sSNA (grey – raw and black – rectified and integrated) [arbitrary unit (a.u.)], rectified PNA (a.u.), PNf and PNamp before and after injection of glutamate (Glu), PBS or orexin A (50 pmol). (B) Grouped data of maximum cardiovascular and respiratory effects following PBS or orexin A (12.5, 25, 50 and 100 pmol). Peak effects are shown as absolute (MAP, HR, PNf) or percentage (sSNA, PNamp) change from respective basal values. (C) Grouped time course effects of PBS or orexin A (50 pmol) on sSNA and PNamp. (D) Microinjection sites in the RVLM and a section showing an injection site stained following an injection of rhodamine microbeads. LHS, left hand side; RHS;right hand side; NA, nucleus ambiguuus; sp5, spinal trigeminal tract; IOL, inferior olive; Py, pyramidal tract. Data are expressed as mean ± SEM. Numbers of animals are shown in parentheses. ***P < 0.001, **P < 0.01, *P < 0.05, significantly different from PBS. bpm, beats per minute for HR or bursts per minute for PNf.
Orexin A injected bilaterally (12.5, 25, 50 and 100 pmol, per side) in the RVLM evoked a significant increase in PNamp without any effect on phrenic nerve frequency (PNf) (Figure 2A,B). The maximum increase in PNamp (n= 6, P < 0.001) was elicited by 50 pmol orexin A (Figure 2B). No significant change in PNamp or PNf was observed after injection of PBS (vehicle) (Figure 2A,B).
In some experiments (n= 5), bilateral injection of orexin A (50 or 100 pmol, per side) in the RVLM evoked a long-lasting increase in sSNA and PNA (Figure 2A,C) that mimicked the pattern of long term facilitation (LTF) of sSNA and PNA induced by acute intermittent hypoxia (Baker-Herman and Mitchell, 2002; Dick et al., 2007) or by intrathecal injection of orexin A (Shahid et al., 2011). All injection sites were centred in the RVLM (Figure 2D).
Effects of orexin receptor antagonism or agonism on cardio-respiratory responses mediated by orexin A
In the absence of orexin A, SB334867 (1 nmol, per side) did not affect MAP, HR, sSNA, PNamp or PNf (n= 5) (data not shown). On the other hand, the cardio-respiratory effects of orexin A (50 pmol per side) in the RVLM were significantly reduced by prior bilateral injection of SB334867 (P < 0.01,; n= 5, Figure 3).
Figure 3.
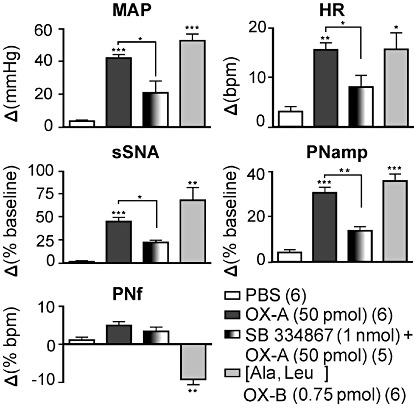
Effects of SB334867 and [Ala11, D-Leu15]orexin B ([Ala,Leu]OX-B) on orexin A-induced cardiorespiratory effects in the RVLM. Bar charts showing group data of maximum cardiovascular and respiratory effects following injection of PBS, orexin A (OX-A, 50 pmol), SB334867 [1 nmol + orexin A (50 pmol)] or [Ala11, D-Leu15]orexin B (0.75 pmol). Peak effects are shown as absolute (MAP, HR, PNf) or percentage (sSNA, PNamp) change from respective basal values. Data are expressed as mean ± SEM. Numbers of animals are shown in parentheses. ***P < 0.001, **P < 0.01, *P < 0.05 significantly different from PBS [except SB334867 (1 nmol) + orexin A (50 pmol)], which was compared with orexin A (50 pmol)). bpm, beats per minute for HR or bursts per minute for PNf.
Bilateral microinjection of the OX2 receptor agonist, [Ala11, D-Leu15]orexin B (0.75 pmol, per side; n= 6), into the RVLM significantly increased MAP, HR, sSNA and PNamp to an extent that was similar or slightly higher in magnitude with the responses to orexin A (50 pmol) (Figure 3). The only exception was a decrease in PNf as compared with PBS (Figure 3).
Effects of orexin A in the RVLM on cardio-respiratory coupling
Peri-phrenic averaging of the sympathetic nerve activity revealed an inspiratory and post-inspiratory peak of sSNA (Figure 4A). The AUC of both inspiratory and post-inspiratory peaks of sSNA was significantly increased by bilateral injection of 50 pmol orexin A (P < 0.05; n= 4, Figure 4B) compared with injection of PBS.
Figure 4.
