Abstract
We compared Ixodes ricinus questing density, the infestation of rodents by immature stages, and the diversity of Borrelia burgdorferi sensu lato (sl) in questing ticks and ticks collected from rodents in two Lyme borreliosis (LB)-endemic areas in Switzerland (Portes-Rouges [PR] and Staatswald [SW]) from 2003 to 2005. There were variations in the seasonal pattern of questing tick densities among years. Questing nymphs were globally more abundant at PR than at SW, but the proportion of rodents infested by immature ticks was similar (59.4% and 61%, respectively). Questing tick activity lasted from February to November with a strong decline in June. The seasonal pattern of ticks infesting rodents was different. Ticks infested rodents without decline in summer, suggesting that the risk of being bitten by ticks remains high during the summer. Rodents from SW showed the highest infestation levels (10±21.6 for larvae and 0.54±1.65 for nymphs). The proportion of rodents infested simultaneously by larvae and nymphs (co-feeding ticks) was higher at SW (28%) than at PR (11%). Apodemus flavicollis was the species the most frequently infested by co-feeding ticks, and Myodes glareolus was the most infective rodent species as measured by xenodiagnosis. At PR, the prevalence of B. burgdorferi sl in questing ticks was higher (17.8% for nymphs and 32.4% for adults) than at SW (10.4% for nymphs and 24.8% for adults), with B. afzelii as the dominant species, but B. garinii, B. burgdorferi sensu stricto, and B. valaisiana were also detected. Rodents transmitted only B. afzelii (at PR and at SW) and B. bavariensis (at SW) to ticks, and no mixed infection by additional genospecies was observed in co-feeding ticks. This implies that co-feeding transmission does not contribute to genospecies diversity. However, persistent infections in rodents and co-feeding transmission contribute to the perpetuation of B. afzelii in nature.
Key Words: Borrelia burgdorferi sensu lato, Co-feeding transmission, Rodents
Introduction
In Europe, Lyme borreliosis (LB) is caused by different Borrelia species belonging to the B. burgdorferi sensu lato (sl) complex. These infectious agents are maintained in natural zoonotic cycles involving wild vertebrates and ticks, which are obligate blood-feeding arthropods. In Europe, Ixodes ricinus is the main vector of B. burgdorferi sl (Piesman and Gern 2004). Both biotic (host species, density and behavior, and vegetation structure), and abiotic factors (local climatic conditions), play a role in modeling the seasonal activity of ticks through the years (Randolph et al. 2002; Randolph 2004; Perret et al. 2004). This seasonal pattern of the density of I. ricinus questing ticks was reported to be variable between years, and to vary from a unimodal pattern with maximum density in spring to a bimodal pattern reaching its maximum density in spring and in autumn. In Switzerland, both bimodal and unimodal patterns were shown to occur (Perret et al. 2000; Jouda et al. 2004a, 2004b; Burri et al. 2007; Morán Cadenas et al. 2007a).
At least nine Borrelia species, B. burgdorferi sensu stricto (ss), B. garinii, B. afzelii, B. valaisiana, B. lusitaniae, B. spielmanii, B. bissettii, B. bavariensis, and B. finlandensis have been identified in I. ricinus (Hanincova et al. 2003b; Rauter and Hartung 2005; Richter et al. 2006; Hulinska et al. 2007; Margos et al. 2009; Casjens et al. 2011).
Studies on the ecology of LB have demonstrated that the efficient persistence of Borrelia pathogens in endemic areas requires the involvement of reservoir hosts. Small-sized mammals, Apodemus mice, and Myodes (Clethrionomys) voles in particular, are often infested by immature I. ricinus stages, and have been described as competent reservoirs for B. burgdorferi sl, as are other mammals, like dormice, hedgehogs, rats, squirrels, hares, ground-frequenting birds like passerines and pheasants, and lizards (Piesman and Gern 2004). Specific associations between hosts, ticks, and Borrelia species are observed in nature. For example, I. ricinus ticks can acquire B. afzelii and B. bavariensis after feeding on rodents (Humair et al. 1995, 1999; Huegli et al. 2002; Margos et al. 2009), and B. afzelii and B. burgdorferi ss are associated with red squirrels (Humair and Gern 1998). In contrast to B. afzelli, which is adapted to rodents, most variants of B. garinii and B. valaisiana present a resistance to the avian complement and are considered bird-associated Borrelia ecotypes (Olsén et al. 1995; Kurtenbach et al. 1998; Humair et al. 1998). These preferential associations affect the genospecies distribution in an area in relation to the composition of the local fauna.
Larvae become infected if at their first blood meal they encounter a Borrelia reservoir host, since transovarial transmission of B. burgdorferi sl is a rare phenomenon (Bellet-Edimo et al. 2005). Nymphs from this cohort of larvae will be infected because of the maintenance and the transfer of the pathogen at the following stages. Borrelia may also pass directly from infected (usually nymphs) to uninfected ticks (usually larvae) when ticks are feeding together on vertebrate hosts before spirochetes have disseminated in the host and produced a systemic infection. This way of transmission is called co-feeding (Gern and Rais 1996).
Here we compared and analyzed seasonal patterns of questing tick activity and infestation of rodents by immature stages in two LB-endemic areas (Staatswald [SW] and Portes-Rouges [PR]) located on the Swiss Plateau. We particularly focused on the simultaneous presence of I. ricinus larvae and nymphs co-feeding on rodents. Co-feeding transmission of LB spirochetes has been described in experimental models (Gern and Rais 1996), but very little information is available on this transmission pathway under natural conditions. Finally, we examined the distribution of B. burgdorferi sl genospecies in questing ticks, ticks that detached from rodents, and xenodiagnostic ticks.
Materials and Methods
Collection of ticks and rodents
The present study took place at two different sites on the Swiss Plateau. The first site, SW (Ins, Canton Bern, 433 m elevation, 46°92′N, 07°07′E) is a mixed forest (Pruno-Fraxinetum) established on a marl. The second site, PR (Bois de l'Hôpital, Neuchâtel, Canton Neuchâtel, 47°01′N, 06°56′E), is a mixed forest (Rhamno-Quercetum) established on a thin soil layer on a rather stony ground (limestone) layer, and is under the climatic influence of the lake of Neuchâtel.
I. ricinus ticks were collected by flagging low vegetation on a monthly basis from March 2003 to November 2005, within the same week at both sites. Contacts between the flag and the litter layer could occur only during winter months, when low vegetation was less dense. Sampling was not undertaken under windy, snowy, or rainy conditions. The 1-m2 flag was examined every 15 m over 360 m at SW and 1049 m at PR along the same transects. Ticks were identified for species, stage, and sex, and maintained at relative humidity close to 95% at room temperature until processing for isolation of spirochetes (within 7–10 days). Questing tick density was expressed as the number of ticks per 100 m2. An annual value for tick density called cumulated tick density (CTD) was obtained by integrating the linearly interpolated curve of the measured questing tick densities over 1 year (Eisen et al. 2003; Jouda et al. 2004a; Burri et al. 2007; Morán Cadenas et al. 2007a).
