Abstract
Global changes such as deforestation, climate change, and invasive species have the potential to greatly alter zoonotic disease systems through impacts on biodiversity. This study examined the impact of the invasive pathogen that causes sudden oak death (SOD) on the ecology of Lyme disease in California. The Lyme disease bacterium, Borrelia burgdorferi, is maintained in the far western United States by a suite of animal reservoirs including the dusky-footed woodrat (Neotoma fuscipes) and deer mouse (Peromyscus maniculatus), and is transmitted by the western black-legged tick (Ixodes pacificus). Other vertebrates, such as the western fence lizard (Sceloporus occidentalis), are important tick hosts but are not reservoirs of the pathogen. Previous work found that higher levels of SOD are correlated with greater abundance of P. maniculatus and S. occidentalis and lower N. fuscipes abundance. Here we model the contribution of these tick hosts to Lyme disease risk and also evaluate the potential impact of SOD on infection prevalence of the tick vector. By empirically parameterizing a static model with field and laboratory data on tick hosts, we predict that SOD reduces an important index of disease risk, nymphal infection prevalence, leading to a reduction in Lyme disease risk in certain coastal woodlands. Direct observational analysis of the impact of SOD on nymphal infection prevalence supports these model results. This study underscores the important direct and indirect impacts of invasive plant pathogens on biodiversity, the transmission cycles of zoonotic diseases, and ultimately human health.
Key Words: Borrelia burgdorferi, Disease ecology, Indirect effects, Nymphal infection prevalence, Phytophthora ramorum, Tick
Introduction
For vector-borne zoonotic diseases, understanding the causes of variation in disease risk can be challenging. Vectors (e.g., mosquitoes and ticks) and vertebrate reservoirs of pathogens can respond in complex ways to ecosystem disturbances such as exotic species invasions, fragmentation, and climate change (Allan et al. 2003; Suzan et al. 2008). In this study, we describe the contribution of specific species to Lyme disease risk in an oak woodland community and then evaluate how the risk of human exposure to Lyme disease, caused by the bacterium Borrelia burgdorferi sensu stricto (Bb), changes in response to a dramatic and novel disturbance in California by the invasive forest pathogen Phytophthora ramorum.
Lyme disease is the most commonly reported vector-borne zoonotic disease in North America, with nearly 25,000 new cases a year (Centers for Disease Control and Prevention 2008). In the far western United States, the vector of the disease to humans is the western black-legged tick, Ixodes pacificus (Furman and Loomis 1984). There are three post-egg life stages in all ixodid ticks: the larva, nymph, and adult. Each of these stages takes a single blood meal before molting to the next stage. Larval and nymphal I. pacificus generally feed on small to medium-sized mammals, birds, and especially lizards, whereas the adults feed on larger mammals such as deer and carnivores (raccoons, foxes, and skunks; Burgdorfer et al. 1985). Because transovarial transmission of Bb is very rare (Schoeler and Lane 1993), ticks acquire the Lyme disease pathogen during one of these three blood meals.
The natural reservoirs of Bb in California include the dusky-footed woodrat (Neotoma fuscipes), the California kangaroo rat (Dipidomys californicus), the deer mouse (Peromyscus maniculatus), and the western gray squirrel (Sciurus griseus; Lane and Brown 1991; Lane et al. 1999; Lane et al. 2005; Brown et al. 2006). However, these species can vary dramatically in their reservoir competency. Other important tick hosts, such as the western fence lizard (Sceloporus occidentalis) and southern alligator lizard (Elgaria multicarinata), have zero reservoir competency and actually reduce the Bb infection prevalence in nymphal I. pacificus ticks (Lane and Quistad 1998; Wright et al. 1998). This antibiotic effect is accomplished through the alternative complement pathway of the lizard's immune system that kills the bacteria in the midgut diverticula of the feeding nymphal tick (Kuo et al. 2000).
We focus on the nymphal stage of I. pacificus because it is the most important stage for transmitting Bb to humans. This is because larvae are uninfected and the adults are readily detected on skin and rarely feed on humans for the minimum time necessary to transmit the pathogen (Clover and Lane 1995).
Phytophthora ramorum, the causative agent of sudden oak death (SOD), is an invasive plant pathogen in coastal California and Oregon that is causing a major disturbance to oak woodlands in the far western United States and presents a unique opportunity to study the impact of a disturbance on the ecology of Lyme disease in real time (Garbelotto et al. 2003; Rizzo and Garbelotto 2003). In addition to death, SOD also changes the structure of forests such that there is more coarse woody debris, more open canopy, and reduced density of the tree species that are highly susceptible to the disease. Some previously abundant species such as coast live oak (Quercus agrifolia) and tanoak (Lithocarpus densiflorus), which have suffered mortality rates up to 55% and 70%, respectively (Brown and Allen-Diaz 2009; Swei et al. 2011a). The current distribution of SOD overlaps extensively with the distribution of Lyme disease in California. To understand the potential impact of SOD-induced forest change on Lyme disease risk, we parameterized a quantitative tick infection prevalence model of how the tick and pathogen are naturally maintained using data on how the vertebrate species involved in the maintenance of Lyme disease pathogens will respond to SOD. We chose to model nymphal infection prevalence (NIP) as our metric of Lyme disease risk because this metric is often correlated with Lyme disease incidence rates in humans (LoGiudice et al. 2003; Connally et al. 2006).
