Abstract
BACKGROUND AND PURPOSE
The transcription factor, Krüppel-like factor 4 (KLF4), plays an important role in regulating the proliferation of vascular smooth muscle cells. This study aimed to examine the effect of rapamycin on the expression of KLF4 and the role of KLF4 in arterial neointimal formation.
EXPERIMENTAL APPROACH
Expression of KLF4 was monitored using real-time PCR and immunoblotting in cultured vascular smooth muscle cells. and in rat carotid arteries in vivo after balloon injury. Adenovirus-mediated overexpression and siRNA-mediated knockdown of KLF4 were used to examine the role of KLF4 in mediating the anti-proliferative role of rapamycin. KLF4-regulated genes were identified using cDNA microarray.
KEY RESULTS
Rapamycin induced the expression of KLF4 in vitro and in vivo. Overexpression of KLF4 inhibited cell proliferation and the activity of mammalian target of rapamycin (mTOR) and its downstream pathways, including 4EBP-1 and p70S6K in vascular smooth muscle cells and prevented the neointimal formation in the balloon-injured arteries. KLF4 up-regulated the expression of GADD45β, p57kip2 and p27kip1. Furthermore, knockdown of KLF4 attenuated the anti-proliferative effect of rapamycin both in vitro and in vivo.
CONCLUSIONS AND IMPLICATIONS
KLF4 plays an important role in mediating the anti-proliferative effect of rapamycin in VSMCs and balloon-injured arteries. Thus, it is a potential target for the treatment of proliferative vascular disorders such as restenosis after angioplasty.
Keywords: rapamycin, transcription, smooth muscle cells, restenosis, gene therapy
Introduction
Coronary artery disease is a leading cause of morbidity and mortality in the Western countries as well as in Asia. Currently, percutaneous transluminal coronary angioplasty (PTCA) is widely used to treat severe arterial stenosis. However, restenosis has been a major drawback, occurring in 10–40% of the patients receiving angioplasty (Nobuyoshi et al., 1988; Serruys et al., 1988). Although the pathophysiological mechanisms remain to be fully elucidated, it is generally recognized that, in response to a range of cytokines generated including platelet-derived growth factor (PDGF) and fibroblast growth factor, the proliferation and migration of vascular smooth muscle cells (VSMCs) play central roles in the neointimal hyperplasia (Ferns et al., 1991; Jawien et al., 1992; Agrotis et al., 2004). Rapamycin (sirolimus), a cytostatic macrocyclic lactone with anti-proliferative properties, has been used in drug-eluting stents to reduce the risk of restenosis (Marx et al., 1995; Morris et al., 1995; Gallo et al., 1999; Sousa et al., 2001). Rapamycin binds to cytosolic FKBP-12 and inhibits the protein kinase mTOR (mammalian target of rapamycin), which regulates the initiation of protein translation by phosphorylating its downstream targets such as p70 S6 kinase (p70S6K) (Heitman et al., 1991; Marks, 1996; Inoki and Guan, 2006). Recently, the therapeutic effects of rapamycin have also been related to its ability to modulate the transcriptional program (Zohlnhofer et al., 2004). However, the transcription factor(s) that mediate the anti-proliferative effect of rapamycin in VSMCs remain to be characterized.
Krüppel-like factors (KLF) are a family of evolutionarily conserved zinc finger-containing transcription factors. KLF4, or gut-enriched KLF, was initially considered to be an epithelial-specific transcription factor functioning in the terminal differentiation and regulation of gut and skin epithelium (Garrett-Sinha et al., 1996; Shields et al., 1996; Segre et al., 1999). However, KLF4 is also expressed in vascular tissues including endothelial cells (ECs), VSMCs and in macrophages (Yet et al., 1998; Adam et al., 2000; King et al., 2003; Feinberg et al., 2005). In ECs, gene expression of KLF4 is induced by laminar shear stress and was shown to negatively regulate endothelial activation in response to pro-inflammatory stimuli (Hamik et al., 2007). However, it appears to have a pro-inflammatory effect in macrophages, suggesting a cell type-specific role in inflammation (Feinberg et al., 2005; Alder et al., 2008). In VSMCs, KLF4 is rapidly induced by TGF-β and PDGF-BB (Adam et al., 2000; King et al., 2003). In addition, overexpression of KLF4 suppressed VSMC differentiation markers such as myocardin and contributed to the phenotypic control of PDGF-BB (Liu et al., 2005). However, deletion of KLF4 accelerated neointimal formation following vascular injury in mice through reducing cellular proliferation, indicating that KLF4 is an important transcription factor in the vascular response to injury (Yoshida et al., 2008). Nonetheless, the regulatory mechanisms underlying KLF4 expression during vascular remodelling are largely unknown. In the present study, we have found that KLF4 expression was increased by rapamycin in VSMCs and balloon-injured rat arteries and we have explored the role of KLF4 in mediating the anti-proliferative effect of rapamycin in the context of vascular injury.
Methods
Adenoviral vectors and plasmids
To generate the recombinant adenovirus expressing KLF4 (Ad-KLF4), a cDNA fragment containing the full-length coding region of mouse KLF4 was subcloned from pMT3-KLF4 (a generous gift from Dr Vincent Yang, Emory University) into pAdlox and recombined with an E1- and E3-deleted adenovirus-5 genome DNA in a Cre-recombinase-expressing 293 cell line. The expression of the inserted gene was driven by a 7× tet operon/minimal CMV promoter, which was further under the control of tetracycline-controlled transactivator (tTA). The adenoviruses were plaque-purified and titrated in 293 cells (Wang et al., 2002). The expression plasmids encoding HA-TSC2, HA-TSC2 1-1080, mTOR and the kinase-dead mTOR (mTOR-KD) were previously described (Inoki et al., 2002; 2003).
Animals
All animal care and experimental procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996) and were approved by the Animal Research Committee of Peking University Health Science Center. Sprague–Dawley rats were given standard rat chow and water ad libitum and maintained on a 12 h light/12 h dark schedule at 22–25°C with 45–65% humidity. Animals were killed by overdose of sodium pentobarbital (200 mg·kg−1, i.p.).