Small mammals were live-trapped monthly from March 2003 to November 2005 during the same week of questing tick collection, using 50 wooden box traps placed every 5 m along the questing tick collection transects. Traps were placed between 4 and 7 pm, and collected the next morning between 7 and 9 am. The traps were baited with grains, pieces of apple, and during winter months straw was added. Captured individuals were brought into the laboratory and caged individually over a pan of water until the feeding ticks dropped off. Engorged ticks were collected, counted, and maintained as described above until the molt was completed.
Xenodiagnosis
About 1 week after capture, rodents were identified for species and sexed. A subset of rodents that had been captured in spring, summer, fall, and winter were submitted to xenodiagnosis 3–4 days after all field-derived ticks detached. About 100 xenodiagnostic I. ricinus larvae, derived from our laboratory colony and free of spirochetal infection, were placed on the heads of the rodents and allowed to engorge until repletion. Replete ticks were collected daily from each host in a pan of water and maintained under the same conditions as those described for field-derived ticks, until they molted. Finally, the rodents were released at the exact trapping site, around 20 days after capture. Ticks collected from rodents and xenodiagnostic ticks were examined for Borrelia after their molt.
Isolation of spirochetes from I. ricinus
Questing ticks, ticks collected on rodents, and xenodiagnostic ticks were briefly soaked in 70% ethanol and individually squashed with sterilized forceps in tubes containing modified BSK-II medium (Sinsky and Piesman 1989). The culture tubes were screened by darkfield microscopy for spirochetes after 7 and 14 days of incubation at 34°C, and 1.5 mL of positive spirochete subcultures were washed by centrifuging and double washing with 1 mL of PBS-MgCl2. Each pellet was suspended in 50 μL of ultra-filtered water and DNA was extracted after incubation at 100°C for 10 min, according to the procedure of Postic and associates (1994). Thermolysates were stored at −20°C until use for polymerase chain reaction (PCR). Cultures that were contaminated were not further investigated and appear as untyped Borrelia.
Identification of Borrelia species
PCR and restriction fragment length polymorphism (RFLP), as described by Postic and colleagues (1994), were used to identify the Borrelia species. Briefly, the variable spacer region between two repeated genes encoding for ribosomal 23S and 5S was amplified using primers P1 (5′-CTGCGAAGTTCGCGGGAGA-3′) and P2 (3′-TCCTAGGCATTCACCATA-3′) in a Tgradient Thermocycler 96 (Whatman Biometra, Göttingen, Germany). Negative and positive controls were included in each PCR. In negative controls, water replaced DNA samples. PCR products were separated by electrophoresis in a 1% agarose gel and stained with ethidium bromide. Positive PCR products were digested overnight with MseI restriction endonuclease at 37°C, and then separated by electrophoresis in a 16% acrylamide gel for 1 h 30 min at 120 V. Digested DNA was stained with ethidium bromide.
Statistical analysis
Rodent species distribution at both sites was compared by Fisher's exact test, tick infestation levels on rodents was compared with an analysis of variance (ANOVA) with logarithmic transformation, and correlations between numbers of larvae and nymphs feeding together on individual hosts were determined with Spearman's rank correlation. The Pearson's chi-square test was used to compare the proportion of rodents infested by nymphs, and the prevalence of infection in ticks, and finally the distribution of B. burgdorferi sl species was analyzed with the goodness of fit test. Statistical results were considered significant if the p value was less than 0.05. All statistical analyses were performed using S-Plus 6.2 for Windows.
Results
Questing ticks
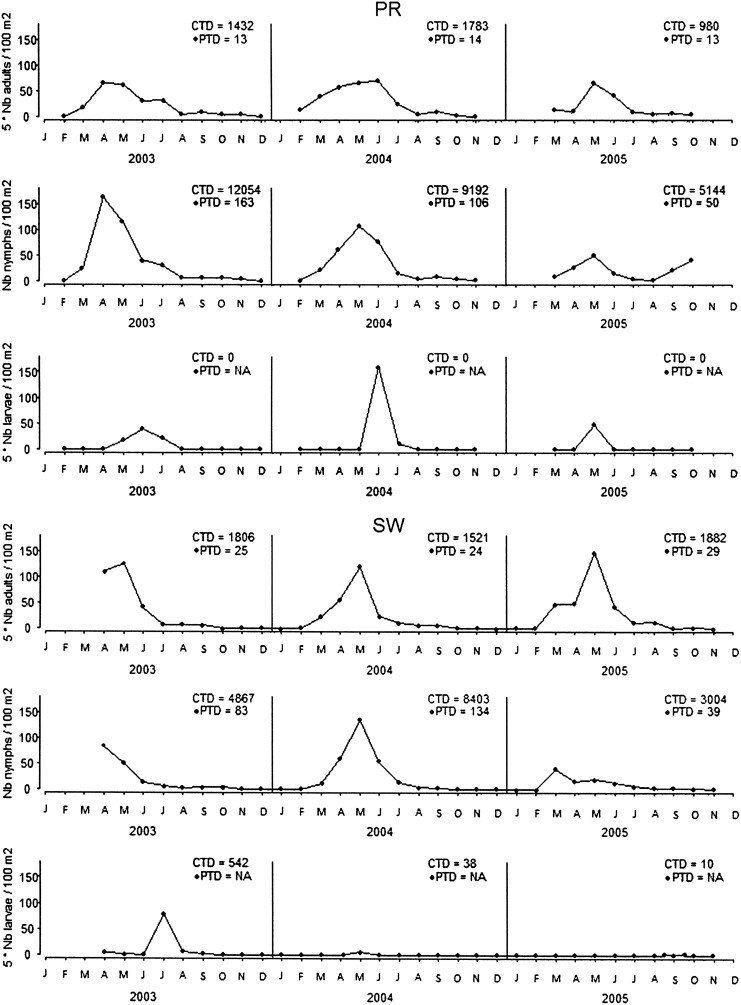
All questing ticks collected by flagging low vegetation at the two sampling sites belonged to I. ricinus. A total of 614 I. ricinus larvae, 9101 nymphs, and 1147 adults were obtained at PR, and 62 larvae, 1712 nymphs, and 540 adults at SW. Questing nymphs were globally more abundant at PR than at SW for the 3-year period, with the highest CTD and the highest peak tick density (PTD) reached in 2003, with 12,054 nymphs per 100 m2/year and 163 nymphs per 100 m2, respectively (Fig. 1). Questing adults showed the highest CTD and PTD at SW in 2005, with 1882 adults per 100 m2/year and 29 adults per 100 m2, respectively. There were variations in the seasonal pattern of questing tick densities among years at both sites, and questing tick activity lasted from February to November at PR, and from March to October at SW, with a strong decline in June at both sites (Fig. 1).
