Abstract
Background
The effect of reactive oxygen species (ROS) on platelet function in coronary heart disease (CHD) is complex and poorly defined. Platelet aggregation studies in healthy volunteers have demonstrated contrasting results when platelets are exposed to ROS. We investigated the effect of ROS on whole blood aggregation (WBA) and the endothelial cell-platelet interaction in patients with CHD.
Methods and Results
ROS generated by xanthine and xanthine oxidase caused a concentration-dependent inhibition of WBA in blood from healthy donors and patients with CHD. In patients with CHD, 100 μM xanthine and 100 mU/ml xanthine oxidase inhibited WBA in response to 3 μg/ml collagen by 28.9% (95% CI 15.9%-41.8%, p < 0.001) and in response to 5 μM ADP by 36.0% (95% CI 9.6%-62.4%, p = 0.005). Using nitrotyrosine expression, platelets isolated from patients with CHD were found to be susceptible to peroxynitrite damage. The addition of 1 × 105 cultured endothelial cells inhibited WBA in response to 3 μg/ml collagen by 31.2% (95% CI 12.2%-50.2%, p < 0.05) and in response to 5 μM ADP by 31.6% (95% CI 2.5-60.7%, p < 0.05). Addition of xanthine and xanthine oxidase did not alter this effect, however pre-treatment of endothelial cells with a nitric oxide synthase inhibitor (L-NAME) partly reversed the inhibition.
Conclusion
ROS inhibit WBA in blood from patients with CHD. Whilst endothelial cells also inhibit WBA, the effect is attenuated by L-NAME, suggesting that nitric oxide is likely to remain an important protective mechanism against thrombosis in CHD.
Keywords: Platelet aggregation, Platelets, Oxidative stress, Reactive oxygen species, Endothelium, Coronary heart disease
Introduction
Oxidative stress is recognised as an important mediator of atherothrombotic events in cardiovascular disease [1]. The classic paradigm of atherothrombosis involves the formation of platelet-rich thrombus overlying damaged endothelium, which may result from oxidative injury. In addition to other factors, reactive oxygen species (ROS) directly participate in the regulation of platelet activation and thrombus formation, however the direct effects of ROS on platelets are reportedly varied. Previous platelet studies in healthy individuals have reported both pro- and anti-aggregatory effects when platelets are exposed to exogenous ROS [2]. Platelets themselves also generate ROS through several intracellular sources such as NADPH oxidase, cyclooxygenase, uncoupled endothelial nitric oxide synthase (eNOS), xanthine oxidase (XO) and mitochondrial respiration (reviewed extensively in [2]) and this appears to be important during recruitment, adhesion and aggregation as well as during activation to agents such as collagen [3].
As healthy endothelium normally inhibits thrombosis, the interactions between ROS, endothelial cells (ECs) and platelets are integral to the pathogenesis of cardiovascular events. Although there is good evidence that oxidative stress damages the endothelium in vivo, which predisposes individuals to thrombosis, the close interactions between ROS and ECs are not well understood. WBA offers the most physiological setting in which to examine platelet aggregation as it closely replicates the milieu in which clinical thrombosis occurs, where other blood constituents, including erythrocytes [4], leucocytes [5] and plasma-derived substances [6,7] are involved. ECs normally inhibit platelet aggregation by release of nitric oxide (•NO) [8], prostacyclin [9], and the activity of endothelial ectonucleotidases [10], although in the presence of heightened oxidative stress, some of these effects may be deficient. Our aims were to quantify the effects of ROS on WBA and to assess the influence of ROS on the EC-platelet interaction in patients with coronary heart disease (CHD).
Material and methods
Study population
This study was approved by the West Glasgow and University of Strathclyde ethics committees. All participants were provided with a Participant Information Sheet and gave their informed written consent. All CHD patients recruited to the study attended the Western Infirmary Glasgow for out-patient cardiology appointments, were over 18 years old and were receiving chronic oral aspirin therapy. Patients were excluded if there was a history of myocardial infarction within 3 months, if they were unable to give informed consent or if they were taking any other antithrombotic therapy. For comparison, blood samples were collected from healthy donors with no history of CHD.
Chemiluminescence
Lucigenin chemiluminescence was used to confirm generation of ROS (specifically superoxide anion [O2-•]) as a consequence of the xanthine/xanthine oxidase (X/XO) reaction [11]. Briefly, 900 μl of PBS and 100 μl lucigenin solution (Sigma-Aldrich, Dorset, UK) were prewarmed in a sample cuvette at 37 °C, then xanthine and XO (Sigma-Aldrich, Dorset, UK) were added before transferring immediately to a chemiluminometer (Berthold, Germany). The chemiluminescence signal was recorded after 120 seconds. Xanthine and XO were dissolved in 10 mM NaOH and PBS respectively, and tested at a range of concentrations likely to generate ROS. As lucigenin itself can interfere with O2-• generation, thus affecting chemiluminescence [12], 5 μM lucigenin was used to minimise this interference.
Whole blood aggregometry
Venous blood was withdrawn into 3.5 ml Vacuette® tubes containing 3.2% sodium citrate (1:9 volume). Blood was tested up to 3 hours following venepuncture. WBA was measured using an impedance aggregometer (Chrono-log, Model 590). 500 μl citrated whole blood was diluted 1:1 with 500 μl normal saline in a plastic sample cuvette and prewarmed for 5 minutes at 37 °C with a stirring speed of 900 rpm. An electrode containing two fine palladium wires was inserted, allowing the platelets in the whole blood to adhere to the wires, forming a uniform monolayer. A small voltage difference was applied across the wires, and the electrical impedance was measured. In the absence of an aggregatory agonist, the impedance between the two wires became constant after 2 minutes, producing a stable baseline which was calibrated using the chart recorder controls. When an agonist is added to the cuvette, platelets and other constituents in the blood become activated and start to aggregate, coating the palladium wires on the electrode, causing a corresponding increase in electrical impedance. This change in impedance is directly proportional to the extent of aggregation and was measured 5 minutes after addition of each agonist. 3 μg/ml collagen and 5 μM adenosine diphosphate (ADP) (Labmedics Limited, Manchester, UK) were chosen as platelet agonists, corresponding to their EC50 under control conditions. Individual aggregation values for each experiment were calculated as a percentage of control conditions. In experiments to investigate the effect of ROS, blood was incubated with X/XO for 1 minute prior to adding either agonist. In additional control experiments, XO alone was incubated prior to the agonist.