Balloon injury was performed as described previously (Clowes et al., 1983; Tang et al., 2008). Briefly, male Sprague–Dawley rats (total number = 42) weighing 350 to 400 g were anaesthetized with sodium pentobarbital (60 mg·kg−1, i.p.). To make sure the adequacy and depth of anaesthesia of animals, respiratory pattern, mucous membrane colour and reactions to manipulation were monitored and toe pinch method was used. During the procedure, body temperature was monitored by a rectal temperature probe and maintained at 37 ± 1°C by means of a heated operating table. The left common carotid artery was injured with a 2F Fogarty balloon catheter inserted from the external carotid artery. Rapamycin was perivascularly delivered with 30% pluronic gel (100 µg per artery). DMSO containing gel was used as control. Uninjured arteries were treated similarly.
Ultrasound biomicroscopy
At different time points following the arterial injury, vascular remodelling was assessed in vivo by using an ultrasound biomicroscopy system (Vevo770, Visualsonics, Toronto, Canada) as previously described (Clowes et al., 1983; Razuvaev et al., 2008). Briefly, rats were anaesthetized with pentobarbital sodium (60 mg·kg−1, i.p.), and the hair on the jugular section was removed. A 17.5 MHz scanning head (RMV-707B) with a 15 to 45 MHz frequency band was used. The real-time images were captured. The same person did all examinations and performed the measurements, without knowledge of the treatments. Intimal–medial thickness (IMT) and the lumen size were measured with the VEVO 770TM analysis software, IMT was defined as the distance from the lumen–intima interface to the media–adventitia interface. Lumen diameter was defined by the distance between the intima–lumen interface of the nearwall and the lumen–intima interface of the farwall.
Cell culture
Rat aortic VSMCs were isolated from thoracic aorta of male Sprague–Dawley rats (killed by sodium pentobarbital, 200 mg·kg−1, i.p., n= 3) and grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics.
Cell proliferation assays
VSMCs were synchronized by serum starvation (0.1% FBS) for 24 h and, then, stimulated with 10% FBS for 24 h. Harvested cells were fixed with 70% ethanol and stained with propidium iodide (50 µg·mL−1) for 10 min. Cell cycle distribution was analysed with a FACScan (Becton-Dickinson, Franklin Lakes, NJ, USA). To detect the vascular cell replication rate in vivo, rats were i.p. injected with BrdU (25 mg·kg−1) at 18 h and 2 h before killing (sodium pentobarbital, 200 mg·kg−1, i.p.). The arteries were perfusion-fixed with 4% paraformaldehyde in PBS under a pressure of 100 mmHg and the segments were snap-frozen in OCT embedding compound. The cryosections of arterial segments were denatured with 1.5 N HCl and subjected to immunofluorescent staining.
Western blotting
Total protein lysates were prepared from VSMCs with the lysis buffer (50 mM Tris–HCl, pH 7.5, 15 mM EGTA, 100 mM NaCl, 0.1% Triton X-100 supplemented with protease inhibitor cocktail) and resolved on SDS-PAGE. Cytoplasmic proteins were extracted with the use of hypotonic lysis buffer (10 mM Tris–HCl, pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 0.5% NP-40, supplemented with protease inhibitor cocktail), and nuclear proteins were extracted with the use of a high-salt buffer (20 mM Tris–HCl, pH 7.5, 1.5 mM MgCl2, 420 mM NaCl, 10% glycerol, 0.2 mM EGTA, supplemented with protease inhibitor cocktail). Immunoblotting was performed with appropriate primary antibodies and a horseradish peroxidase (HRP)-conjugated secondary antibody followed by ECL detection (Amersham Biosciences, Piscataway, NJ, USA).
Microarray analysis
RNA samples were analysed with Affymetrix U230 2.0 arrays, which contain over 31 000 probe sets representing 30 000 gene transcripts. RNA labelling, hybridization, washing and scanning of the microarray were performed following the manufacturer's specifications (Affymetrix, Santa Clara, CA, USA). The results were analysed with GeneChip Operating Software 1.4. Primary analysis of raw cell data files was performed using the dChip platform. Control RNA was considered baseline and changes more than twofold were considered significant.
Quantitative reverse-transcriptase-PCR (qRT-PCR)
Total RNA was isolated from VSMCs and arterial segments with Trizol Reagent (Invitrogen, Grand Island, NY, USA). cDNA was synthesized with the use of Superscript III reverse transcriptase and oligo-(dT) primer. qPCR was performed with the iQ™ SYBR Green PCR Supermix in the DNA Engine Opticon real-time system (Bio-Rad, Hercules, CA, USA), with GAPDH used as an internal control. The primer sequences are as follows: KLF4, 5′-CTGAACAGCAGGGACTGTCA-3′ (forward), 5′-GTGTGGGT GGCTGTTCTTTT-3′ (reverse); GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′ (forward), 5′-TCCACCACCCTGTTGCTGTA-3′ (reverse); p57kip2, 5′-TCTGAGCAGGTCTCTGAGCA (forward), 5′-CAGGAGCCACGTTAGGAGAG (reverse); GADD45β, 5′-GACAACGCGGTTCAGAAGAT (forward), 5′-TGACAGTTCGTGACCAGGAG (reverse).
Small-interfering RNA (siRNA)-mediated gene knockdown
The siRNAs targeting rat KLF4 mRNA (NM_053713.1) were synthesized with the sense sequence siKLF4-1416, 5′-ACCUUGCCUUACACAUGAATT-3′; siKLF4-298, 5′-GGACCUAGACUUUAUCCUUTT-3′. The siRNA against an irrelevant sequence derived from the Thermotoga maritimia genome was used as a control. The double-strand RNAs (100 nM) were transfected into VSMCs with Lipofectamine 2000 (Invitrogen). The control siRNA was used at the same dose. For in vivo use, 15 µg of the siRNA dissolved in 30% pluronic gel solution was perivascularly delivered to the rat carotid arteries immediately after injury as described previously (Simons et al., 1992).
Statistical analysis
All values are expressed as means ± SEM. Student's t-test (paired groups) or one-way anova followed by Newman–Keuls test (multi-group comparisons) were used to analyse the statistical significance of differences. P < 0.05 was considered significant. Non-quantitative results were representative of at least three independent experiments.