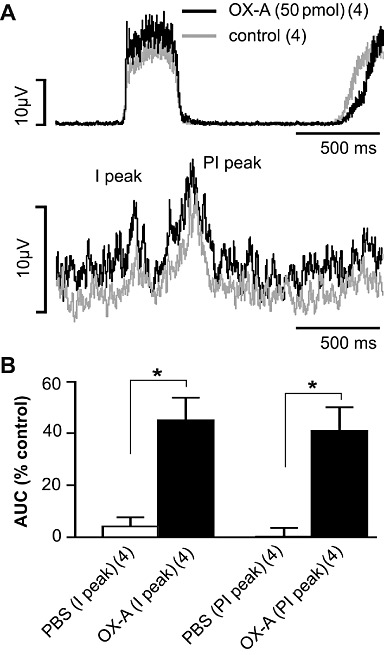
Effect of orexin A on phrenic nerve discharge-related rhythmicity of sSNA in the RVLM. (A) Phrenic-triggered average of sSNA before and after bilateral orexin A (OX-A; 50 pmol, each side) injection. (B) Grouped data illustrating the effects of PBS or orexin A (50 pmol, n= 4) on I and PI peaks of sSNA. The AUC of both inspiratory (I) and post- inspiratory (PI) peaks are increased following bilateral orexin A injection. Values are expressed as mean ± SEM. Numbers of animals are shown in parentheses. *P < 0.05, significantly different from PBS. Both PBS and orexin A values were normalized to the control period before injections.
Effects of orexin A in the RVLM on the somato-sympathetic reflex
Intermittent stimulation of the sciatic nerve resulted in two characteristic excitatory peaks in sSNA with latencies of 84 ± 6 ms and 186 ± 7 ms, before microinjection (n= 5, Figure 5A). The latencies of the peaks were not significantly altered by PBS [89 ± 5 ms and 190 ± 7 ms, n= 5, non-significant (ns)], or by orexin A (88 ± 5 ms and 189 ± 8 ms, n= 5, ns), injection. Bilateral orexin A injection (50 pmol per side) in the RVLM markedly attenuated the AUC of both excitatory peaks by 32% of baseline (P < 0.01; n= 5, Figure 5B).
Figure 5.
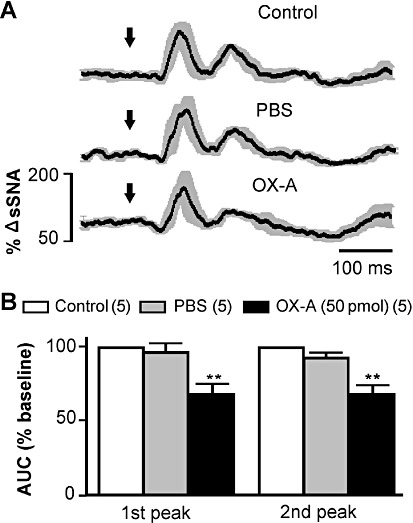
Effect of bilateral microinjection of orexin A (OX-A) on somato-sympathetic reflex. (A) Grouped effect of sciatic nerve-evoked stimulation of sSNA at control period and after injection of PBS and orexin A. Data are mean (black) ± SE (grey). Arrows indicate the time of stimulation. (B) Group data illustrating the effects of PBS or orexin A (50 pmol) on the AUC of 1st and 2nd sympatho-excitatory peaks. Numbers of animals are shown in parentheses. **P < 0.01, significantly different from control.
Effects of orexin A in the RVLM on baroreflex
In five animals, the changes in sSNA were plotted against the changes in MAP evoked by i.v. injection of SNP and phenylephrine. Bilateral RVLM microinjection of orexin A (50 pmol per side) significantly enhanced the reflex sympatho-inhibitory responses evoked by phenylephrine (Figure 6A). Orexin A significantly increased the upper plateau, range of sSNA, operating range and maximum gain of the sSNA without significantly altering the lower plateau, the threshold level, midpoint and the saturation levels of MAP as compared with control (Figure 6B and Table 1).
Figure 6.
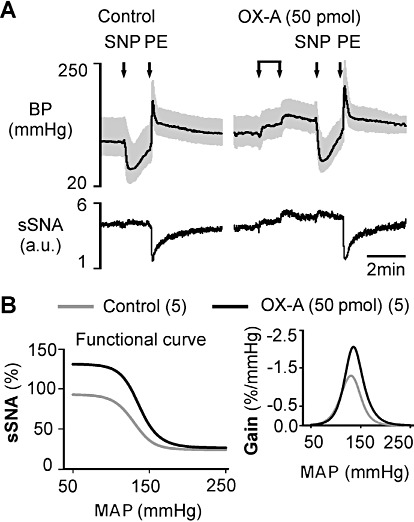
Effect of bilateral orexin A (OX-A) injection in the RVLM on the arterial baroreflex evoked by i.v. injection of sodium nitroprusside (SNP) or phenylephrine hydrochloride (PE). (A) Representative experimental recording of the effect of changes in BP on sSNA due to SNP or phenylephrine before (control) or after orexin A injection. (B) Average sympathetic baroreflex function curves generated for data before (control) or after orexin A (50 pmol) injection (numbers of animals are shown in parentheses). Trace at right represents baroreflex gain for sSNA (error bars are omitted for clarity – see Table 1). The range and gain of the reflex are significantly increased.