Endothelial cell-platelet interaction
ECs were scraped from the pulmonary artery of fresh ex-vivo pig hearts from a local abattoir. ECs were then cultured in large vessel EC growth medium package (TCS Cellworks, Buckinghamshire, UK) and grown to confluence in cell culture flasks. ECs were collected from the flasks using TrypLE Express (Invitrogen Corporation, Paisley UK) and spun down in a centrifuge at 10,000 rpm for 5 minutes. The supernatant was extracted and the ECs resuspended in 1 ml blank EC medium. The number of ECs in 1 ml suspension was determined using a haemocytometer. 1 × 105 ECs were added to the sample cuvette using an appropriate volume of cell suspension and made up to 500 μl with normal saline, to which 500 μl whole blood was added. In control experiments, an equivalent volume of blank cell medium was added to the cuvette in place of the cell suspension. WBA was tested as previously described and the effect of ECs was determined in the absence and presence of X/XO, and following 5 minutes pre-treatment of ECs with Nω-nitro-L-arginine methyl ester (L-NAME) (Sigma-Aldrich, Dorset, UK), an inhibitor of •NO synthase.
Platelet nitrotyrosine expression
In order to establish the susceptibility of platelets to oxidative stress, washed platelets were prepared according to the method of Cardoso et al. [13]. Baseline nitrotyrosine (NT) expression and the effect of oxidative stress was studied by incubating platelets for 10 minutes at room temperature with 200 μM peroxynitrite (ONOO-), 100 mU/ml XO alone or a combination of 100 μM xanthine and 100 mU/ml XO. Samples for western blotting were prepared by sedimenting the platelets, homogenising the pellet in lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.25% (w/v) Na-deoxycholate, 1% (v/v) TX-100, 1 x protease inhibitor cocktail) (Merck) and determining protein concentration using Coomassie Plus Protein Assay Reagent (Perbio, Rockford, IL, USA). Samples were heated at 70 °C for 20 minutes prior to gel loading. All samples were run at the same time to control for inter-gel variation. SDS-PAGE was performed using the NuPAGE system (Invitrogen) with 4–12% tris–acetate gels followed by blotting onto a nitrocellulose membrane (Invitrogen) by the Bradford method. NT was detected using rabbit anti-nitrotyrosine primary antibody (diluted 1:5000, Upstate, Ca, USA) and goat anti-rabbit horseradish peroxidase conjugate as secondary (Transduction Laboratories). Following antibody incubation, membranes were treated with ECL reagent (Pierce) and exposed onto film. Detected bands were analysed densitometrically and corrected for background. GAPDH antibody (diluted 1:40000) was used to ensure equal protein loading in all wells.
Statistical analysis
All data are expressed as mean ± SEM unless otherwise stated. Groups were compared using repeated measures analysis of variance and post-hoc Dunnett's test. Statistical significance was confirmed at p < 0.05. Statistical analysis was performed using the SPSS statistical software package 14.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of study participants
33 patients with CHD were recruited and their baseline characteristics are shown in Table 1. There was a high prevalence of cardiovascular risk factors and regular medications prescribed. All CHD patients were taking oral aspirin but no other antithrombotic medication. In total, 16 healthy donors were recruited (mean [SD] age, 41.6 [17.2] years; 62.5% male) and none had a history of cardiovascular disease or were taking any antithrombotic medication at the time of the study.