FIG. 1.
Seasonal pattern of Ixodes ricinus questing tick density at Portes-Rouges (PR) and Staatswald (SW; CTD, cumulated tick density; PTD, peak tick density).
Infestation of rodents by ticks
During this study, 632 rodents of three species (A. flavicollis, A. sylvaticus, and M. glareolus) and two Sorex araneus shrews were captured over 3150 trap nights (Table 1). Shrews were not examined for tick infestation because they were dead when the traps were checked. Rodents were less frequently trapped at PR (0.11 rodent/trap night; n=165 rodents) than at SW (0.30 rodent/trap night; n=467). Over the 3-year study at PR each of the three rodent species represented one-third of all captured animals, whereas at SW M. glareolus was clearly the dominant species (55%), followed by A. flavicollis (35%) (Fisher's exact test, p<0.001).
Table 1.
Infestation of Small Mammals with Larval and Nymphal Ixodes ricinus at Portes-Rouges (PR) and Staatswald (SW)
| |
PR |
||||
|---|---|---|---|---|---|
| |
|
Larvae |
Nymphs |
||
| Host species | No infested/examined hosts | No | Mean no per host±SD | No | Mean no per host±SD |
| A. flavicollis | 36/59 (61%) | 453 | 7.7±15 | 11 | 0.19±0.66 |
| A. sylvaticus | 30/52 (58%) | 238 | 4.6±7 | 20 | 0.38±1.03 |
| M. glareolus | 32/54 (59%) | 166 | 3.1±5.1 | 3 | 0.06±0.23 |
| Total | 98/165 (59.4%) | 857 | 5.2±10.4 | 34 | 0.21±0.72 |
| |
SW |
||||
|---|---|---|---|---|---|
| |
|
Larvae |
Nymphs |
||
| Host species | No infested/examined hosts | No | Mean no per host±SD | No | Mean no per host±SD |
| A. flavicollis | 112/162 (69%) | 2786 | 17.2±30.2 | 139 | 0.86±2.16 |
| A. sylvaticus | 24/50 (48%) | 495 | 9.9±25.6 | 26 | 0.52±2.04 |
| M. glareolus | 149/255 (58%) | 1386 | 5.4±10.1 | 85 | 0.33±1.06 |
| Total | 285/467 (61%) | 4667 | 10±21.6 | 250 | 0.54±1.65 |
SD, standard deviation.
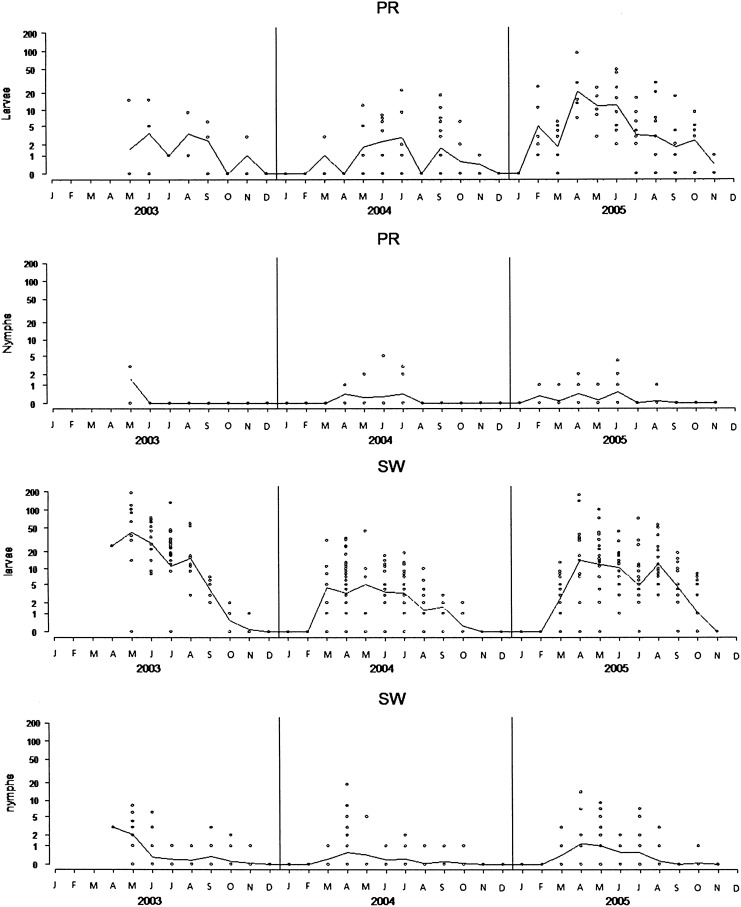
A total of 5808 I. ricinus ticks (5524 larvae [95.1%] and 284 nymphs [4.9%]) detached from captured rodents (Table 1). The seasonal pattern of ticks infesting rodents was different from the seasonal pattern of questing tick activity (Figs. 1 and 2). Ticks were observed on rodents from February–March to November without a decline in summer (Fig. 2). At the two sites, the proportion of rodents infested by immature ticks was similar (PR: 59.4% and SW: 61%; Table 1). However, rodents from SW showed the highest infestation levels, reaching 10±21.6 for larvae and 0.54±1.65 for nymphs. Each rodent species was infested by significantly more larvae at SW than at PR (ANOVA with logarithmic transformation, p=0.033 for M. glareolus, p<0.001 for A. sylvaticus, and p<0.001 for A. flavicollis), and nymphs represented 5.1% of ticks infesting rodents at SW compared to 3.8% at PR (Table 1). At PR, the larval infestation level among the three rodent species did not show any significant difference, whereas at SW, M. glareolus displayed a significantly lower level of infestation by larvae than the two Apodemus species (ANOVA with logarithmic transformation, p<0.001). At both sites, the bank vole was the rodent species showing the lowest infestation load (Table 1).
FIG. 2.
Annual distribution of the median number of I. ricinus immature stages per rodent at Staatswald (SW) and Portes-Rouges (PR). Each circle indicates an individual rodent.