In a multi-year study, Swei and associates (2011a) investigated the impact of SOD on small mammals and lizards in Californian oak woodlands and found that three species were encountered in high enough abundance and with sufficient tick burdens to affect Bb infection of I. pacificus ticks: N. fuscipes, P. maniculatus, and S. occidentalis. Swei and colleagues (2011a) found that the abundance of P. maniculatus and S. occidentalis were positively correlated with SOD damage to trees, and N. fuscipes was negatively correlated with SOD. In this study we characterize the contribution of these important tick hosts to tick infection prevalence, and examine how SOD is likely to affect disease risk.
Materials and Methods
Field sites were located in SOD-infested areas in Marin County, California, north of San Francisco. Field plots were established in two sites: China Camp State Park (CCSP; 38° 0’9.50”N; 122°28’2.53”W) in San Rafael, and in the Marin Municipal Water District (MMWD) Sky Oaks headquarters (37°58’5.39”N; 122°36’15.20”W). Full sampling design and plot descriptions can be found in Swei and associates (2011a).
Swei and co-workers (2011a) found that the abundance of P. maniculatus and S. occidentalis were positively correlated with SOD damage to trees, and N. fuscipes was negatively correlated with SOD. In this study we gathered data from 2006 to 2008 on vertebrates, ticks, and the B. burgdorferi prevalence to model species-specific contributions to and impact on NIP. This model has been shown to reliably estimate NIP given proper parameterization (LoGiudice et al. 2008). We then combined the parameterized NIP model with the findings of the vertebrate response to SOD (Swei et al. 2011a) to predict the impact of SOD on Lyme disease risk (see Appendix). We evaluated the model predictions with field observations of NIP.
Small mammal and lizard populations were monitored from 2006–2008 across all host plots using mark-recapture or mark-resight methods, respectively (Swei et al. 2011a). Our vertebrate surveys focused on species that, at the time of our study, were demonstrated to be important for Lyme disease ecology in California, and therefore did not focus on fossorial animals or birds. Tick burden and infection status with B. burgdorferi was assessed for all captured animals (see Swei et al. 2011a for handling methods). In 2006, lizard tick burden data were not available, so tick burden was estimated as the average of 2007 and 2008 values.
Nymphal tick sampling
Questing juvenile ticks were sampled by drag cloth sampling using a 1-m2 white flannel cloth in the forest understory. Sampling was conducted during the peak questing activity of juvenile I. pacificus sub-adults, from March to May between 10 a.m. and 4 p.m. On each plot, five randomly selected 100-m transects were sampled for ticks. Each plot was sampled twice per year from 2006 to 2008 during the peak I. pacificus questing season. All collected ticks were stored in 70% ethanol for laboratory identification of species and age class.
Host infectivity and tick molting success
Tick hosts were infested with larval I. pacificus in the laboratory (Mather et al. 1989; Brown and Lane 1996) to determine tick molting probability, and the transmission percentage to molted nymphal ticks from each species. N. fuscipes and P. maniculatus naturally infected with B. burgdorferi were transported to the University of California (UC) Berkeley, animal care facilities for xenodiagnosis. A limited number of infected animals were recaptured and placed in cages in a biosafety level 3 facility at UC Berkeley. In 2006, 9 wild-caught Bb-infected N. fuscipes and 8 P. maniculatus were used for laboratory transmission studies. In 2007, 3 additional N. fuscipes were included in the laboratory study. After an acclimation period, the animals were sedated (Hahn et al. 2005), and approximately 100 unfed larval ticks were placed on the animals, which were held in cages suspended over a bin of water (Eisen et al. 2003). Fed ticks were collected from the water bath daily and kept in holding chambers until they molted into nymphs (4–12 months. Then they were placed in 95% ethanol for infection testing by polymerase chain reaction (PCR) and sequencing.
Transmission potential and infection percentage of lizards were assumed to be 0 (Lane and Quistad 1998; Kuo and Lane 2000). The molting success of I. pacificus larvae to nymphs following feeding on S. occidentalis was taken from the literature (Table 1; Slowik and Lane 2009).
Table 1.
Infection Prevalence, Sample Size, and Larval Tick Burdens of Small Mammals Encountered in the Study
| |
2006 |
2007 |
||||
|---|---|---|---|---|---|---|
| n | Infection % | Mean tick burden (SE) | N | Infection % | Mean tick burden (SE) | |
| M. californicus | 14 | 7.14 | 1.5 (0.54) | 5 | 0 | 0.2 (0.20) |
| N. fuscipes | 324 | 17.24 | 3.56 (0.23) | 180 | 18.47 | 5.64 (0.47) |
| P. maniculatus | 342 | 13.00 | 2.61 (0.19) | 119 | 13.45 | 2.52 (0.42) |
| P. truei | 0 | NA | NA | 4 | 0 | 2 (1.18) |
| R. rattus | 4 | 0 | 7.33 (3.18) | 4 | 0 | 3 (1.55) |
| Sorex species. | 0 | NA | NA | 1 | 0 | 0 |
| S. occidentalis | 206 | 0 | 19.51 (0.97)a | 349 | 0 | 25.44 (1.42) |
Calculated as mean of 2007 and 2008 burdens due to unavailable data.
Data are presented for 2006 and 2007.
SE, standard error; NA, not available.