Table 1.
Baseline characteristics of CHD patients.
| (n = 33) | |
|---|---|
| Patients’ characteristics | |
| Age, mean (SD), years | 63.4 (8.9) |
| Male, n (%) | 26 (78.8) |
| Current smoker, n (%) | 9 (27.3) |
| Hypertension, n (%) | 18 (54.5) |
| Hypercholesterolaemia, n (%) | 23 (69.7) |
| Diabetes mellitus, n (%) | 11 (33.3) |
| Family history of premature CHD, n (%) | 13 (39.4) |
| Previous myocardial infarction, n (%) | 13 (39.4) |
| Previous stroke, n (%) | 2 (6.1) |
| Previous PCI or CABG, n (%) | 15 (45.5) |
| Impaired LV function, n (%) | 10 (30.3) |
| Heart failure, n (%) | 3 (9.1) |
| Drug treatment | |
| Aspirin, n (%) | 33 (100.0) |
| Clopidogrel, n (%) | 0 (0.0) |
| Statin, n (%) | 31 (94.0) |
| ACE inhibitor or ARB, n (%) | 25 (75.8) |
| Beta-blocker, n (%) | 25 (75.8) |
| Calcium channel blocker, n (%) | 12 (36.4) |
| Diuretic, n (%) | 8 (24.2) |
| Nitrate, n (%) | 18 (54.5) |
| Nicorandil, n (%) | 12 (36.4) |
Confirmation of O2-• production
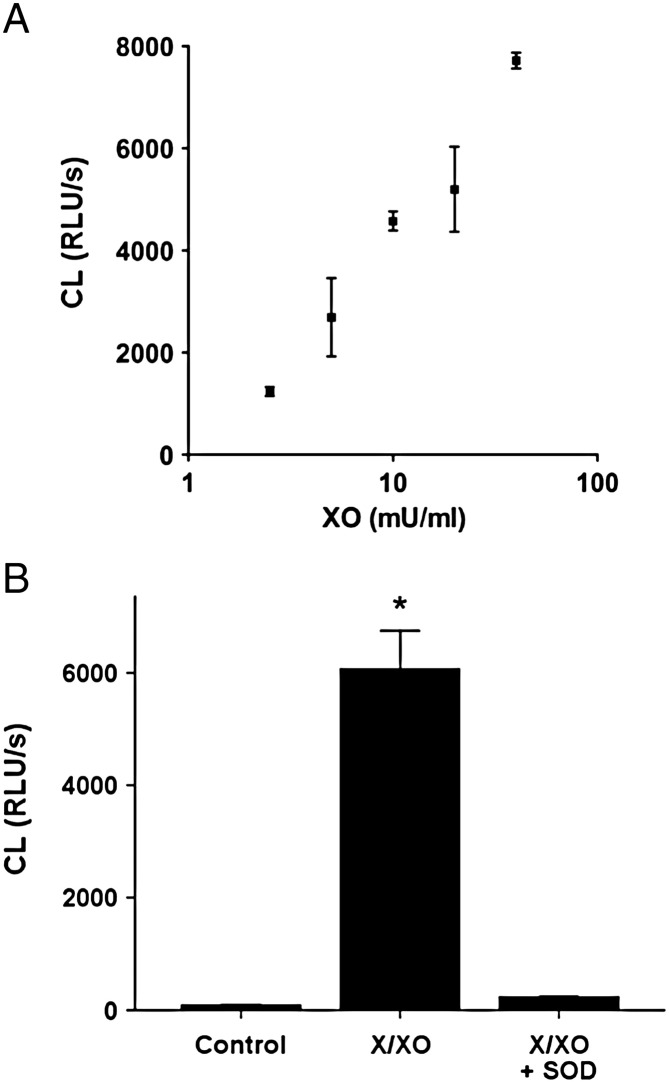
There was a highly significant positive correlation (r = 0.94, p = 0.006) between chemiluminescence and XO concentration in the presence of a constant concentration of 100 μM xanthine (Fig. 1A). 50 U/ml superoxide dismutase (SOD) completely abolished chemiluminescence in the presence of 100 μM xanthine and 10 mU/ml XO (p < 0.001), indicating that O2-• was the principal active molecule generated under the conditions of these experiments (Fig. 1B).
Fig. 1.
A, The effect of xanthine and XO on lucigenin chemiluminescence. XO caused a concentration-dependent increase in chemiluminescence due to formation of O2-•. The amount of luigenin-enhanced chemiluminescence was very strongly correlated with XO concentration, using constant 100 μM xanthine (R = 0.94, p = 0.006). B, The effect of SOD on chemiluminescence induced by X/XO. Chemiluminescence due to 100 μM xanthine + 10 mU/ml XO was completely abolished by 50 U/ml SOD, * p < 0.001 vs. control. CL = chemiluminescence; RLU/s = relative light units per second.
Effects of ROS on WBA
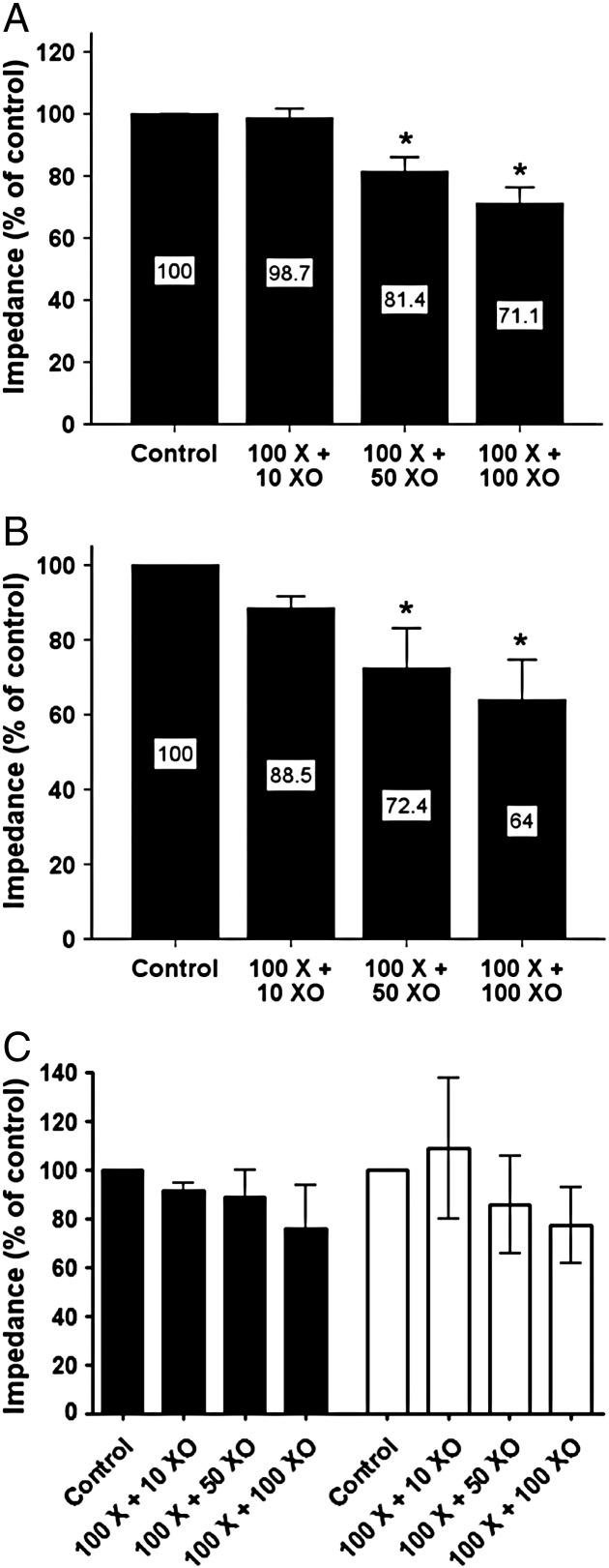
There was no significant difference in baseline aggregation to collagen (15.4 ± 0.8 Ω [n = 23] vs. 15.9 ± 1.5 Ω [n = 12]) or ADP (9.3 ± 0.9 Ω [n = 23] vs. 7.8 ± 1.5 Ω [n = 10]) between CHD patients and healthy donors, respectively (both p = NS). The effect of oxidative stress on WBA in 14 CHD patients was determined using the X/XO reaction. The concentration of xanthine was kept constant at 100 μM, while the concentration of XO was tested at 10, 50 and 100 mU/ml to engender incremental levels of oxidative stress, previously confirmed using chemiluminescence. ROS produced by X/XO caused a concentration-dependent inhibition of WBA in blood from CHD patients, for both collagen and ADP (Figs. 2A and B, respectively). 100 μM xanthine and 100 mU/ml XO inhibited WBA in response to collagen by 28.9% (95% CI 15.9% - 41.8%, p < 0.001) and in response to ADP by 36.0% (95% CI 9.6% - 62.4%, p = 0.005). Blood from healthy donors also demonstrated concentration-dependent inhibition of WBA in response to X/XO although this failed to reach statistical significance (n = 4; Fig. 2 C), while addition of XO alone did not affect WBA to either agonist (data not shown).