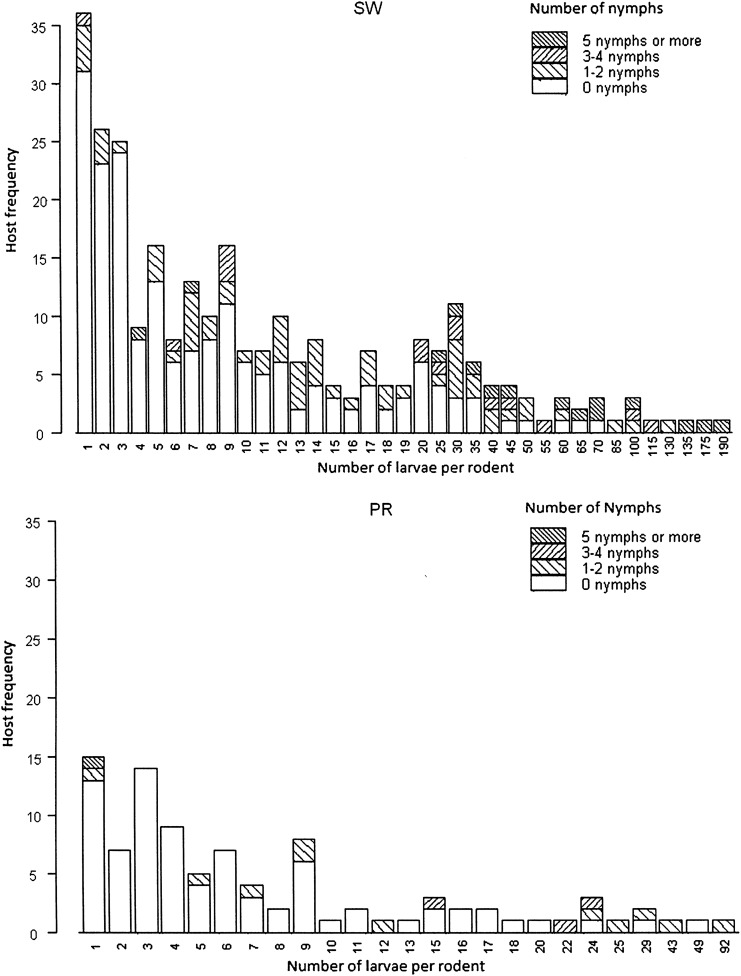
At both sites, a significant positive correlation was observed between the numbers of larvae and nymphs feeding together (co-feeding ticks) on individual hosts (Spearman's rank correlation, p<0.001). Considering each site, the proportion of individuals infested by at least one nymph did not vary among years (Pearson's chi-square test, SW: p=0.062, PR: p=0.91). The probability of finding feeding nymphs on a host increased with larval infestation, particularly at SW (Fig. 3). The proportion of rodents concurrently infested by larvae and nymphs was higher at SW (19.5%, 91/467; M. glareolus: 13.7%, 35/255; A. flavicollis: 29.6%, 48/162; A. sylvaticus: 16%, 8/50) than at PR (9.09%, 15/165; M. glareolus: 5.6%, 3/54; A. flavicollis: 8.5%, 5/59; A. sylvaticus: 13.5%, 7/52). Considering only March to September (the months with the highest tick infestations), there were clearly more rodents with co-feeding ticks at SW (88/310, 28%) than at PR (13/113, 12%) (Pearson's chi-square test, p<0.001), and the proportion of rodents infested by nymphs was also significantly higher at SW (91/310, 29%) than at PR (15/113, 13%) (Pearson's chi-square test, p<0.001). A. flavicollis was the species most frequently infested by co-feeding ticks considering both sites (Pearson's chi-square test, p=0.002).
FIG. 3.
Coincident aggregated frequency distributions of I. ricinus larvae and nymphs on rodents.
Borrelia infection in questing ticks
A total of 638 Borrelia isolates were obtained from 2073 I. ricinus nymphs and 1055 adults collected by flagging vegetation at both sites (Table 2). More ticks were infected at PR (22.3%, 478/2148) than at SW (16.3%, 160/980), but the difference was not significant. However, at each site, adult ticks were significantly more infected than nymphs (Pearson's chi-square test, PR: p<0.001, SW: p=0.005; Table 2). Four Borrelia species, B. afzelii, B. garinii, B. burgdorferi ss, and B. valaisiana, were identified in questing ticks at both sites. Mixed infections were observed in 23 questing ticks (3.6%; B. afzelii and B. burgdorferi ss in 6 nymphs and 6 adults, B. garinii and B. valaisiana in 2 nymphs and 4 adults, B. garinii and B. afzelii in 2 nymphs and 2 adults, and B. garinii and B. burgdorferi ss in 1 adult).
Table 2.
Distribution of Borrelia Species in Questing Ixodes ricinus Ticks Collected at Portes-Rouges (PR) and Staatswald (SW)
| |
|
|
Distribution of Borrelia species (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| Site | Stage | No infected/tested ticks (%) | BA | BG | BB | BV | Bsp | Mixed |
| PR | N | 267/1497 (17.8) | 127 (47.6) | 62 (23.2) | 43 (16.1) | 11 (4.1) | 18 (6.8) | 6 (2.2) |
| A | 211/651 (32.4) | 71 (33.6) | 62 (29.4) | 31 (15.2) | 28 (13.3) | 6 (2.8) | 13 (6.2) | |
| Total | 478/2148 (22.3) | 198 (41.4) | 124 (25.9) | 74 (15.5) | 39 (8.2) | 24 (5) | 19 (4) | |
| SW | N | 60/576 (10.4) | 32 (53.3) | 11 (18.3) | 3 (5) | 3 (5) | 10 (16.7) | 1 (1.7) |
| A | 100/404 (24.8) | 31 (31) | 27 (27) | 19 (19) | 11 (11) | 9 (9) | 3 (3) | |
| Total | 160/980 (16.3) | 63 (39.4) | 38 (23.8) | 22 (13.8) | 14 (8.7) | 19 (11.9) | 4 (2.5) | |
N, nymphs; A, adults; BA, Borrelia afzelii; BG, Borrelia garinii; BB, Borrelia burgdorferi sensu stricto; BV, Borrelia valaisiana; Bsp, untyped Borrelia.
Species distribution was not uniform at the two sites and in tick stages (Goodness of fit test, p≤0.001), but the Borrelia species distribution in nymphs and in adults was not different between PR and SW. B. afzelii was the dominant species in questing I. ricinus nymphs and adults. B. afzelii and B. burgdorferi ss were more frequently detected in questing nymphs at PR than at SW (PR: 63.7%, SW: 58.3%), and B. burgdorferi ss was significantly more frequently detected in nymphs from PR (16.1%) than from SW (5%, Pearson's chi-square test, p=0.04). If we compare the species distribution at each site between questing nymphs and adults, we observe a significant difference at both PR (Pearson's chi-square test, p<0.001) and SW (p=0.011). B. afzelii was significantly more frequent than B. garinii but only in nymphs (Pearson's chi-square test, p=0.003 for PR, and p<0.001 for SW).