Genetic analysis and infection identification
Mammals were live-trapped and two 2-mm ear punch biopsies were collected for lab testing before being released at the point of capture (Swei et al. 2011a). All mammal and tick samples were extracted and tested for infection with Bb sensu stricto following the nested PCR protocol of Lane et al. and associates (2005). Ticks were first screened by real-time PCR (Swei et al. 2011a). All positive samples were sequenced following the protocol provided in Lane et al. and colleagues (2005). Mammal infection data were determined for 2006 and 2007, and tick infection data were collected for 2006–2008.
Modeling vertebrate contribution to nymphal infection prevalence
Model parameters (see Eq. 1 in the Appendix) were calculated for vertebrates that we encountered as significant tick hosts or pathogen reservoirs (Swei et al. 2011a). The functional relationship between host species abundance and NIP was modeled for each species by varying the abundance (Ni) of each species from 0 to 100 individuals per 1-hectare plot while holding all other parameters and mean species densities constant. To reflect empirical observations of an inverse relationship between N. fuscipes and P. maniculatus in 2006 (Swei et al. 2011a), a host-density-dependent model was included only in the 2006 model simulation. Using the observed relationship between N. fuscipes and P. maniculatus, we incorporated negative slopes to account for the inverse relationship (Swei et al. 2011a). No lizard density dependence was included in the model simulation in either year.
Impact of sudden oak death on Lyme disease risk
The vertebrate response results of Swei and colleagues (2011a) were implemented to simulate the qualitative impact of SOD on NIP. Data from 2006 were used as a baseline for the simulations. All simulations excluded density-dependent interactions to generate conservative estimates because density-dependence was not observed in all years (Swei et al. 2011a). Our model used vertebrate abundance, vertebrate infection prevalence, reservoir competency, and tick burdens on vertebrates to predict NIP. These model predictions were then compared with the field-measured infection prevalence of questing nymphal I. pacificus ticks on each plot in each year.
Observed impact of sudden oak death on nymphal infection prevalence
We used a generalized linear mixed effect model (GLMM) to evaluate the direct impact of SOD on the observed NIP. Observed values of NIP from field collections were compiled for all plots from 2006–2008. Impact of SOD on forest structure parameters were summarized by principal components analysis (PCA) into four variables: PC 1–4 (Swei et al. 2011a). These four principal components were included as independent variables in a GLMM using a binomial error distribution for proportion data. Site was included as a fixed factor and plot was a random factor. All model combinations were tested and the best supported model was determined using an Akaike information criterion score (AIC).
Results
Vertebrate tick burdens
Larval tick burdens were greatest on the non-reservoir host S. occidentalis, with burdens in 2007 reaching 25.44±1.42 (mean±standard error [SE]; Table 1). In 2008, the larval tick burden was 14.13±2.43 (mean±SE). The mean of 2007 and 2008 larval burdens (19.51) was used to parameterize the larval burden for 2006 because data were not available for that year.
Mean larval burdens on small mammals were lower than lizard burdens. Among the mammals evaluated, N. fuscipes had the highest total larval burden (Table 1). Species abundance and tick burden data showed that of the species we captured, the most important tick hosts were S. occidentalis, N. fuscipes, and P. maniculatus, in that order. The other vertebrates encountered in this study were not present in significant numbers or were not numerically important hosts for larval I. pacificus (Table 1).
N. fuscipes and P. maniculatus had the highest prevalence of Bb infection among the small mammals we encountered. These results confirm the importance of N. fuscipes, and to a lesser extent P. maniculatus, relative to other potential pathogen reservoirs in this study. Other species that were captured had very low or zero infection prevalence (Table 1).
Host infectivity and tick molting success
Transmission of Bb to molted nymphal ticks (γ; see Appendix) was more than twice as high in N. fuscipes as P. maniculatus (Table 2). In contrast, tick molting success (S) was higher from P. maniculatus than N. fuscipes (Table 2). However, both these species had lower tick molting success than S. occidentalis (Slowik and Lane 2009).
Table 2.
Parameter Values Used in the Baseline Nymphal Infection Prevalence Model
| |
2006 |
2007 |
2006 |
2007 |
|
2006 |
2007 |
|
|---|---|---|---|---|---|---|---|---|
| N | B | S | π | γ | ||||
| N. fuscipes | 23.86 | 13.29 | 3.56 | 5.64 | 0.54 | 0.172 | 0.181 | 0.40 |
| P. maniculatus | 26.33 | 10.09 | 2.61 | 2.52 | 0.75 | 0.13 | 0.135 | 0.17 |
| S. occidentalis | 20.64 | 35.82 | 19.51a | 24.89 | 0.93b | 0b | 0b | 0b |
2006 larval burden on S. occidentalis was estimated from the mean values from 2007 and 2008.
Values taken from the literature (Slowik and Lane 2009).
Field and lab parameters from 2006 and 2007 of abundance per hectare (N), body burden (B), molting rate (S), infection prevalence (π), and probability of transmission to molted nymphs (γ) were used. Transmission experiment results were averaged over the course of the study (2006–2007). All parameter estimates were derived herein except for S. occidentalis molting and transmission rates, which were taken from the literature.