Fig. 2.
The effect of ROS on WBA in response to platelet agonists. Xanthine (μM) + XO (mU/ml) caused a concentration-dependent inhibition of WBA in response to 3 μg/ml collagen (A), 5 μM ADP (B) in blood from CHD patients and also in healthy donors [black bars = collagen; white bars = ADP] (C), * p < 0.05 vs. control.
Endothelial cell-platelet interaction studies
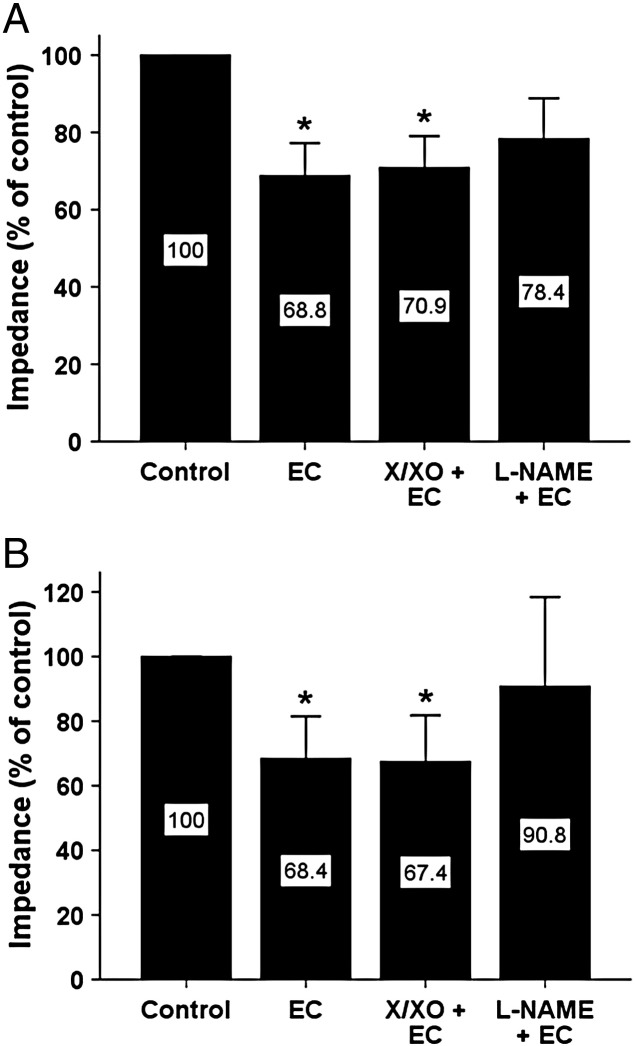
The effects of cultured ECs on WBA in 9 CHD patients are shown in Fig. 3A and B. The addition of 1 x 105 ECs caused a significant decrease in WBA in response to collagen by 31.2% (95% CI 12.2% - 50.2%, p < 0.01) and ADP by 31.6% (95% CI 2.5- 60.7%, p < 0.05). The inhibitory effect of ECs on WBA remained significant after pre-treatment of ECs with 100 μM xanthine and 10 mU/ml XO in response to collagen (29.1% [95% CI 10.1% - 48.1%, p < 0.01]) and ADP (32.6% [95% CI 3.4- 61.7%, p < 0.05]). However, pre-treatment with 100 μM L-NAME attenuated the inhibitory effect of ECs, which was no longer statistically significant in response to collagen (21.6% [95% CI − 7.2% - 50.5%, p = NS]) or ADP (9.2% [95% CI − 44.7% - 63.1%, p = NS]).
Fig. 3.
The effect of ECs alone and after pretreatment with X/XO or L-NAME on WBA in blood from CHD patients. Cultured ECs inhibited WBA in response to 3 μg/ml collagen (A) and 5 μM ADP (B). Inhibition was not affected by 100 μM xanthine and 10 mU/ml XO but was no longer significant after pre-treatment of ECs with 100 μM L-NAME, * p < 0.05 vs. control.
