Abstract
HIV risk behaviors, susceptibility to HIV acquisition, progression of disease after infection, and response to anti-retroviral therapy all vary by age. In those living with HIV, current effective treatment has increased the median life expectancy to > 70 years of age. Biologic, medical, individual social and societal issues change as one ages with HIV infection, but there has been only a small amount of research in this field. Therefore, the Office of AIDS Research of the National Institutes of Health commissioned a working group to develop an outline of the current state of knowledge and areas of critical need for research in HIV and Aging; the working groups’ findings and recommendations are summarized in this report. Key overarching themes identified by the group included: multi-morbidity, poly-pharmacy and the need to emphasize maintenance of function; the complexity of assessing HIV vs. treatment effects vs. aging vs. concurrent disease; the inter-related mechanisms of immune senescence, inflammation and hypercoagulability; the utility of multi-variable indices for predicting outcomes; a need to emphasize human studies to account for complexity; and a required focus on issues of community support, caregivers and systems infrastructure. Critical resources are needed to enact this research agenda and include expanded review panel expertise in aging, functional measures and multi-morbidity, as well as facilitated use and continued funding to allow long-term follow-up of cohorts aging with HIV.
Keywords: Aging, HIV/AIDS, Co-morbidity, Research Priorities
Executive Summary
The development and application of effective antiretroviral therapy (ART) for HIV has allowed many infected persons to live to an older age. In addition, an increasing proportion of incident HIV infections are occurring in older adults as members of this age group are the least likely to practice safe sex and late-life changes in the reproductive tract and immune system may enhance susceptibility to HIV acquisition in seniors. Thus, by 2015, half the people living with HIV infection in the United States will be 50 years of age or over. Research in Sub-Saharan Africa suggests that these trends are also occurring in more resource limited settings (1,2). Further, there is an emerging consensus that HIV and/or its treatment affecs the process of aging and/or the development of illnesses typically associated with advanced age. When compared to behaviorally and demographically similar HIV-uninfected individuals, people with HIV infection, even those receiving effective ART with suppression of virus to levels below typical detection limits, experience excess morbidity and mortality (3,4). On average, a 20 year old initiating ART may have already lost 1/3 of the expected remaining years of life compared to demographically similar HIV-uninfected persons (5).
While AIDS-defining illnesses are increasingly rare in those with ART-suppressed HIV, the list of HIV-associated, Non-AIDS (HANA) conditions is growing. A common theme among currently identified HANA conditions is their association with advancing age and chronic inflammation. These include cardiovascular disease (6), a number of infectious and noninfectious cancers (7,8), osteopenia/osteoporosis (9), liver disease (10), renal disease (11,12) and neurocognitive decline. It is uncertain whether people with HIV infection develop these conditions earlier in their life course because the aging process itself is accelerated (i.e., is HIV speeding pathways of aging in every organ?) (13) or whether HIV is an additive risk factor (i.e., is HIV similar to high cholesterol which does not make one “age” faster, but increases the risk of cardiovascular events?).
Any comparison between people with and without HIV infection must be accomplished with careful study design as these populations tend to differ in a number of behavioral and biologic factors that are known to affect the aging process. People aging with HIV infection are more likely to continue substance use (tobacco, alcohol, opioids, and other psychoactive substances) (14). People with HIV infection are also more likely to be co-infected with chronic viruses such as hepatitis C, which interacts with HIV or with alcohol use to lead to more rapid cirrhosis and more rapid development of hepatocellular carcinoma. People with HIV infection differentially represent sexual and racial minorities with constrained economic and social resources. As a result, issues of homelessness, food insecurity, and social isolation may exacerbate substance use and complicate the aging process broadly – physically, emotionally, and socially.
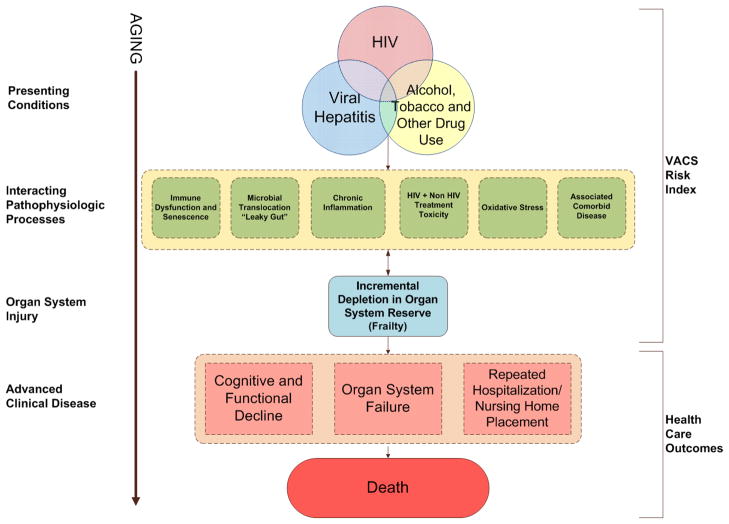
The pathophysiology leading to morbidity and mortality among those aging with HIV is only beginning to be elucidated. Evidence from the Strategies for Management of Antiretroviral Therapy (SMART) trial and other observational studies suggests that HIV infection and ART influence morbidity and mortality through effects on inflammation, treatment-related toxicity (which includes abnormal fat distribution, renal and kidney dysfunction and neuropathy), interactions with other chronic viral infections and co-morbid diseases typically associated with advanced age. This complex and often subtle pathophysiology also interacts with prolonged substance use and other psychosocial and health behaviors more commonly experienced by those with HIV infection. As a result, aging HIV-infected persons exhibit an excess burden of co-morbid conditions and the premature onset of a number of clinical symptoms and syndromes that are often associated with advanced aging, multi-morbidity, poly-pharmacy, limited reserve and functional (physical and cognitive) decline (Figure 1). Addressing these aspects of heath care is the primary domain of the subspecialty of Geriatric Medicine which can help inform the research agenda for HIV and the clinical management of those aging with HIV infection (15).
Figure 1.
One model of integrated pathophysiology of aging with HIV infection. The sequelae of residual immune dysfunction (inflammation/hypercoagulation), concomitant viral infections, ART side effects, and multi-morbidity accelerate the development of HIV-associated, non-AIDS (HANA) and other common diseases of aging. This leads to premature functional decline, vulnerability to additional illnesses and death. Social isolation of older HIV-infected adults and care giving issues further complicate management and impair quality of life. From (17) with permission.
Recognizing the issues and needs of the evolving HIV-infected population and the potential insights provided through the pathophysiology of aging (Gerontology) and the care of older adults (Geriatrics), September 18th has been designated National HIV/AIDS and Aging Awareness Day since 2008, and a White House Conference on HIV and Aging was held in 2010 (16). However, the extent to which Geriatric principles can be directly applied to HIV-infected individuals is unknown. While parallels with other chronic diseases such as cardiovascular disease or diabetes are compelling, several factors set apart those aging with HIV. At the moment, the population aging with HIV infection is predominantly middle aged. Many geriatric syndromes of greatest concern in the general population, including dementia, frailty, and falls, are not common among those under 65 years of age and are frequently seen only in those 80+ years of age. Thus, we may not fully appreciate the importance of these conditions until a larger proportion of the population of those aging with HIV infection reaches older thresholds. The Geriatric concepts of multi-morbidity, personalized care, maximizing function, and deriving integrated management strategies are, however, very likely relevant to the care of the growing middle to older age adult population with HIV.
To further address the issues of aging with HIV infection, the NIH Office of AIDS Research assembled a working group with the goal of assessing what is known and unknown, and what the priorities should be for research at the interface of HIV, aging, and multi-morbidity. The task force met in a face-to-face meeting in April 2011, and assigned breakout groups to address four specific areas – 1) Triggers and Underlying Mechanisms of Aging in those with HIV; 2) Biomarkers/Prognostic Indices of Aging and Illness; 3) Design and Conduct of Observational and Intervention Studies; and 4) Societal, Mental Health, Behavioral and Care giving Issues. The working group force findings are summarized in this white paper.
Several common themes emerged during the discussions:
Multi-morbidity, poly-pharmacy and functional assessment – Aging with HIV infection is typically characterized by the development of multiple chronic diseases and treatments. The consequences of HIV infection and its treatment on aging is best addressed by strategies that focus on preserving function rather than curing disease. The evidence base of Geriatric Medicine and gerontology provides a strong foundation for studying these issues in those aging with HIV.
The complexity of HIV vs. Treatment Effects vs. Aging vs. Disease – It is the nature of science to design studies that isolate specific risk factors. In those aging with HIV, it is nearly impossible to separate these factors. Even if it were possible, study designs that isolate unique factors may not be necessary or desired as none of these factors occur in isolation. However, it is imperative that studies be appropriately designed and use control groups that can accurately reflect populations of similar complexity. Particularly relevant is the need for study populations and control groups to gather relevant information on use of alcohol, tobacco, and psychoactive drugs, mental health, co-morbid illness, and functional status (both physical and cognitive).
The utility of multi-variable indices in predicting outcomes – In complex, multi-morbid illness it is often necessary to utilize well-validated indices to better predict which patients need specific interventions or are at high-risk for specific complications. Integration of clinical measures, common laboratory values and emerging biomarkers is likely to be more robust than relying on simple, traditional, HIV-specific variables such as CD4 count or viral load.
Immune senescence, inflammation and hypercoagulability – These appear to be unifying mechanisms that may lead to the greater incidence of disease in persons aging with HIV, and should be a focus for future research drawing on the substantial database already available from HIV-uninfected cohorts.
Emphasis on human studies – though animal studies, particularly nonhuman primate studies, have a role to play in this research, the lack of animal models that can appropriately reproduce complexities of HIV, ART, aging, social context, multi-morbidity and functional outcomes of relevance indicate that human studies should be the primary focus of research in this area.
The need to address issues of community support, caregivers and systems infrastructure –social/behavioral issues and isolation of many persons aging with HIV infection require unique solutions for this population, and dedicated resources to be deployed in the community.
Resources Required to Meet Critical Needs in this Area
Study Section Expertise – Additional expertise is needed in HIV-focused study sections to appropriately evaluate studies of aging. Though ad hoc reviewers can evaluate individual proposals, it is vital that multiple, standing members elevate the discussion and expand the knowledge base of the whole group to most effectively evaluate new research proposals.
Facilitated use of current and future cohorts – a number of HIV-infected cohorts have been established with NIH funding (e.g. the Multicenter AIDS Cohort Study (MACS), the Women’s Interagency HIV Study (WIHS), the Veterans Aging Cohort Study (VACS)) that have and will greatly facilitate the study of issues raised in this document. It is critical that these study participants continue to be followed and that variables related to aging be collected in a rigorous manner. Further, HIV-uninfected cohorts being accrued at this time (e.g. the Aspirin in Reducing Events in the Elderly (ASPREE) trial, the Systolic Blood Pressure Intervention Trial (SPRINT), and the VACS) should include HIV-infected subjects and variables/specimens collected to allow evaluation of HIV and aging. AIDS Clinical Trial Group (ACTG) and other HIV-focused clinical research sites should be encouraged to become enrolling sites for these crucial studies on aging and health.