Response of nymphal infection prevalence to vertebrate abundance
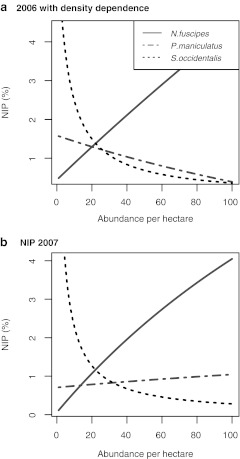
The contribution of N. fuscipes and S. occidentalis to NIP was not qualitatively different between years, despite differences in species density and infection prevalence of N. fuscipes (Fig. 1). However, P. maniculatus switches from a negative relationship with NIP in 2006, when a negative density-dependent interaction with N. fuscipes was included, to a positive relationship in 2007 (Fig. 1). The model predicted that NIP would increase asymptotically with N. fuscipes abundance with the parameters for both years (Fig. 1). In contrast to N. fuscipes, S. occidentalis has an exponentially negative impact on NIP, reducing NIP to near 0% as lizard abundance reached 100 animals per plot (Fig. 1). The negative density-dependent interaction in 2006 changed the functional relationship of P. maniculatus on NIP such that N. fuscipes was the only species contributing to NIP in this system. However, when a negative interaction was not included in 2007, both P. maniculatus and N. fuscipes contribute positively to NIP.
FIG. 1.
Nymphal infection prevalence (NIP) estimates of Neotoma fuscipes, Peromyscus maniculatus, and Sceloporus occidentalis, as each species' density is adjusted from 0 to 100 animals/hectare based on parameter values from (a) 2006, for which a density-dependent relationship between P. maniculatus and N. fuscipes was incorporated into the model simulation, and (b) 2007, for which no density-dependence was included because lower overall densities of small mammals did not lead to an empirically detectable inverse relationship.
Impact of sudden oak death on Lyme disease?
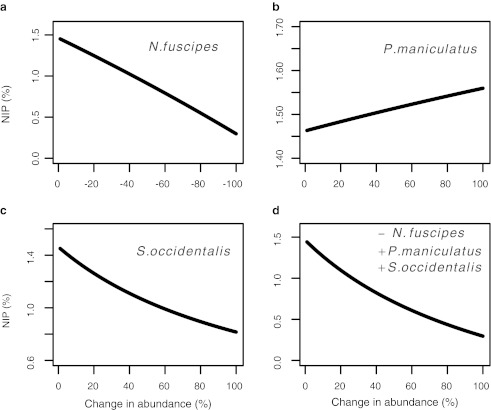
Fitting the NIP model (see Eq. 1 in the Appendix) with the empirical results of the Swei group (2011a) led to the prediction that NIP should decrease as a result of increased abundance of S. occidentalis, and decreased abundance of N. fuscipes (Fig. 2a-c). While decreasing N. fuscipes from 100% to 0% had a greater impact on reducing NIP, increasing S. occidentalis abundance decreased NIP at a faster rate before reaching an asymptote (Fig. 2c and d). Increasing the relative abundance of P. maniculatus from 0% to 100% had a small positive effect on NIP (Fig. 2b). When all three changes in abundance (increased P. maniculatus and S. occidentalis, and decreased N. fuscipes) were incorporated into the model, NIP was found to decrease at a rate similar to when only S. occidentalis abundance was changed (Fig. 2d).
FIG. 2.
Nymphal infection prevalence (NIP) based on parameter values in 2006 as sudden oak death-related changes. Seen here are the decreases in the relative abundance of (a) Neotoma fuscipes, increases in the relative abundance of (b) Peromyscus maniculatus, and (c) decreases in the relative abundance of Sceloporus occidentalis. The combined effect on all three species changing simultaneously is shown in (d).
Observed impact of sudden oak death on tick density and infection prevalence
A total of 4676 nymphal I. pacificus ticks were collected from 2006–2008 and tested for infection with Bb sensu stricto. We found a mean NIP of 7.83%±0.89. NIP was particularly high in 2006, at 10.94%, and was lower in 2007 and 2008 (7.18% and 5.39%, respectively).
Nymphal infection prevalence model validation
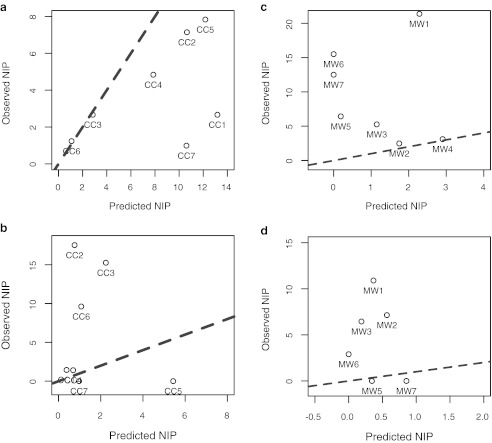
The NIP model predictions were evaluated empirically for all plots and years. The model accurately predicted observed NIP at CCSP in 2006 (r2=0.9895; p<0.001; see Appendix), but it tended to underestimate NIP at CCSP in 2006 and MMWD in both years (Fig. 3b–d; p>0.05).
FIG. 3.
Plot of observed nymphal infection prevalence (NIP) against model-predicted values of NIP at (a) China Camp State Park (CCSP; CC1–CC7) in 2006, (c) Marin Water Municipal District (MMWD; MM1–MM7) in 2006, (b) CCSP in 2007, and (d) MMWD in 2007. A one-to-one line is imposed on the plots as a dashed line.
