Abstract
The oxidative effect of nicotine was investigated using androgen biomarkers of redox status and wound healing in fibroblasts; using the antioxidant glutathione for confirmation of responses. Cultures of human gingival (HGF) and periosteal fibroblasts (HPF) were incubated with substrates 14C-testosterone/14C-4-androstenedione in the presence or absence of serial concentrations of nicotine (N100-500), glutathione (G1–5) and their combinations, in medium. At 24 h the medium was solvent extracted for metabolites, separated by TLC and quantified using radioisotope scanning. Nicotine caused significant inhibition in yields of the physiologically active metabolite 5α-dihydrotestosterone (DHT) in HGF and HPF, overcome to varying degrees by the anti-oxidant glutathione (n = 6; p<0.01, one way ANOVA); this is suggestive of moderation of an oxidative mechanism induced by nicotine. Down-regulation of 5α-reductase activity by nicotine resulting in reduced yields of DHT was overcome by glutathione. Overcoming oxidative stress in a redox environment is applicable to treatment outcome.
We have investigated oxidative stress-inducing effects of nicotine and its modulation by glutathione in a cell culture model of human gingival and oral periosteal fibroblasts which may be extrapolated to an oxidative stress-inducing environment of periodontitis in smokers. Two radiolabelled androgen substrates 14C-testosterone and 14C-4-androstenedione and their metabolites serve as redox markers of wound healing. Redox activity of cells in response to their environment has important implications on periodontal disease. The actions of glutathione, nicotine and the androgen metabolite 5α-dihydrotestosterone (DHT) as a redox marker are addressed in the introduction as background relevant to our study.
Assaying for redox markers in response to nicotine in fibroblasts as an index of oxidative stress is a pertinent area for investigation, with regard to potential oxidative effects of nicotine and its modulation by the anti-oxidant glutathione. Using DHT in this context as a marker of oxidative stress and wound healing in parallel is a novel application, considering the prevalence of androgen receptor expression and activity in gingival and periosteal fibroblasts. This experimental model could be applied to a facet of periodontal disease progression amongst smokers1,2,3, in the context of mechanisms that induce reduced antioxidant capacity. Periodontitis is initiated by the host inflammatory response to bacterial plaque leading to oxidative damage of the supporting structures of teeth and eventual tooth loss.
Evaluation of reduced and oxidized glutathione in gingival crevicular fluid (GCF) of periodontitis patients prior to and after non-surgical periodontal therapy has demonstrated that levels of glutathione were compromised in periodontitis4; compared with levels in health. While treatment did not fully restore glutathione concentrations in crevicular fluid, the redox balance was restored between its reduced and oxidized forms; suggestive of an abnormal redox equilibrium in periodontitis due to oxidative stress. Antioxidants as adjuncts to root surface debridement have been shown to improve treatment outcome in periodontitis patients5. In the context of periodontal disease and systemic comorbidities6,7, antioxidants could be beneficial in overcoming delayed wound healing induced by an imbalance in redox status. The stress-inducible protein heme oxygenase-1 expressed in human gingival fibroblasts exposed to nicotine was overcome by the addition of the glutathione precursor N-acetyl-L-cysteine8. Low levels of antioxidants such as glutathione accompanied by raised levels of markers of free radical damage play an important role in delayed wound healing.
Topical application of the nitric oxide (NO) donor S-nitrosoglutathione (GSNO) known to exert beneficial effects on wound healing resulted in improved rates of wound contraction, re-epithelialization, decreased inflammation, increased collagen fibre density and organization; and decreased neovascularization when applied to ischaemic wounds, compared with controls9. These findings demonstrate the efficacy of topical application of GSNO in the treatment of ischaemic wounds, comparable to an environment of oxidative stress in periodontitis. There is therapeutic potential for its use as a topical GSNO-containing hydrogel to enhance wound healing in an environment driven by oxidative stress, which could be beneficial to periodontitis patients.
Androgens are effective biomarkers of oxidative stress and wound healing. The rationale for their use in our investigation include the demonstration of androgen responsive genes associated with oxidative stress10. DHT-activated androgen receptor proteins directly regulate glutathione S-transferases11, functioning as important redox regulators.
Alteration of antioxidant proteins could contribute to oxidative stress, due to loss of equilibrium between reactive oxygen species and antioxidant responses. DHT, the biologically active steroid metabolite of testosterone, is an effective marker of oxidative stress with inherent antioxidant properties12,13,14. DHT induces anti-apoptotic proteins and attenuates oxidative stress in a redox environment15; it ameliorates oxidative effects of H2O2 by activating catalase and down-regulating p38MAPK, JNK 1 SAPK and NF-kappaB (a stress-activated protein kinase family), via androgen receptors16, making it an effective marker of oxidative actions and redox interactions in the context of this study. Previous work has established that androgen metabolites are suitable biomarkers of healing responses in the context of alkaline phosphatase (ALP) activation17 and inhibition by the ALP inhibitor levamisole18; and also of oxidative stress7,19,20. In addition, there is a significant distribution of androgen receptors in gingival and periodontal tissue fibroblasts21,22,23.
Assays for DHT, using 14C-testosterone/14C-4-androstenedione as substrates in gingival and oral periosteal fibroblast cell cultures in response to nicotine and glutathione would be a suitable index of redox status in a simulated model of selected parameters; to study mechanisms of action of nicotine relevant to periodontitis subjects who smoke and their treatment outcome.
The specialized nature of periosteal tissue comprising fibroblasts, osteoblasts and progenitor cells, provides optimal potential for tissue engineering due to its location and ability to differentiate24,25. Its osteogenic potential is used extensively for these applications. The versatility and potential for cell commitment of periosteal derived cells, established fibroblasts and osteoblasts subjected to both adipogenic and osteogenic culture conditions have been studied26. It is relevant that heterogeneous populations of periosteal cells and fibroblasts were able to express both osteoblast-like and adipocyte-like markers with similar potential. This raises a pertinent question regarding the progenitor potential of fibroblasts within a multi-lineage tissue such as the periosteum. Expanded periosteal or fibroblast cultures may not need enrichment or sorting by molecular markers in order to provide applications for tissue engineering, as indicated in this study.