Table 1.
Parameters describing baroreflex control of sSNA after bilateral microinjection of orexin A (OX-A) (50 pmol)
| Lower plateau (%) | Upper plateau (%) | Mid point (mmHg) | Maximum gain (%/mmHg) | Range of SNA (%) | Threshold level (mmHg) | Saturation level (mmHg) | Operating range (mmHg) | |
|---|---|---|---|---|---|---|---|---|
| Control | 24.1 ± 6.9 | 93.2 ± 9.1 | 131.2 ± 3.2 | −1.4 ± 0.2 | 57.3 ± 8.5 | 93.7 ± 6.5 | 168.8 ± 5.3 | 68.3 ± 10.2 |
| OX-A (50 pmol) | 26.5 ± 7.6 (ns) | 131.0 ± 4.6** | 138.0 ± 3.1 (ns) | −2.1 ± 0.3** | 100.4 ± 5.2* | 99.3 ± 3.6 (ns) | 176.6 ± 7.5 (ns) | 104.7 ± 16.2* |
Values are mean ± SEM (n= 5). Maximum gain is the slope of the sigmoid curve of best fit at the MAP corresponding to steepest part of the curve.
P < 0.01,
P < 0.05 significantly different from control. ns, non-significant.
Effects of orexin A in the RVLM on chemoreflex
Activation of peripheral chemoreceptors with brief hypoxia evoked an increase in MAP, sSNA, HR, PNamp and PNf (Figure 7A). Peak effects occurred near the end of stimulus and recovered rapidly to baseline. Bilateral injection of orexin A (50 pmol per side) in the RVLM significantly increased the sympatho-excitatory response by 23% while attenuating the tachycardia by 43%, without any significant alteration in the pressor response (P < 0.01 for either; n= 4, Figure 7B). Orexin A caused significant attenuation of peak amplitude of PNamp and peak PNf during hypoxia by 33% and 24% of baseline, respectively (P < 0.001;n= 4; Figure 7B).
Figure 7.
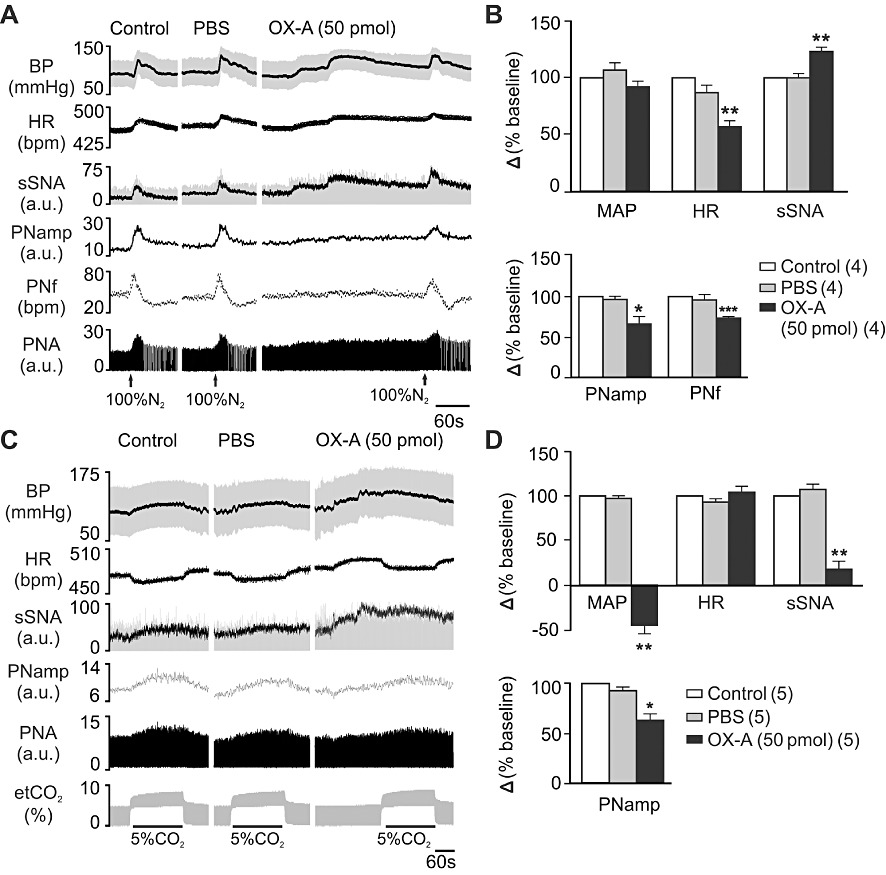
Effect of bilateral orexin A (OX-A) injection in RVLM on the cardiovascular and respiratory response to hypoxia and hypercapnia. (A) Effect on the peripheral chemoreceptor reflex activated by brief hypoxia with 100% N2 for 12–14 s – experimental recording of hypoxic episodes at control period and after PBS or orexin A injection. (B) Grouped data of peak effects on cardiovascular (MAP, HR and sSNA) (top bar chart) and respiratory function (PNamp and PNf) (bottom bar chart) after injection of PBS and orexin A (50 pmol, n= 4) in response to brief hypoxia. (C) Effect on central chemoreceptor reflex activated by hypercapnia with 5% CO2 in O2 for 3 min – experimental recording of hypercapnic episodes at control period and after PBS or orexin A injection. (D) Comparison of peak effects on cardiovascular (MAP, HR and sSNA) and respiratory function (PNamp) after injection of PBS and orexin A (50 pmol, n= 5) in response to hypercapnia. Values are expressed as mean ± SE. Numbers of animals are shown in parentheses. ***P < 0.001, **P < 0.01, *P < 0.05, significantly different from control. bpm, beats per minute for HR or bursts per minute for PNf.