Borrelia infection in ticks naturally infesting rodents
A total of 296 Borrelia isolates were obtained from 1074 molted ticks (28.4%) that detached from 130 rodents (PR: n=56; SW: n=74; Table 3). B. afzelii was the dominant species (n=278, 94%), and B. bavariensis (identified by reverse line blotting according to Gern et al. 2010, data not shown) was observed once at SW. No infection with more than one Borrelia species was detected in molted ticks that engorged on rodents. The infection prevalence in ticks that detached from rodents trapped at PR reached 28.9% (114/395), and 39.3% (22/56) of rodents were infested by infected ticks, whereas at SW more than 50% (55.4%, 41/74) of rodents were infested by infected ticks with an infection prevalence in ticks similar to that observed at PR (26.8%, 182/679; Table 3). The rodent species the most frequently infested by infected ticks was A. sylvaticus (47.6%, 10/21) at PR and A. flavicollis (65%, 26/40) at SW. Infection rates of larvae (examined as nymphs) that detached from rodents varied according to rodent species, from 18.6% to 37.6% at PR, and from 21.6% to 31.9% at SW (Table 3).
Table 3.
Infection Prevalence and Genospecies Distribution of Borrelia burgdorferi sensu lato in I. ricinus Feeding on Different Rodent Species Captured at Portes-Rouges (PR) and Staatswald (SW)
| |
|
Hosts |
Nymphs |
Adults |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Host species | Site | No with infected ticks /tested (%) | No infected/tested (%) | BA | Bbav | Bsp | No infected/tested (%) | BA | Bbav | Bsp |
| A. flavicollis | PR | 5/17 (29.4) | 43/157 (27.4) | 42 | 0 | 1 | 2/2 (100) | 1 | 0 | 1 |
| SW | 26/40 (65) | 80/370 (21.6) | 71 | 1 | 8 | 21/47 (44.7) | 18 | 0 | 3 | |
| A. sylvaticus | PR | 10/21 (47.6) | 47/125 (37.6) | 45 | 0 | 2 | 3/6 (50) | 3 | 0 | 0 |
| SW | 2/6 (33.3) | 16/72 (22.2) | 16 | 0 | 0 | 9/12 (75) | 9 | 0 | 0 | |
| M. glareolus | PR | 7/18 (38.9) | 19/102 (18.6) | 17 | 0 | 2 | 0/3 | 0 | 0 | 0 |
| SW | 13/28 (46.4) | 53/166 (31.9) | 53 | 0 | 0 | 3/12 (25) | 3 | 0 | 0 | |
| Total | PR | 22/56 (39.3) | 109/384 (28.4) | 104 | 0 | 5 | 5/11 (45.5) | 4 | 0 | 1 |
| SW | 41/74 (55.4) | 149/608 (24.5) | 140 | 1 | 8 | 33/71 (46.5) | 30 | 0 | 3 | |
BA, Borrelia afzelii; Bbav, Borrelia bavariensis; Bsp, untyped Borrelia.
To examine the effect of co-feeding ticks (nymphs and larvae feeding simultaneously on rodents) on the percentage of Borrelia-infected larvae, we compared the infection of larvae feeding on hosts infested by co-feeding ticks with that of larvae feeding on hosts infested by larvae only. At PR a significantly higher infection rate (44%, 62/141) was observed among larvae feeding on hosts infested by both immature stages than on hosts infested by larvae only (18.8%, 42/223; Pearson's chi-square test, p<0.001). The difference at SW was not significant (115/459, 25.1% versus 26/149, 17.4%, Pearson's chi-square test, p=0.07). If we compared the infection rate of larvae feeding on Apodemus spp. or on M. glareolus at both sites, according to the infestation pattern of rodents (with co-feeding nymphs versus without), the difference was significant at PR for Apodemus spp. (51.7%, 62/120 versus 15.4%, 25/162; Pearson's chi-square test, p≤0.001), and for M. glareolus at SW (44.2%, 42/95 versus 15.5%, 11/71; Pearson's chi-square test, p≤0.001). However, the difference was not significant for Apodemus spp. at SW (20.1%, 73/364 versus 19.2%, 15/78), and a comparison was not possible for M. glareolus at PR because none of the captured voles was simultaneously infested by larvae and nymphs.
Borrelia infection in xenodiagnostic ticks
A xenodiagnosis performed on a subset of captured rodents (PR: n=23; SW: n=38) showed that B. afzelii (180/209, 86.1%) was clearly the dominant species transmitted from rodents to ticks (Table 4). B. bavariensis (6/209, 2.9%) was also observed in xenodiagnostic ticks, but only in ticks from rodents captured at SW (Table 4). More than 60% of rodents captured at both sites (PR: 69.6%, 16/23; SW: 60.5%, 23/38) transmitted spirochetes to 28.1% (81/288) of xenodiagnostic ticks at PR, and 20.8% (128/616) at SW. Rodent infectivity for xenodiagnostic ticks varied between sites and rodent species. At both sites, M. glareolus was the most infectious rodent species since 77% of them transmitted infection to 39% of xenodiagnostic ticks. In contrast, A. sylvaticus was the less infectious species, and its infectivity was particularly low at SW, where only 28.6% of them transmitted spirochetes to only 6.7% of ticks (Table 4).
Table 4.
Infection Prevalence and Genospecies Distribution of Borrelia burgdorferi sensu lato in Xenodiagnostic Ixodes ricinus Ticks Fed on different rodent species captured at Portes-Rouges (PR) and Staatswald (SW)
| |
|
Hosts |
Xenodiagnostic ticks |
|||
|---|---|---|---|---|---|---|
| Host species | Site | No with infected ticks/tested (%) | No infected/tested (%) | BA | Bbav | Bsp |
| A. flavicollis | PR | 4/6 (66.7) | 23/75 (30.7) | 19 | 0 | 4 |
| SW | 14/23 (60.9) | 83/389 (21.3) | 67 | 6 | 10 | |
| A. sylvaticus | PR | 9/12 (75) | 31/157 (19.7) | 26 | 0 | 5 |
| SW | 2/7 (28.6) | 8/120 (6.7) | 7 | 0 | 1 | |
| M. glareolus | PR | 3/5 (60) | 27/56 (48.2) | 26 | 0 | 1 |
| SW | 7/8 (87.5) | 37/107 (34.6) | 35 | 0 | 2 | |
| Total | PR | 16/23 (69.6) | 81/288 (28.1) | 71 | 0 | 10 |
| SW | 23/38 (60.5) | 128/616 (20.8) | 109 | 6 | 13 | |
BA, Borrelia afzelii; Bbav, Borrelia bavariensis; Bsp, untyped Borrelia.