HIV and aging in the larger context of the HIV research portfolio
Although the priority for research on HIV and aging vs. other important issues in HIV research is a question well beyond the purview of this working group, the working group members believe the issues outlined here are high priority as more than 50 percent of individuals affected by HIV in the U.S will be age 50 years or greater by 2015. A common theme from the geriatric literature is that proactive prevention of geriatric syndromes is preferred to interventions aimed to reverse diseases which have already occurred; hence, interventions aimed at addressing concerns outlined in this report will need to be implemented soon if the large population of aging HIV-infected adults are to benefit. Further, as outlined in the White House Conference (16), issues regarding HIV in aging people is a high priority area among a large and diverse community of advocacy groups active in the area of HIV. Finally, as was true of immunology research in the early days of HIV, studies in this area of AIDS research are likely to have dramatic impact on other fields. Compression of disease development and functional decline in HIV-infected individuals offers a unique model of multi-morbidity that should be applicable to many populations (e.g. the aged, dialysis patients, chronic inflammatory conditions such as rheumatoid arthritis). Thus, research on multiple, coexistent conditions and the preservation of function in aging HIV-infected individuals is likely to pay dividends for the wider field of healthcare. Specific areas of active research in the larger community that may benefit from knowledge gained studying HIV and aging include the biology of inflammation and disease development, the identification and validation of clinically relevant biomarkers, the comparative effectiveness of various interventions to optimize clinical outcomes and preserve function in complex populations, and the role of community-based research and systems-based delivery of healthcare.
Part 1: Mechanisms and Triggering of Functional Decline/Aging in HIV-infected Persons
Background – Statement of the Problem
Human aging is associated with substantial changes in both the innate and adaptive immune response and occur at the phenotypic, functional, and molecular levels. It is now recognized that immune changes that occur during normal aging may occur earlier in chronically HIV-1 infected individuals. There also may be synergistic effects of aging and HIV in this population resulting in complex pathologies and precipitous immune decline. Studies focused specifically on the impact of aging and HIV on immune function will therefore likely remain central to research in this area.
A number of priority areas of research exist. Research in the areas of aging and immune dysfunction in HIV-infected persons should focus on mechanisms and triggers in human populations, although the use of primate models for combined aging and HIV/SIV research may prove to be useful. Most studies on aging and HIV have utilized peripheral blood which contains approximately 2 percent of the host immune cells. It is critical for HIV and aging research to expand work to the analysis of tissue sites including bone marrow, gut mucosa, and lymph nodes. There are also significant needs in developing imaging technologies to evaluate sites such as the central nervous system since these areas are not easily accessible for study. Imaging tools such as advanced computed tomography (CT), functional magnetic resonance imaging (MRI), and position emission tomography (PET) may provide platforms for expanded assessment of multiple tissues. These tools will clearly provide important resources for pathogenesis studies and will be likely to improve the clinical evaluation of antiretroviral-induced immune reconstitution (18–30).
Cellular senescence is an important mechanism that contributes to organismal aging. There are numerous aspects of immune and cellular senescence that have been documented in the elderly but only a few of them have been extended to studies in HIV-infected individuals (31,32). The senescent phenotype is complex and involves the activation of gene programs that have important clinical implication for the prevention of cancer, but are thought to also result in cumulative damage to tissues and organs.
Many of the pathways known to affect cellular aging may be affected by HIV or its treatment. Shortening of telomeric sequences and structural changes to the telomere are believed to be major drivers of the senescence program in all individuals. Telomere length decreases with age in all hematopoietic lineages that have been studied, including circulating CD38 hematopoietic precursor cells, neutrophils, and naïve and memory T cells. HIV-associated inflammation and/or exposure to nucleoside analogues have been associated with changes in these and other key biologic pathways (33–40).
Emerging data from a number of groups suggest that HIV-infected individuals demonstrate features of immunologic aging including accumulation of terminal stage, effector CD8+ T cells (most often identified by the absence of CD28 expression) with shortened telomeres, absence of telomerase activity, and limited T cell proliferation potential. The biologic consequence for the accumulation of these cells is controversial, but their presence has been associated with clinical problems in seniors including reduced vaccine responses, bone loss, neurocognitive decline and cardiovascular disease.
It is also important to note that the immunology of aging is thought to be influenced by other chronic viral pathogens, particularly cytomegalovirus (CMV). CMV infection is characterized by the oligoclonal expansion of a CMV specific T cell population that comprises a large fraction of the T cell repertoire. It is not clear how the human host can compensate for so much anti-CMV T cell reactivity over so many years without having persistent problems in immune control. The impact of HIV disease on this process is not known, but emerging data suggest that these co-infections interact in a potential negative manner (41–44).
The role of dysregulation of the immune system among those with HIV requires careful study. Specifically, age-associated T cell changes include an increase in the number of regulatory T cells both within the CD4 and CD8 subsets, and a generalized chronic inflammatory state (which may reflect the combined effects of senescent T cell cytokine secretion), a shift in the balance of Th1, Th2, Th17, and T-regulatory responses, and alterations in cells of the innate immune system. This so-called “inflammaging” is associated with frailty and increased mortality risk in the very old, HIV-uninfected population. The role that this process has in causing HIV-associated morbidity remains only theoretical and deserves investigation.
Humoral immunity is affected by aging as well, as evidenced by the dramatic decline in antibody responses to a variety of vaccines and markedly increased risk of pathogens typically controlled by antibody-based mechanisms (e.g. Streptococcus pneumoniae). The major intrinsic B cell defects include a reduction in both somatic hypermutation of the immunoglobulin genes and in class switching, which undoubtedly contribute to the blunted titer and affinity of antibodies in the elderly. Aging is also associated with diminished numbers and sizes of the lymph node germinal centers, which may impact affinity maturation and antibody quality, as well as a reduction in the number of B cells migrating out of the bone marrow. All of these immune alterations merit investigation in HIV-infected persons, both young and old, and are clearly relevant to vaccine development.
There is only minimal information on innate immunity changes associated with HIV/AIDS, but recent information from studies in the elderly can provide a preliminary road-map for future HIV studies. Most prominent among the age-associated changes are functional alterations in Toll-Like Receptors (TLR), a family of invariant pattern recognition receptors specific for highly conserved portions of pathogens. There is evidence of age-associated defects in TLR-induced production of IL-6 and TNF-alpha, particularly in response to engagement of TLR1/2, as well as a generalized defect in TLR-induced CD80 upregulation in monocytes from older individuals (45). The proinflammatory cytokine production by cells of the monocyte/macrophage lineage is hypothesized to be influenced by the complex interplay of immunologic, hormonal, and neuroendocrine factors. Circulating levels of adipokines and adrenal hormones are altered during aging. These systemic influences on both innate and adaptive immunity are key areas that have not yet been addressed with respect to HIV/AIDS.
Other cell types within the innate immune system that undergo age-related changes include natural killer (NK) cells, which show defects in cytotoxicity and signal transduction, and natural killer T (NKT) cells, which have some overlapping phenotypic features with senescent CD8 T cells. In both cases, research in the context of HIV is lacking and should be considered a priority. Diminished neutrophil function that accompanies aging (e.g. reduced phagocytic capacity and intracellular killing) clinically manifested by high morbidity and mortality due to bacterial infections is another area worthy of more extensive research. Finally, studies on dendritic cells, which bridge the gap between innate and adaptive immunity, are clearly critical both in terms of immune function in general, and development of vaccine formulations that compensate for immunosenescence effects.
We can also learn a significant amount of information on aging and how it applies to HIV by studying other clinical conditions. In rheumatoid arthritis, lymphocytes display a phenotype consistent with accelerated immune aging including accelerated telomere attrition, accumulation of differentiated effector T cell populations that have lost the CD28 marker and contraction in T cell receptor repertoire diversity. Compared to normal age-matched controls, T lymphocytes from patients with rheumatoid arthritis have increased numbers of DNA double-strand breaks and increased spontaneous apoptosis rates; repair of radiation-induced DNA breaks is reduced and delayed. There is significant information that can be gained for the aging and HIV fields by studies on autoimmune disease.
Priority Research Areas
Studies of innate and adaptive immunity
Studies of cellular measures of aging and how nuclear instability, telomere shortening, and activation of DNA repair mechanisms participate in normal immune aging and accelerated aging in HIV disease
Studies to more fully understand the role of CMV and other chronic viruses on inflammation and immune senescence with and without HIV co-infection
Development of imaging tools to more fully evaluate the impact of immune aging in the CNS and other tissue sites less accessible for sampling
Specific Knowledge Gaps
Cellular Mechanisms of DNA Damage and Aging
Mechanisms of telomere shortening in immune cells in normal and accelerated immune aging. What is the contribution of replicative history vs. defective telomere protection vs. failure in telomere elongation?
Breakage of non-telomeric DNA. Do DNA repair mechanisms deteriorate with progressive age and how does insufficient DNA repair affect immune cell survival and regeneration?
DNA damage responses. How does sensing of DNA damage and the attempt to repair alter the functionality of aging immune cells?
Senescence-associated inflammation. Are chronic DNA damage responses in immune cells connected to the activation of gene programs similar to the senescence-associated secretory pattern in senescent fibroblast? Is this a source of inflammatory mediators sustaining the subclinical inflammation of aging?
Innate and Adaptive Immunity Changes
Human models for understanding immunosenescence and immune-mediated pathologies (e.g., bone, cardiovascular disease) and further validation of primate models
Multiple immune compartments: bone marrow, gut, etc. samples, not just blood samples
Innate immune system changes: Toll-like receptors, dendritic cells, NKT and NK cells, neutrophils
Neuroendocrine involvement in age-related immune changes
Chronic Viral Illness and Immune Senescence
Conduct a comprehensive longitudinal studies of CD8 and CD4 T cell responses to multiple CMV proteins, including associations with CMV load, other herpes virus T cell responses, and immune activation and translocation factors
Define the putative anatomical sites (throat, genital tract, urinary tract, GI tract) and cell harboring latent/persistent CMV infection of HIV-uninfected and HIV-infected, ART suppressed persons – viral DNA, RNA, protein, infectious virus – and their relationship to anti-CMV immunity
Delineate whether CMV infection has more profound effects on aging in populations where the vast majority is infected from a very early age, e.g., Southern US African Americans; Asia, China, and Africa
Determine genetic associations of host factors with CMV infection and immunity
Conduct clinical studies to address whether antiviral drugs can impede persistently active CMV infection and delay immune senescence
Develop vaccines for prevention of CMV infection and their effects on aging.