Platelet nitrotyrosine expression
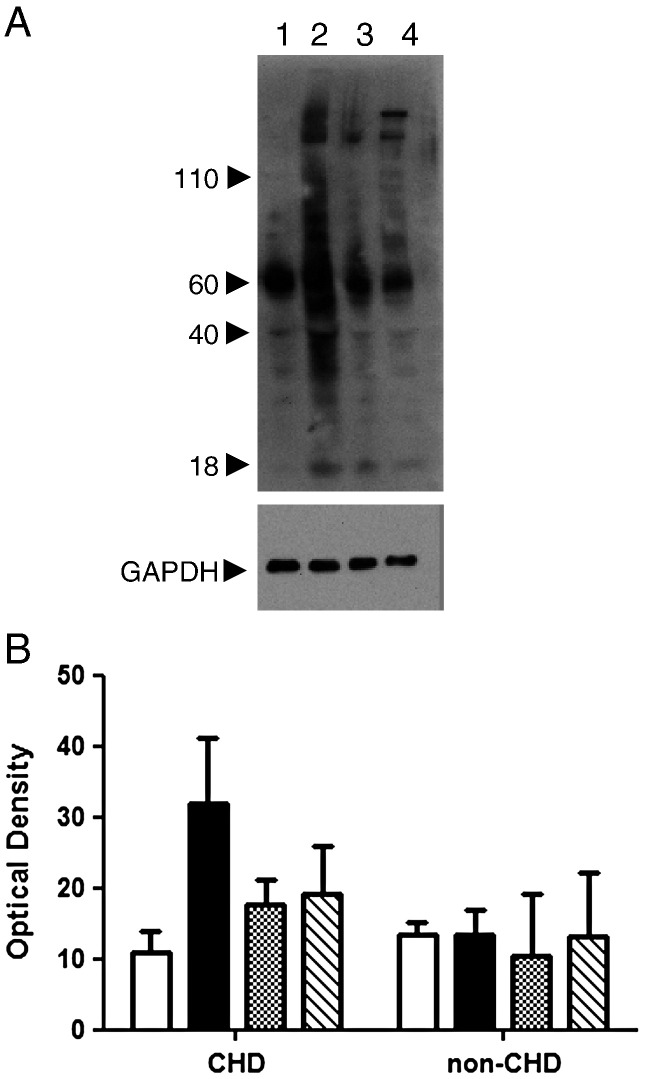
Baseline expression of NT in platelets from CHD patients and healthy donors was similar (densitometric expression of all detected bands was 10.93 ± 3.0 in CHD patients [n = 3] vs. 13.5 ± 1.7 in healthy donors [n = 2], p = NS). In washed platelets from 7 CHD patients, ONOO- caused an increase in platelet NT expression, whereas XO and X/XO had a smaller effect on NT expression compared to baseline (Fig. 4B). In healthy donors, none of the three treatments resulted in a significant increase in NT expression (Fig. 4B); however caution should be exercised as only two samples were studied.
Fig. 4.
The expression of nitrotyrosine in washed platelets. A, Representative blot showing baseline NT expression (lane 1), effect of ONOO- (lane 2), XO alone (lane 3) and X/XO (lane 4). GAPDH was used to ensure equal protein loading in each lane. B, Bar chart showing effect of the three treatments on NT expression in CHD patients and healthy donors (columns left to right represent baseline, ONOO-, XO, X/XO).
Discussion
Recently, evidence has accumulated that ROS are heavily involved in the regulation of platelet function, although their precise role is complex [2]. Therefore, clarifying the actions of ROS on WBA in patients with CHD is important to extend our knowledge of the pathogenesis of acute atherothrombotic events [14].
Effect of ROS on platelet aggregation
The principal aim of the current study was to examine, for the first time, the effects of ROS on WBA in patients with clinically proven CHD. Our results have shown that ROS, generated by the X/XO reaction, caused a concentration-dependent inhibition of WBA in blood derived from patients with CHD. This was clearly demonstrated in response to the biologically relevant agonists, collagen and ADP. While we confirmed using chemiluminescence that O2-• was generated by X/XO, we propose that secondary molecules may have exerted a major influence.
In addition to producing O2-•, the X/XO reaction also leads to downstream production of H2O2 [15], which modulates platelet function [2]. Additionally, although resting platelets do not produce •NO, this is formed following stimulation with platelet agonists [16] and can react with O2-• to produce ONOO- [17]. ONOO-, unlike O2-•, is cell permeable and would readily cross the platelet membrane. This highly reactive molecule is known to be a potent oxidant and additional contributor to platelet aggregation [18]. O2-• generated within platelets should also be considered. It is reported that O2-• generation in response to collagen occurs with a delay of 3–5 minutes and since we measured aggregation after 5 minutes exposure, O2-• generated by the platelet may also have contributed to the effects we observed. It is very difficult to estimate how the magnitude of platelet-derived O2-• compares to that generated by X/XO in our experiments although it has been reported that O2-• formation by platelets is similar to that by ECs [2]. We anticipate that platelet-derived O2-• is likely to be quickly dismutated to form antiaggregatory molecules such as H2O2 or lead to generation of ONOO-, both of which are important mediators of aggregation and platelet function.
The hitherto reported effects of ROS on platelet function are variable and depend on a variety of factors, particularly the type and concentration of ROS and the milieu in which they act. Using washed platelets in buffer, exposure to X/XO led to irreversible aggregation to subthreshold levels of ADP, although there was no effect on collagen-induced platelet aggregation [19]. In another study, collagen induced O2-• release from platelets, which stimulated ADP-dependent platelet recruitment to preformed thrombus, but did not in itself cause platelet aggregation [19]. Others have shown that addition of X/XO to washed platelets led to release of the surrogate marker serotonin, thought to occur as a result of platelet aggregation [20]. This effect was blocked by SOD suggesting that O2-• was the relevant molecule involved. The O2-• generator pyrogallol increased thrombin-induced platelet aggregation, an effect which was also blocked by SOD [21], and increased arachidonic acid-induced platelet aggregation, an effect which was inhibited by dipyridamole [22]. Put together, these data suggest the more likely effect of O2-• on platelet aggregation is stimulatory, albeit mild in some cases. With regard to potential mechanisms, Sill et al. demonstrated that intra-platelet increases in oxidant status capable of activating platelet glycoprotein IIb/IIIa receptors were iron-dependent, and neither O2-• (generated by X/XO) nor H2O2 delivered externally to platelets produced this effect [23].