A pro-oxidant fibroblast phenotype may be elicited in an environment enriched with bacterial LPS and nicotine. Fibroblasts derived from a chronically inflamed tissue source used in our investigation would be a suitable model for studying androgen responsiveness, as a biomarker of healing. It is pertinent to study the mechanisms involved by using the antioxidant glutathione in a cell culture model to demonstrate possible oxidant effects of nicotine in this context.
The aim of this investigation is to establish the effects of nicotine and glutathione on steroid markers of redox status and wound healing in cultured human gingival and oral periosteal fibroblasts; and the antioxidant role of glutathione in this context, based on clinical and scientific evidence of their actions as addressed in the introduction.
Results
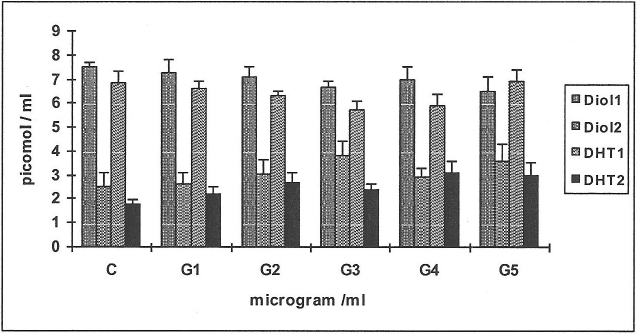
Establishing effective concentrations of nicotine (Figure 1)
When 14C-testosterone was incubated with HGF and serial concentrations of nicotine, the substrate was metabolised mainly to the diols, DHT and 4-androstenedione. The metabolic yields of DHT only from each of the substrates 14C-testosterone and 14C-4-androstenedione are shown in Figure 1 as DHT1 and DHT2 respectively, being the effective metabolite. In response to nicotine (N) there was inhibition in the yields of DHT1 of 18–35% at N100-500 (Figure 1, n = 4; p<0.01), demonstrating reduced 5α-reductase activity while the values for 4-androstenedione and the diols progressively increased from 26–47% in response to N100-400 (results not shown); this appears to be a compensatory mechanism of other enzyme systems.
Figure 1. Establishing effective concentrations of nicotine.
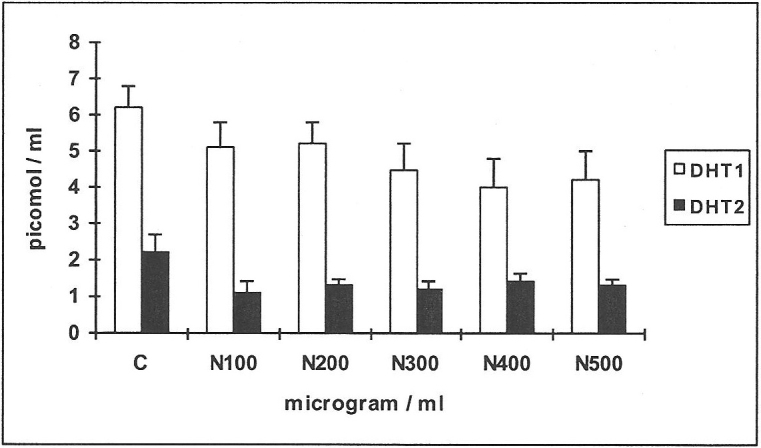
Four cell-lines of human gingival fibroblasts (HGF) were incubated with serial concentrations of nicotine N100-500 μg/ml, using the androgen substrates 14C-testosterone/14C-4-androstenedione and the metabolites formed were DHT1 and DHT2 respectively. Mean values and standard deviations are shown in all figures; C: controls.
When 14C-4-androstenedione was used as substrate (Figure 1), it was metabolised mainly to the diols, testosterone (T), DHT and androstanedione (A). Values for DHT2 only are shown, being the effective metabolite. There was 40–50% reduction in the yields of DHT2 at N100-500 (n = 4; p<0.01). There was not much change in the values for testosterone and the diols or A. Based on these observations for yields of DHT1 and DHT2 an effective concentration of 250μg / ml of nicotine was used in subsequent experiments.
Effects of serial concentrations of glutathione on the metabolism of 14C-testosterone and 14C-4-androstenedione by human gingival fibroblasts (Figure 2)
Figure 2 demonstrates the main metabolites Diol1, DHT1; and Diol2, DHT2 formed from each of the substrates 14C-testosterone and 14C-4-androstenedione respectively, tested with 4 cell-lines each. There was no appreciable difference between control and test incubations, with small increases in the yields of certain metabolites; this is in accordance with previous studies, suggestive of a positive trend in anabolic responses to glutathione. In view of the fact that the effects of glutathione (G) are more likely to be demonstrated in response to oxidative challenge (shown in the experiments below), serial concentrations of G1-4 were used in subsequent experiments in the presence of an effective concentration of nicotine.
Figure 2. Effects of serial concentrations of glutathione on the metabolism of 14C-testosterone and 14C-4-androstenedione.
Four cell-lines of human gingival fibroblasts (HGF) were incubated with 1-5 μg/ml glutathione (G1–5), with each substrate 14C-testosterone and 14C-4-androstenedione; the metabolites formed were Diol1, DHT1; and Diol2, DHT2 respectively.