Activation of central chemoreceptors with hypercapnia evoked an increase in MAP, sSNA, PNamp and a decrease in HR (Figure 7C). Orexin A (50 pmol per side) markedly attenuated the effect of hypercapnia on MAP by 143% (P < 0.01) and sSNA by 82% (P < 0.01) without any significant alteration in the bradycardia response (n= 5, Figure 7D). The effects of hypercapnia on peak PNamp was also reduced following orexin A (50 pmol per side) injection in the RVLM (P < 0.05; n= 5, Figure 7D).
Discussion
The principal new findings of the present study are: (i) OX1 and OX2 receptors are expressed in both C1 and non-C1 neurones of the RVLM; (ii) microinjection of orexin A into the RVLM causes sympatho-excitation, hypertension and tachycardia; (iii) orexin A microinjection significantly increases PNamp without affecting PNf; (iv) the respiratory modulation of sSNA is dramatically increased after microinjection of orexin A; (v) both OX1 and OX2 receptors are involved in the cardio-respiratory responses to orexin A; (vi) both peaks of somato-sympathetic reflex are attenuated by orexin A; (vii) sympathetic baroreflex sensitivity is increased following orexin A microinjection; (viii) the sympatho-excitatory response to hypoxia is potentiated, but there is a decrease in the HR, and PNA, response; and (ix) the cardiovascular and respiratory responses to hypercapnia are attenuated by orexin A.
Both C1 and non-C1 neurones in the RVLM play a role in the tonic and reflex control of sympathetic vasomotor tone (Lipski et al., 1995; 1996; Pilowsky and Goodchild, 2002; Guyenet, 2006; Pilowsky et al., 2009). The results of this study reveal that both OX1 and OX2 receptors are expressed in a discrete population of C1 and non-C1 neurones throughout the rostro-caudal pole of the RVLM, some of which may be bulbospinal and barosensitive. This study also demonstrates that varicose orexin A-immunoreactive fibres are present in the RVLM and are in close proximity to TH-immunoreactive neurones, consistent with the findings of Machado et al. (2002). The findings suggest that OX1 and OX2 receptors are activated under physiological or pathological conditions to modulate the activity of cardio-respiratory neurones in the RVLM. OX1 and OX2 receptors are found throughout the brainstem in regions involved in cardio-respiratory regulation, including: the NTS, RVMM, nucleus ambiguus, pre-Bötzinger complex (Trivedi et al., 1998; Marcus et al., 2001; Sunter et al., 2001; Cluderay et al., 2002; Ciriello and de Oliveira, 2003). The presence of OX1 and OX2 receptors within C1 neurones in the rostral 600 µm of the RVLM is consistent with the idea that these receptors are expressed by bulbospinal baro-sensitive, sympatho-excitatory neurones (Brown and Guyenet, 1984; Lipski et al., 1995; 1996; Schreihofer and Guyenet, 1997), and that orexin A is involved in regulating sympathetic outflow.
We suggest that the hypertensive and tachycardiac effects of centrally administered orexin A, as reported in previous studies (Chen et al., 2000; Machado et al., 2002), are likely to be due to an increase in sympathetic vasomotor tone mediated by the RVLM. In previous studies, orexin A microinjection into the RVLM resulted in hypertension and tachycardia for 25–30 min in anaesthetized rats (Chen et al., 2000) and for about 7 min in an conscious rats (Machado et al., 2002). We observed a significant, sympathetically mediated, hypertension and tachycardia after bilateral microinjection of orexin A into the RVLM that lasted for ∼10 min. There were several differences between this study and earlier work; firstly, the rats used in this study were vagotomized, secondly, the rats used here were significantly larger (∼425 g vs. ∼280 g), and thirdly, the doses used in this study, based on an initial dose-response study, were higher (1 mM vs. ∼250 µM.). The doses and weights used here were similar to those used in our earlier study of the effects of orexin A in the spinal cord (Shahid et al., 2011).