Measured impact of sudden oak death on nymphal infection prevalence
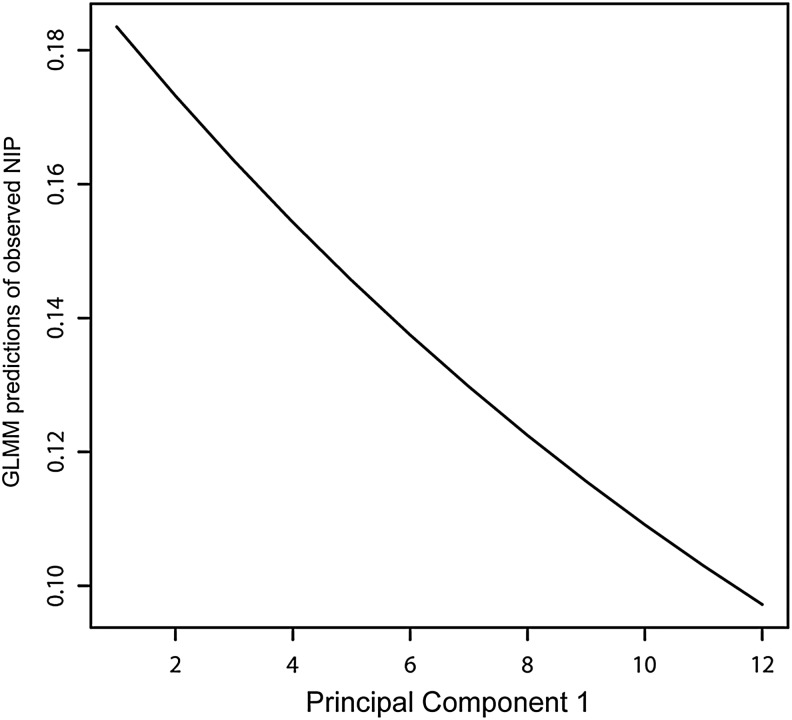
GLMM analysis of data from 2006–2008 found that observed NIP was negatively correlated with SOD (Table 3). The top three models were statistically equivalent (see Appendix), so the least parameterized model was the winning model and included PC 1 and Site as fixed effects (Table 3). Using the range of values for PC 1 in our data, CCSP as the baseline site, and holding all other model components constant, the relationship of PC 1 with NIP is shown in Figure 4.
Table 3.
Generalized Linear Mixed Effect Models of Sudden Oak Death (SOD) on Observed Nymphal Infection Prevalence
| Model component | Parameter | Nymphal infection prevalence (z value) |
|---|---|---|
| Intercept | α | −2.50 (−20.95)*** |
| SOD (PC 1) | β1 | −0.11 (−2.09)* |
| Site | β2 | 0.36 (2.27)* |
p Value: *0.05; ***0.001.
SOD was summarized previously using principal components analysis (Swei et al. 2011a). Interpretations of principal components are provided in the table based on the parameters that were significantly loaded on each component (Swei et al. 2011a). The model for nymphal infection prevalence is: Yi=NIPi ∼ Binomial(nymphsi, πi) for i=1…n, where n=(number of plots) * (number of years)=42, nymphsi is the number of nymphal ticks screened for Bb on survey i, and πi is the probability of infection with Bb. E(Yi)=πi×nymphsi, var(Yi)=nymphsi×πi×(1–πi); logit(πi)=α+β1×PC1+β2×PC2+β3×PC3+β4×PC4+β5×Site+γj[i]+ψk[i], where γj and ψk are random effects. The random effect of Plot is γj[i] ∼ N(0,σplot) for j=1…J, where J=number of plots=14. The random effect of Year is ψk[i] ∼ N(0,σyear) for k=1…K, where=number of years=3. The final model included PC 1, and Site as a fixed effect. Model selection was determined by Akaike information criterion score values. The z value is the Wald statistic.
FIG. 4.
Field-observed nymphal infection prevalence (NIP) was analyzed in a generalized mixed effect model (GLMM) with principal components of sudden oak death as independent variables. The model-predicted correlation between principal component 1 and NIP is shown using site China Camp State Park as a baseline, and assuming all other model parameters are fixed. The values for principal component 1 reflect the range of values in our plot-level data.
Discussion
This study describes the contribution of three important tick hosts in an oak woodland community to nymphal tick infection prevalence, a key metric of Lyme disease risk (LoGiudice and Ostfeld 2003; Connally and Ginsberg 2006). The study then addresses the impact of an invasive forest pathogen on the risk of an important vector-borne zoonotic disease. Swei and associates (2011a) showed that sudden oak death (SOD) affects tick hosts in species-specific ways, increasing the abundance of deer mice and fence lizards, and decreasing the abundance of woodrats. We used the vertebrate response data of Swei's group (2011a) to qualitatively predict the impact of SOD on NIP. Our modeling of NIP accurately predicted observed NIP in some plots and years, but not in others. The lack of fit suggests that either unknown (untrappable) hosts are important in the transmission of Lyme disease, or that parameter values are dynamic, undermining our effort to model disease prevalence using mean parameter estimates. However, an imperfectly parameterized model should still generate reasonable predictions of the qualitative, directional response of NIP (Schmidt and Ostfeld 2001), and is our best estimate for the impact of SOD on Lyme disease given a simple three-species host community. Furthermore, the GLMM analyses of the impact of SOD on observed NIP are qualitatively consistent with our model's prediction that disturbance from SOD negatively affected NIP in affected oak woodlands. However, an experimental removal of lizards found a significant decrease in the density of infected nymphs (Swei et al. 2011b). The ecological role of lizards in Lyme disease transmission is complex. On the one hand they kill B. burgdorferi in feeding ticks, but because they also host large numbers of juvenile ticks, lizards are critical for maintaining sizable populations of juvenile ticks.