Address these same issues in other chronic viral infections (e.g., hepatitis C virus (HCV)) that may accelerate immune senescence and/or immune exhaustion
Imaging
Gaps are widespread in using imaging tools for brain characterization in aging HIV infected individuals
MR technology is the most promising, widely available, with potential for fine detail and functional assessment
PET scanning may provide unique insights to microglial activation that could drive brain aging
Imaging could be applied to other organs not amenable to direct sampling, although it is less commonly used in this way
Approach will need to be multicenter, and to provide controls, might be linked with large national studies underway for early detection of Alzheimer’s disease early
Types of Research Funding to Emphasize and Available Cohorts
Focus should be on investigator-initiated research. We should also take advantage of existing cohorts or research networks for supplemental funding opportunities (See Part 3 - Aging with HIV Infection: Multimorbidity and Clinical Research). There are other cohorts outside the HIV field that could be utilized to provide normative data for age-matched or senior populations. Examples include the Alzheimer’s Disease Neurological Initiative (ADNI) that is collecting a very large population data base and ASPREE, currently accruing senior subjects. It will be critical to continue to accrue subjects and data in cohorts aging with HIV (e.g. the VACS, the MACS, and the WIHS) as comparator groups. Women’s issues require particular attention as there are substantial sex differences in immune response, menopause leads to changes in immune response, vaginal/cervical changes in epithelium and secretions that may influence HIV acquisition and/or progression.
Part 2 – Biomarkers and Clinical Indices as Predictors/Surrogate Outcome Markers
The objective of this section is to identify and prioritize areas of research relevant to the characterization and validation of biomarkers and clinical indices associated with the development of conditions that lead to morbidity and mortality.
Background – Statement of the Problem
Although combination antiretroviral therapy is highly effective in suppressing HIV replication, it does not fully restore health. When compared to HIV-uninfected persons, long-term treated HIV-infected adults have excess risk of a number of HIV-associated, non-AIDS (HANA) conditions, including many typically associated with advancing age (e.g., cardiovascular disease, kidney disease, liver disease, osteoporosis, cancer, and cognitive impairment) (46). The mechanisms accounting for this excess risk likely include persistent immune dysfunction/inflammation, treatment toxicity, co-infections such as CMV, HCV and hepatitis B virus (HBV) and traditional risk factors (including use of alcohol, tobacco, and psychoactive drugs) (15,47,48).
Many studies of biomarkers reported to date have included untreated and treated individuals, despite the fact that HIV replication is known to be major a determinant of inflammation, immune function and overall health. Even those studies that focused on long-term treated individuals rarely controlled for the degree of viral suppression. Further, use of alcohol, tobacco, and psychoactive drugs affects both biomarker behavior and the risk of non-AIDS morbidity and mortality; the lack of precise data regarding these factors in most cohorts prevents a careful assessment regarding mechanisms.
The impact of HIV and its treatment on the biology of aging is also likely an important factor in premature development of typical age-associated diseases (see Figure 1). HIV infection results in chronic activation/dysfunction of the innate and adaptive immune responses, leading to inflammation and perhaps the development of most non-AIDS conditions. HIV infection also causes a loss of effective immune surveillance, which may contribute to cancer and other complications. The cumulative burden of these complications reduces a person’s ability to compensate and respond to many events, which collectively results in onset of frailty and other geriatric syndromes (15, 17, 48).
Advances in our knowledge of these areas will almost certainly be driven by the use of biomarkers. Many putative HIV-associated biomarkers have established prognostic significance in HIV-uninfected populations. To what degree these biomarkers or indices have unique performance characteristics in HIV-infected adults independent of established risk factors is often unknown because of the difficulties in identifying cohorts of behaviorally and demographically similar infected and uninfected adults. The interaction between certain biomarkers appears to be different in HIV-infected and HIV-uninfected infected individuals. In the SMART study, C reactive protein (CRP) only exhibited an IQR odds ratio of 3.1, much lower than what one might expect given the IL-6 data. In the Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM), CRP was considerably lower than anticipated in HIV and HCV co-infected individuals than in either controls or in HIV mono-infected patients. In a combined analysis from WIHS and MACS, the initiation of effective antiretroviral therapy was associated with the expected decline in IL-6 and D-dimer, but an apparent increase in CRP (49). It is likely that some of these unusual trends and interactions relate to the complex effect HIV and its treatment and co-infection with other viruses, such as HCV, has on the ability of the liver and perhaps other organs to regulate biomarker levels.
There is accumulating evidence that the behavior of certain biomarkers in the context of treated HIV disease is different from that in general population. Many (but not all) biomarkers associated with inflammation (e.g., IL-6, sCD14) and end-organ diseases common in seniors (e.g., cystatin C as a marker of declining renal function) are higher in antiretroviral-treated adults than uninfected adults (50). The association between these biomarkers and subsequent morbidity/mortality appears to be stronger in those with HIV infection compared HIV-uninfected individuals. For example, in the SMART studies, the fully adjusted odds ratios of mortality (fourth versus first quartile; IQR) in HIV disease were 8.0, 12.4, and 41.2 for sCD14, IL-6 and D-dimer, respectively (47); these odds ratios are higher than those observed in the general population. The ability of these biomarkers to predict outcome has been observed in most patient populations, including those with preserved immune function (51).
The durability in terms of prognostic significance of certain biomarkers also appears to be unique among HIV-infected adults. In the Evaluation of Subcutaneous Interleukin-2 in a Randomized International Trial (ESPIRIT) study, high plasma D-dimer was associated with continued excess risk four to seven years after the measurement of a single level. This stability in terms of prognosis is years longer than that which has been observed in the general population.
Despite the tremendous amount of research in this area, the precise role these biomarkers have in the causal pathway is largely unknown. The ability of these markers and indices to provide prognostic significance beyond traditional markers (e.g., CD4+ T cell count, HIV RNA level, and routine cardiovascular risk markers) is also largely unknown. Finally, how a clinician should use these emerging markers or measures of multiple co-morbidities has yet to be addressed in any prospective clinical study. It is expected that high-quality basic, translational and clinical research in this area will advance our understanding of the pathogenesis of HIV infection during treatment It is also expected that research in this area could inform clinical management of HIV disease and perhaps even of other diseases that might be affected by similar pathways (e.g., chronic inflammation and “aging”).
Biomarker development
Biomarkers progress through several stages: a) initial discovery; b) translation into small human populations; c) application to larger more generalizable populations; and d) testing for clinical relevance. A handful of these are then incorporated into larger, more comprehensive clinical indices like the Framingham Index for cardiovascular disease. For eventual clinical applicability, biomarkers and indices would likely begin by demonstrating significant associations with the specific outcome of interest, using standard epidemiological criteria. The most promising ones would demonstrate consistent and independent associations with important clinical outcomes across a broad and representative population. Further, these associations should be independent of other biomarkers already in routine clinical use. Tests of prediction generally compare a known set of clinical risk factors, such as the Framingham equation for coronary heart disease, with and without the addition of the new biomarker. Metrics for determining added value include the c-statistic (area under the curve) and the Net Reclassification Index (52, 53).
Biomarkers and clinical/biomarker indices intended for use as surrogate outcomes are held to an even higher standard, as they become proxies for the underlying disease process. Not only must the biomarker or index indicate increased risk factor for adverse outcomes, but they must rise and fall in step with the disease process. The impact of clinical intervention on the target disease must be captured by proportional changes on the candidate biomarker and differentiate between two treatments of differing benefit. If the differential effects of treatment options on mortality are reflected by parallel changes in the biomarker, then it is considered a good surrogate outcome and can be used as an endpoint in clinical trials. The measurement of HIV RNA in plasma is a well-accepted example of a surrogate marker.
What is known and not known about current biomarkers and clinical indices that predict outcomes in aging, HIV-infected subjects?
A brief summary regarding what is known and not known about existing non-specific integrated biomarkers and indices is provided below. Given the effects of HIV infection on immune function, much of the emphasis in the past several years have been on inflammatory biomarkers.
IL-6. IL-6 is a major autocrine, paracrine and endocrine cytokine mediator of a wide variety of functions including, but in no way limited to, liver protein synthesis (e.g., albumin, thereby helping regulate colloid osmotic pressure, amphipathic drug transport, fatty acid metabolism, etc.), adaptive immune function (in particular T Helper cell biasing) and innate immune function (e.g., monocyte/macrophage differentiation and function). IL-6 is produced by many cells. The origin of plasma IL-6 is not clear and probably variable depending on health and age. IL-6 levels are strongly associated with near-term mortality in the elderly in general, and with mortality among well-controlled HIV seropositive individuals. Additional biomarkers to explore include sIL-6R and sGP-130, since both of these modify IL-6 effective levels and functions. For example, genome wide association studies (GWAS) of IL-6 reveals the IL6R gene as an important regulator of circulating IL-6 levels.
C Reactive Protein (CRP) and other acute phase reactants. CRP is an acute phase protein produced by hepatocytes (and several other cell types), perhaps limiting its use in those with underlying liver disease. CRP is a member of the innate immune system and a member of a class of proteins known as pentraxins; others in humans are Serum Amyloid P (SAP), and Pentraxin-3 (PTX-3) both of which are measurable in serum/plasma. In SMART, both CRP and SAP were measured (since both predict CVD and mortality in the elderly), but only CRP predicted mortality in SMART. SAP is also produced predominantly by the liver, and possibly more affected by the suppression of liver protein synthesis in HIV disease than CRP was. Additional biomarkers to explore include human pentraxin, PTX-3; other acute phase proteins (including serum amyloid A, which in SMART showed an IQR OR identical to CRP), and other members of the innate immune system including circulating cytokines such as IFN-γ (and IFN-responsive proteins such as IP-10), circulating members of other innate systems such as complement, and the expression of cell-based innate mediators such as TLRs, gamma-delta TCRs, and others.
D-dimer. D-dimer is a fibrin degradation product produced from fibrin (the main product of plasma coagulation) by the fibrinolytic system whose ultimate enzyme is plasmin. Therefore D-dimer is an integrated measure of both coagulation and fibrinolysis, although more reflective of the extent of the former rather than the capacity of the latter. D-dimer predicts venous thrombosis at all ages. D-dimer also predicts CVD and mortality in middle-aged and elderly people, more powerfully in the latter. In SMART D-dimer showed a remarkable ability to predict future mortality with an IQR of approximately 40. Other summary measures to explore include plasmin-anti-plasmin complex (PAP), and specific markers of plasmin function such as fibrino peptides. The causes of the D-dimer increase in HIV disease have been hypothesized to include increased bacterial translocation (see sCD14, below); alternatives have not been explored in any detail to date. Such alternatives include coagulant imbalance (studied by examining individual coagulation factors), fibrinolytic insufficiency (examined by studying individual fibrinolytic factors), and platelet activation (difficult to study in frozen samples, or even in fresh samples, but some approaches exist). Also the role or procoagulant genetic variation has not been explored. The hazard ratios and stability of D-dimer are remarkable and unique to HIV disease. It is expected that ongoing work with diabetes mellitus (which may be a microthrombotic process) might prove highly informative regarding the potential of interventions to modify D-dimer among those individuals with HIV infection.
Soluble CD14 (sCD14) binds to lipopolysaccharides (LPS) and is expressed on the surface of monocytes and macrophages and is released into the circulation after activation of these cells and/or after direct exposure to LPS. The level of sCD14 in plasma has been strongly associated with mortality independent of other factors (including LPS) in treated HIV disease. Other options for monocyte/macrophage activation include sCD-133 and LPS binding protein (LBP).