Pre-sympathetic neurones in the RVLM region are close to, and intermingled with, neurones in the Bötzinger region and pre-Bötzinger complex (Pilowsky et al., 1990; Sun et al., 1994; Kanjhan et al., 1995), and receive projections from the respiratory column (Sun et al., 1994.). Here we find that PNamp is increased following orexin A microinjection into the RVLM, while PNf remained unchanged. This result agrees with previous studies demonstrating that orexin A in the pre-Bötzinger complex increased amplitude without affecting breathing rate (Young et al., 2005). We also observed a long term facilitation (LTF) of sSNA and PNA in some of the experiments following orexin A microinjection into the RVLM which supports earlier studies suggesting a role for orexin A in LTF in the brain (Mileykovskiy et al., 2002; Walling et al., 2004; Wayner et al., 2004; Terada et al., 2008; Toyama et al., 2009). One possible explanation is that orexin A triggers the release of excitatory neurotransmitters (glutamate or noradrenaline) in the spinal cord and respiratory column, to cause LTF of SNA and PNA (Shi et al., 1988).
RVLM neurones are influenced by central respiratory activity (Guyenet et al., 1990; Miyawaki et al., 1996; Pilowsky et al., 1996), and sympathetic nerve discharge shows a respiratory rhythmogenicity in relation to PNA (Adrian et al., 1932; Guyenet, 2000). orexin receptor activation caused a significant increase in the inspiratory and post-inspiratory burst in sSNA with an increase on baseline activity suggesting that orexin receptor activation enhanced the respiratory-related, excitatory synaptic drive to RVLM neurones.
In order to determine if orexin A was involved in the tonic regulation of neurones in the RVLM, and to find out which type of orexin receptor mediated the central effects of orexin A, the selective OX1 receptor antagonist, SB334867 (pKb OX1= 7.2), and the selective OX2 (400 times more potent compared with OX1) receptor agonist, [Ala11, D-Leu15]orexin B, were injected into the RVLM. SB334867 itself did not affect baseline cardio-respiratory parameters, suggesting that OX1 receptors are not constitutively, or tonically, active. SB334867 reduced orexin A-induced cardio-respiratory responses by about 50%. Furthermore, [Ala11, D-Leu15]orexin B induced sympatho-excitation, hypertension, tachycardia and increases respiratory drive, in a similar manner to orexin A. Taken together, the results suggest that both OX1 and OX2 receptors mediated the central effects of orexin A, confirming results from a previous in vitro study (Huang et al., 2010). Huang et al. (2010) suggested a minor role of OX1 receptors on orexin A-induced depolarization of RVLM neurones in the brainstem slice preparation. This discrepancy may be due to the lower dose of SB334867 used compared with other studies (Deng et al., 2007; Shih and Chuang, 2007), or developmental differences between the neonate and the adult animal. In this study we also found that activation of OX2 receptors increased PNamp, but decreased PNf. We speculate that activation of orexin receptors decreases the activity of inhibitory Bötzinger neurons, resulting in an increase in PNamp. This increase in amplitude may be counteracted by a reflex decrease in frequency by the unaffected pre-Bötzinger complex which controls respiratory rhythm. Further studies will be required to clarify this issue.
In order to maintain cardiovascular homoeostasis, RVLM neurones integrate information from many peripheral afferent neurones, including: somatic receptors, baroreceptors and chemoreceptors (Dampney, 1994; Sun, 1995; Pilowsky et al., 2009). Peptide neurotransmitters modulate the different reflex responses of RVLM neurones via pre- or post-synaptic pathways (Miyawaki et al., 2002; Makeham et al., 2005; Burke et al., 2008; Kashihara et al., 2008; Abbott and Pilowsky, 2009). The somato-sympathetic reflex evoked by sciatic nerve stimulation was significantly attenuated by orexin A microinjection into the RVLM. This result suggests that the attenuation of somato-sympathetic reflex was not an indirect effect of raised sympathetic activity following orexin A injection into the RVLM. The mechanism by which orexin A exerts its effect on the somato-sympathetic reflex is likely to be complicated, as OX1 receptors were found to be localized both pre- and post-synaptically.
The reflex responses of sSNA to baroreceptor loading and unloading induced by vasoactive drug administration were markedly enhanced by orexin A microinjection into the RVLM. This response is not due to an increase in sSNA following orexin A injection, as injection of phenylephrine was equally able to reduce sSNA to the same low levels seen in the control situation. Exogenous orexin A increased the upper plateau of the baroreflex curve as well as the maximum gain and operating range, suggesting that orexin A in the RVLM regulates baroreflex control of sSNA by increasing disinhibition of RVLM neurones or by activation of barosensitive RVLM neurones.