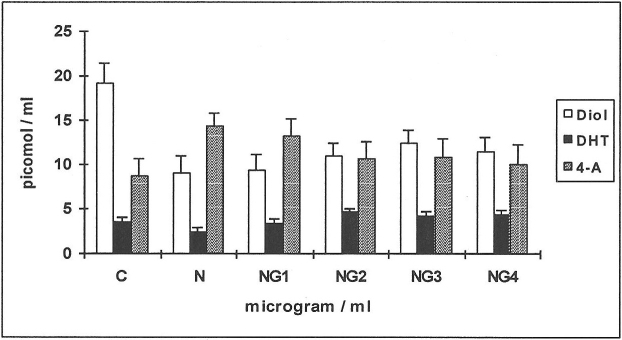
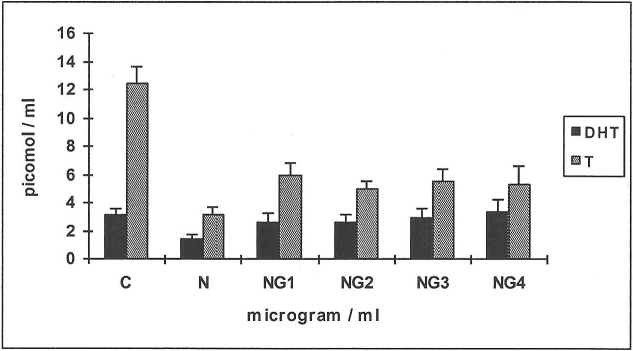
Effects of an effective concentration of nicotine (N 250 μg/ml) in the presence or absence of glutathione (G 1–4 μg/ml) on the metabolism of 14C-testosterone and 14C-4-androstenedione in HGF (Figures 3 & 4)
When 14C-testosterone was used as substrate (Figure 3) there was a 2-fold reduction in the production of D (a metabolite of DHT) when compared to controls in response to N with 33% less inhibition when G was added (NG2,3,4). The production of DHT was decreased by 33% in response to N while the values were similar to controls at NG1 and increased further by 30% at NG2, 3 and 4 (n = 6; p<0.01). The yield of 4-A increased by 60% in response to N (n = 6; p<0.01) and decreased to values similar to controls in combination with high concentrations of G; in keeping with 17β-hydroxysteroid dehydrogenase activity which complements 5α-reductase activity and DHT synthesis.
Figure 3. Effects of nicotine (N) and glutathione (G) on the metabolism of 14C-testosterone in HGF.
The yields of Diol, DHT and 4-androstenedione (4-A) are shown for the control ( C ) and in response to testing agents. Means values from 6 human gingival fibroblast (HGF) cell-line incubations are shown, for the substrate 14C-testosterone. N = 250 μg/ml; G1-4: 1–4 μg/ml.
When 14C-4-androstenedione was used as substrate (Figure 4), N caused a 2.7-fold reduction in the production of DHT (n = 6; p<0.01) while values returned to those similar to controls at NG2-4. There were no significant changes in the values of T and D. The values for A increased over controls by 32% at NG1-2, decreasing to control values at NG4 in keeping with enzymic pathways.
Figure 4. Effects of nicotine (N), and glutathione (G) on the metabolism of 14C-4-androstenedione in HGF.
The yields of Diol, DHT and androstanedione (A) are shown for the control (C) and in response to testing agents. Means from 6 human gingival fibroblast (HGF) cell-line incubations are shown, for the substrate 14C-4-androstenedione. N = 250 μg/ml; G1–4: 1–4 μg/ml.
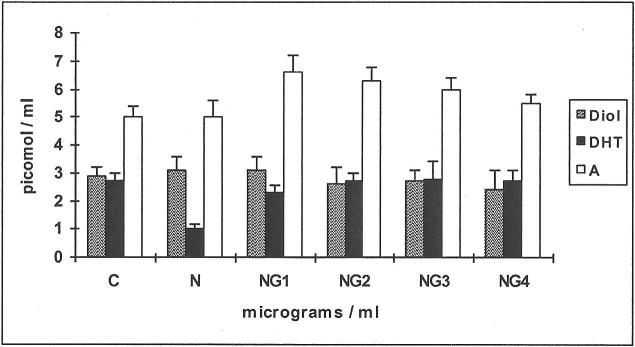
Effects of an effective concentration of nicotine (N 250 μg/ml) in the presence or absence of glutathione (G 1–4 μg/ml) on the metabolism of 14C-testosterone and 14C-4-androstenedione by HPF (Figures 5 & 6)
When 14C-testosterone was used as substrate (Figure 5), the main metabolites formed were Diols, DHT and androstanedione (A), of which DHT and A are shown.
Figure 5. Effects of nicotine (N) and glutathione (G) on the metabolism of 14C-testosterone in HPF.
The yields of DHT and androstanedione (A) are shown for the control ( C )and in response to testing agents. Means from 6 human periosteal fibroblast (HPF) cell-line incubations are shown, for the substrate 14C-testosterone. N = 250 μg/ml; G1–4: 1–4 μg/ml.
The production of D was decreased by 38% (19 pmol compared with 32 for control) when N alone was added. Further decreases of up to 37% (16–12 pmol) over N alone occurred at NG1–4 (n = 6; p<0.01, results not shown).
Yields of DHT showed a decrease of 31% when N alone was added, increasing by 26% at NG1,2 and reaching values similar to C at NG3,4 (n = 6; p<0.01).
Yields of 4-A did not show much change. Yields of A showed a decrease of 38% when N alone was added, a further decrease of 37% over N alone occcurred at NG1 and remained at the same values up to NG4.
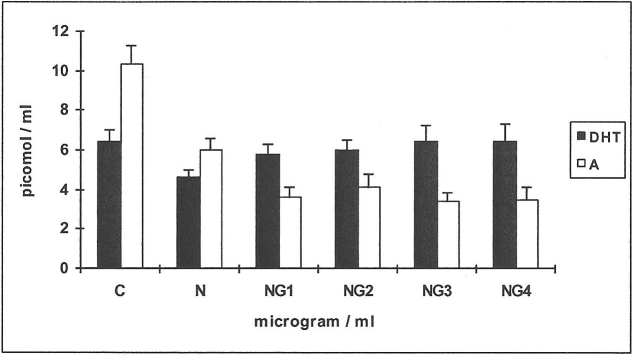
When 14C-4-androstenedione was used as substrate (Figure 6), there was not much change in the yields of D (results not shown).