Materials
Antibodies against KLF4 and BrdU were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), antibodies against mTOR, phosphorylated mTOR (Ser2448), S6K, phosphorylated S6K (Thr389), 4EBP1 (eukaryotic translation initiation factor 4E binding protein 1), phosphorylated 4EBP1 (Thr70), histone, PCNA were from Cell Signaling Technology (Danvers, MA, USA). Rapamycin, recombinant PDGF-BB and antibody against GAPDH and p27kip1 were from Sigma-Aldrich, Grand Island, NY, USA.
Results
Rapamycin induced KLF4 expression in vitro and in vivo
To examine the effect of rapamycin on gene expression of KLF4, VSMCs were treated with rapamycin (100 ng·mL−1) or vehicle (DMSO) for 12 and 24 h. qRT-PCR showed that mRNA level of KLF4 was significantly increased by rapamycin (Figure 1A). Western blotting showed that rapamycin increased KLF4 expression also at the protein level (Figure 1B).
Figure 1.
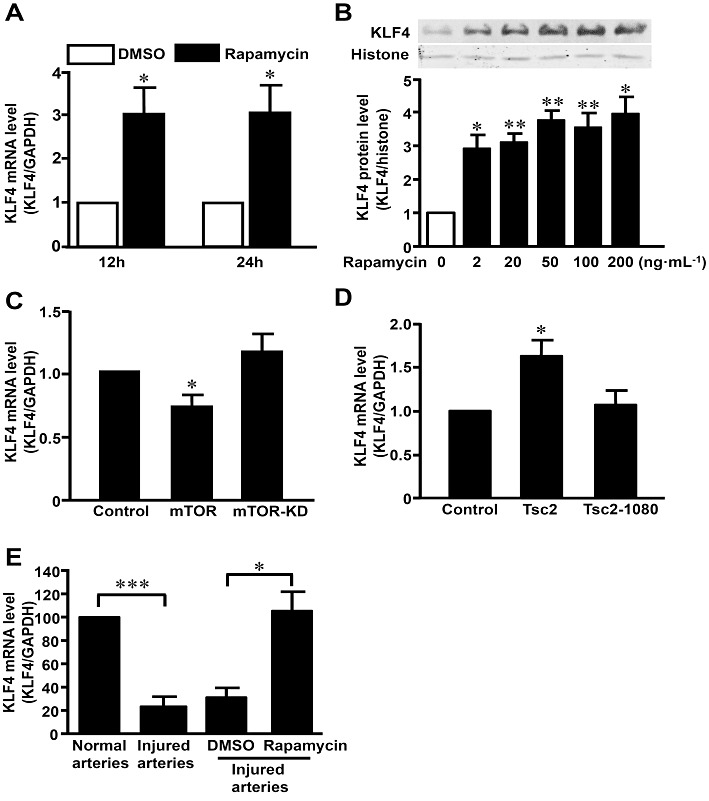
KLF4 was up-regulated by rapamycin in vitro and in vivo. (A) VSMCs were treated with rapamycin (100 ng·mL−1) or control (DMSO) for 12 and 24 h. KLF4 mRNA level was assessed with qRT-PCR and expressed as fold induction compared with the controls after normalizing to GAPDH. (B) After treatment with a range of concentrations of rapamycin (2–200 ng·mL−1) for 24 h, nuclear extracts were immunoblotted with antibodies against KLF4 and histone. Data are representative of five independent experiments. (C) VSMCs were transfected with plasmids expressing mTOR, mTOR-KD or control plasmid. Total RNA was extracted 36 h later and subjected to qRT-PCR for KLF4. (D) KLF4 mRNA levels were detected in VSMCs transfected with TSC2, TSC2-1080 or control plasmid. Total RNA was extracted 36 h later and subjected to qRT-PCR for KLF4. (E) Rat carotid arteries were balloon-injured. Total RNA was extracted 72 h later and subjected to qRT-PCR for KLF4 (n= 6 for each group). Balloon-injured arteries were treated perivascularly with pluronic gel containing rapamycin (100 µg per artery) or DMSO, for 72 h before RNA extraction (n= 3 for each group). *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from control.
Rapamycin exerts its anti-proliferative effect via the suppression of mTOR. To investigate the role of mTOR in the regulation of KLF4 expression, VSMCs were transfected with plasmids expressing wild-type mTOR or the kinase-dead mutant (mTOR-KD). The results showed that expression of mTOR, but not mTOR-KD, decreased gene expression of KLF4 in VSMCs (Figure 1C). Conversely, overexpression of the tumour suppressor tuberous sclerosis complex (TSC)-2, which functions as a complex with TSC1 to negatively regulate the activity of mTOR, increased the gene expression of KLF4. However, TSC-2-1-1080, a truncated mutant lacking the GTPase-activating domain, had little effect (Figure 1D). The results suggested that KLF4 induction by rapamycin might involve inhibition of the mTOR pathway.
To further examine the effect of rapamycin on KLF4 in vivo, rat carotid arteries were injured and treated perivascularly with pluronic gel containing rapamycin or vehicle (DMSO). qRT-PCR showed that KLF4 expression was decreased in injured arteries (n= 6 for each group, P < 0.01), and rapamycin significantly up-regulated KLF4 (n= 3 for each group, P < 0.05) (Figure 1E). Similarly, rapamycin also increased KLF4 in uninjured arteries (Figure S1) (n= 3 for each group, P < 0.05).
Overexpression of KLF4 inhibited mTOR activation and induced the expression of p27kip1
To explore the functional role of KLF4 in mediating the effect of rapamycin on VSMCs, we constructed a tetracycline-regulated adenovirus expressing KLF4 (AdKLF4). As shown in Figure 2A, overexpression of KLF4 was observed in VSMCs co-infected with AdKLF4 and AdtTA, a virus expressing the tetracycline-responsive transactivator, which drives the tetracycline-controlled expression of KLF4. Furthermore, immunofluorescence confirmed the nuclear localization of the overexpressed KLF4 (Figure 2B).
Figure 2.