Neurones in the ventral respiratory column and the RVLM are activated directly, and indirectly, by hypoxia. (Sun and Reis, 1994; De Paula and Branco, 2005; Alheid and McCrimmon, 2008). Orexin A microinjection into the RVLM enhanced the sympatho-excitatory response to hypoxia while reducing tachycardiac and phrenic responses. Enhanced sympatho-excitatory, but reduced tachycardiac effects, suggest that orexin A activates different population of presympathetic RVLM neurones projecting to different levels of the spinal cord. On the other hand, attenuated respiratory responses to hypoxia following orexin A microinjection into the RVLM suggest that orexin A may act on inhibitory Bötzinger neurones that project to other respiratory nuclei in the brainstem and spinal cord.
The reflex sympathetic response to hypercapnia is initiated by central chemoreceptors in the RTN, which then activate presympathetic neurones in the RVLM (Guyenet, 2000; Guyenet et al., 2010). Orexin A microinjection in the RVLM markedly attenuated cardio-respiratory responses to hypercapnia. It is not known whether chemosensitive RTN neurones make monosynaptic or polysynaptic connection with bulbospinal RVLM neurones. The inhibition of cardiovascular response to hypercapnia suggests the possible activation of GABAergic interneurones in the RVLM by orexin A.
Our findings show that OX1 and OX2 receptors are present in both C1 and non-C1 neurones in the RVLM and activation of these receptors in the RVLM causes sympathetically mediated hypertension, tachycardia and increase in respiratory drive. Orexin receptor activation in the RVLM reduces the somato-sympathetic reflex, but increases barosensitivity. Sympatho-excitatory responses to hypoxia and hypercapnia are enhanced and inhibited, respectively, by orexin receptor activation in the RVLM. Orexin A and orexin B play a key role in the stabilization of wakefulness, and are thought to be arousal-promoting peptides. Orexin ablated mice have disrupted sleep patterns (Sakurai, 2007)and orexin neurones are activated just before awakening, remain activated during wakefulness and cease firing during sleep (Kuwaki and Zhang, 2010). Active waking causes increase in HR, MAP, respiration and locomotor activity. However, the neural mechanisms and pathways that mediate the pressor and tachycardic responses to arousal are poorly understood. The presence of orexin receptors in C1 bulbospinal sympatho-excitatory neurones of the rostral RVLM and the effects of orexin A in the RVLM on basal cardio-respiratory parameters and adaptive reflexes suggest that orexin receptor activation plays a key role in mediating the sympatho-excitatory responses to arousal. Finally, the results suggest that drugs affecting central orexinergic pathways may be a useful target for the pharmacological treatment of disorders such as hypertension.
Acknowledgments
Work in the authors' laboratory is supported by grants from the National Health and Medical Research Council of Australia (457080, 457069, 604002), Australian Research Council (DP110102110) and Macquarie University. I.Z. Shahid and A.A. Rahman are supported by a Macquarie University Research Excellence Scholarship. The authors are grateful to Dr M.S.Y. Lung for assistance with immunohistochemistry and Dr A.Y. Fong for assistance with microscopy.
Glossary
- AUC
area under the curve
- HR
heart rate
- IHC
immunohistochemistry
- LTF
long term facilitation
- MAP
mean arterial pressure
- NTS
nucleus tractus solitarius
- OX1
orexin receptor 1
- OX2
orexin receptor 2
- PNA
phrenic nerve activity
- PNamp
phrenic nerve amplitude
- PNf
phrenic nerve frequency
- RTN
retrotrapezoid nucleus
- RVLM
rostral ventrolateral medulla
- RVMM
rostral ventromedial medulla
- SB334867
(N-(2-methyl-6-benzoxazolyl)-N′-1,5-naphthyridin-4-yl-urea
- SNP
sodium nitroprusside
- SPN
sympathetic preganglionic neurones
- sSNA
splanchnic sympathetic nerve activity
- TH
tyrosine hydroxylase
Conflict of interest
The authors have no conflict of interest to declare.