The addition of X/XO to platelets in platelet rich plasma (PRP) has led to different results. In healthy volunteers, X/XO caused potent inhibition of platelet aggregation in response to ADP, collagen and U-46619 (a thromboxane mimetic) [24]. These findings were attributed to an overproduction of H2O2, stimulation of guanylate cyclase and an increase in cGMP. This inhibitory effect was blocked by catalase, but not by SOD, suggesting that H2O2 was a crucial inhibitory ROS. The inhibitory effect of H2O2 on ADP-induced aggregation has also been confirmed in other studies [25,26]. There are reports that lower concentrations of H2O2 may enhance subthreshold collagen and arachidonic acid-induced aggregation, but not ADP-induced aggregation [27,28]. The influence of ONOO- on isolated platelets has been varied, causing both pro-aggregatory [29] and anti-aggregatory effects [30,31], but studies in PRP consistently show that ONOO- inhibits collagen, ADP, and thrombin-induced aggregation [29,32]. Subsequent reports suggested that the •NO-dependent inhibitory effects of ONOO- occurred at lower concentrations than the pro-aggregatory effects [33].
Taking all of these data into consideration, the actions of ROS on platelet function are diverse, depend on the molecule involved, and are concentration-dependent. Furthermore, assessment of platelet function must take into account the environment in which aggregation is stimulated. Although washed platelets can provide information regarding the specific actions of substances on platelets, more physiological effects can be replicated in plasma or whole blood, where other blood constituents and endogenous antioxidants are represented that may antagonise the stimulation of platelets induced by some ROS [34]. Our results indicate that ROS (generated by X/XO) cause a concentration-dependent inhibition of platelet aggregation in whole blood derived from patients with CHD. We propose that this was caused by the generation of secondary inhibitory ROS such as H2O2 and ONOO-, possibly related to endogenous substances present in whole blood. However, the exact mechanism of this effect should be clarified in future studies.
Effect of ROS on the endothelial cell-platelet interaction
For the first time, we have demonstrated that healthy ECs maintain the ability to inhibit platelet aggregation in whole blood derived from patients with CHD. A study by Kader et al. [35] found that transfecting cultured ECs with eNOS reduced human platelet aggregation in co-incubation experiments; suggesting that •NO production is likely to mediate the anti-aggregatory effect. In addition, a previous study by our group found that incubating whole blood with a ring of endothelium-containing blood vessel could reduce platelet aggregation when the endothelium was stimulated pharmacologically to produce •NO [36]. As well as •NO, ECs may inhibit thrombosis through the actions of prostacyclin [37] and the activity of an endothelial ecto-ADPase present on the surface of ECs, identified as CD39 [38,39]. It was reported that depolarization of ECs led to O2-• production, which inactivated the ecto-ADPase, thereby decreasing the inhibitory properties of ECs on ADP-induced aggregation [40]. We have shown that the addition of exogenous ROS using X/XO failed to influence the inhibitory properties of healthy ECs. We propose that this resulted from an equilibrium of negative and positive effects, such as inactivation of EC-derived antiplatelet mechanisms (NO, ecto-ADPase) counterbalanced by the production of secondary ROS which oppose aggregation, as described. We have shown that the inhibitory effect of healthy ECs is decreased by the pretreatment of ECs with L-NAME. This supports the notion that isolated removal of EC-derived •NO from the blood decreases its anti-aggregatory capacity, which implies an increased risk of thrombosis where endothelial dysfunction occurs.
Platelet nitrotyrosine expression
ONOO- is cell permeable [41] and would readily cross the platelet membrane when formed. Using immunoblotting, we measured total NT expression in washed platelets. CHD patients did not express higher levels of NT in platelets. Very few other studies have measured this but in a canine model of acute coronary syndromes, platelet NT was increased [42] and this could be normalised with tetrahydrobiopterin; suggesting that platelet •NO formation is important in regulating NT expression under baseline conditions. Following treatment with ONOO-, NT expression was raised in CHD patients, but not in healthy donors. Recent data has demonstrated that some supplements such as L-carnitine can protect platelets against the nitrating effects of ONOO- [43] and it is possible that platelet levels of endogenous antioxidants were higher in healthy donors. Similarly, the O2-• generating X/XO system or XO alone, which would likely generate O2-• anions, also caused a small increase in platelet NT expression in CHD patients, likely through an interaction with platelet-derived •NO. Healthy donors showed no such increase, perhaps indicating that •NO generation was lower and that platelets were in a non-activated state compared to CHD. Such pre-activation has been noted previously in patients with CHD [44].
Limitations
Direct comparison of results between CHD patients and healthy controls in this study should take into consideration that groups were not age or sex matched, in order to facilitate recruitment. All CHD patients were taking regular oral antiplatelet medication (soley aspirin, to provide consistency within the CHD group) which would be expected to decrease WBA relative to healthy donors, none of whom were receiving antithrombotic therapy. The main focus of this study was to investigate the effects of ROS on platelets derived from patients with CHD.
Conclusion
ROS generated by X/XO produced a concentration-dependent inhibitory effect on WBA in blood obtained from patients with CHD. This concurs with previous studies in healthy individuals using X/XO as a source of ROS and is likely to be a consequence of the formation of highly reactive molecules such as H2O2 and ONOO- which have inhibitory effects that appear to predominate. We have also shown that exogenous delivery of reactive molecules, especially ONOO-, is capable of causing damaging effects to proteins within platelets. Healthy ECs maintain the ability to carry out their physiological role to inhibit platelet aggregation in blood derived from atherosclerotic human populations, even after short term exposure to ROS, suggesting that strategies to preserve endothelial function and •NO bioavailability are likely to be beneficial. However, prolonged damage to the endothelium may diminish the capacity of ECs to generate •NO, which would mitigate protection against thrombosis and so may lead to a worse clinical outcome.
Conflict of interest statement
All authors report no conflict of interest.
Acknowledgements
This work was supported by a British Heart Foundation Junior Research Fellowship (FS/05/096/19933).