Discussion
During this 3-year study, we focused our attention on several parameters related to questing ticks and to infestation of small-sized mammals (A. flavicollis, A. sylvaticus, and M. glareolus) described as reservoirs for B. burgdorferi sl in two LB endemic areas located on the Swiss Plateau.
Questing ticks and ticks naturally infesting rodents
In Switzerland, the seasonal pattern of questing tick density over time (phenology) presents both bimodal (peaks in spring and autumn) and unimodal (peak in spring) patterns (Perret et al. 2000; Jouda et al. 2004a, 2004b; Burri et al. 2007; Morán Cadenas et al. 2007a). In the present study, both profiles were observed. Variability in tick density (CTD) between years was noticed as previously reported in temporal studies on tick density in Europe (Randolph et al. 2002, 2004; Jouda et al. 2004a; Morán Cadenas et al. 2007a; Perret et al. 2000).
Overall, questing nymph density was higher at PR than at SW, contrasting with the intensity of rodent infestation, that was higher at SW. This might be partly explained by differences in vegetation type between the two study sites. In fact, at SW dense vegetation, brambles, fallen trees and branches were present along the sampling transect, differing from the more homogeneous vegetation found at PR. In the inhomogeneous habitat at SW the number of sub-adult questing ticks could have been underestimated because the flag did not penetrate to the leaf-litter surface or to the lowest vegetation level (Carroll and Schmidtmann 1992; Vassallo et al. 1992), which could have biased our data (Burri et al. 2011). Another possible explanation could be that immature ticks have different questing behavior in different areas, for example, questing closer to the ground as suggested by Kurtenbach and associates (2006). This would lead to an increase of contacts between immature tick stages and rodents, and to a decrease in the success of collection of questing ticks by flagging vegetation as observed in the present study.
At both sites, a discrepancy was observed between the seasonal activity patterns of questing ticks and of ticks infesting rodents. In fact, immature ticks feeding on rodents were frequently detected from February to November at both sites, whereas questing tick populations were drastically decreasing in summer. The seasonal pattern of tick infestations of rodents shows that ticks were in fact active for a much greater part of the year than was indicated by flagging the vegetation. According to these observations, monitoring of questing tick activity by flagging vegetation alone does not appear to be a good indicator for the seasonal risk of tick bites in humans. This is corroborated by recent observations in Switzerland describing a contrast between seasonal questing tick activity and the seasonal pattern of tick bites seen in humans, which mostly occur when questing tick density has declined during the summer (Huegli et al. 2009; Federal Office for Public Health, 2010). It remains to be elucidated whether some questing ticks quest lower on vegetation in summer, but are still picked up by humans during their activity in tick biotopes. This behavior of I. ricinus ticks would be in accord with a study showing that nymphs quest lower on vegetation when desiccation risks are high (Randolph and Storey 1999).
Rodent trapping success was higher at SW (0.30 rodents/trap night) than at PR (0.11 rodents/trap night). The proportion of hosts infested by ticks was similar at both sites, but the infestation load was higher at SW than at PR. On average, more larvae were attached to Apodemus mice than to Myodes voles, as previously described by other authors (Humair et al. 1993; Kurtenbach et al. 1995; Gray et al. 1999; Hanincova et al. 2003a; Michalik et al. 2005). Nevertheless xenodiagnoses demonstrated that M. glareolus was the most infective rodent species at both sites, which corroborates results obtained by Humair and colleagues (1999), although these authors did not screen ticks for Borrelia infection after the molt as we did. This is interesting because it is known that ticks fed on voles do not feed or molt successfully due to acquired resistance to ticks (Dizij and Kurtenbach 1995). Nevertheless, our results and those reported by Kurtenbach and associates (1994) show that when ticks attached to voles are analyzed after their molt, vole contribution to the infection of ticks appears to be higher than mouse contribution.
If we compare our data with those reported by Humair and associates (1993) at SW in the early nineties, the larval infestation loads on rodents recorded between the two periods are similar. Nevertheless, during the present study higher infestation levels by nymphs were observed (0.54±1.65 nymphs/host compared to 0.2±0.8 nymphs/host in the Humair study). Similarly, Borrelia-infected ticks were infesting a higher proportion of rodents in the present study (M. glareolus: 46.4%, A. sylvaticus: 33.3%, and A. flavicollis: 65%), compared to data from the Humair study (M. glareolus: 25% and A. flavicollis: 20%), except for A. sylvaticus (43%). Apparently the situation changed at SW, as rodents harbor more nymphs and they carry more infected ticks than they did two decades ago.
The lower tick infestation rate of rodents trapped at PR compared to SW might be explained by differences in questing tick behavior as mentioned above, and by results from previous studies at PR demonstrating the importance of red squirrels (Sciurus vulgaris) as hosts for immature ticks (Humair and Gern 1998; Morán Cadenas et al. 2007b). This suggests that at PR sub-adult ticks feed more frequently on squirrels than on rodents. All the more so since a higher prevalence of squirrel-associated species, B. burgdorferi ss and B. afzelii, was detected in questing nymphs at PR than at SW. However, additional environmental conditions at both sites that were not investigated in the present study might influence the infestation of rodents by immature tick stages.
Borrelia genospecies
Four Borrelia genospecies were detected in questing ticks (B. afzelii, B. burgdorferi ss, B. garinii, and B. valaisiana): this large diversity of Borrelia genospecies reflects the diversity of hosts (rodents, birds, and medium and large mammals) playing a role at both sites in the tick developmental cycle and in the maintenance of B. burgdorferi sl. However, only two genospecies (B. afzelii and B. bavariensis) were identified in field-derived ticks attached to rodents as well as in xenodiagnostic ticks. This illustrates once again the host specificity displayed by Borrelia species, and confirms that rodents transmit B. afzelii and B. bavariensis to feeding ticks (Hu et al. 1997; Huegli et al. 2002). This contrasts with results obtained by Richter and associates (1999), who observed B. afzelii, B. garinii, and B. burgdorferi ss in ticks that fed on rodents. The most striking feature of these results is the high prevalence of B. garinii in Borrelia-infected xenodiagnostic ticks that fed on field-derived Rattus norvegicus and A. flavicollis (52% and 47%, respectively). It remains to be elucidated whether currently these DNA samples would not be identified as B. bavariensis, formerly B. garinii OspA serotype 4. Interestingly, B. bavariensis was observed only at SW, and this observation substantiates the presence of this Borrelia species at that site (Hu et al. 2001), although B. bavariensis has recently been detected in questing ticks at PR (unpublished data). Our results corroborate the first description of rodents, more specifically Apodemus mice, as reservoirs for B. bavariensis (Huegli et al. 2002). Currently, Apodemus species are the only identified reservoir host for B. bavariensis, a Borrelia species frequently identified in patients with neuroborreliosis.