T cell activation (CD38, HLA-DR). The frequency of T cells expressing CD38 (which is a cell surface ectoenzyme involved in regulating cell activation) and HLA-DR (Major Histocompatibility Complex (MHC) class II receptor) is markedly elevated in untreated HIV infection and associated with mortality independent of HIV RNA levels and CD4+ T cell counts. The frequency of these actively turning over cells declines with ART but often fails to normalize. The prognostic significance of these cells in treated HIV disease is not known.
T cell senescence (CD28, CD57). After exposure to antigen, T cells differentiate toward a terminally-differentiated state, and in the process down-regulate certain cell receptors such as CD28 and up-regulate certain receptors such as CD57. A population of well-differentiated “senescent” cells that have limited proliferative potential and are potentially pro-inflammatory and apoptosis-resistant accumulates over time. A higher frequency of these cells — including those that are CD28− and/or CD57+ — has been associated with cardiovascular disease in HIV-infected adults, and is believed to be causally associated with morbidity in very old HIV-uninfected individuals.
HIV burden. Although specific to HIV disease, it is possible that measures of residual HIV burden during apparently effective antiretroviral therapy may provide useful information to understand why some biomarkers remain abnormal during treatment. These measures may also prove to have prognostic and therapeutic significance independent of these other biomarkers.
Estimated Glomerular Filtration Rate (eGFR). Renal function is multi-faceted and the endocrine (e.g. vitamin D metabolism, erythropoietin production) and other kidney functions may not be well-represented by glomerular filtration alone. For example, eGFR and albuminuria are independent predictors of cardiovascular risk in those with chronic kidney disease (54). However, eGFR can be readily obtained from standard blood chemistry panels and is an important component in multi-variate models of mortality and hospitalization in HIV-infected persons (15, 17, 48, 55). The role of decreased glomerular function vs. other renal functions perhaps implicated by eGFR is an important area for future research.
Red blood cell indices. A number of studies have noted the importance of anemia and RBC size/shape (typically measured by mean cell volume (MCV) and red blood cell distribution width (RDW), respectively) as important indicators of mortality and hospitalization risk in multivariate analyses (17). This is reminiscent of the epidemiologic link between these indices and outcomes in those with end-stage renal disease on dialysis and those with cancer-related anemia in whom clinical trials of merely elevating hemoglobin through erythropoietin injections sometimes had adverse consequences. Thus, understanding specific mechanisms within this field is essential.
FIB 4 and aminotransferase / platelet ratio index (APRI). These composite markers of liver injury have been repeatedly shown to indicate risk of liver fibrosis among those with HIV and HCV infections (56). FIB 4 is also clearly associated with risk of all cause mortality and strongly predictive of incident hepatocellular cancer (53, 57).
The Veterans Aging Cohort Study Risk Index (VACS Index) integrates a number of routine clinical biomarkers currently followed as individual indicators in HIV management (55, 56). It includes HIV-1 RNA, CD4 count, hepatitis C co-infection, FIB 4, eGFR, anemia, and age and is substantially more predictive of all causes of mortality than an index restricted to HIV-1 RNA, CD4 count, and age. Of note, the VACS Index reflects both positive and negative effects of ART over time (58–60), is correlated to IL-6, D-dimer, and sCD14(61), and is also predictive of Medical Intensive Care Unit Admission (61). After adjusting for the VACS Index, IL-6 does not substantially improve the discrimination of all cause mortality (61). While the VACS Index predicts all cause mortality more accurately than D-dimer or sCD14, the addition of either marker results in improved risk classification.
Clinical Indices derived in HIV-uninfected subjects. Perhaps the most broadly studied multi-component index in medicine is the Framingham index, and this has been used in HIV-infected populations as well. While it predicts cardiovascular events, the thresholds may vary from HIV-uninfected populations and may under-estimate risk and HIV-specific indices (e.g., DAD CV index) that may be more accurate (62–64) Other than the VACS Index studies of routine clinical biomarkers, there is a paucity of data regarding the role of markers of tissue function and injury in the context of HIV disease. Examples of tissue-specific biomarkers which may require validation in HIV disease include cystatin C (a measure of kidney function and perhaps inflammation), albuminuria (kidney function), fibrinogen (coagulation system), tissue factor (coagulation system), HDL/LDL (metabolic function), soluble vascular cell adhesion molecule-1 (vascular function), E-selectin (vascular function), insulin levels (metabolic function), adionectin (metabolic function), telomere length (aging), CSF tau (CNS), amyloid (CNS), and APO-E status (CNS).
Priority Research Areas
The priority areas for focus in the development of biomarkers and clinical prognostic indices are: (1) identifying biomarkers indicative of residual disease; (2) characterizing the link between chronic inflammation and immunodeficiency during treatment-mediated viral suppression on biomarkers and outcomes; and (3) comparing and contrasting the risk and modifiable risk factors for non-AIDS conditions among those with and without chronic HIV infection. Some examples of specific high-priority questions are listed below.
Specific Knowledge Gaps
Prognostic Significance in HIV-Infected vs. Uninfected Individuals
Do biomarkers in HIV-infected adults have the same prognostic significance as they do in uninfected adults (e.g., liver dysfunction may alter levels of specific biomarkers and their ability to predict subsequent disease)?
Do treatment-mediated changes in biomarkers have the same significance in HIV and other diseases (e.g., does a statin-mediated change in cholesterol have the same implications for HIV-infected and uninfected adults)?
Candidate Genes and Pathways
What candidate genes and pathways lead to poor outcomes despite effective antiretroviral therapy?
Innate and Adaptive Immunity
What are useful markers associated with innate and adaptive immunity that correlate with important clinical outcomes?
Do interventions targeted at modifying coagulation (e.g., aspirin) in those with evidence of hypercoagulable states (e.g, elevated D-dimer) improve clinical outcomes?
Novel Biomarkers
Are there other biomarkers associated with fibrosis, liver function, and coagulation more specific for this disease process than D-dimer?
Process of Identification-Validation-Therapeutics
The process of going from pathogenesis to biomarker identification/validation (in cross-sectional and longitudinal studies) and then to therapeutics is being pursued in multiple disease states and HIV-infected patients should be included in these cohorts (e.g., in ASPREE).
Types of Research Funding to Emphasize and Available Cohorts
A greater investment in novel cohorts aimed at studies on specific end-organ diseases is needed. Most cohorts have focused on collecting clinical information and specimens which can inform issues related to HIV disease. Novel approaches including multi-disciplinary teams should be formed to develop needed cohorts to advance our knowledge in cardiovascular function, metabolism and other chronic diseases, especially those that have a high impact on HIV-infected individuals either because of their association with HIV infection itself or because of their severity and prevalence among those with HIV. For example, hepatitis C is common among those with HIV and has a different disease trajectory in the face of HIV co-infection.
Most of the early work in novel cohorts is expected to be observational. Initially analyses may be cross-sectional and involve the identification of biomarkers associated with important clinical outcomes that can be measured in stored samples. This work should then rapidly move into longitudinal studies to characterize how modifiable a biomarker may be and to identify treatments and behavior changes associated with improvements in biomarker levels over time. These analyses would be invaluable to the design of strategic intervention studies and to inform ongoing clinical management.
Eventually, randomized clinical trials will need to fully test the utility of the biomarker--possibly as ancillary studies to ongoing clinical trials. Then full scale intervention studies aimed at preserving end-organ function could be launched using the biomarker or a panel or index of biomarkers as the primary outcome (much as earlier antiretroviral treatment studies used CD4 cell count and HIV-1 RNA). As an example, drugs that protect kidney disease from inflammation-associated or treatment-associated injury could be studied in the context of smaller, focused studies that use accepted biomarkers of injury as an outcome.
A key related objective of future research is to determine when we can apply principles developed in non-HIV infected adults to those with HIV disease. Examples might include angiotensin-converting-enzyme (ACE) inhibitors in protecting against kidney disease, or bisphosphonates for osteoporosis. Validated markers from the general population known to be mediators of outcome could be investigated in HIV disease to determine that these markers are at least responsive to an intervention.
Finally, the most important work yet to be done is the process of translating biomarker research from pathogenesis-oriented studies into the clinic. Such work will require phase II and eventually phase III clinical trials. Resources may need to shift from pilot correlative studies to larger definitive interventional studies in which a therapeutic intervention improves health. The role of a biomarker in mediating this therapeutic effect can only be defined in such resource-intensive studies. Although multiple interventions might be considered, it is reasonable to first advance into clinical studies therapies which in other disciplines have proven to be safe, well-tolerated, and are aimed at unifying hypotheses (e.g., inflammation). Also of high priority is the study of drugs which affect development of common non-AIDS conditions, but which might be affected by HIV disease. As an example, adults with insulin resistance and metabolic syndromes appear to derive remarkable clinical benefit from the use of oral insulin-sparing interventions. Such drugs could conceivably have a number of positive benefits in HIV-infected adults with subclinical metabolic conditions, including chronic inflammation, early insulin resistance, and the presence of the metabolic syndrome.
Part 3 - Aging with HIV Infection: Multi-morbidity and the Clinical Research Agenda
Background – Statement of the Problem
HIV Associated Non-AIDS (HANA) conditions are increasingly common and associated with aging and chronic inflammation. These include cardiovascular disease, a number of infectious and noninfectious cancers (7,8), osteopenia/osteoporosis(9), liver cirrhosis (10), and renal disease (11, 12). It remains to be seen whether people with HIV infection develop these conditions earlier in their life course or are simply at greater risk of developing aging associated conditions at ages observed among those without HIV infection (13).
Of many useful geriatric concepts, three are particularly relevant to intervention research among those aging with HIV infection: frailty (which encompasses the concepts of multi-morbidity and functional decline and vulnerability to illness); chronic inflammation; and personalized care. These concepts are highly related to each other in that all three recognize the complex overlapping effects of multi-morbidity in aging, the need to measure cumulative injury from multiple causes, and the need to identify modifiable mediators of common pathways of injury.
Frailty is a syndrome characterized in HIV-uninfected adults, typically in their 7th, 8th, or 9th decade, by muscle weakness, weight loss, fatigue, and low levels of physical activity signifying a decreased ability to withstand further injury (65). It indicates vulnerability to diverse adverse health outcomes including treatment toxicity, functional decline, hospitalization, surgical complications, and mortality. While few middle aged individuals with HIV infection demonstrate the full frailty phenotype (66), there is a concern that those with HIV infection may have significantly decreased physiologic reserve at younger ages than those without HIV infection. Association studies among older HIV uninfected patients have demonstrated high levels of inflammatory mediators, high levels of cortisol, activated sympathetic nervous system, and activated clotting cascades in frail compared to non-frail older adults.