Increased abundance of lizards, which are refractory to the Lyme disease pathogen, provided the main driving force of this impact in our model. At the population level, lizards feed more larval ticks than do either mice or woodrats, and fail to infect the ticks they feed. Consequently, increases in lizard abundance had the strongest influence on reducing NIP in our overall model than did increasing abundance of mice or decreasing abundance of woodrats.
We identified N. fuscipes as an important reservoir of B. burgdorferi, whose abundance was linearly, positively correlated with nymphal infection prevalence. Although P. maniculatus can also transmit B. burgdorferi to feeding ticks, in years with heavy competition with N. fuscipes, increased abundance of mice actually led to lower NIP due to reductions in the abundance of the more competent reservoir host, N. fuscipes. In our simulations without competition between the two rodent species, P. maniculatus was positively correlated with NIP, but only weakly so. Our model results indicate that even in the absence of competition, N. fuscipes has a considerably stronger impact on NIP than does P. maniculatus. One of the reasons for this lower competency is that infected P. maniculatus mice transmit B. burgdorferi to a lower percentage of feeding larval ticks.
We found a great deal of complexity in the transmission dynamics of Lyme disease when competition between hosts was considered. Swei and colleagues (2011a) reported a strong inverse relationship between N. fuscipes and P. maniculatus in 2006, when rodent densities were high, but not in 2007, when they were lower. This suggests that a density threshold is necessary for competition for limited resources to occur (Aloiau et al. 1997, Bilenca and Kravetz 1999). A critical result from our study is that some hosts (e.g., P. maniculatus) can alternate between acting as an amplifying host for Bb, and acting as a dilution host that reduces Bb prevalence, depending on ecological interactions (Fig. 1; 2006 versus 2007). A similar alternation of roles was also documented for short-tailed shrews (Blarina brevicauda) and masked shrews (Sorex cinereus) in the northeastern U.S.A. (Ostfeld et al. 2006).
A previous study (Salkeld and Lane 2010) found that western gray squirrels and western fence lizards are the most important tick hosts at sites in Mendocino County, California, squirrels because they are a primary pathogen reservoir, and lizards because they are a refractory tick host. Their model also found that predicted NIP tended to be lower than observed NIP, and postulated that this may be due to low estimates of mammal infection prevalence due to false-negative diagnostic tests. A key distinction of our study is that the study by Salkeld and Lane (2010) was a retrospective study that characterized the host community by combining data across different years and plots, whereas we sought to model the host community in a spatially and temporally explicit way [i.e., data from one plot in year (t) were used to predict NIP on the same plot in the following year (t+1), thus following a single cohort of ticks from larvae (year=t) to nymphs (year=t+1)]. Furthermore, our study was the first attempt to characterize the host community for the Lyme disease pathogen in California in such a way, and illustrates the complexity that dynamically changing host abundance has on Lyme disease risk.
Transmission of Lyme disease and the maintenance of tick populations are governed by host communities that vary by region and habitat. Our NIP model parameterization predicted observed NIP for some plots and years, but made inaccurate predictions for other plots and years, suggesting that the roles of other less apparent hosts should be pursued. Despite quantitative predictions that were sometimes not supported by data, the primary qualitative result of our model—that NIP will decrease with increasing SOD damage—is supported by the data. Our results also demonstrate that a simplistic model of pathogen transmission with only three host species can in some cases predict pathogen prevalence, and that although I. pacificus most certainly feeds on many other species, only a few species play an important role. It remains to be seen how SOD will affect those areas with a different assemblage of tick hosts, and how incorporating density-dependence of tick burdens as host densities change will affect our predictions. However, because of the consistent importance of S. occidentalis throughout California, it is likely that if the SOD-induced disturbance increases the abundance of lizards, NIP will be similarly decreased in other SOD-impacted areas.
Appendix
Results of Generalized Linear Models of All Candidate Models Used in the Evaluation of Observed Nymphal Infection Prevalence (NIP) and Principal Components of Sudden Oak Death
| Candidate model | ΔAIC | |
|---|---|---|
| 1. | NIP∼ PC1+PC3+PC4+Site | 0 |
| 2. | NIP∼ PC1+Site | 0.5* |
| 3. | NIP∼ PC1+PC2+PC3+Site | 1.6 |
| 4. | NIP∼ PC1+PC2+Site | 2.5 |
| 5. | NIP∼ PC4+Site | 2.5 |
| 6. | NIP∼ PC1+PC4 | 2.5 |
| 7. | NIP∼ PC1+PC2PC4 | 2.6 |
| 8. | NIP∼ PC1 | 2.7 |
| 9. | NIP∼ PC1+PC2 | 3.1 |
| 10. | NIP∼ PC2 | 3.4 |
| 11. | NIP∼ PC2+PC4+Site | 4.4 |
| 12. | NIP ∼ PC3 | 85.1 |
| 13. | NIP∼ PC1+PC3 | 85.6 |
| 14. | NIP∼PC3+Site | 86.4 |
| 15. | NIP∼ PC1+PC3+Site | 86.6 |
| 16. | NIP∼ PC2+PC3 | 86.7 |
| 17. | NIP∼ PC3+PC4 | 87 |
| 18. | NIP∼ PC1+PC2+PC3 | 87.1 |
| 19. | NIP ∼ PC4 | 87.1 |
| 20. | NIP∼ PC1+PC3+PC4 | 87.5 |
| 21. | NIP∼ PC2+Site | 87.9 |
| 22. | NIP∼PC2+PC3+Site | 88.4 |
| 23. | NIP∼ PC3+PC4+Site | 88.4 |
| 24. | NIP∼PC2+PC3+PC4 | 88.5 |
| 25. | NIP∼ PC2+PC4 | 88.7 |
| 26. | NIP∼ PC1+PC2+PC3+PC4 | 88.9 |
| 27. | NIP∼ PC1+PC4+Site | 88.9 |
| 28. | NIP∼ PC2+PC3+PC4+Site | 89.9 |
| 29. | NIP∼ PC1+PC2+PC3+PC4+Site | 90.5 |
| 30. | NIP∼ PC1+PC2+PC4+Site | 90.9 |
Delta Akaike information criterion score (AIC) values are given and the winning model is shown with an asterisk. Model comparison found no statistically significant difference between models 1–3, so the least parameterized model was chosen as the best model and is indicated with an asterisk (PC1–PC4 from Swei et al. 2011a).