Figure 6. Effects of nicotine (Ν) and glutathione (G) on the metabolism of 14C-4-androstenedione in HPF.
The yields of DHT and testosterone (T) are shown for the control (C) and in response to testing agents. Means from 6 HPF cell-line incubations are shown, for the substrate 14C-4-androstenedione. N = 250 μg/ml; G1–4: 1–4 μg/ml.
Yields of T showed a decrease of 400% when N alone was added (n = 6; p<0.01), increasing to approximately 200% over N alone at NG1-4 (n = 6; p<0.01).
Yields of DHT showed a decrease of 200% when N alone was added (n = 6; p<0.01), a substantial increase of 83% at NG1 over N alone, with a gradual increase up to NG4 (240% over N alone; n = 6; p<0.01) reaching values marginally above C (13%).
Yields of A did not show much change, over controls in response to the testing agents (results not shown).
Discussion
Using two radiolabelled steroid substrates reinforces the implications of yields of biologically active metabolites in response to the testing agents and trends that are shown as a result of metabolic activity in cell culture. The results of this investigation demonstrated that human periosteal and gingival fibroblasts metabolized 14C-T mainly to 5α-dihydrotestosterone (DHT), diols (D) and 4-androstenedione (4-A), while 14C-4-A was mainly metabolised to DHT and testosterone (T), both with controls and testing agents in accordance with enzymic pathways. 14C-4-A as a substrate is first metabolised to small amounts of testosterone and subsequently reduced to DHT by 5α-reductase18,32. It is relevant that when 14C-testosterone was used as substrate, the yield of DHT was nearly 2-fold greater for HPF than HGF which could have some bearing on enhanced anabolic and antioxidant responses of HPF when compared with HGF.
DHT is considered to be the most potent natural AR natural ligand33 with a 2-10-fold greater potency than testosterone in androgen-responsive tissues and binds to androgen receptor. Testosterone is irreversibly converted to DHT by 5α-reductase. DHT exerts anti-apoptotic effects and prevents H2O2 - induced apoptotic cell death by activation of catalase, downregulation of p38 MAPK, JNK/SAPK, and NF-kappaB via the androgen receptor. These effects are blocked by flutamide treatment which confirms mediation of these actions via androgen receptor34 and the efficacy of DHT as a marker of oxidative stress.
Yields of DHT from both androgen substrates were significantly reduced in response to nicotine. The physiologically weaker androgen metabolites were sometimes increased when the main metabolite DHT decreased. This demonstrates the reversible pathway between testosterone and 4-androstenedione via 17β-hydroxysteroid dehydrogenase activity, in preference to 5α-reduction, resulting in decreased yields of the physiologically active metabolite DHT, relevant to wound healing. The anti-oxidant glutathione was able to overcome the inhibitory effect of nicotine, demonstrated in the yields of DHT with both substrates, in both HGF and HPF. Again it is relevant that with HGF when 14C-testosterone was used as substrate, yields of the weaker androgen 4-androstenedione increased in response to nicotine and subsequently reduced in response to glutathione, complementing the 5α-reductase pathway for yields of DHT.
The antioxidant role of glutathione is further bourne out in other studies where GSH levels peaked on day 14 during cutaneous wound healing in rats enhanced with EGF, when compared with an untreated group35. EGF may function like an antioxidant by scavenging toxic oxidation products in the healing wound. In addition, it could contribute to early healing and assist wound healing therapies. Similarly, topical use of esterified glutathione, an established antioxidant could potentially minimize effects of oxidative stress with raised intracellular levels of GSH and accelerate wound healing by optimizing the capacity of fibroblasts and keratinocytes. In a rat ischaemic wound model, topical treatment with glutathione decreased oxidative stress leading to re-establishment of the MMP-1/TIMP-1 ratio which resulted in regular extracellular matrix production and re-epithelization36. These actions have applications in the clinical management of ischemic wounds, also applicable to periodontitis, which may be cautiously extrapolated from the results of this investigation, as we have used a marker of oxidative stress and wound healing; within limitations of the study model.
Glutathione-S-transferase (GST) enzymes play a role in scavenging endogenous oxidants. Altered enzyme activity resulting from inherited polymorphisms, results in increased oxidative stress. The variant Val allele at the GSTP1 105 codon was associated with a significantly increased risk of surgical complications following microvacular surgical reconstruction. It implies that GSTP1 codon 105 polymorphism may be a marker for risk of wound complications microvascular reconstruction37, emphasizing its important role in wound healing. The antioxidant and wound healing roles of glutathione are demonstrated in our investigation using DHT as a redox marker with effects on wound healing; these results could have potential applications in gingival and periosteal fibroblasts in vivo in response to nicotine.
It is relevant that cytogenetic damage resulting from exposure of human gingival fibroblasts to nicotine was significantly reduced in response to the glutathione precursor N-acetyl- cysteine38. In our experimental model GSH was able to overcome oxidative effects of nicotine.
Oxidative stress can alter the balance between genetic expression of pro-inflammatory mediators and anti-oxidant enzymes; resulting in apoptotic markers of oxidative stress which have been demonstrated in lymphocytes of diabetics with chronic non-healing wounds, with reduced levels of catalase and glutathione39. In our experimental study DHT serves as an effective marker of redox status in response to oxidant/antioxidant stimuli in cell culture and its inherent antioxidant and other matrix enhancing actions would be effective in wound healing responses relevant to periodontitis. The ability of androgen metabolites to stimulate ALP activity is relevant to their antioxidant role in contributing to healing responses and their efficacy as markers of oxidative stress.