References
- 1.Loscalzo J. Oxidant stress: a key determinant of atherothrombosis. Biochem Soc Trans. 2003;31(Pt 5):1059–1061. doi: 10.1042/bst0311059. [DOI] [PubMed] [Google Scholar]
- 2.Krotz F., Sohn H.Y., Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler Thromb Vasc Biol. 2004;24(11):1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 3.Wachowicz B., Olas B., Zbikowska H.M., Buczynski A. Generation of reactive oxygen species in blood platelets. Platelets. 2002;13(3):175–182. doi: 10.1080/09533710022149395. [DOI] [PubMed] [Google Scholar]
- 4.Rocca B., FitzGerald G.A. Simply Read: Erythrocytes Modulate Platelet Function: Should We Rethink the Way We Give Aspirin? Circulation. 1997;95(1):11–13. doi: 10.1161/01.cir.95.1.11. [DOI] [PubMed] [Google Scholar]
- 5.Afshar-Kharghan V., Thiagarajan P. Leukocyte adhesion and thrombosis. Curr Opin Hematol. 2006;13(1):34–39. doi: 10.1097/01.moh.0000190107.54790.de. [DOI] [PubMed] [Google Scholar]
- 6.Griffin J.H., Fernandez J.A., Deguchi H. Plasma lipoproteins, hemostasis and thrombosis. Thromb Haemost. 2001;86(1):386–394. [PubMed] [Google Scholar]
- 7.Ruggeri Z.M. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003;1(7):1335–1342. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 8.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 9.De La Cruz J.P., Moreno A., Guerrero A., de La Cuesta F.S. Antiplatelet effects of prostacyclin and nitric oxide in patients with type I diabetes and ischemic or edematous retinopathy. Platelets. 2001;12(4):210–217. doi: 10.1080/09537100120058748. [DOI] [PubMed] [Google Scholar]
- 10.El Omar M.M., Islam N., Broekman M.J., Drosopoulos J.H.F., Roa D.C., Lorin J.D. The ratio of ADP- to ATP-ectonucleotidase activity is reduced in patients with coronary artery disease. Thromb Res. 2005;116(3):199–206. doi: 10.1016/j.thromres.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Zhu H., Kuppusamy P., Roubaud V., Zweier J.L., Trush M.A. Validation of Lucigenin (Bis-N-methylacridinium) as a Chemilumigenic Probe for Detecting Superoxide Anion Radical Production by Enzymatic and Cellular Systems. J Biol Chem. 1998;273(4):2015–2023. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- 12.Tarpey M.M., White C.R., Suarez E., Richardson G., Radi R., Freeman B.A. Chemiluminescent detection of oxidants in vascular tissue. Lucigenin but not coelenterazine enhances superoxide formation. Circ Res. 1999;84(10):1203–1211. doi: 10.1161/01.res.84.10.1203. [DOI] [PubMed] [Google Scholar]
- 13.Cardoso M.H., Morganti R.P., Lilla S., Murad F., de Nucci G., Antunes E. The role of superoxide anion in the inhibitory effect of SIN-1 in thrombin-activated human platelet adhesion. Eur J Pharmacol. 2010;627(1–3):229–234. doi: 10.1016/j.ejphar.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 14.Iakovou I., Schmidt T., Bonizzoni E., Ge L., Sangiorgi G.M., Stankovic G. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 15.Asai R., Nishino T., Matsumura T., Okamoto K., Igarashi K., Pai E.F. Two Mutations Convert Mammalian Xanthine Oxidoreductase to Highly Superoxide-productive Xanthine Oxidase. J Biochem (Tokyo) 2007;141(4):525–534. doi: 10.1093/jb/mvm054. [DOI] [PubMed] [Google Scholar]
- 16.Radomski M.W., Palmer R.M., Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990;87(13):5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pryor W.A., Squadrito G.L. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol Lung Cell Mol Physiol. 1995;268(5):L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 18.Olas B., Wachowicz B. Role of reactive nitrogen species in blood platelet functions. Platelets. 2007;18(8):555–565. doi: 10.1080/09537100701504087. [DOI] [PubMed] [Google Scholar]
- 19.Krotz F., Sohn H.Y., Gloe T., Zahler S., Riexinger T., Schiele T.M. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood. Aug. 1 2002;100(3) doi: 10.1182/blood.v100.3.917. 917–24 2002;100:917–924. [DOI] [PubMed] [Google Scholar]
- 20.Handin R.I., Karabin R., Boxer G.J. Enhancement of platelet function by superoxide anion. J Clin Invest. 1977;59(5):959–965. doi: 10.1172/JCI108718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvemini D., de Nucci G., Sneddon J.M., Vane J.R. Superoxide anions enhance platelet adhesion and aggregation. Br J Pharmacol. 1989;97(4):1145–1150. doi: 10.1111/j.1476-5381.1989.tb12572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De La Cruz J.P., Garcia P.J., Sanchez de la C. Dipyridamole inhibits platelet aggregation induced by oxygen-derived free radicals. Thromb Res. 1992;66(4):277–285. doi: 10.1016/0049-3848(92)90278-i. [DOI] [PubMed] [Google Scholar]
- 23.Sill J.C., Proper J.A., Johnson M.E., Uhl C.B., Katusic Z.S. Reactive oxygen species and human platelet GP IIb/IIIa receptor activation. Platelets. 2007;18(8):613–619. doi: 10.1080/09537100701481385. [DOI] [PubMed] [Google Scholar]
- 24.Ambrosio G., Golino P., Pascucci I., Rosolowsky M., Campbell W.B., DeClerck F. Modulation of platelet function by reactive oxygen metabolites. Am J Physiol. 1994;267(1 Pt 2):H308–H318. doi: 10.1152/ajpheart.1994.267.1.H308. [DOI] [PubMed] [Google Scholar]