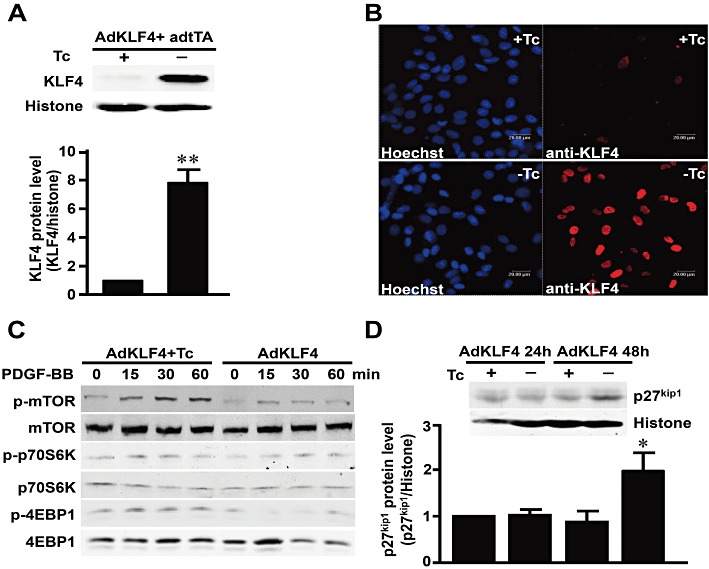
Overexpression of KLF4 inhibited the activation of mTOR. VSMCs were co-infected with AdKLF4 and AdtTA (20 MOI) and maintained in the medium with or without tetracycline (Tc; 0.1 µg·mL−1). (A) Nuclear protein lysates were immunoblotted with antibodies against KLF4 or histone H3 as an internal control. (B) Immunofluorescent staining was performed using a primary antibody against KLF4 and detected with a rhodamine-conjugated secondary antibody. Nuclei were counterstained with Hoechst 33258. (C) VSMCs were treated with PDGF-BB (20 ng·mL−1) for the indicated time, and total proteins were immunoblotted with antibodies against mTOR, p70S6K, 4EBP1 or their phosphorylated forms. Data shown are representative of three independent experiments. (D) Nuclear protein lysates were immunoblotted with antibody against p27kip1 and histone H3. Data are representative of three independent experiments. **P < 0.01, *P < 0.05, significantly different from VSMCs infected with AdtTA and AdKLF4 in the presence of Tc.
As KLF4 was up-regulated by rapamycin and down-regulated by overexpression of mTOR in VSMCs, we examined whether induced expression of KLF4 could also affect mTOR activation. VSMCs were infected with AdKLF4 and adtTA for 48 h and stimulated with PDGF-BB. PDGF-BB was chosen because of its high affinity to three types of PDGF receptors and its pathophysiological role in restenosis (Raines, 2004). Western blotting showed that KLF4 overexpression markedly attenuated the PDGF-induced phosphorylation of mTOR and its downstream targets 4E-BP1 and p70S6K (Figures 2C and S2).
As previous studies showed that p27kip1 was essential for the anti-proliferation effect of rapamycin, we also examined whether overexpression of KLF4 could affect the expression of p27kip1. VSMCs were infected with AdKLF4 and AdtTA for 48 h. Western blotting showed that KLF4 overexpression induced p27kip1 (Figure 2D). The result suggested that induction of KLF4 might enhance the role of rapamycin through inhibiting activation of mTOR and up-regulating p27kip1.
KLF4 inhibited the proliferation and intimal hyperplasia in injured arteries
To explore the functional role of KLF4 in neointimal hyperplasia, rat carotid arteries were balloon-injured and adenovirally transduced to express KLF4 or LacZ as a control. KLF4 transgene expression in the vessel wall was confirmed with immunohistochemical staining (Figure 3A). Intimal cell proliferation was evaluated with BrdU incorporation 7 days after balloon angioplasty, when the intimal proliferation peaked. The results showed that BrdU-incorporated intimal cells were decreased by approximately 50% in the KLF4-transduced arteries compared with the LacZ-transduced arteries (Figure 3A), indicating that KLF4 inhibited intimal SMC proliferation in vivo.
Figure 3.
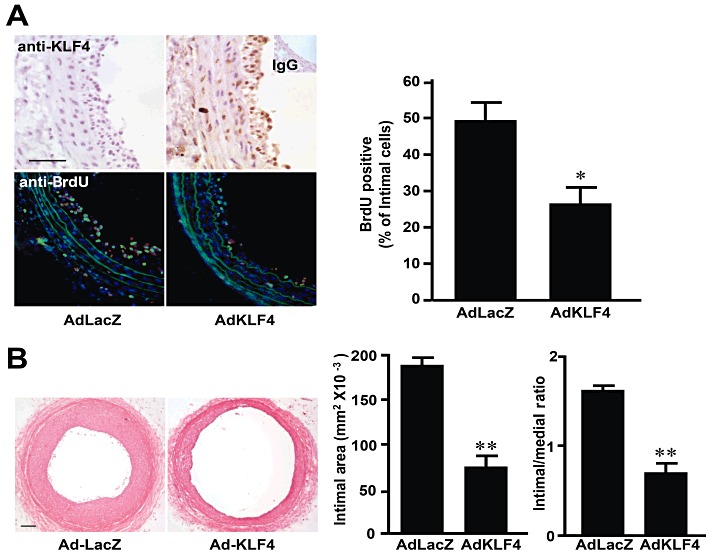
Overexpression of KLF4 inhibited neointimal formation. (A) Rat carotid arteries were balloon injured and infected with AdtTA and AdKLF4 or AdLacZ. Vessel segments were harvested 7 days later for immunohistochemical staining of KLF4 (a rabbit IgG was used as negative control) and BrdU. The bar graph indicates the percentage of BrdU-positive cells in the neointima (n= 4 for each group). (B) Rat carotid arteries were balloon-injured and co-infected with AdtTA and AdKLF4 or with AdLacZ. Vessel segments were harvested 21 days later and cross-sections were stained with haematoxylin–eosin to evaluate neointimal formation. Intimal and medial areas were measured and expressed as mean ± SEM of neointimal area (mm2) and intima-to-media ratio (I/M) (n= 11 for Lac Z, n= 10 for KLF4) (Scale bar =100 µm, **P < 0.01, *P < 0.05, significantly different from values with AdLacZ).
IMT and vascular lumen sizes were examined by ultrasound biomicroscopy on days 7, 14 and 21 after gene delivery. Overexpression of KLF4 effectively inhibited the increase of IMT and preserved the lumen size, compared with the vector control (Table S1). The animals were killed on day 21 after gene delivery, for morphometric analysis of the cross-sections of the injured carotid arteries. The results (Figure 3B) revealed that the carotid arteries infected with the control adenovirus exhibited pronounced neointimal formation, while those infected with AdKLF4 had a significantly reduced neointimal hyperplasia (intimal areas: 184.4 ± 9.3 versus 72.1 ± 12.9 mm2 × 10−3, P < 0.01, I/M ratio: 1.60 ± 0.62 versus 0.69 ± 0.11, P < 0.01, n= 11 for control, n= 10 for KLF4). No difference in medial area was observed between the two groups (data not shown). These results demonstrated that KLF4 suppressed neointimal hyperplasia in injured arteries.