Co-feeding transmission
In many host-parasite systems, parasites are spatially distributed in clusters on the vertebrate hosts (Shaw et al. 1998). When infected (usually nymphs) and uninfected (usually larvae) ticks feed simultaneously in close proximity, pathogens may directly pass from infected to uninfected ticks, even in the absence of a systemic infection in hosts. This transmission pathway, which was previously described for Thogoto (Jones et al. 1987) and tick-borne encephalitis (TBE) (Alekseev and Chunikhin 1990; Labuda et al. 1992) viruses, has also been reported for LB spirochetes (Gern and Rais 1996; Hu et al. 2003). During our study, a significant difference was observed between the two sites with regard to the proportion of rodents simultaneously infested by larvae and nymphs. In fact, at SW many more of the hosts (28%) were infested with co-feeding larvae and nymphs compared to PR (12%).
Although co-feeding transmission has been described for LB spirochetes in the laboratory (Gern and Rais 1996; Hu et al. 2003), its role in nature is not well known for B. burgdorferi sl. We observed that at PR larvae feeding simultaneously with nymphs on captured rodents were significantly more often infected than larvae feeding without nymphs. In particular, this was the case for Apodemus mice at this site. In contrast, at SW, there was no significant difference when all rodent species were considered. But when each species was examined separately a significant difference was observed for larvae feeding on M. glareolus, when they were co-feeding with nymphs, but not on Apodemus spp. mice. How can we explain this? First, at SW more larvae infested rodents than at PR, and the percentage of hosts infested by nymphs (29%) was significantly higher than at PR (13%). This indicates that at SW, a higher percentage of hosts were probably already systemically infected, if one assumes that after the bite of infected nymphs the rodents, in particular Apodemus mice, remain infectious throughout their lives (Gern et al. 1994). Moreover, it has been reported that the infectivity for ticks of systemically-infected rodents increases with successive infestations by larvae (Gern et al. 1994). Hence, most larvae attached to Apodemus mice at SW were probably acquiring infection from rodents that were already infected, even when they were feeding with nymphs. This may explain why at SW larvae attached to Apodemus mice did not display a difference in infection rate when they were feeding alone or simultaneously with nymphs. The situation was different for M. glareolus at SW or Apodemus mice at PR; their infestation level was lower than for Apodemus at SW. In fact, at PR Apodemus mice had rare contacts with nymphs (globally only 13% of rodents carried nymphs), and at both sites M. glareolus displayed the lowest infestation level, with the lowest mean numbers of larvae and of nymphs. The consequence is that persistent or systemic infections were probably less frequent among these individuals. Larvae that were feeding with nymphs on these rodents had a higher likelihood to feed on hosts that were not systemically infected compared to larvae feeding on Apodemus mice at SW. In this situation the effect of co-feeding transmission on the larval infection rate is more visible without the “background” created by infection due to persistent or systemic infection. This indicates that the perpetuation of B. afzelii depends both on persistent infection in rodents and on co-feeding transmission on hosts, and is modulated by the frequency of contacts between hosts and both larvae and nymphs, which varies among sites.
Here, although more than 28% of rodents at one site were infested by co-feeding larvae and nymphs, no infection by more than one Borrelia species was detected in ticks that fed on these rodents. In fact, only B. afzelii was identified in co-feeding ticks. This is interesting because it has been shown previously in the laboratory that other Borrelia species can be transmitted by co-feeding ticks, like B. burgdorferi ss (Gern and Rais 1996; Hu et al. 2003), B. garinii, and B. valaisiana (Hu et al. 2003). This indicates that conditions in nature create a situation where only B. afzelii is transmitted, probably because of host complement (Kurtenbach et al. 1998). Our observation also suggests that co-feeding transmission on rodents does not contribute to the presence of infections by more than one Borrelia species in questing ticks as we observed here, and does not participate in maintaining Borrelia genospecies diversity. Nevertheless, we recently described the role of co-feeding in maintaining Borrelia diversity at the intra-specific level (Pérez et al. 2011). In fact, we observed that co-feeding transmission contributed in nature to promoting and maintaining B. afzelii OspC genotype diversity within local tick populations.
Since co-feeding transmission of TBE virus (TBEV) is important for the maintenance of TBEV in endemic areas (Randolph et al. 1999), it is interesting to note that SW, the site with the highest proportion of hosts infested by co-feeding ticks, has been known as a TBE-endemic area since 1984 (Wyler and Matile 1984), whereas TBE cases have never been reported at PR. As an interesting side note, a prospective study recently suggested that the proportion of rodents with co-feeding ticks might be one of the factors that distinguish a TBEV focus from a non-TBEV focus (Burri et al. 2011).
Acknowledgements
We would like to thank N. Tonetti and C. Holzer for the identification of B. bavariensis, and Jacqueline Moret for her precious help with the statistical analysis. The results presented here are part of the PhD thesis of one of the authors (D.P.). This work was financially supported by the Swiss National Science Foundation (grant no. 3200-100657). Finally, we would like to thank the two referees for their very useful comments.
Author Disclosure Statement
No competing financial interests exist.