References
- Abbott SBG, Pilowsky PM. Galanin microinjection into rostral ventrolateral medulla of the rat is hypotensive and attenuates sympathetic chemoreflex. Am J Physiol. 2009;296:R1019–R1026. doi: 10.1152/ajpregu.90885.2008. [DOI] [PubMed] [Google Scholar]
- Adrian ED, Bronk DW, Phillips G. Discharges in mammalian sympathetic nerves. J Physiol (Lond) 1932;74:115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (5th edn.) 2011;164:S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DL, Guyenet PG. Cardiovascular neurones of brain stem with projections to spinal cord. Am J Physiol. 1984;247:R1009–R1016. doi: 10.1152/ajpregu.1984.247.6.R1009. [DOI] [PubMed] [Google Scholar]
- Burke PG, Li Q, Costin ML, McMullan S, Pilowsky PM, Goodchild AK. Somatostatin 2A receptor-expressing presympathetic neurones in the rostral ventrolateral medulla maintain blood pressure. Hypertension. 2008;52:1127–1133. doi: 10.1161/HYPERTENSIONAHA.108.118224. [DOI] [PubMed] [Google Scholar]
- Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol. 2000;278:R692–R697. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- Ciriello J, de Oliveira CV. Cardiac effects of hypocretin-1 in nucleus ambiguus. Am J Physiol. 2003;284:R1611–R1620. doi: 10.1152/ajpregu.00719.2002. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Li Z, De Oliveira CVR. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res. 2003;991:84–95. doi: 10.1016/j.brainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paula PM, Branco LGS. Glutamatergic receptors of the rostral ventrolateral medulla are involved in the ventilatory response to hypoxia. Respir Physiol Neurobiol. 2005;146:125–134. doi: 10.1016/j.resp.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- Dick TE, Hsieh Y-H, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. Neural structures that mediate sympathoexcitation during hypoxia. Respir Physiol. 2000;121:147–162. doi: 10.1016/s0034-5687(00)00125-0. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Darnall RA, Riley TA. Rostral ventrolateral medulla and sympathorespiratory integration in rats. Am J Physiol. 1990;259:1063–1074. doi: 10.1152/ajpregu.1990.259.5.R1063. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Abbott SBG, Depuy SD, Fortuna MG, Kanbar R. Central CO2 chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol. 2010;108:995–1002. doi: 10.1152/japplphysiol.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S-C, Dai Y-WE, Lee Y-H, Chiou L-C, Hwang L-L. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther. 2010;334:522–529. doi: 10.1124/jpet.110.167791. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Lipski J, Kruszewska B, Rong W. A comparative study of pre-sympathetic and Botzinger neurons in the rostral ventrolateral medulla (RVLM) of the rat. Brain Res. 1995;699:19–32. doi: 10.1016/0006-8993(95)00814-7. [DOI] [PubMed] [Google Scholar]
- Kashihara K, McMullan S, Lonergan T, Goodchild AK, Pilowsky PM. Neuropeptide Y in the rostral ventrolateral medulla blocks somatosympathetic reflexes in anesthetized rats. Auton Neurosci. 2008;142:64–70. doi: 10.1016/j.autneu.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Kuwaki T, Zhang W. Orexin neurones as arousal-associated modulators of central cardiorespiratory regulation. Respir Physiol Neurobiol. 2010;174:43–54. doi: 10.1016/j.resp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X-B, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Goodchild AK, Seyedabadi M, Pilowsky PM. Preprotachykinin A mRNA is colocalized with tyrosine hydroxylase-immunoreactivity in bulbospinal neurons. Neuroscience. 2005;136:205–216. doi: 10.1016/j.neuroscience.2005.07.057. [DOI] [PubMed] [Google Scholar]
- Lipski J, Kanjhan R, Kruszewska B, Smith M. Barosensitive neurons in the rostral ventrolateral medulla of the rat in vivo: morphological properties and relationship to C1 adrenergic neurons. Neuroscience. 1995;69:601–618. doi: 10.1016/0306-4522(95)92652-z. [DOI] [PubMed] [Google Scholar]
- Lipski J, Kanjhan R, Kruszewska B, Rong W. Properties of presympathetic neurones in the rostral ventrolateral medulla in the rat: an intracellular study ‘in vivo’. J Physiol (Lond) 1996;490:729–744. doi: 10.1113/jphysiol.1996.sp021181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado BH, Bonagamba LGH, Dun SL, Kwok EH, Dun NJ. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept. 2002;104:75–81. doi: 10.1016/s0167-0115(01)00351-2. [DOI] [PubMed] [Google Scholar]