The etiology of frailty is thought to be age-related and multi-systemic in nature. However, as among those without HIV infection, a likely driving force behind this vulnerability to injury is chronic inflammation (67–69). Chronic inflammation and hypercoagulability occur when the body is exposed to chronic antigen stimulation through a number of possible mechanisms including chronic viral infection and microbial translocation. It is thought to play an important role in many diseases of aging including cardiovascular and rheumatologic diseases. Biomarkers of chronic inflammation and hypercoagulability such as IL-6, sCD14, and D-dimer are elevated among those with HIV infection compared with demographically matched uninfected controls and are higher still among HIV-infected individuals with unsuppressed levels of HIV-1 RNA (70,71). These biomarkers were correlated with non-AIDS events observed in the SMART study. Thus, the concept appears relevant for those aging with HIV infection. As noted in the prior section, those aging with HIV have many reasons to have chronic antigen stimulation including infection with HIV and common co-infection with HCV, HBV, HPV, and CMV; microbial translocation from HIV (72) and from alcohol (73), and oxidative stress from smoking, obesity, and ART toxicity. These factors must also be considered when evaluating biomarkers of chronic inflammation.
Interest in understanding chronic inflammation and hypercoagulability and its role in the excess burden of disease experienced among those aging with HIV infection stems from a desire to evaluate diverse therapeutic approaches shown to mitigate inflammation in other settings. These include pharmacologic treatment and behavioral interventions. If these interventions are to be grouped and prioritized effectively, we need a means of comparing their effectiveness for important patient outcomes. Outcomes of primary interest remain morbidity, mortality, and cost. However, clinical and experimental biomarkers might offer important insight and prove useful intermediate outcomes for some studies, especially if they are considered as part of an integrated risk index such as the VACS Index (see prior section) (52).
The concept of personalized care rests on the observation that those at greatest risk of a particular disease often reap the largest benefit from screening and prevention thereby justifying the risks and/or costs associated with the intervention (screening test or preventative therapy). It requires an accurate assessment of individual risk that accounts for all important sources of risk. An example of this approach is the use of the Framingham Index in cardiovascular disease management. The Framingham Index is used to determine the patient’s individual risk of disease, to indicate whether or not diagnostic tests such as a stress test are indicated, and to tailor the approach to hypertension and cholesterol treatment, and to monitor progress over time. Patients at low risk of cardiovascular disease are not given further diagnostic workup nor treated with cholesterol lowering agents even if the cholesterol is elevated since this would more likely result in toxicity rather than in averting a very low probability cardiovascular event. Similarly, hypertension is managed more aggressively in those with diabetes than without. In both cases, this risk assessment allows providers to identify those most likely to benefit from an intervention and those for whom toxicity, polypharmacy, or simply being distracted from more beneficial interventions is a greater concern. Of note, newly developed biomarkers of cardiovascular disease are compared with the current standard (the Framingham Index) rather than in isolation (52). Only biomarkers that substantially improve the accuracy of the Index are likely to be adopted whether they are based on genetics, proteomics, biochemistry, biometrics (e.g., blood pressure), or patient behaviors (53).
Priority Research Areas
Studies comparing decreased organ system reserve (frailty), its consequences among HIV-infected and uninfected aging individuals, and modifiable risk factors
Studies informing the characterization of the comparative effectiveness of multiple and diverse intervention options including both behavioral and pharmacologic interventions
Studies focused on personalized medicine in which guideline driven care is compared to an approach that determines care based on an individualized assessment of risks and preferences
Specific Knowledge Gaps
Comparative Research to Inform Prioritization in the Face of Complexity
The number of interventions shown effective among those aging without HIV infection that may have benefit among those aging with HIV is large and diverse. Their applicability to this population will likely evolve as the population with HIV continues to age. Most require substantial time and resources. Further, with every intervention chosen for implementation, time and attention is taken from other clinical activities. Several kinds of outcomes must be considered to inform this prioritization:
Both benefits and harms (including costs) of interventions should be evaluated using metrics that allow integrated comparison across studies to optimize care provided. Comprehensive indices (e.g., VACS Index) could be used as a surrogate outcome allowing comparative analyses across a wide range of behavioral and pharmacologic interventions.
Some patients may strongly prefer a simplified regimen and behavioral change to more medications with potential toxicities. Thus, patient preferences should also be increasingly considered as an outcome in intervention work.
Special populations: the relative balance of benefit and harm and patient preference may vary in important ways among special populations. Efforts are needed to characterize these differences to inform tailored interventions.
Guideline Driven Versus Individualized Care
The geriatric literature has called into question the wisdom of following all applicable primary and secondary prevention guidelines among those with multi-morbidity, polypharmacy, and reduced life expectancy (74). These arguments likely apply to those aging with HIV infection. For example, the incremental benefit of aspirin, bisphosphonates, vitamin D, and/or testosterone for osteoporosis based on low BMD among men with HIV has yet to be demonstrated. The cost of competing attention to guidelines needs to be characterized in terms of what is not accomplished in the clinical encounter.
Synergistic Interventions
Interventions that have several types of beneficial effects need to be comprehensively evaluated to characterize their multiple beneficial effects.
Smoking and alcohol cessation (benefits may include adherence to ART, decreased organ system injury, and improved exercise tolerance)
Maintenance of normal BMI (benefits may include improved Framingham risk, improved liver function, and improved exercise tolerance)
Weight bearing and aerobic exercise
Falls prevention (increased exercise tolerance, decreased fractures, avoided hospitalizations)
Treatment for HCV infection (improved VACS Index, decreased inflammatory biomarkers, improved functional status)
Provider Behavioral Interventions
Medication reconciliation, adherence interventions, and efforts to reduce complexity of a patient’s treatment regimen may avoid ART treatment interruptions, prevent liver, renal, and bone marrow toxicity, and improve adherence.
ART Adjustment vs. Conventional Risk Factor Mitigation
The relative importance of adjusting ART medications with possible toxic side effects versus addressing conventional risk factors requires careful evaluation. For example, given the substantial excess risk of myocardial infarction among those with HIV infection compared with demographically similar uninfected controls, the benefits of conventional management of risk factors according to the Framingham index versus ART adjustments should be explored in observational and randomized studies.
Types of Research Funding to Emphasize and Available Cohorts
There is a need for methodologic studies, observational data analyses, and carefully designed clinical randomized trials:
Methodologic Questions
Framingham and NHANES data have limited generalizability to those with HIV infection due to behavioral and demographic differences. Who are the appropriate demographically and behaviorally matched controls for these studies?
Beyond all cause mortality, what are appropriate outcomes to compare across diverse intervention and observational studies?
How could these outcomes be employed across a variety of studies and compared?
Can risk indices be adapted to those with HIV infection? Do they qualify as surrogate endpoints? What are their limitations in this regard?
Does the Framingham index “work” among those with HIV infection?
When we compare disease outcomes other than death among those with and without HIV infection, how should adjustment for substantial differences in competing risk of death be accomplished?
Observational Studies and Randomized Clinical Trials
Better observational data are needed characterizing polypharmacy, nutritional status, and multi-morbidity among those with HIV infection and comparing its associated outcomes among those with HIV infection and demographically and behaviorally similar controls. This information can then immediately inform intervention studies.
For example:
Observational Studies
-
What are the common clusters of conditions experienced by those with HIV infection and how does their co-occurrence influence response to treatment and outcomes?
How do liver and/or renal disease influence the risk of cardiovascular disease?
Are the adverse effects of hypertension accentuated enough among those with HIV infection to justify more aggressive management (akin to that used in diabetes)?
Should smoking cessation be paired with other behavioral interventions (alcohol cessation, physical conditioning, health coaching) to improve quit rates?
Are patients who started ART at lower CD4 cell counts at higher risk for long-term morbidity?
Are there special populations of patients at particularly higher or lower risk for these conditions (i.e., women, older MSMs, those addicted to drugs or alcohol)?
How do common non HIV treatments (e.g., acetaminophen and liver cirrhosis, proton pump inhibitors, and osteoporosis) influence risk of HANA conditions?
What is the role of nutritional deficiencies and long-term, low-level malabsorption in development of chronic illness/HANA conditions
By studying “natural experiments” (i.e. studies in which the treatment assignment is haphazard and not made by the investigator) in observational data, we can determine what interventions and intervention strategies may appear the most promising among those aging with HIV infection.
-
How much improvement is there in the risk of mortality when HIV-infected individuals
Stop smoking
Control their blood pressure
Stop or decrease alcohol consumption
Lose weight (or avoid excess weight gain after ART)
Exercise
Stop taking liver or renal toxic medications (for HIV-related and/or non-HIV-related conditions)
Conversely, how much is the risk of mortality increased when smoking, drinking, or toxic medications are increased?
Are there differences in adipose tissue deposits with weight “re-gain” after initiation of ART vs. weight maintenance with earlier initiation?
Will specific nutritional or exercise interventions change the character of weight “re-gain” and alter risk associated with specific body composition?
Are there differences in those starting ART now vs. those who have had the experience of serial monotherapies or lipotoxic agents (e.g., stavudine)?
Randomized Clinical Trials
Ultimately randomized clinical trials will need to test central approaches to aging with HIV infection. These will likely need to focus on key strategies rather than individual interventions and, likely, should represent a combination of behavioral and therapeutic approaches. The wisdom of combining behavioral and therapeutic approaches has already been demonstrated in the treatment of depression, alcohol addiction, and smoking cessation. For example,
Initiation of ART as a “teachable moment” at which patients might be open to other health interventions such as exercise, smoking cessation, and blood pressure control compared to delaying these interventions until the patient is adherent to ART.
Comparison of individually tailored and adapted care to guideline (HIV and primary care) driven care.
Optimization of liver outcomes among those with HIV and HCV coinfection which include approaches to screening for fibrosis (FIB 4), among those with evidence of liver injury. A trial may randomize people to receive alcohol cessation, exercise, dietary modifications, and medication review for toxic medications +/− HCV treatment, or other interventions targeted to reduce hepatic fibrosis.
Tailored care to the individual may randomize people to usual care versus medical management guided by the VACS Risk Index, Frailty Phenotype, or other integrated risk assessments for total morbidity and mortality and evidence based care to address early modifiable factors including early signs of cardiovascular, pulmonary, liver, renal, and bone or bone marrow injury.
Studies focused on a particular source of injury (e.g., low bone mineral density) should consider adverse consequences of treatment including decreased adherence to other therapies, toxicity, and costs.
Small proof of concept trials should be used to identify interventions that reduce HIV-associated inflammation and/or immune activation. Those interventions with promise could then be moved into larger studies aimed at improving multi-morbidity and survival.
Specific Issues in women aging with HIV
An increasing number of women with HIV will now live through the menopausal transition and beyond. Given what is already known about menopause and estrogen deficiency in HIV-uninfected populations, particularly as it relates to cardiovascular disease, metabolic syndrome, bone health, and cognition, understanding how menopause and HIV, including its treatment, may interact is critical for successfully reducing the disease burden for women aging with HIV. With emerging evidence that long term HIV infection and its treatment may increase risk for the same co-morbidities associated with reproductive aging, a greater understanding of the effect of sex hormones on the trajectories of HIV disease progression and development of HANA co-morbidities is important to both prevent and treat these conditions more effectively.
Part 4 - Societal Infrastructure, Mental Health/Substance Abuse, and Care giving Issues
Background – Statement of Problem
Due largely to the advances of antiretroviral therapy (ART), in industrialized countries HIV has been transformed into a manageable, chronic condition leading to the emergence of a large population aging with this disease. However, the quality of life in adults aging with HIV is in question due to medical complications, co-morbidities, polypharmacy, substance use, poorer mental health, social isolation, as well as stigmatization from healthcare providers and society at larger (75).