KLF4 up-regulated an anti-proliferative gene profile in SMCs
To identify the potential targets of KLF4 that mediated the inhibition of vascular remodelling, gene profiles were compared between the AdKLF4- or mock-infected SMCs with cDNA array by using Affymetrix U230 2.0 gene chips (Figure S3). Among the KLF4-regulated genes, proliferation-related genes including two cell cycle regulators, cyclin-dependent kinase inhibitor 1c (p57kip2) and growth arrest and DNA damage inducible gene β (GADD45β), were validated in vitro and in vivo. As shown in Figure 4A and B, overexpression of KLF4 significantly induced the expression of p57kip2 and GADD45β in cultured SMCs and in balloon-injured arteries. It is thus suggested that induction of these anti-proliferative genes may contribute to the suppressive effects of KLF4 on neointimal formation.
Figure 4.
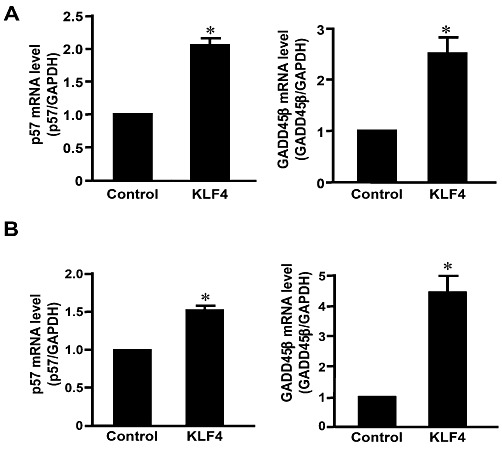
KLF4 regulated cell cycle-related genes in SMCs. (A) RNA was isolated from SMCs infected with AdKLF4 or mock for 36 h. Gene expression of p57kip2 and GADD45β were validated with qRT-PCR. (B) Rat carotid arteries were balloon-injured and infected with AdKLF4 or AdLacZ with Ad tTA. RNA was extracted from the vessels 72 h after infection and subjected to qRT-PCR. *P < 0.05, **P < 0.01, significantly different from control group. n= 3 for each group.
RNA interference of KLF4 enhanced the proliferation of VSMCs
To examine whether endogenous KLF4 plays a role in VSMC proliferation, we transfected VSMCs with KLF4 siRNAs (two sets of siRNA targeting different regions of KLF4 mRNA were used to ensure efficient gene silencing) or control siRNA. KLF4 siRNA efficiently decreased KLF4 at both mRNA and protein levels (Figure 5A,B). Knockdown of endogenous KLF4 significantly increased the serum-induced DNA synthesis in VSMCs (Figure 5C).
Figure 5.
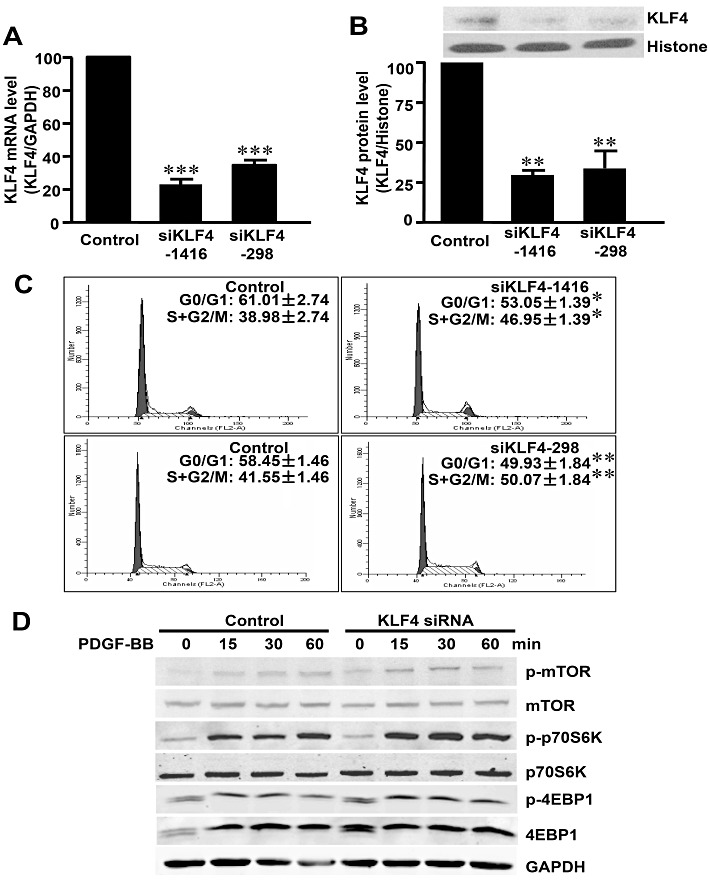
Knockdown of KLF4 increased the proliferation of VSMCs. VSMCs were transfected with KLF4 siRNAs (siKLF4-1416 or siKLF4-298) or control siRNA (100 nM each) for 48 h. KLF4 expression level was assessed with qRT-PCR (A) and Western blotting (B). (C) Synchronized VSMCs were transfected with two independent siRNA, siKLF4-1416 or siKLF4-298, and harvested for cell cycle analysis 24 h after serum stimulation with flow cytometry. (D) SMCs were transfected with KLF4 siRNA (siKLF4-1416) or control siRNA for 48 h and then were stimulated with PDGF-BB with indicated times. Total protein lysate was immunoblotted with antibodies against mTOR, p70S6K, 4EBP1 and their phosphorylated forms. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from siRNA control group.
To elucidate the effect of KLF4 on the activation of mTOR, VSMCs were transfected with KLF4 siRNA or control siRNA for 48 h and stimulated with PDGF-BB. Knockdown of KLF4 potentiated the PDGF-BB induced phosphorylation of mTOR and its downstream p70S6K and 4EBP1 (Figure 5D). These results suggested that endogenous KLF4 played an important role in the proliferation of VSMCs, by inhibiting their proliferation and suppressing activation of the mTOR pathway, induced by PDGF-BB.