Studies have linked the anabolic effects of androgens on connective tissues and bone to alkaline phosphatase (ALP) activity. Enhanced AR expression in differentiated osteoblasts increases mineralization, while knockdown of AR expression prevents androgen-induced mineralization. The gene family associated with tissue- nonspecific alkaline phosphatase (TNSALP) and several members of small integrin binding ligand N-linked glycoprotein (SIBLING) have been identified as androgen target genes required for androgen receptor (AR)-mediated bone formation40. Thus, androgen/AR signalling pathways play an essential role in bone formation by coordinating the expression of genes associated with phosphate regulation. Periosteal and gingival fibroblasts used in our investigation demonstrate suitable antioxidant effects in response to glutathione after exposure to nicotine; these effects are also relevant to wound healing responses in the context of the marker DHT used in the study.
There are several mechanisms that could contribute to reduced yields of DHT in response to nicotine, including its oxidant effects, counteracted by the antioxidant glutathione; as indicated below. This has some bearing on the yields of DHT, relevant in this context as a biomarker of healing and oxidative stress.
Nicotine has been identified as a significant risk factor for several diseases being a major toxic component of cigarette smoke. The role of reduced glutathione (GSH) in nicotine-induced organ toxicity41 has shown that the protective effect of GSH was exerted by modulation of the biochemical marker enzyme lactate dehydrogenase, lipid peroxidation and augmentation of the antioxidant defence system. Documented literature suggests a redox imbalance in smokers which may be an important factor in mediating tissue damage in several tissues and that antioxidants are effective in overcoming the oxidative stress-inducing effects of nicotine42,43,44. The actions of nicotine closely identify with responses induced by an oxidative biomarker45. Nicotine also has direct effects on wound healing. There is documentation of nicotine-induced inhibition of myofibroblast differentiation in human gingival fibroblasts in vitro46. This supports the hypothesis that decreased wound contraction by myofibroblasts could contribute to delayed wound healing in smokers.
The effect of nicotine on various activities of human periodontal ligament fibroblasts in vitro, including a dose-dependent inhibition of alkaline phosphatase activity has been demonstrated47. Our data indicate that gingival and oral periosteal fibroblasts constitute an effective cell culture model to study the oxidative responses to nicotine as detailed in the introduction; androgen metabolites are an effective index of redox activity in response to oxidative stress34,40 in gingival and periosteal fibroblasts due to their stimulatory effects on bone and connective tissue turnover. Previous work has demonstrated that ALP enhanced the yields of DHT which were inhibited by nicotine and the ALP inhibitor levamisole18, indicating that these effects could be linked to oxidative effects of nicotine in the context of the findings of this investigation.
Similarly the oxidative effects of nicotine could contribute to modulation of ALP activity, overcome by the antioxidant glutathione, with direct implications on healing, drawing a parallel comparison with the results of our investigation, using steroid hormone biomarkers. The findings of our investigation are pertinent to the detrimental effects of nicotine on periodontal wound healing in smokers, using steroid hormone biomarkers, which may be overcome by the anti-oxidant glutathione.
In view of the ability of DHT to induce mRNA levels for alpha1 (I) collagen, osteopontin, osteocalcin and especially alkaline phosphatase48,40; and the ability of inflamed gingival tissue preparations to metabolise androgens better than tissue from healthy samples27,28, a role for DHT as a biomarker of repair is reinforced in the context of the study model used here.
Considering the distinct influences of individual agents and their combinations, the findings of this study exclude the possibility of non-specific apoptotic changes. Mechanisms of wound healing are essential for determining both the progression of disease and response to treatment. In view of the proven oxidant effects of nicotine on tissues exposed to it, its down-regulation of androgen biomarkers and recovery in response to the anti-oxidant glutathione, it is suggestive of oxidant/antioxidant interactions in disease progression and repair; especially in view of the fact that ALP activity seems to be influenced by the redox status of the environment induced by nicotine45,49. There is a possible link between potential oxidant effects of nicotine demonstrated in this study and its modulation of ALP mediated actions47, collagen and ECM formation in fibroblasts50, which could contribute to the effects of nicotine on wound healing, applicable to the cells used in our investigation. It affected yields of DHT, a biomarker of redox status and repair, with potential implications on healing in an oxidative stress-inducing environment, due to their ability to activate relevant genes and their direct antioxidant capacity. Critical stages of wound healing are down-regulated in a redox environment; these effects could be attenuated by glutathione. We have used a novel marker which is a regulator of oxidative stress and wound healing in parallel. Using human gingival and oral periosteal fibroblasts in this experimental model we demonstrate oxidative effects of nicotine overcome by glutathione; these findings also have implications on wound healing using cells that are relevant to these processes in periodontal tissues of smokers. These mechanisms open interesting possibilities for the application of suitable agents for optimizing healing responses in an oxidative milieu, relevant to that induced by nicotine.
Methods
50μCi/ml each of the radiolabelled androgens 14C-testosterone/14C-4-androstenedione (specific radioactivity 58 mCi/mmol) were obtained from Amersham International, Amersham, U.K. Organic solvents for isolation of steroid metabolites and for thin layer chromatography (TLC), were provided by Merck Ltd., Dagenham, Essex, U.K. Eagle's MEM, L-glutamine, antibiotic solution (penicillin and streptomycin) and sodium bicarbonate used for incubation of cell cultures were all provided by Invitrogen Ltd., Paisley, Scotland. Glutathione and nicotine used in the incubations were obtained from Sigma Chemical Co. Ltd., Poole, Dorset, U.K.
Gingival tissue was isolated from chronically inflamed sites with probing pocket depths of 6–8 mm which bled on probing; during pocket elimination periodontal surgery following initial phase periodontal treatment. Ethical permission was granted by King's College Hospital Health Care Trust and consent was obtained from patients in keeping with requirements for ethical approval of surgical procedures and tissue isolation carried out. Tissue isolated as part of the surgical procedures is usually discarded; it was processed anonymously. Human gingival fibroblast explants were derived from the chronically inflamed gingival tissue samples of 6 periodontal patients (3 males and 3 females) with no history of smoking. Human periosteal fibroblast explants were derived from periosteal tissue attached to bone, isolated during muco-gingival surgery, from 6 patients (3 males and 3 females). They were non-smokers and all patients ranged in age from 30–50 years.