Likewise, the burgeoning population of older adults with HIV also faces considerable psychosocial challenges with regard to social engagement and interaction, adequacy of informal social supports and care giving resources, and utilization of community-based services to meet their growing needs. Numerous studies have documented that those aging with HIV tend to have limited and inadequate social networks, especially with regard to traditional familial support (76). These truncated social networks result from a variety of reasons, including HIV-stigma and resultant social isolation due to rejection from social network members, as well as self-protective withdrawal. Social network insufficiency may also be attributed to histories of incarceration and use of alcohol, tobacco, and psychoactive drugs. Such isolation has been linked to high levels of loneliness and depression and insufficiency of instrumental and emotional support from both family and friends. These limited social networks also have negative implications for care giving resources for an aging population with HIV since caregivers are typically drawn from these networks. As a result, this population will need to rely on formal community-based services in the near future, yet this is complicated by frequent service barriers (e.g., knowledge and access to services, ability to afford services) as well as cultural competency issues of mainstream service providers interacting with an HIV-infected population (77). Furthermore, some community-based resources, such as faith-based organizations, may have resources to meet the needs of older adults with HIV, but are likely underutilized due to stigma issues as well as a lack of understanding of the service needs of these older adults (78).
The primary objective of this component of the research program is to enhance our understanding of the socio-behavioral influences on aging with HIV in order to enhance positive outcomes (e.g., quality of life, adherence, and “successful aging”). Aging is a complex process physically, mentally, and socially which takes place within societal infrastructures such as the medical community; this complexity increases with HIV disease. Those who have aged with HIV are often very different from those who have contracted HIV in later life. Additionally, HIV affects those from a variety of social and ethnic backgrounds, some who have more competencies and resources to cope with the disease (79). Therefore, exploring the basis for health disparities among those aging with HIV remains a priority. Finally, because of the complex nature of aging with this disease, a trans-disciplinary, multi-professional team approach is encouraged to investigate how to improve functioning and quality of life for these individuals.
Although many topics can be studied in relation to aging with HIV, objectives focus on, but are not limited to, the topics outlined below. Future research projects should use a wide variety of scientific methods to address these objectives including cross-sectional and longitudinal survey designs, translational research, and secondary data analysis. Studies should also place special emphasis on participation of women, minorities, and rural populations.
Priority Research Areas
Studies on HIV prevention in older adults
Studies on the effects and management of co-morbidities in older adults with HIV
Studies on the mechanisms of successful aging in older adults with HIV
Studies on the cognitive effects of older adults with HIV and possible interventions
Studies on the mental health effects of older adults with HIV and possible interventions
Studies on substance use in older adults with HIV
Studies on networks and care giving of older adults with HIV
Studies on community resources and infrastructure issues affecting older adults with HIV
Studies focused on issues of women aging with HIV, at risk for HIV or providing care for those with HIV
Specific Knowledge Gaps
HIV Prevention in Older Adults
It is uncertain what older adults know about HIV and safer sex. In addition, for those who have the knowledge, little is known about the barriers to practicing safer sex. In a time in older adulthood when there may be divorce, widowhood, or even incapacitation of a spouse due to a disease, opportunities for new sexual partners may emerge.
This vulnerability is further complicated in many normal older adults by poorer cognitive functioning, health disparities, depression, mental illness, and substance use. Studies investigating these sensitive and complex issues are needed along with innovative prevention, education, and intervention research programs.
Specifically, how do older adults negotiate safe sexual practices with partners? What do older adults know about preventing transmission of HIV and other sexually transmitted diseases?
What types of educational programs would be most effective in preventing transmission in this population? What are the specific factors that contribute to risky sexual behaviors and what interventions are effective in reducing such risk?
Many women over 50 remain sexually active but do not appreciate their HIV disease risk as contraceptive choice no longer plays a role in sexual decision making. Do physical changes in the vaginal-cervical environment do and systemic hormonal level changes confer greater risk for HIV acquisition? This has major implications for targeting HIV prevention messages for this population historically excluded in HIV prevention campaigns.
Effects and Management of Co-morbidities
A diagnosis of HIV entails learning new information about viral load, CD4+ lymphocyte count, and a host of other health information (80). Yet, with increasing age, co-morbidities are more frequent; this means that older adults with HIV will need to acquire and integrate additional medical information in order to manage HIV with co-morbidities (81).
How older adults with HIV do so is not well understood, especially in view of cognitive problems, depression, polypharmacy, substance use, and other complications (82).
How effective are older adults in integrating information about HIV with information about other co-morbidities they have? What are complications that older adults with HIV have in managing multiple diseases?
What are the common polypharmacy issues that confront this population and what interventions can be used to address them?
Successful Aging in Older Adults with HIV
There are many adults with HIV who are aging well with this disease despite certain personal and physical losses (83). Not only is it important to examine the problems associated with living with HIV, it is also important to encourage and translate research findings for those not successfully aging.
Studies with an emphasis on positive psychology, mindfulness, hardiness, resilience, social support, self-efficacy, and spirituality are encouraged. Why are some older adults with HIV more resilient and hardy than others?
What interventions can increase hardiness and resilience in older adults with HIV to improve their ability to successfully age? How are these positive traits and perceptions related to self-management of health and medication adherence?
Cognitive Issues in Older Adults with HIV
Cognition is threatened in many older adults with HIV (84); however, there may be several causes for such declines such as increased depression and anxiety, more social isolation, poverty, substance use, not being employed, inflammation, co-morbidities, etc. (85).
Many cognitive studies recruit adults who may be unemployed, have mental health and substance use issues, and have poor education (86). Such studies also need appropriate non-HIV infected controls (i.e., matched on education and income) (87). Although it is important to study cognition in this group, studies are also needed to examine those who are also successfully cognitively aging who may still be working and thereby be stimulated cognitively.
Few cognitive interventions have been attempted in older adults with HIV, especially those with cognitive problems (88). Studies are needed to examine these issues and investigate novel ways of protecting or improving cognitive and everyday functioning (e.g., driving, medication adherence) in this population.
Cognitive decline in women aging with HIV - a better understanding of cognitive function, including sex/gender-related predictors of neurocognitive impairment in women aging with HIV will require: 1) identification and validation of gender sensitive screening and assessment tools, 2) comparisons with age, race, and risk matched HIV uninfected female and HIV infected male cohorts, and 3) accurate measurement of potential mediator/moderator variables including menopausal staging, substance use, mental health, and gender specific exposures (sexual abuse history, etc).
Mental Health Issues in Older Adults with HIV
Mental health issues remain a significant problem; some studies show that depression is as high at 50 percent and anxiety as high as 20 percent in older adults with HIV (81). Other mental health issues such as bipolar disorder, schizophrenia, and substance use contribute to and exacerbate everyday functioning, medical management, and sexual decision making.
How do such mental health issues impact medical compliance and medication adherence in older adults with HIV?
What interventions are effective in reducing the incidence of mental illness which increases the functionality of older adults with HIV?
What is the relative impact of perceived vs. actual social isolation on mental health?
Substance Use in Older Adults with HIV
Substance use has played a critical role in the HIV epidemic, through direct transmission behaviors (e.g., sharing injection drug use equipment) and through the influence of substance use (drugs and alcohol) on increasing risky sexual behaviors (89). What is the influence of substance use on HIV transmission in older adults, including heterosexuals and men who have sex with men?
Among older adults living with HIV, what is the influence of substance use on medication adherence, co-morbidities, and treatment outcomes?
What are some potential interventions addressing substance use (including illegal drugs, alcohol, and smoking) and its impact on adherence and retention in HIV care?
Misuse of prescription drugs can easily happen in a medically vulnerable population. How can this impact HIV prevention and care in older adults?
Networks and Care giving in Older Adults with HIV
The limited and fragile friend-centered social networks of many older adults with HIV has been well-documented, but we lack an understanding of how these networks have evolved over time and how they will change with the aging of this population.
Meanwhile, stigma remains a powerful factor for those living with HIV, including those who are older. Older adults with HIV may be multiply stigmatized by their HIV serostatus, sexual orientation, race, gender, age, or other factors (e.g., substance use) (82).
To what extent have histories of incarceration and use of alcohol, tobacco, and psychoactive drugs impact on social network composition prior to HIV diagnosis?
What are the immediate and long-term effects of an HIV diagnosis on social network composition? How do these social networks change as these individuals age with HIV? To what extent is social network insufficiency the direct result of stigmatizing and discriminating behaviors on the part of others?
Who provides care giving assistance in times of need (i.e., family, friends, or paid helpers), and what is the preferred source of assistance for this population?
What types of interventions could be implemented to increase opportunities for socialization and decrease isolation in this population?
Community Resources Affecting Older Adults with HIV
At a time of retrenchment on the part of government and private funders in supporting services for people living with HIV, it is paramount that we effectively leverage existing resources to meet the needs of this growing and aging population.
What factors are associated with older adults with HIV turning from informal supports to non-medical community-based services?
What are the support preferences of older adults with HIV and how do they differ from older adults in general? What types of education, training, and capacity-building programs are most effective in assisting senior service providers to meet the needs of those aging with HIV?
How can underutilized community-resources, such as faith-based organizations, be enlisted to meet the support and care needs of older adults with HIV despite institutional barriers of stigma and a lack of awareness of this population in their midst?
Infrastructure Issues Affecting Older Adults with HIV
Given their high rates of physical and mental health co-morbidities, and their lack of informal social supports, older adults with HIV can be expected to have growing service needs in the coming decades. However, they are likely to experience service barriers when attempting to engage community-based providers.
How the nation’s infrastructure, or lack thereof, can provide services and care for this growing population is not clear and needs to be investigated at several levels (community, statewide, regionally, nationally).
Another infrastructure concern is that service providers may not have the knowledge or skills to effectively interact with those older adults with HIV. Do medical professionals have an adequate understanding of the polypharmacy issues surrounding this population? What are the service needs of older adults with HIV, sources of support for such needs, and the extent of unmet need for services?
What types of service delivery options are associated with more favorable clinical and psychosocial outcomes? What are the types and extent of service barriers for the older adult with HIV? What types of interventions are most efficacious at reducing barriers to service?
Specific issues in HIV/Aging for women
Traditional Gender Roles in Older Women with HIV - Women with or at risk for HIV infection are a particularly disempowered group in that they tend to be poor, African American or Latina, and often have a history of trauma. Investigating the degree to which women identify with traditional gender role norms, such as prioritizing care for others over self- care, silencing the needs of the self to avoid relational conflict and loss, and subordinating themselves to male partners has been associated with poor outcomes in many chronic diseases. Also, women utilizing active coping strategies, such as seeking education, being persistent, and making meaning (i.e. the act of “making sense” of an experience) have been shown to have better quality of life and health outcomes. Understanding internalized gender roles and their relationships to coping strategies is particularly important because the quality of women’s relationships with their sexual partners, family members and providers affects how they contract and cope with HIV and adhere to HIV treatment.