Nymphal infection prevalence (NIP) was calculated using a formula modified from LoGiudice et al. (2003):
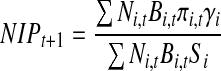 |
[Eq. 1] |
where Ni is the density of host species i, Bi is the average larval tick body burden on species i (no. of larval ticks/individual), πi is the probability that a host of given species is infected, Si is the proportion of successfully molted nymphs from species i, and γi is the infection prevalence of molted nymphal ticks from species i. Testing molted nymphal ticks rather than blood fed larval ticks is advantageous because the undigested vertebrate blood in larval tick samples can interfere with and inhibit polymerase chain reactions (PCR) and therefore infection detection.
The parameters N, B, and π were parameterized for each species on our plots such that values in year t predict NIP in t+1. Parameters S and γ were assumed to be species-specific physiological parameters that are constant among years and as such were averaged for each species with laboratory xenodiagnosis data (LoGiudice et al. 2003).
Acknowledgments
We thank Tina Cheng, Natalie Reeder, Esther Omi-Olsen, and Joyce Kleinjan, for efforts in the field and lab. This research was funded by the National Science Foundation, Ecology of Infectious Diseases (0525755), the National Science Foundation predoctoral fellowship, the Mammal Society grant-in-aid, and Sigma Xi. This work was supported in part by grant RO1AI022501 from the National Institute of Allergy and Infectious Diseases to R.S.L. All protocols were approved by China Camp State Park, Marin Municipal Water District, the California Department of Fish and Game, and the University of California–Berkeley Animal Care and Use Committee (protocol R092-B).
Author Disclosure Statement
No competing financial interests exist.
References
- Allan BF. Keesing F. Ostfeld RS. Effect of forest fragmentation on Lyme disease risk. Conserv Biol. 2003;17:267–272. [Google Scholar]
- Aloiau BG. Post DM. Horne EA. Interspecific competition for food between white-footed mice and eastern woodrats. Prairie Nat. 1997;29:249–256. [Google Scholar]
- Bilenca DN. Kravetz FO. Seasonal changes in microhabitat use and niche overlap between Akodon azarae and Calomys laucha (Rodentia, Muridae) in agroecosystems of central Argentina. Stud Neotrop Fauna Environ. 1999;34:129–136. [Google Scholar]
- Brown LB. Allen-Diaz B. Forest stand dynamics and sudden oak death: Mortality in mixed-evergreen forests dominated by coast live oak. For Ecol Manag. 2009;257:1271–1280. [Google Scholar]
- Brown RN. Lane RS. Reservoir competence of four chaparral-dwelling rodents for Borrelia burgdorferi in California. Am J Trop Med Hyg. 1996;54:84–91. doi: 10.4269/ajtmh.1996.54.84. [DOI] [PubMed] [Google Scholar]
- Brown RN. Peot MA. Lane RS. Sylvatic maintenance of Borrelia burgdorferi (Spirochaetales) in northern California: Untangling the web of transmission. J Med Entomol. 2006;43:743–751. doi: 10.1603/0022-2585(2006)43[743:smobbs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W. Lane RS. Barbour AG, et al. The western black-legged tick Ixodes pacificus, a vector of Borrelia burgdorferi. Am J Trop Med Hyg. 1985;34:925–930. doi: 10.4269/ajtmh.1985.34.925. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Surveillance for Lyme disease–United States, 1992–2006. Morb Mortal Wkly Rep. 2008;57:1–9. [Google Scholar]
- Clover JR. Lane RS. Evidence implicating nymphal Ixodes pacificus (Acari: Ixodidae) in the epidemiology of Lyme disease in California. Am J Trop Med Hyg. 1995;53:237–240. doi: 10.4269/ajtmh.1995.53.237. [DOI] [PubMed] [Google Scholar]
- Connally NP. Ginsberg HS. Mather TN. Assessing peridomestic entomological factors as predictors for Lyme disease. J Vector Ecol. 2006;31:364–370. doi: 10.3376/1081-1710(2006)31[364:apefap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Eisen L. Dolan MC. Piesman J, et al. Vector competence of Ixodes pacificus and I. spinipalpis (Acari: Ixodidae), and reservoir competence of the dusky-footed woodrat (Neotoma fuscipes) and the deer mouse (Peromyscus maniculatus), for Borrelia bissettii. J Med Entomol. 2003;40:311–320. doi: 10.1603/0022-2585-40.3.311. [DOI] [PubMed] [Google Scholar]
- Furman DP. Loomis EC. The Ticks of California. Berkeley: University of California Press; 1984. [Google Scholar]