Previous workers have shown that although gingival tissues from healthy males metabolized testosterone better than that of healthy females, chronically inflamed gingiva from both sexes did not show any difference in testosterone metabolism27,28. Based on this evidence, the present study sample was not categorized for the sexes. But the samples were not pooled, maintaining individual cultures and experiments were set up for each cell-line independently, with its own controls; sample numbers include males and females.
Confluent monolayer cultures of human gingival fibroblasts (HGF) and human oral periosteal fibroblasts (HPF) of the 5th-9th passage were established in Eagle's Minimum Essential Medium (MEM), in 24 well multiwell plates for all experiments. This was done by trypsinising (0.25%) fully confluent fibroblasts in 25 cm2 flasks (2.2×106 cells) for distribution amongst 24 wells of a multiwell plate. After the 3rd passage, cells appear to be stable in culture29. Metabolic activity in differentiated cells do not appear to be affected by passage number30, even after extended in vitro culturing. The passage number of cells used was appropriate for the purpose of our investigation.
Establishing effective concentrations of nicotine
For the purpose of establishing effective concentrations of nicotine, incubations were performed with confluent monolayer cultures of 4 of the established cell-lines of HGF in Eagle's MEM using 14C-testosterone/14C-4-androstenedione as substrate, in 24 well multiwell dishes. Serial concentrations of nicotine N100–N500 μg/ml were used in order to establish its effective concentration.
Effects of serial concentrations of glutathione on the metabolism of 14C-testosterone and 14C-4-androstenedione by human gingival fibroblasts
Serial concentrations of Glutathione at 1–5 μg/ml were incubated in the presence or absence of the substrates 14C-testosterone/14C-4-androstenedione, with 4 cell lines of HGF, in order to establish the effect of the antioxidant alone on the metabolism of these substrates, compared with controls.
Mediation of androgen metabolism by an optimal concentration of nicotine and serial concentrations of glutathione in cultured gingival and periosteal fibroblasts
Incubations were performed with 6 cell-lines of fibroblasts in Eagle's MEM, using 14C-testosterone/14C-4-androstenedione as substrates with an effective concentration of nicotine established previously (N250 μg/ml), in the presence or absence of serial concentrations of glutathione (G, 1–4 μg/ml). This series was performed with 6 cell-lines each of HGF and HPF.
For all experiments, at the end of a 24 h incubation period, the medium was solvent extracted with ethyl acetate (2 ml, twice). The radiolabelled androgen metabolites were subsequently evaporated to dryness in a vortex evaporator and solubilized in chloroform prior to separation by thin layer chromatography in a benzene:acetone solvent system (4∶1 v/v). The separated metabolites were tentatively identified using mobilities of cold standards, disclosed in iodine, showing coincident images with radiolabelled samples using autoradiography, for DHT, 4-androstenedione, androstanedione and the diols; and quantified using a radioisotope scanner. Further confirmation of the identity of steroid metabolites was established by carrying out gas chromatography/mass spectrometry (GC-MS)19,20.
Characterization of steroid metabolites by gas chromatography - mass spectrometry
As 5α-dihydrotestosterone (DHT) is the most significant biologically active metabolite in stimulating fibroblast matrix synthetic activity, it was characterized as follows. Several incubations were performed with human gingival fibroblasts and unlabelled testosterone (10−6 M). After extraction, the identity of 5α-DHT as a metabolite in the dried extracts was confirmed by combined capillary gas chromatography - mass spectrometry (g.c-m.s; courtesy of Professor A.I Mallet, St. Thomas' Hospital, London, UK). The derivatized biological material as the pentafluorobenzyloxime trimethylsilylether (PFBO/TMS) had a molecular ion (557) and mass spectral fragmentation pattern identical to those of authentic PFBO/TMS ether of 5α-DHT, but at lower levels due to smaller concentrations of the steroid. Characteristic ions were noted, for example at m/z values of 542 [M-15]+ due to loss of a methyl group; 467 [M-90]+ due to loss of TMS ether; 452 [M-90-15]+ due to loss of TMS ether plus a methyl group and at an m/z value of 360, due to loss of the pentafluorobenzyloxime group. All these procedures have been described in detail31.
Statistical analysis
The mean values and standard deviations for metabolic yields from four (Figures 1 and 2; n = 4) or six (Figures 3–6; n = 6) such cell-lines are shown in the Results section. The cell-lines were not pooled and experiments were set up with individual cell-lines. Significance testing was done using one way ANOVA. The unit of analysis was the subject, where the control incubation in the absence of testing agents served as the comparison for the cell-lines studied. Since individual controls were set up for each cell-line, the data is representative of the number of cell-lines studied.
Author Contributions
Both authors contributed to text and figures, based on a dissertation of a former postgraduate student at King's College London Dental Institute, supervised by the corresponding author.
References
- Bergström J. Tobacco smoking and chronic destructive periodontal disease. Odontology. 92, 1–8 (2004). [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Wilson R. F., Hasan A. S. & Scott D. A. Mechanisms of action of environmental factors–tobacco smoking. J. Clin. Periodontol. 32, 180–195 (2005). [DOI] [PubMed] [Google Scholar]
- Walter C., Kaye E. K. & Dietrich T. Active and passive smoking: assessment issues in periodontal research. Periodontol 2000. 58, 84–92 (2012). [DOI] [PubMed] [Google Scholar]
- Grant M. M., Brock G. R., Matthews J. B. & Chapple I. L. Crevicular fluid glutathione levels in periodontitis and the effect of non-surgical therapy. J. Clin. Periodontol. 37, 17–23 (2010). [DOI] [PubMed] [Google Scholar]
- Kudva P., Tawkhira S. T. & Shekhawat N. K. Effect of green tea catechin, a local drug delivery system as an adjunct to scaling and root planing in chronic periodontitis patients. J. Indian. Soc. Periodontol. 15, 39–45 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soory M. & El-Shinnawi U. Diagnostic value of acute phase proteins in periodontal, psychosomatic and cardiometabolic diseases: Response to treatment. In: FranciscoVeas Ed. Acute phase proteins as early non-specific biomarkers of human and veterinary diseases. In Tech. Chapter 3, p 43–68 (2011).