The demands of the aging process may mean self-care may become even more important in maintaining quality of life at older ages than it is at younger ages. Further, “meaning making” may be an especially important coping strategy for older women because of their developmental life stage. Being able to derive some sense from their many years of past experience may be especially important compared to its importance for younger women who more often are looking ahead to the future.
Acknowledgments
Sources of Support: Office of AIDS Research, National Institutes of Health
Working Group Members
Mark Brennan-Ing, Ph.D., Senior Research Scientist, AIDS Community Research Initiative of America (ACRIA), ACRIA Center on HIV and Aging, 230 West 38th Street, 17th Floor, New York, NY 10018, 212-924-3934, ext. 131, mbrennan@acria.org
David B. Clifford, M.D., Melba and Forest Seay Professor of Clinical, Neuropharmacology in Neurology, Washington University School of Medicine, Box 8111, 660 South Euclid, Saint Louis, MO 63110, 314-747-8423, cliffordd@neuro.wustl.edu
Mardge H. Cohen, M.D., Professor of Medicine, Rush University, Women Interagency HIV Study (WIHS), Medical Director, Women’s Equity in Access to Care and Treatment (WE-ACTx), 2255 W Harrison, Chicago, IL 60612, 312-925-5660, mardge.cohen@gmail.com
Judith Currier, M.D., M.Sc., Professor of Medicine, Chief, Division of Infectious Diseases, Associate Director, Clinical AIDS Research and Education (CARE) Center, David Geffen School of Medicine at University of California, Los Angeles, 9911 W. Pico Blvd., Suite 980, Los Angeles, CA 90035, 310-557-2273, jscurrier@mednet.ucla.edu
Steven G. Deeks, M.D., Professor of Medicine, University of California, San Francisco, Ward 84, Building 80, 995 Potrero Avenue, San Francisco, CA 94110, 415-476-4082 x404, sdeeks@php.ucsf.edu
Sherry Deren, Ph.D., Director, Center for Drug Use and HIV Research, Senior Research Scientist, New York University College of Nursing, 726 Broadway, 10th Floor, New York, NY 10003, 212-998-9015, shd2@nyu.edu
Rita B. Effros, Ph.D., Professor of Pathology and Laboratory Medicine, David Geffen School of Medicine, University of California, Los Angeles, 10833 Le Conte Avenue, Los Angeles, CA 90095-1732, 310-825-0748, reffros@mednet.ucla.edu
Kelly Gebo, M.D., M.P.H., Associate Professor of Medicine, Epidemiology, Division of Infectious Diseases, Johns Hopkins University School of Medicine, 1830 E. Monument Street, Room 435, Baltimore, Maryland 21287, 410-502-2325, kgebo@jhmi.edu
Jörg J. Goronzy, M.D., Ph.D., Professor of Medicine, Department of Immunology and Rheumatology, Stanford University School of Medicine, 269 Campus Drive West, Mail Code 5166, Stanford, CA 94305-5166, 650-723-9027, jgoronzy@stanford.edu
Kevin P. High, M.D. , M.S., Co-Chair, Professor of Medicine and Translational Science, Chief, Section on Infectious Diseases, Associate Dean, Clinical Research, Wake Forest University Baptist Medical Center, 100 Medical Center Boulevard, Winston Salem, NC 27157-1042, 336-716-4584, khigh@wfubmc.edu
Amy C. Justice, M.D., Ph.D., Professor of Medicine, Yale University, Section Chief of General Internal Medicine, Veteran Affairs Connecticut Healthcare System, 950 Campbell Avenue (11-ACSL-G), West Haven, Connecticut 06516, 203-932-5711 x3541, amy.justice2@va.gov
Alan Landay, Ph.D., Professor and Chairman, Department of Immunology/Microbiology, Rush University Medical Center, 1735 W. Harrison Street, Room 616 Cohn, Chicago, IL 60612-3823, 312-942-2849, alanday@rush.edu
Jules Levin, Executive Director, National AIDS Treatment Advocacy Project (NATAP), 580 Broadway, Suite 1010, New York, NY 10012, 212-219-0106, JuLev@aol.com
Paolo G. Miotti, M.D., Executive Secretary, Natural History and Epidemiology Coordinator, Office of AIDS Research, Office of the Director, National Institutes of Health, DHHS, 5635 Fishers Lane, Suite 4000, Rockville, MD 20852, 301-435-7699, pm122m@nih.gov
Robert J. Munk, Ph.D., Project Coordinator, AIDS InfoNet, P.O. Box 810, 34A Percilla Lane, Arroyo Seco, NM 87514, 575-776-8032, bobmunk@ix.netcom.com
Heidi Nass, J.D., Madison, WI, 608-263-9219, hmnass@uwalumni.com
Charles R. Rinaldo, Jr., Ph.D., Professor and Chairman, Department of Infectious Diseases and Microbiology, University of Pittsburgh, Graduate School of Public Health, A427 Crabtree Hall, 130 DeSoto Street, Pittsburgh, PA 15261, Ph: 412-624-3928, Fax: 412-624-4953, rinaldo@pitt.edu
Michael G. Shlipak, M.D., M.P.H., General Internal Medicine Division Chief, Veteran Affairs Medical Center, Professor of Medicine, Epidemiology & Biostatistics, University of California, San Francisco, 4150 Clement St. Rm. 111A1, 415-221-4810 x3381, San Francisco, CA 94143, Michael.shlipak@ucsf.edu
Russell Tracy, Ph.D., Professor of Pathology and Biochemistry, University of Vermont College of Medicine, 208 South Park Drive, Suite 2, Colchester, VT 05446, 802-656-8968, russell.tracy@uvm.edu
Victor Valcour, M.D., Associate Professor of Geriatric Medicine, Division of Geriatric Medicine and Department of Neurology, Memory and Aging Center, University of California, San Francisco, 350 Parnassus Avenue, Suite 905, San Francisco, CA 94143-1207, 415-476-3746, vvalcour@memory.ucsf.edu
David E. Vance, Ph.D., Associate Professor, School of Nursing, University of Alabama at Birmingham, 1701 University Boulevard, Room 456, Birmingham, AL 35294-1210, 205-934-7589, devance@uab.edu
Paul Volberding, M.D., Co-Chair, Professor and Vice Chair, University of California, San Francisco, Chief of the Medical Service, San Francisco Veterans Affairs Medical Center, 4150 Clement Street, VAMC 111, San Francisco, CA 94121, 415-750-2037, Paul.volberding@ucsf.edu
Jeremy D. Walston, M.D., Raymond and Anna Lublin Professor of Geriatric Medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Suite 1A.62, 5501 Hopkins Bayview Circle, Baltimore, MD 21224, 410-550-1003, jwalston@jhmi.edu
References
- 1.Negin J, Wariero J, Cumming RG, Mutuo P, Pronyk PM. High rates of AIDS-related mortality among older adults in rural Kenya. J Acquir Immune Defic Syndr. 2010;55:239–44. doi: 10.1097/QAI.0b013e3181e9b3f2. [DOI] [PubMed] [Google Scholar]
- 2.Negin J, Cumming RG. HIV infection in older adults in sub-Saharan Africa: extrapolating prevalence from existing data. Bull World Health Organ. 2010;88:847–53. doi: 10.2471/BLT.10.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, Vaeth M, Obel N. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 4.Losina E, Schackman BR, Sadownik SN, Gebo KA, Walensky RP, Chiosi JJ, Weinstein MC, Hicks PL, Aaronson WH, Moore RD, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the United States: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49:1570–8. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freiberg MS, McGinnis K, Butt AA, Goetz M, Brown S, Oursler KA, Rodriguez-Barradas M, Gibert C, Rimland D, Justice AC, et al. HIV is associated with clinically confirmed myocardial infarction after adjustment for smoking and other risk factors. 18th Conference on Retroviruses and Opportunistic Infections; 2011. p. Abs. 809. [Google Scholar]
- 7.Silverberg MJ, Chao C, Leyden WA, Xu L, Tang B, Horberg MA, Klein D, Quesenberry CP, Jr, Towner WJ, Abrams DI. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337–45. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverberg MJ, Lau B, Jing Y, D’Souza G, Engels EA, Gill J, Goedert JJ, Kirk GD, Justice AC, Dubrow R. Risk of anal cancer in HIV-infected patients and HIV-infected controls in North America. 12th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies; 2010. p. Abs. 306. [Google Scholar]
- 9.Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B, Fraenkel L, Mattocks K, Rimland D, Rodriguez-Barradas MC, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011;6:e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-Infected Person. Ann Intern Med. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- 11.Lucas GM, Mehta SH, Atta MG, Kirk GD, Galai N, Vlahov D, Moore RD. End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS. 2007;21:2435–43. doi: 10.1097/QAD.0b013e32827038ad. [DOI] [PubMed] [Google Scholar]
- 12.Fischer MJ, Wyatt CM, Gordon K, Gibert C, Brown ST, Rimland D, Rodriguez-Barradas M, Justice AC, Parikh CR and the VACS Project Team. Hepatitis C is associated with a high prevalence of chronic kidney disease and mortality among veterans with HIV. HSR&D National Meeting. 2009:Abs 3026. [Google Scholar]
- 13.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010;153:452–60. doi: 10.1059/0003-4819-153-7-201010050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green TC, Kershaw T, Lin H, Heimer R, Goulet JL, Kraemer KL, Gordon AJ, Maisto SA, Day NL, Bryant K, et al. Patterns of drug use and abuse among aging adults with and without HIV: A latent class analysis of a US Veteran cohort. Drug Alcohol Depend. 2010;110:208–220. doi: 10.1016/j.drugalcdep.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: What is known and future research directions. Clin Inf Dis. 2008;47:542–53. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://www.whitehouse.gov/blog/2011/09/16/national-hivaids-and-aging-awareness-day-perspective-national-institutes-health
- 17.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 18.Raichle ME. A brief history of human brain mapping. Trends in Neurosciences. 2008;32:118–26. doi: 10.1016/j.tins.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Behar R, Wiley C, McCutchan JA. Cytomegalovirus polyradiculoneuropathy in acquired immune deficiency syndrome. Neurology. 1987;37:557–61. doi: 10.1212/wnl.37.4.557. [DOI] [PubMed] [Google Scholar]
- 20.Venneti S, Lopresti BJ, Wang G, Bissel SJ, Mathis CA, Meltzer CC, Boada F, Capuano S, III, Kress GJ, Davis DK, et al. PET imaging of brain macrophages using the peripheral benzodiazepine receptor in a macaque model of neuroAIDS. J Clin Invest. 2004;113:981–9. doi: 10.1172/JCI20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenny-Avital ER, Hoffman J, Katz M, Leider J, Apfelroth S. Cerebrospinal fluid HIV load in diverse clinical circumstances. Clinical Infectious Diseases. 2008;46:1938–40. doi: 10.1086/588555. [DOI] [PubMed] [Google Scholar]
- 22.Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Human Brain Mapping. 2009;30:1120–32. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, McCutchan JA, Ellis RJ HIV Neurobehavioral Research Center. HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. Journal of Infectious Diseases. 2010;201:336–40. doi: 10.1086/649899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortega M, Lee T, Thomas J, Ho P, Clifford D, Vaida F, Ances B. Prolonged HAART causes caudate volume reduction. 18th Conference on Retroviruses and Opportunistic Infections; 2011. p. Abs 442. [Google Scholar]