Knockdown of KLF4 attenuated the anti-proliferative role of rapamycin in vitro and in vivo
To explore the possibility that KLF4 mediated the anti-proliferative role of rapamycin, we examined the effect of rapamycin upon KLF4 siRNA transfected-VSMCs and injured arteries. We found that knockdown of KLF4 attenuated the suppressive effect of rapamycin on VSMC proliferation (Figure 6A) and the expression of proliferation cell nuclear antigen (PCNA), a well-established marker for cell proliferation (Figure 6B). These results suggested that knockdown of KLF4 reduced the sensitivity of VSMCs to the anti-proliferative effect of rapamycin. We further examined the role of endogenous KLF4 in mediating the anti-proliferative effect of rapamycin in vivo. Perivascular delivery of KLF4 siRNA effectively decreased the KLF4 mRNA level in injured carotid arteries (Figure S4) and resulted in increased BrdU incorporation in injured arteries and neointimal formation (BrdU incorporation ratio: 43.8% ± 1.8% vs. 55.8% ± 4.7%, P < 0.05, n= 3 for each group. intimal/medial ratio: 0.27 ± 0.03 vs. 0.46 ± 0.04, P < 0.001, n= 3 for each group). KLF4 siRNA attenuated the inhibitory effects of rapamycin on intimal proliferation in injured arteries (BrdU incorporation ratio: 8.0% ± 1.5% vs. 32.0% ± 4.1%, P < 0.01, n= 3 for each group. intimal/medial ratio: 0.13 ± 0.01 vs. 0.22 ± 0.01, P < 0.05, n= 3 for each group) (Figure 6C,D). Taken together, these results suggested that endogenous KLF4 functioned as a negative regulator of VSMC proliferation and the induction of KLF4 contributed to the anti-proliferative action of rapamycin in vitro and in vivo.
Figure 6.
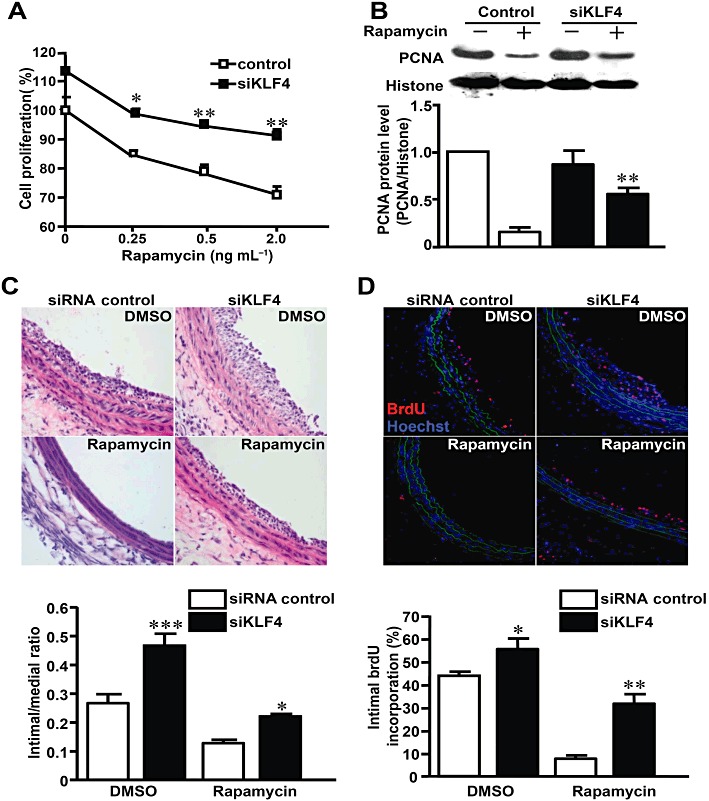
Knockdown of KLF4 suppressed the effect of rapamycin in vitro and in vivo. (A) After transfection with siRNAs, VSMCs were stimulated with 10% FBS and exposed to different concentrations of rapamycin or DMSO for 48 h. Cell numbers were counted and expressed as percentage of the DMSO-treated and control siRNA-transfected group. Comparison was made between siKLF4 and control siRNA groups at various concentrations of rapamycin. (B) After transfection with siRNAs, VSMCs were stimulated with 10% FBS in the presence or absence of rapamycin. Nuclear extracts were immunoblotted with antibodies against PCNA and histone 24 h later. (C) Rat carotid arteries were balloon-injured and treated with perivascular pluronic gel containing siKLF4 or control siRNA (15 µg per artery) and rapamycin (100 µg per artery) or DMSO. The injured arteries were harvested 7 days later and stained with haematoxylin–eosin. (D) BrdU incorporation was assessed 7 days later using anti-BrdU antibody (TRITC). Nuclei were stained with Hoechst 33258. The bar graph indicates the percentage of BrdU-positive cells in the neointima (n= 3 for each group). *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from siRNA control group.
Discussion
In this study, we describe the novel finding that rapamycin induced expression of KLF4 in vitro and in vivo. We also demonstrated a critical role of KLF4 in mediating the anti-proliferative effects of rapamycin on VSMC proliferation.
Rapamycin is an anti-proliferative agent, widely used to inhibit post-angioplasty restenosis. In addition to a well-recognized action on protein translational machinery via the suppression of mTOR-mediated signal pathways, recent studies indicated that the anti-proliferative effect of rapamycin may also involve the regulation of transcriptional mechanisms controlling cellular proliferation (Zohlnhofer et al., 2004). However, the transcription factor(s) that mediate the anti-proliferative effects of rapamycin remain less characterized. Here, our results show that rapamycin induced KLF4 in VSMCs and arteries (Figures 1B,E and S1). However, because of the low abundance of endogenous KLF4 protein in arteries, the effect of rapamycin on KLF4 protein level in arteries still needs further confirmation. Our results indicated that induction of KLF4 may represent a novel, transcriptional regulation step, in the mediation of the pharmacological actions of rapamycin.