- Tilakaratne A. & Soory M. Osteoblastic responses to LPS, glucose-oxidised LDL and minocycline: Therapeutic targets for periodontal and cardiometabolic diseases. Recent Pat. Endocr. Metab. Immune Drug Discov. 6,73–84 (2012). [DOI] [PubMed] [Google Scholar]
- Chang Y-C., Lai C-C., Lin L-F., Ni W-F. & Tsai C-H. The up-regulation of heme oxygenase-1 expression in human gingival fibroblasts stimulated with nicotine. J. Periodont. Res. 40, 252–7 (2005). [DOI] [PubMed] [Google Scholar]
- Georgii J. L., Amadeu T. P., Seabra A. B., de Oliveira M. G. & Monte-Alto-Costa A. Topical S-nitrosoglutathione-releasing hydrogel improves healing of rat ischaemic wounds. J. Tissue Eng. Regen. Med. 5, 612–9 (2011). [DOI] [PubMed] [Google Scholar]
- Pang S. T., Dillner K., Wu X., Pousette A., Norstedt G. & Flores-Morales A. Gene expression profiling of androgen deficiency predicts a pathway of prostate apoptosis that involves genes related to oxidative stress. Endocrinology. 43, 4897–4906 (2002). [DOI] [PubMed] [Google Scholar]
- Imperlini E. et al. Androgen receptor signalling induced by supraphysiological doses of dihydrotestosterone in human peripheral blood lymphocytes. Proteomics. 10, 3165–3175 (2010). [DOI] [PubMed] [Google Scholar]
- Xu Z. R., Hu L., Cheng L. F., Qian Y. & Yang Y. M. Dihydrotestosterone protects human vascular endothelial cells from H2O2 - induced apoptosis through inhibition of caspase-3, caspase-9 and p38 MAPK. Eur. J. Pharmacol. 643, 254–9 (2010). [DOI] [PubMed] [Google Scholar]
- Ganesan K., Tiwari M., Balachandran C., Manohar B. M. & Puvanakrishnan R. Estrogen and testosterone attenuate extracellular matrix loss in collagen-induced arthritis in rats. Calcif. Tissue Int. 83, 354–64 (2008). [DOI] [PubMed] [Google Scholar]
- Demirbag R., Yilmaz R. & Erel O. The association of total antioxidant capacity with sex hormones. Scand. Cardiovasc. J. 39, 172–6 (2005). [DOI] [PubMed] [Google Scholar]
- Giretti M. S. & Simoncini T. Rapid regulatory actions of sex steroids on cell movement through the actin cytoskeleton. Steroids. 73, 895–900 (2008). [DOI] [PubMed] [Google Scholar]
- Lee S. H., Heo J. S., Lee M. Y. & Han H. J. Effect of dihydrotestosterone on hydrogen peroxide-induced apoptosis of mouse embryonic stem cells. J. Cell Phys. 216, 269–75 (2008). [DOI] [PubMed] [Google Scholar]
- Davey R. A. & Morris H. A. Effects of estradiol and dihydrotestosterone on osteoblast gene expression in osteopenic ovariectomized rats. J. Bone Miner. Metab. 23, 212–8 (2005). [DOI] [PubMed] [Google Scholar]
- Soory M. & Suchak A. Effects of alkaline phosphatase and its inhibitor levamisole on the modulation of androgen metabolism by nicotine and minocycline in human gingival and oral periosteal fibroblasts. Arch. Oral Biol. 48, 69–76 (2003). [DOI] [PubMed] [Google Scholar]
- Soory M. & Suchak A. The effects of human mast cell products and of phenytoin on androgen 5α-reductase expression in human gingival fibroblasts. Arch. Oral Biol. 46, 847–855 (2001). [DOI] [PubMed] [Google Scholar]
- Tilakaratne A. & Soory M. Modulatory effects of indomethacin on androgen metabolism in human gingival and oral periosteal fibroblasts. Steroids. 66, 857–63 (2001). [DOI] [PubMed] [Google Scholar]
- Fujita T., Kawata T., Tokimasa C. & Tanne K. Influence of oestrogen and androgen on modelling of the mandibular condylar bone in ovariectomised and orchiectomised growing mice. Arch. Oral Biol. 46, 57–65 (2001). [DOI] [PubMed] [Google Scholar]
- Lu H. K., Tseng C. C., Lee Y. H., Li C. L. & Wang L. F. Flutamide inhibits nifedipine- and interleukin-1 beta-induced collagen overproduction in gingival fibroblasts. J. Periodontal Res. 45, 451–7 (2010). [DOI] [PubMed] [Google Scholar]
- Parkar M. H., Newman H. N. & Olsen I. Polymerase chain reaction analysis of oestrogen and androgen receptor expression in human gingival and periodontal tissue. Arch. Oral Biol. 41, 979–83 (1996). [DOI] [PubMed] [Google Scholar]
- Hutmacher D. W. & Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 9, Suppl 1, S45–64 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang X., Awad H. A., O'Keefe R. J., Guldberg R. E. & Schwarz E. M. A perspective: engineering periosteum for structural bone graft healing. Clin. Orthop. Relat. Res. 466, 1777–87 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsdorf E. J., Jones L. M., Carter D. R. & Jacobs C. R. The periosteum as a cellular source for functional tissue engineering. Tissue Eng. Part A 15, 2637–42 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojanotko A., Nienstedt W. & Harri M. P. Metabolism of testosterone by human healthy and inflamed gingiva in vitro. .Arch. Oral Biol. 25, 481–484 (1980). [DOI] [PubMed] [Google Scholar]