- Makeham JM, Goodchild AK, Pilowsky PM. NK1 receptor activation in rat rostral ventrolateral medulla selectively attenuates somato-sympathetic reflex while antagonism attenuates sympathetic chemoreflex. Am J Physiol. 2005;288:R1707–R1715. doi: 10.1152/ajpregu.00537.2004. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of Orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Muscle tone facilitation and inhibition after orexin-A (Hypocretin-1) microinjections into the medial medulla. J Neurophysiol. 2002;87:2480–2489. doi: 10.1152/jn.2002.87.5.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T, Minson J, Arnolda L, Chalmers J, Llewellyn-Smith I, Pilowsky P. Role of excitatory amino acid receptors in cardiorespiratory coupling in ventrolateral medulla. Am J Physiol. 1996;271:R1221–R1230. doi: 10.1152/ajpregu.1996.271.5.R1221. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Goodchild AK, Pilowsky PM. Rostral ventral medulla 5-HT1A receptors selectively inhibit the somatosympathetic reflex. Am J Physiol. 2001;280:R1261–R1268. doi: 10.1152/ajpregu.2001.280.5.R1261. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Goodchild AK, Pilowsky PM. Activation of mu-opioid receptors in rat ventrolateral medulla selectively blocks baroreceptor reflexes while activation of delta opioid receptors blocks somato-sympathetic reflexes. Neuroscience. 2002;109:133–144. doi: 10.1016/s0306-4522(01)00439-0. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Padley JR, Kumar NN, Li Q, Nguyen TB, Pilowsky PM, Goodchild AK. Central command regulation of circulatory function mediated by descending pontine cholinergic inputs to sympathoexcitatory rostral ventrolateral medulla neurons. Circ Res. 2007;100:284–291. doi: 10.1161/01.RES.0000257370.63694.73. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky P, Arnolda L, Chalmers J, Llewellyn-Smith I, Minson J, Miyawaki T, et al. Respiratory inputs to central cardiovascular neurons. Ann N Y Acad Sci. 1996;783:64–70. doi: 10.1111/j.1749-6632.1996.tb26707.x. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002;20:1675–1688. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Jiang C, Lipski J. An intracellular study of respiratory neurons in the rostral ventrolateral medulla of the rat and their relationship to catecholamine-containing neurons. J Comp Neurol. 1990;301:604–617. doi: 10.1002/cne.903010409. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Lung MSY, Spirovski D, McMullan S. Differential regulation of the central neural cardiorespiratory system by metabotropic neurotransmitters. Philos Trans R Soc Lond B Biol Sci. 2009;364:2537–2552. doi: 10.1098/rstb.2009.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman AA, Shahid IZ, Fong AY, Hammond AM, Pilowsky PM. Vasostatin I (CgA17–76) vasoconstricts rat splanchnic vascular bed but does not affect central cardiovascular function. Auton Neurosci. 2011a doi: 10.1016/j.autneu.2011.08.023. doi: 10.1016/j.autneu.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Rahman AA, Shahid IZ, Pilowsky PM. Intrathecal neuromedin U induces biphasic effects on sympathetic vasomotor tone, increases respiratory drive and attenuates sympathetic reflexes in rat. Br J Pharmacol. 2011b;164:617–631. doi: 10.1111/j.1476-5381.2011.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA, Pilowsky PM. Intrathecal orexin A increases sympathetic outflow and respiratory drive, enhances baroreflex sensitivity and blocks the somato-sympathetic reflex. Br J Pharmacol. 2011;162:961–973. doi: 10.1111/j.1476-5381.2010.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Lewis DI, Coote JH. Effects of activating spinal alpha-adrenoreceptors on sympathetic nerve activity in the rat. J Auton Nerv Syst. 1988;23:69–78. doi: 10.1016/0165-1838(88)90168-3. [DOI] [PubMed] [Google Scholar]
- Shih CD, Chuang YC. Nitric oxide and GABA mediate bi-directional cardiovascular effects of orexin in the nucleus tractus solitarii of rats. Neuroscience. 2007;149:625–635. doi: 10.1016/j.neuroscience.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Sun MK. Central neural organization and control of sympathetic nervous system in mammals. Prog Neurobiol. 1995;47:157–233. doi: 10.1016/0301-0082(95)00026-8. [DOI] [PubMed] [Google Scholar]
- Sun MK, Reis DJ. Intrathecal kynurenate but not benextramine blocks hypoxic sympathoexcitation in chemodenervated anesthetized rats. J Auton Nerv Syst. 1994;47:141–150. doi: 10.1016/0165-1838(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Sun QJ, Pilowsky P, Minson J, Arnolda L, Chalmers J, Llewellyn-Smith IJ. Close appositions between tyrosine hydroxylase immunoreactive boutons and respiratory neurons in the rat ventrolateral medulla. J Comp Neurol. 1994;340:1–10. doi: 10.1002/cne.903400102. [DOI] [PubMed] [Google Scholar]
- Sunter D, Morgan I, Edwards CMB, Dakin CL, Murphy KG, Gardiner J, et al. Orexins: effects on behavior and localisation of orexin receptor 2 messenger ribonucleic acid in the rat brainstem. Brain Res. 2001;907:27–34. doi: 10.1016/s0006-8993(01)02344-7. [DOI] [PubMed] [Google Scholar]
- Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, et al. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- Toyama S, Sakurai T, Tatsumi K, Kuwaki T. Attenuated phrenic long-term facilitation in orexin neuron-ablated mice. Respir Physiol Neurobiol. 2009;168:295–302. doi: 10.1016/j.resp.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LHT, Guan X-M. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Walling SG, Nutt DJ, Lalies MD, Harley CW. Orexin-A infusion in the locus ceruleus triggers norepinephrine (NE) release and NE-induced long-term potentiation in the dentate gyrus. J Neurosci. 2004;24:7421–7426. doi: 10.1523/JNEUROSCI.1587-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides. 2004;25:991–996. doi: 10.1016/j.peptides.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, et al. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]