Previous groups have found that certain types of cells exhibited a decreased sensitivity to rapamycin (Dumont et al., 1995; Sugiyama et al., 1996). Here, we report, for the first time, that KLF4 may affect the sensitivity to rapamycin in VSMCs and injured arteries (Figure 6A,D). This effect may be explained by a reciprocal inhibition between mTOR and KLF4. This interaction is supported by our observation that expression of KLF4 was suppressed by mTOR but not by its kinase-dead mutant (Figure 1C), and was increased by TSC2, a suppressor of mTOR (Figure 1D). Moreover, KLF4 inhibited the phosphorylation of mTOR and its downstream targets p70S6K and 4E-BP1 (Figures 2C and S2). As mTOR is activated in PDGF-stimulated VSMCs and in restenosis, suppression of mTOR may be involved in the anti-proliferative effect of KLF4 in VSMCs (Tang et al., 2008).
We also found that overexpression of KLF4 increased p27kip1 (Figure 2D), which is consistent with the previously reported effect of rapamycin (Marx and Marks, 2001; Moss et al., 2010). Conversely, siRNA-mediated knockdown of KLF4 increased the activity of mTOR as well as its downstream targets (Figure 5D). Taken together with the in vivo finding that knockdown of KLF4 attenuated the anti-proliferative effect of rapamycin (Figure 6D), it appears that induction of KLF4 contributes to the anti-proliferative effect and that the mechanisms, among others, may involve a suppression of mTOR by KLF4.
Furthermore, we asked whether KLF4 was essential for the inhibition of mTOR downstream pathways by rapamycin. We compared the effects of rapamycin on 4EBP1 phosphorylation stimulated by PDGF-BB in VSMCs transfected with KLF4 siRNA or scrambled control (Figure S5). The results show that phosphorylation of 4EBP1 was increased in the KLF4-silenced cells in the presence of rapamycin, whereas the effect of rapamycin was only partially diminished. Thus, KLF4 may have a contributing rather than a pivotal role in blocking the pathways downstream of mTOR activation. In this regard, our microarray study demonstrated that GADD45 and p57kip2, among others, may also be involved in mediating the anti-proliferative action of rapamycin.
Pro-inflammatory processes including the activation of macrophages and VSMCs also play an important role in the development of restenosis after angioplasty (Bayes-Genis et al., 2002; Shagdarsuren et al., 2009). The anti-inflammatory effect of rapamycin also contributes to its pharmacological actions (Zohlnhofer et al., 2004) but KLF4 may promote the activation of monocytes/macrophages and prevent the differentiation of VSMCs (Liu et al., 2005; Feinberg et al., 2007; Yoshida et al., 2008). In addition, the effect of KLF4 on VSMC migration, also a key step in the intimal hyperplasia, remains to be studied.
In conclusion, the present study demonstrated that the transcription factor KLF4 was induced by rapamycin and played an important role in mediating the anti-proliferative effect of rapamycin on VSMCs. It is suggested that KLF4 is a potential target for the development of novel approaches to the treatment of proliferative vascular disorders, such as restenosis after angioplasty.
Acknowledgments
This study was supported by the grants from the National Natural Science Foundation of China (30670848, 30890041 and 30910280), the Ministry of Science and Technology (2010CB912500) and the National Health Institute (HL106579).
Glossary
- Ad-KLF4
adenovirus expressing KLF4
- ECs
endothelial cells
- GADD45β
growth arrest and DNA damage inducible gene β
- IMT
intimal–medial thickness
- KLF4
Krüppel-like factor 4
- mTOR
mammalian target of rapamycin
- mTOR-KD
kinase-dead mTOR
- p27kip1
cyclin-dependent kinase inhibitor 1b
- p57kip2
cyclin-dependent kinase inhibitor 1c
- p70S6K
p70 S6 kinase
- PDGF
platelet-derived growth factor
- PTCA
percutaneous transluminal coronary angioplasty
- tTA
tetracycline-controlled transactivator
- VSMCs
vascular smooth muscle cells
- 4EBP1
eukaryotic translation initiation factor 4E binding protein 1
Conflicts of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Effect of rapamycin on KLF4 expression of normal arteries. Rat carotid arteries were isolated and pluronic gel, containing DMSO or rapamycin (100 μg perartery), applied perivascularly. Total RNA was isolated and subjected to qRT-PCR to validate the expression of KLF4 and GAPDH24 h later (n = 3 for each group, *P < 0.05).
Figure S2 Quantification of Western blotting in Figure 2C. For the Western blotting in Figure 2C, band intensities were scanned, quantified with NIH Image J and expressed as fold of control. Data are mean ± SEM from three to four independent experiments. *P < 0.05, ***P < 0.001, compared with control SMCs stimulated with PDGF-BB for 30 min. #P < 0.05, ###P < 0.001, compared with control SMCs stimulated with PDGF-BB for 60 min. NS, not significant.
Figure S3 Microarray analysis of KLF4-overexpressed SMCs. Scatter plot shows the differential gene expression in SMCs with or without KLF4 overexpression.
Figure S4 SiRNA-mediated KLF4 knockdown in rat carotid arteries. Rat carotid arteries were balloon injured and perivascularly treated with pluronic gel containing siKLF4 or control siRNA (15 μg per artery). Total RNA was extracted 72 h later and subjected to qRT-PCR for KLF4. *P < 0.05, vs. control siRNA, n = 3.
Figure S5 Effect of rapamycin on the downstream targets of mTOR. SMCs were transfected with KLF4 siRNA or control siRNA for 24 h and pretreated with rapamycin or DMSO for 30 min. Cells were then stimulated with PDGF-BB. Levels of 4EBP1 and its phosphorylated forms were detected with Western blotting using GADPH as internal control. *P < 0.05, compared with control SMCs stimulated with PDGF-BB, #P < 0.05, compared with control SMCs treated with rapamycin and PGDF-BB.