- Sooriyamoorthy M. & Gower D. B. Phenytoin stimulation of testosterone metabolism in inflamed human gingival fibroblasts. Biochem. Soc. Trans. 17, 1020–1021 (1989). [DOI] [PubMed] [Google Scholar]
- Pradel W., Mai R., Gedrange T. & Lauer G. Cell passage and composition of culture medium effects proliferation and differentiation of human osteoblast-like cells from facial bone. J. Physiol. Pharmacol. 59 (Suppl 5), 47–58 (2008). [PubMed] [Google Scholar]
- Mauro A., Buscemi M. & Gerbino A. Immunohistochemical and transcriptional expression of matrix metalloproteinases in full-term human umbilical cord and human umbilical vein endothelial cells. J. Mol. Histol. 41, 367–77 (2010). [DOI] [PubMed] [Google Scholar]
- Soory M. Bacterial steroidogenesis by periodontal pathogens and the effect of bacterial enzymes on steroid conversions by human gingival fibroblasts in culture. J. Periodontal Res. 30,124–31 (1995). [DOI] [PubMed] [Google Scholar]
- Soory M. & Tilakaratne A. The modulation of androgen metabolism by phenytoin, oestradiol and tamoxifen in human gingival fibroblasts. J. Clin. Periodontol., 30, 556–61 (2003). [DOI] [PubMed] [Google Scholar]
- Krum S. A. Direct transcriptional targets of sex steroid hormones in bone. J. Cell Biochem. 112, 401–408 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Heo J. S., Lee M. Y. & Han H. J. Effect of dihydrotestosterone on hydrogen peroxide-induced apoptosis of mouse embryonic stem cells. J. Cell Phys. 216, 269–275 (2008). [DOI] [PubMed] [Google Scholar]
- Kalay Z. & Cevher S. C. Oxidant and antioxidant events during epidermal growth factor therapy to cutaneous wound healing in rats. Int. Wound J. 10.1111/j.1742-481X.2011.00895. Epub ahead of print (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopal C., Deveci M., Oztürk S. & Sengezer M. Effects of topical glutathione treatment in rat ischemic wound model. Ann. Plast. Surg. 58, 449–55 (2007). [DOI] [PubMed] [Google Scholar]
- Zevallos J. P., Hanasono M. M., Li G., Wei Q. & Sturgis E. M. Glutathione-S-transferase polymorphisms and complications of microvascular head and neck reconstruction. Arch. Facial Plast. Surg. 12, 373–8 (2010). [DOI] [PubMed] [Google Scholar]
- Argentin G. & Cicchetti R. Genotoxic and antiapoptotic effect of nicotine on human gingival fibroblasts. Toxicol. Sci. 79, 75–81 (2004). [DOI] [PubMed] [Google Scholar]
- Arya A. K., Pokharia D. & Tripathi K. Relationship between oxidative stress and apoptotic markers in lymphocytes of diabetics with chronic non healing wound. Diabetes Res. Clin. Pract. [Epub ahead of print] PMID, 21872354 (2011). [DOI] [PubMed] [Google Scholar]
- Kang H-Y. et al. Altered TNSALP expression and phosphate regulation contribute to reduced mineralization in mice lacking androgen receptor. Mol. Cell Biol. 28, 7354–67 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S. K. & Roy S. Role of reduced glutathione in the amelioration of nicotine-induced oxidative stress. Bull. Environ. Contam. Toxicol. 84, 385–389 (2010). [DOI] [PubMed] [Google Scholar]
- Helen A., Krishnakumar K., Vijayammal P. L. & Augusti K. T. Antioxidant effect of onion oil (Allium cepa. Linn) on the damages induced by nicotine in rats as compared to alpha-tocopherol. Toxicol. Lett. 116, 61–68 (2000). [DOI] [PubMed] [Google Scholar]
- Sener G., Sehirli A. O., Ipci Y., Cetinel S., Cikler E. & Gedik N. Chronic nicotine toxicity is prevented by aqueous garlic extract. Plant Foods Hum. Nutr. 60, 77–86 (2005). [DOI] [PubMed] [Google Scholar]
- Kalpana C. & Menon V. P. Curcumin ameliorates oxidative stress during nicotine-induced lung toxicity in Wistar rats. Ital. J. Biochem. 53, 82–6 (2004). [PubMed] [Google Scholar]
- Walker A., Uduppa K. B. & Chowdhury P. Mitogenic and functional responses by nicotine and hydrogen peroxide in AR42J cells: A comparative study. Tob. Ind. Dis. 4, 5 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y. & Svoboda K. H. Nicotine Inhibits Myofibroblast Differentiation in Human Gingival Fibroblasts. J. Cell Biochem. 95, 1108–1119 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannopoulou C., Geinoz A. & Cimasoni G. Effects of nicotine on periodontal ligament fibroblasts in vitro. J. Clin. Periodontol. 26, 49–55 (1999). [DOI] [PubMed] [Google Scholar]
- Davey R. A. M., Hahn C. N., May B. K. & Morris H. A. Osteoblast Gene Expression in Rat Long Bones: Effects of Ovariectomy and Dihydrotestosterone on mRNA Levels. Calcif. Tissue Int. 67, 75–79 (2000). [DOI] [PubMed] [Google Scholar]
- Qiao D., Seidler F. J. & Slotkin T. A. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol. Appl. Pharmacol. 206, 17–26 (2005). [DOI] [PubMed] [Google Scholar]
- Giannopoulou C., Roehrich N. & Mombelli A. Effect of nicotine-treated epithelial cells on the proliferation and collagen production of gingival fibroblasts. J. Clin. Periodontol. 28, 769–775 (2001). [DOI] [PubMed] [Google Scholar]