- 25.Belisario M.A., Tafuri S., Di Domenico C., Squillacioti C., Della M.R., Lucisano A. H(2)O(2) activity on platelet adhesion to fibrinogen and protein tyrosine phosphorylation. Biochim Biophys Acta. 2000;1495(2):183–193. doi: 10.1016/s0167-4889(99)00160-3. [DOI] [PubMed] [Google Scholar]
- 26.Stuart M.J., Holmsen H. Hydrogen peroxide, an inhibitor of platelet function: effect on adenine nucleotide metabolism, and the release reaction. Am J Hematol. 1977;2(1):53–63. doi: 10.1002/ajh.2830020108. [DOI] [PubMed] [Google Scholar]
- 27.Pignatelli P., Pulcinelli F.M., Lenti L., Gazzaniga P.P., Violi F. Hydrogen peroxide is involved in collagen-induced platelet activation. Blood. 1998;91(2):484–490. [PubMed] [Google Scholar]
- 28.Pratico D., Iuliano L., Ghiselli A., Alessandri C., Violi F. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis. 1991;21(3):169–174. doi: 10.1159/000216222. [DOI] [PubMed] [Google Scholar]
- 29.Moro M.A., Darley-Usmar V.M., Goodwin D.A., Read N.G., Zamora-Pino R., Feelisch M. Paradoxical fate and biological action of peroxynitrite on human platelets. Proc Natl Acad Sci U S A. 1994;91(14):6702–6706. doi: 10.1073/pnas.91.14.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowak P., Wachowicz B. Studies on pig blood platelet responses to peroxynitrite action. Platelets. 2001;12(6):376–381. doi: 10.1080/09537100120068161. [DOI] [PubMed] [Google Scholar]
- 31.Nowak P., Wachowicz B. Peroxynitrite-mediated modification of fibrinogen affects platelet aggregation and adhesion. Platelets. 2002;13(5–6):293–299. doi: 10.1080/0953770021000007230. [DOI] [PubMed] [Google Scholar]
- 32.Yin K., Lai P.S., Rodriguez A., Spur B.W., Wong P.Y. Antithrombotic effects of peroxynitrite: inhibition and reversal of aggregation in human platelets. Prostaglandins. 1995;50(3):169–178. doi: 10.1016/0090-6980(95)00119-0. [DOI] [PubMed] [Google Scholar]
- 33.Brown A.S., Moro M.A., Masse J.M., Cramer E.M., Radomski M., Darley-Usmar V. Nitric oxide-dependent and independent effects on human platelets treated with peroxynitrite. Cardiovasc Res. 1998;40(2):380–388. doi: 10.1016/s0008-6363(98)00182-5. [DOI] [PubMed] [Google Scholar]
- 34.Maalej N., Albrecht R., Loscalzo J., Folts J.D. The potent platelet inhibitory effects of S-nitrosated albumin coating of artificial surfaces. J Am Coll Cardiol. 1999;33(5):1408–1414. doi: 10.1016/s0735-1097(98)00687-1. [DOI] [PubMed] [Google Scholar]
- 35.Kader K.N., Akella R., Ziats N.P., Lakey L.A., Harasaki H., Ranieri J.P. eNOS-overexpressing endothelial cells inhibit platelet aggregation and smooth muscle cell proliferation in vitro. Tissue Eng. 2000;6(3):241–251. doi: 10.1089/10763270050044425. [DOI] [PubMed] [Google Scholar]
- 36.Greenlees C., Wainwright C.L., Wadsworth R.M. Vasorelaxant and antiaggregatory properties of the endothelium: a comparative study in normocholesterolaemic and hereditary and dietary hypercholesterolaemic rabbits. Br J Pharmacol. 1996;119(7):1470–1476. doi: 10.1111/j.1476-5381.1996.tb16060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ware J.A., Heistad D.D. Platelet-Endothelium Interactions. N Engl J Med. 1993;328(9):628–635. doi: 10.1056/NEJM199303043280907. [DOI] [PubMed] [Google Scholar]
- 38.Marcus A.J., Broekman M.J., Drosopoulos J.H., Islam N., Alyonycheva T.N., Safier L.B. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcus A., Broekman M., Drosopoulos J., Pinsky D., Islam N., Gayle R., III Thromboregulation by endothelial cells: significance for occlusive vascular diseases. Arterioscler Thromb Vasc Biol. 2001;21(2):178–182. doi: 10.1161/01.atv.21.2.178. [DOI] [PubMed] [Google Scholar]
- 40.Krotz F., Sohn H.Y., Keller M., Gloe T., Bolz S.S., Becker B.F. Depolarization of endothelial cells enhances platelet aggregation through oxidative inactivation of endothelial NTPDase. Arterioscler Thromb Vasc Biol. Dec. 1 2002;22(12) doi: 10.1161/01.atv.0000043454.08172.51. 2003–9 2002;22:2003–2009. [DOI] [PubMed] [Google Scholar]
- 41.Keyer K., Imlay J.A. Inactivation of Dehydratase [4Fe-4S] Clusters and Disruption of Iron Homeostasis upon Cell Exposure to Peroxynitrite. J Biol Chem. 1997;272(44):27652–27659. doi: 10.1074/jbc.272.44.27652. [DOI] [PubMed] [Google Scholar]
- 42.Kanaya S., Ikeda H., Haramaki N., Murohara T., Imaizumi T. Intraplatelet Tetrahydrobiopterin Plays an Important Role in Regulating Canine Coronary Arterial Thrombosis by Modulating Intraplatelet Nitric Oxide and Superoxide Generation. Circulation. 2001;104(20):2478–2484. doi: 10.1161/hc4501.098930. [DOI] [PubMed] [Google Scholar]
- 43.Saluk-Juszczak J., Olas B., Wachowicz B., Glowacki R., Bald E. L-carnitine modulates blood platelet oxidative stress. Cell Biol Toxicol. 2010;26(4):355–365. doi: 10.1007/s10565-009-9148-4. [DOI] [PubMed] [Google Scholar]
- 44.Bergandi L., Cordero M., Anselmino M., Ferraro G., Ravera L., Dalmasso P. Altered nitric oxide/cGMP platelet signaling pathway in platelets from patients with acute coronary syndromes. Clin Res Cardiol. 2010;99(9):557–564. doi: 10.1007/s00392-010-0157-3. [DOI] [PubMed] [Google Scholar]