Table S1 Ultrasonic evaluation of carotid arteries
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adam PJ, Regan CP, Hautmann MB, Owens GK. Positive- and negative-acting Kruppel-like transcription factors bind a transforming growth factor beta control element required for expression of the smooth muscle cell differentiation marker SM22alpha in vivo. J Biol Chem. 2000;275:37798–37806. doi: 10.1074/jbc.M006323200. [DOI] [PubMed] [Google Scholar]
- Agrotis A, Kanellakis P, Kostolias G, Di Vitto G, Wei C, Hannan R, et al. Proliferation of neointimal smooth muscle cells after arterial injury. Dependence on interactions between fibroblast growth factor receptor-2 and fibroblast growth factor-9. J Biol Chem. 2004;279:42221–42229. doi: 10.1074/jbc.M408121200. [DOI] [PubMed] [Google Scholar]
- Alder JK, Georgantas RW, 3rd, Hildreth RL, Kaplan IM, Morisot S, Yu X, et al. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J Immunol. 2008;180:5645–5652. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes-Genis A, Campbell JH, Carlson PJ, Holmes DR, Jr, Schwartz RS. Macrophages, myofibroblasts and neointimal hyperplasia after coronary artery injury and repair. Atherosclerosis. 2002;163:89–98. doi: 10.1016/s0021-9150(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- Dumont FJ, Staruch MJ, Grammer T, Blenis J, Kastner CA, Rupprecht KM. Dominant mutations confer resistance to the immunosuppressant, rapamycin, in variants of a T cell lymphoma. Cell Immunol. 1995;163:70–79. doi: 10.1006/cimm.1995.1100. [DOI] [PubMed] [Google Scholar]
- Feinberg MW, Cao Z, Wara AK, Lebedeva MA, Senbanerjee S, Jain MK. Kruppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. J Biol Chem. 2005;280:38247–38258. doi: 10.1074/jbc.M509378200. [DOI] [PubMed] [Google Scholar]
- Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, et al. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;253:1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Gallo R, Padurean A, Jayaraman T, Marx S, Roque M, Adelman S, et al. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99:2164–2170. doi: 10.1161/01.cir.99.16.2164. [DOI] [PubMed] [Google Scholar]
- Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, et al. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Inoki K, Guan KL. Complexity of the TOR signaling network. Trends Cell Biol. 2006;16:206–212. doi: 10.1016/j.tcb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992;89:507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KE, Iyemere VP, Weissberg PL, Shanahan CM. Kruppel-like factor 4 (KLF4/GKLF) is a target of bone morphogenetic proteins and transforming growth factor beta 1 in the regulation of vascular smooth muscle cell phenotype. J Biol Chem. 2003;278:11661–11669. doi: 10.1074/jbc.M211337200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- Marks AR. Cellular functions of immunophilins. Physiol Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- Marx SO, Marks AR. Bench to bedside: the development of rapamycin and its application to stent restenosis. Circulation. 2001;104:852–855. doi: 10.1161/01.cir.104.8.852. [DOI] [PubMed] [Google Scholar]
- Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–417. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- Morris RE, Cao W, Huang X, Gregory CR, Billingham ME, Rowan R, et al. Rapamycin (Sirolimus) inhibits vascular smooth muscle DNA synthesis in vitro and suppresses narrowing in arterial allografts and in balloon-injured carotid arteries: evidence that rapamycin antagonizes growth factor action on immune and nonimmune cells. Transplant Proc. 1995;27:430–431. [PubMed] [Google Scholar]
- Moss SC, Lightell DJ, Jr, Marx SO, Marks AR, Woods TC. Rapamycin regulates endothelial cell migration through regulation of the cyclin-dependent kinase inhibitor p27Kip1. J Biol Chem. 2010;285:11991–11997. doi: 10.1074/jbc.M109.066621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuyoshi M, Kimura T, Nosaka H, Mioka S, Ueno K, Yokoi H, et al. Restenosis after successful percutaneous transluminal coronary angioplasty: serial angiographic follow-up of 229 patients. J Am Coll Cardiol. 1988;12:616–623. doi: 10.1016/s0735-1097(88)80046-9. [DOI] [PubMed] [Google Scholar]
- Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Razuvaev A, Lund K, Roy J, Hedin U, Caidahl K. Noninvasive real-time imaging of intima thickness after rat carotid artery balloon injury using ultrasound biomicroscopy. Atherosclerosis. 2008;199:310–316. doi: 10.1016/j.atherosclerosis.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Luijten HE, Beatt KJ, Geuskens R, de Feyter PJ, van den Brand M, et al. Incidence of restenosis after successful coronary angioplasty: a time-related phenomenon. A quantitative angiographic study in 342 consecutive patients at 1, 2, 3, and 4 months. Circulation. 1988;77:361–371. doi: 10.1161/01.cir.77.2.361. [DOI] [PubMed] [Google Scholar]
- Shagdarsuren E, Djalali-Talab Y, Aurrand-Lions M, Bidzhekov K, Liehn EA, Imhof BA, et al. Importance of junctional adhesion molecule-C for neointimal hyperplasia and monocyte recruitment in atherosclerosis-prone mice-brief report. Arterioscler Thromb Vasc Biol. 2009;29:1161–1163. doi: 10.1161/ATVBAHA.109.187898. [DOI] [PubMed] [Google Scholar]
- Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Edelman ER, DeKeyser JL, Langer R, Rosenberg RD. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992;359:67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Sousa JE, Costa MA, Abizaid A, Abizaid AS, Feres F, Pinto IM, et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103:192–195. doi: 10.1161/01.cir.103.2.192. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Papst P, Gelfand EW, Terada N. p70 S6 kinase sensitivity to rapamycin is eliminated by amino acid substitution of Thr229. J Immunol. 1996;157:656–660. [PubMed] [Google Scholar]
- Tang Z, Wang Y, Fan Y, Zhu Y, Chien S, Wang N. Suppression of c-Cbl tyrosine phosphorylation inhibits neointimal formation in balloon-injured rat arteries. Circulation. 2008;118:764–772. doi: 10.1161/CIRCULATIONAHA.107.761932. [DOI] [PubMed] [Google Scholar]
- Wang N, Verna L, Chen NG, Chen J, Li H, Forman BM, et al. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem. 2002;277:34176–34181. doi: 10.1074/jbc.M203436200. [DOI] [PubMed] [Google Scholar]
- Yet SF, McA'Nulty MM, Folta SC, Yen HW, Yoshizumi M, Hsieh CM, et al. Human EZF, a Kruppel-like zinc finger protein, is expressed in vascular endothelial cells and contains transcriptional activation and repression domains. J Biol Chem. 1998;273:1026–1031. doi: 10.1074/jbc.273.2.1026. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102:1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohlnhofer D, Nuhrenberg TG, Neumann FJ, Richter T, May AE, Schmidt R, et al. Rapamycin effects transcriptional programs in smooth muscle cells controlling proliferative and inflammatory properties. Mol Pharmacol. 2004;65:880–889. doi: 10.1124/mol.65.4.880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