- 25.Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Trojanowski JQ, Shaw LM, Bernstein MA, Aisen PS, Weiner M, Petersen RC, et al. Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology. 2010;75:143–51. doi: 10.1212/WNL.0b013e3181e7ca82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Risacher SL, Shen L, West JD, Kim S, McDonald BC, Beckett LA, Harvey DJ, Jack CR, Jr, Weiner MW, Saykin AJ. Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol Aging. 2010;31:1401–18. doi: 10.1016/j.neurobiolaging.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Barkhof F, Bernstein MA, Cantillon M, Cole PE, DeCarli C, Dubois B, Duchesne S, Fox NC, Frisoni GB, et al. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer’s disease. Alzheimers Dement. 2011;7:474–85. doi: 10.1016/j.jalz.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang GC, Insel PS, Tosun D, Schuff N, Truran-Sacrey D, Raptentsetsang S, Jack CR, Jr, Weiner MW. Identifying cognitively healthy elderly individuals with subsequent memory decline by using automated MR temporoparietal volumes. Radiology. 2011;259:844–51. doi: 10.1148/radiol.11101637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Swaminathan S, Shen L, Risacher SL, Nho K, Foroud T, Shaw LM, Trojanowski JQ, Potkin SG, Huentelman MJ, et al. Genome-wide association study of CSF biomarkers Abeta1–42, t-tau, and p- tau181p in the ADNI cohort. Neurology. 2011;76:69–79. doi: 10.1212/WNL.0b013e318204a397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo RY, Hubbard AE, Shaw LM, Trojanowski JQ, Petersen RC, Aisen PS, Weiner MW, Jagust WJ. Longitudinal Change of Biomarkers in Cognitive Decline. Arch Neurol. 2011;68:1257–1266. doi: 10.1001/archneurol.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akbar AN, Soares MV, Plunkett FJ, Salmon M. Differential regulation of CD8+ T cell senescence in mice and men. Mech Ageing Dev. 2000;121:69–76. doi: 10.1016/s0047-6374(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 32.Appay V, Rowland-Jones S. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 2002;23:580–585. doi: 10.1016/s1471-4906(02)02338-4. [DOI] [PubMed] [Google Scholar]
- 33.Shao L, Goronzy JJ, Weyand CM. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med. 2010;2:415–27. doi: 10.1002/emmm.201000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–56. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nat Cell Biol. 2011;13:506–12. doi: 10.1038/ncb0511-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seviour EG, Lin SY. The DNA damage response: Balancing the scale between cancer and ageing. Aging. 2010;2:900–7. doi: 10.18632/aging.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews NP, Fujii H, Goronzy JJ, Weyand CM. Telomeres and immunological diseases of aging. Gerontology. 2010;56:390–403. doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–46. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: Roles in cellular aging. Mutat Res/Fund and Molec Mech of Mutagenesis. 2012;730:85–89. doi: 10.1016/j.mrfmmm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol. 2011;46:135–40. doi: 10.1016/j.exger.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–22. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol. 2009;21:440–5. doi: 10.1016/j.coi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Brunner S, et al. Persistent viral infections and immune aging. Ageing Res Rev. 2011;10:362–9. doi: 10.1016/j.arr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infections in the US population: NHANES III. Epidemiol Infect. 2009;137:58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw AC, Panda A, Joshi SR, et al. Dysregulation of human Toll-like receptor function in aging. Ageing Res Rev. 2011;10:346–53. doi: 10.1016/j.arr.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22:2409–18. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deeks SG. HIV Infection, Inflammation, Immunosenescence, and Aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palella FJ, Jr, Gange SJ, Benning L, et al. Inflammatory biomarkers and abacavir use in the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study. AIDS. 2010;24:1657–65. doi: 10.1097/QAD.0b013e3283389dfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–22. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006 May 16;113:2335–62. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 53.Cook NR. Assessing the Incremental Role of Novel and Emerging Risk Factors. Curr Cardiovasc Risk Rep. 2010;4:112–9. doi: 10.1007/s12170-010-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson S, Halter JB, Hazzard WR, et al. Prediction, progression and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol. 2009;20:1199–209. doi: 10.1681/ASN.2008080860. [DOI] [PubMed] [Google Scholar]
- 55.Justice AC, McGinnis KA, Skanderson M, Chang CC, Gibert CL, Goetz MB, Rimland D, Rodriguez-Barradas MC, Oursler KK, Brown ST, et al. Towards a combined prognostic index for survival in HIV infection: the role of ‘non-HIV’ biomarkers. HIV Med. 2010;11:143–151. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatolol. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 57.Park LS, Tate JP, Justice AC, Lo RV, III, Lim JK, Brau N, Brown ST, Butt AA, Gibert C, Goetz MB, et al. FIB-4 Index Is Associated with Hepatocellular Carcinoma Risk in HIV-Infected Patients. Cancer Epidemiol Biomarkers Prev. 2011;20:2512–7. doi: 10.1158/1055-9965.EPI-11-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tate JP, Justice AC for the VACS Project Team. Change in a prognostic index for survival in HIV infection after one year on cART by level of adherence. 48th Annual Meeting of the Infectious Diseases Society of America; 2010. [Google Scholar]
- 59.Tate JP, Hughes MD, Justice AC for the VACS Project Team. Do risk factors for mortality change with time on antiretroviral therapy?. 48th Annual Meeting of the Infectious Diseases Society of America; 2010. [Google Scholar]
- 60.Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, Lampe F, Bucher HC, Sterling TR, Crane HM, et al. Performance of the refined VACS Risk Index during the first 12 months of antiretroviral therapy among US and European subjects. 15th International Workshop on HIV Observational Databases; 2011. [Google Scholar]
- 61.Justice AC, Freiberg MS, Tracy R, Tate J, Goetz M, Butt AA, Rodriguez-Barradas M, Gibert C, Oursler KA, Bryant KJ, et al. Does an Index Composed of Clinical Data Reflect Effects of Inflammation, Coagulation, and Monocyte Activation on Mortality Among those Aging with HIV? Clin Infect Dis. 2012 doi: 10.1093/cid/cir989. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edwards-Jackson N, Kerr S, Tieu H, et al. Cardiovascular risk assessment in persons with HIV infection in the developing world comparing three risk equations in a cohort of HIV-infected Thais. HIV Med. 2011;12:510–5. doi: 10.1111/j.1468-1293.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 63.Barros ZM, de Alencar Ximenes RA, Miranda-Filho DB, et al. Comparison between the Framingham and prospective cardiovascular of Munster scores for assessment of coronary heart disease in human immunodeficiency virus positive patients in Brazil. Metab Syndr Relat Disord. 2010;8:489–97. doi: 10.1089/met.2009.0100. [DOI] [PubMed] [Google Scholar]
- 64.Friis-mooler N, Thiebaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse events of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 65.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 66.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–86. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 67.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17:118–23. [PubMed] [Google Scholar]
- 68.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 69.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuller LH, Tracy R, Belloso W, De WS, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La RA, Kuller LH, Pett SL, Ristola M, Ross MJ, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 73.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575–92. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 74.Akgun KM, Pisani MA, Fried TR, Butt AA, Gibert CL, McGinnis K, Huang L, Rimland D, Justice AC, Rodriguez-Barradas MC, et al. Risk Factors for Medical Intensive Care Unit Admission in HIV Infected Veterans. American Thoracic Society; 2010. [Google Scholar]
- 75.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–24. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 76.Shippy RA, Karpiak SE. The aging HIV/AIDS population: Fragile social networks. Aging Ment Health. 2005;9:246–254. doi: 10.1080/13607860412331336850. [DOI] [PubMed] [Google Scholar]
- 77.Okonkwo O, Vance D, Antia L, Smith B, Blanshan S, Heirs K, Bodner E. Service utilization and cognitive complaints in adults with HIV: Results from a state-wide survey. Journal of HIV/AIDS and Social Services. 2008;7:175–194. [Google Scholar]
- 78.Vance DE, Brennan M, Enah C, Smith GL, Kaur J. Religion, spirituality, and older adults with HIV: Critical personal and social resources for an aging epidemic. Clinical Interventions in Aging. 2011;6:101–109. doi: 10.2147/CIA.S16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vance DE, Struzick TC, Masten J. Hardiness, successful aging, and HIV:Implications for social work. Journal of Gerontological Social Work. 2008;51:260–283. doi: 10.1080/01634370802039544. [DOI] [PubMed] [Google Scholar]
- 80.Kalichman SC, Pope H, White D, Cherry C, Amaral CM, Swetzes C, Flanagan J, Kalichman MO. Association between health literacy and HIV treatment adherence: Further evidence from objectively measured medication adherence. Journal of the International Association of Physicians in AIDS Care. 2008;7:317–323. doi: 10.1177/1545109708328130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vance DE, Mugavero M, Willig J, Raper JL, Saag MS. Aging with HIV: A cross-sectional study of co-morbidity prevalenceclinical characteristics across decades of life. J Assoc Nurses AIDS Care. 2011;22:17–25. doi: 10.1016/j.jana.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 82.Vance DE. Aging with HIV: Clinical considerations on an emerging population. American Journal of Nursing. 2010;110:42–47. doi: 10.1097/01.NAJ.0000368952.80634.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vance DE, Bayless H, Kempf MC, Keltner NL, Fazeli PL. Aging, HIV, and Wellness: Augmenting the components of successful aging. Aging Health. 2011;7:435–446. [Google Scholar]
- 84.Ettenhofer ML, Hinkin CH, Castellon SA, et al. Aging, neurocognition, and medication adherence in HIV infection. Am J Geriat Psychiat. 2009;17:281–290. doi: 10.1097/JGP.0b013e31819431bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fazeli PL, Marceaux JC, Vance DE, Slater L, Long CA. Predictors of cognition in adults with HIV: Implications for nursing practice and research. Journal of Neuroscience Nursing. 2011;43:36–50. doi: 10.1097/jnn.0b013e3182029790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vance DE. Implications of positive and negative neuroplasticity on cognition in HIV. Medical Science Monitor. 2010;16:HY3–5. [PubMed] [Google Scholar]
- 87.Hardy D, Vance D. The neuropsychology of HIV/AIDS in older adults. Neuropsychology Review. 2009;19:263–272. doi: 10.1007/s11065-009-9087-0. [DOI] [PubMed] [Google Scholar]
- 88.Vance DE, Wadley VG, Crowe MG, Raper J, Ball KK. Cognitive and everyday functioning in younger and older adults with and without HIV. Clinical Gerontologist. 2011;34:413–426. doi: 10.1080/07317115.2011.588545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Green TC, Kershaw T, Lin H, Heimer R, Goulet JL, Kraemer KL, Gordon AJ, Maisto SA, Day NL, Bryant K, et al. Patterns of drug use and abuse among aging adults with and without HIV: A latent class analysis of a US Veteran cohort. Drug Alcohol Depend. 2010;110:208–20. doi: 10.1016/j.drugalcdep.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]