- Garbelotto M. Rizzo DM. Davidson JM, et al. Phytophthora ramorum: An emerging forest pathogen. Phytopathology. 2003;93:S28. [Google Scholar]
- Hahn N. Eisen RJ. Eisen L, et al. Ketamine-medetomidine anesthesia with atipamezole reversal: practical anesthesia for rodents under field conditions. Lab Animal. 2005;34:48–51. doi: 10.1038/laban0205-48. [DOI] [PubMed] [Google Scholar]
- Kuo MM. Lane RS. Giclas PC. A comparative study of mammalian and reptilian alternative pathway of complement-mediated killing of the Lyme disease spirochete (Borrelia burgdorferi) J Parasitol. 2000;86:1223–1228. doi: 10.1645/0022-3395(2000)086[1223:ACSOMA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lane RS. Brown RN. Wood rats and kangaroo rats, potential reservoirs of the Lyme disease spirochete in California, USA. J Med Entomol. 1991;28:299–302. doi: 10.1093/jmedent/28.3.299. [DOI] [PubMed] [Google Scholar]
- Lane RS. Mun J. Eisen RJ, et al. Western gray squirrel (Rodentia: Sciuridae): A primary reservoir host of Borrelia burgdorferi in Californian oak woodlands? J Med Entomol. 2005;42:388–396. doi: 10.1093/jmedent/42.3.388. [DOI] [PubMed] [Google Scholar]
- Lane RS. Peavey CA. Padgett KA, et al. Life history of Ixodes (Ixodes) jellisoni (Acari: Ixodidae) and its vector competence for Borrelia burgdorferi sensu lato. J Med Entomol. 1999;36:329–340. doi: 10.1093/jmedent/36.3.329. [DOI] [PubMed] [Google Scholar]
- Lane RS. Quistad GB. Borreliacidal factor in the blood of the western fence lizard (Sceloporous occidentalis) J Parasitol. 1998;84:29–34. [PubMed] [Google Scholar]
- LoGiudice K. Duerr S. Newhouse M, et al. Impact of community composition on Lyme disease risk. Ecology. 2008;89:2841–2849. doi: 10.1890/07-1047.1. [DOI] [PubMed] [Google Scholar]
- LoGiudice K. Ostfeld RS. Schmidt KA, et al. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather TN. Wilson ML. Moore SI, et al. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete Borrelia burgdorferi. Am J Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS. Keesing F. Logiudice K. Community ecology meets epidemiology: the case of Lyme disease. In: Collinge SK, editor; Ray C, editor. Disease Ecology: Community Structure and Pathogen Dynamics. Oxford: Oxford University Press; 2006. pp. 28–40. [Google Scholar]
- Rizzo DM. Garbelotto M. Sudden oak death: endangering California and Oregon forest ecosystems. Front Ecol Environ. 2003;1:197–204. [Google Scholar]
- Salkeld DJ. Lane RS. Community ecology and disease risk: lizards, squirrels, and the Lyme disease spirochete in California, USA. Ecology. 2010;91:293–298. doi: 10.1890/08-2106.1. [DOI] [PubMed] [Google Scholar]
- Schmidt KA. Ostfeld RS. Biodiversity and the dilution effect in disease ecology. Ecology (Wash DC) 2001;82:609–619. [Google Scholar]
- Schoeler GB. Lane RS. Efficiency of transovarial transmission of the Lyme disease spirochete, Borrelia burgdorferi, in the western black-legged tick, Ixodes pacificus (Acari: Ixodidae) J Med Entomol. 1993;30:80–86. doi: 10.1093/jmedent/30.1.80. [DOI] [PubMed] [Google Scholar]
- Slowik TJ. Lane RS. Feeding preferences of the immature stages of three western North American ixodid ticks (Acari) for avian, reptilian, or rodent hosts. J Med Entomol. 2009;46:115–122. doi: 10.1603/033.046.0115. [DOI] [PubMed] [Google Scholar]
- Suzan G. Armien A. Mills JN, et al. Epidemiological considerations of rodent community composition in fragmented landscapes in Panama. J Mammal. 2008;89:684–690. [Google Scholar]
- Swei A. Ostfeld RS. Lane RS, et al. Effects of an invasive forest pathogen on abundance of vertebrate hosts in a California Lyme disease focus. Oecologia. 2011a;166:91–100. doi: 10.1007/s00442-010-1796-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swei A. Ostfeld RS. Lane RS, et al. Impact of the experimental removal of lizards on Lyme disease risk. Proc R Soc Biol Sci Ser B. 2011b;278:2970–2978. doi: 10.1098/rspb.2010.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SA. Lane RS. Clover JR. Infestation of the southern alligator lizard (Squamata: Anguidae) by Ixodes pacificus (Acari: Ixodidae) and its susceptibility to Borrelia burgdorferi. J Med Entomol. 1998;35:1044–1049. doi: 10.1093/jmedent/35.6.1044. [DOI] [PubMed] [Google Scholar]