References
- Alekseev AN. Chunikhin SP. Exchange of tickbome encephalitis between Ixodidae simultaneously feeding on animals with subthreshold levels of viraemia. Med Parazitol Parazit Bolezni. 1990;2:48–50. [PubMed] [Google Scholar]
- Bellet-Edimo R. Betschart B. Gern L. Frequency and efficiency of transovarial and subsequent transstadial transmissions of Borrelia burgdorferi in Ixodes ricinus ticks. Bull Soc Neuch Sci Nat. 2005;128:117–125. [Google Scholar]
- Burri C. Bastic V. Maeder G, et al. Microclimate and the zoonotic cycle of tick-borne encephalitis virus in Switzerland. J Med Entomol. 2011;48:615–627. doi: 10.1603/me10180. [DOI] [PubMed] [Google Scholar]
- Burri C. Morán Cadenas F. Douet V, et al. Ixodes ricinus density and infection prevalence of Borrelia burgdorferi sensu lato along a North facing altitudinal gradient in the Rhône Valley (Switzerland) Vector-Borne Zoon Dis. 2007;7:50–58. doi: 10.1089/vbz.2006.0569. [DOI] [PubMed] [Google Scholar]
- Carroll JF. Schmidtmann ET. Tick sweep: modification of the tick drag-flag method for sampling nymphs of the deer tick (Acari: Ixodidae) J Med Entomol. 1992;29:352–355. doi: 10.1093/jmedent/29.2.352. [DOI] [PubMed] [Google Scholar]
- Casjens SR. Fraser-Liggett CM. Mongodin EF, et al. Whole genome sequence of an unusual Borrelia burgdorferi sensu lato isolate. J Bacteriol. 2011;193:1489–1490. doi: 10.1128/JB.01521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizij A. Kurtenbach K. Clethrionomys glareolus, but not Apodemus flavicollis, acquires resistance to Ixodes ricinus L., the main European vector of Borrelia burgdorferi. Paras Immunol. 1995;17:177–183. doi: 10.1111/j.1365-3024.1995.tb00887.x. [DOI] [PubMed] [Google Scholar]
- Eisen RJ. Eisen L. Castro MC, et al. Environmentally related variability in risk of exposure to Lyme disease spirochetes in Northern California: effect of climatic conditions and habitat type. Environm Entomol. 2003;32:1010–1018. [Google Scholar]
- Federal Office for Public Health. La borréliose de Lyme: enquête Sentinella 2008/2009–Le Centre national de référence pour les maladies transmises par les tiques se présente. http://www.bag.admin.ch/dokumentation/publikationen/01435/07914/index.html?lang=fr Bulletin. 2010;22/10:579–582. [Google Scholar]
- Gern L. Douet V. Lopez Z, et al. Diversity of Borrelia genospecies in Ixodes ricinus ticks in a Lyme borreliosis endemic area in Switzerland identified by using new probes for reverse line blotting. Ticks Tick-borne Dis. 2010;1:23–29. doi: 10.1016/j.ttbdis.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Gern L. Rais O. Efficient transmission of Borrelia burgdorferi between cofeeding Ixodes ricinus ticks (Acari: Ixodidae) J Med Entomol. 1996;33:189–192. doi: 10.1093/jmedent/33.1.189. [DOI] [PubMed] [Google Scholar]
- Gern L. Siegenthaler M. Hu CM, et al. Borrelia burgdorferi in rodents (Apodemus flavicollis and A. sylvaticus): duration and enhancement of infectivity for Ixodes ricinus ticks. Eur J Epid. 1994;10:75–80. doi: 10.1007/BF01717456. [DOI] [PubMed] [Google Scholar]
- Gray J. Kirstein F. Robertson J. Borrelia burgdorferi sensu lato in Ixodes ricinus ticks and rodents in a recreational park in south-western Ireland. Exp Appl Acarol. 1999;23:717–729. doi: 10.1023/a:1006233700194. [DOI] [PubMed] [Google Scholar]
- Hanincova K. Schäfer SM. Etti S, et al. Association of Borrelia afzelii with rodents in Europe. Parasitology. 2003a;126:11–20. doi: 10.1017/s0031182002002548. [DOI] [PubMed] [Google Scholar]
- Hanincova K. Taragelova V. Koci J, et al. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl Environm Microbiol. 2003b;69:2825–2830. doi: 10.1128/AEM.69.5.2825-2830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CM. Cheminade Y. Perret JL, et al. Early detection of Borrelia burgdorferi sensu lato infection in Balb/c mice by co-feeding Ixodes ricinus ticks. Int J Med Microbiol. 2003;293:421–426. doi: 10.1078/1438-4221-00285. [DOI] [PubMed] [Google Scholar]
- Hu CM. Humair PF. Wallich R, et al. Apodemus sp rodents, reservoir hosts for Borrelia afzelii in an endemic area in Switzerland. Zentbl Bakt. 1997;185:558–564. doi: 10.1016/s0934-8840(97)80117-x. [DOI] [PubMed] [Google Scholar]
- Hu CM. Wilske B. Lobet Y, et al. Transmission of Borrelia garinii OspA serotype 4 to Balb/c mice by Ixodes ricinus ticks collected in the field. J Clin Microbiol. 2001;39:1169–1171. doi: 10.1128/JCM.39.3.1169-1171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huegli D. Hu CM. Humair PF, et al. Apodemus species mice are reservoir hosts of Borrelia garinii OspA serotype 4 in Switzerland. J Clin Microbiol. 2002;40:4735–4737. doi: 10.1128/JCM.40.12.4735-4737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huegli D. Moret J. Rais O, et al. Tick bites in a Lyme borreliosis highly endemic area in Switzerland. Int J Med Microbiol. 2009;299:155–160. doi: 10.1016/j.ijmm.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Hulinska D. Votypka J. Kriz B, et al. Phenotypic and genotypic analysis of Borrelia spp. isolated from Ixodes ricinus ticks by using electrophoretic chips and real-time polymerase chain reaction. Folia Microbiol. 2007;52:315–324. doi: 10.1007/BF02932085. [DOI] [PubMed] [Google Scholar]
- Humair PF. Gern L. Relationship between Borrelia burgdorferi sensu lato species, red squirrels (Sciurus vulgaris) and Ixodes ricinus in enzootic areas in Switzerland. Acta Trop. 1998;69:213–227. doi: 10.1016/s0001-706x(97)00126-5. [DOI] [PubMed] [Google Scholar]
- Humair PF. Péter O. Wallich R, et al. Strain variation in Borrelia burgdorferi isolated from Ixodes ricinus ticks and rodents collected in the same Swiss localities. J Med Entomol. 1995;32:433–438. doi: 10.1093/jmedent/32.4.433. [DOI] [PubMed] [Google Scholar]
- Humair PF. Postic D. Wallich R, et al. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochete. Zentrblt Bakt Hyg. 1998;287:521–538. [PubMed] [Google Scholar]
- Humair PF. Turrian N. Aeschlimann A, et al. Borrelia burgdorferi in a focus of Lyme borreliosis: The epizootiologic contribution of small mammals. Folia Paras. 1993;40:65–70. [PubMed] [Google Scholar]
- Humair PF. Rais O. Gern L. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and over wintering maintenance. Parasitology. 1999;118:33–42. doi: 10.1017/s0031182098003564. [DOI] [PubMed] [Google Scholar]
- Jones LD. Davies CR. Steele GM, et al. A novel mode of arbovirus transmission involving a nonviremic host. Science. 1987;237:775–777. doi: 10.1126/science.3616608. [DOI] [PubMed] [Google Scholar]
- Jouda F. Perret JL. Gern L. Density of questing Ixodes ricinus nymphs and adults infected by Borrelia burgdorferi sensu lato in Switzerland: spatio-temporal pattern at a regional scale. Vector-Borne Zoonotic Dis. 2004b;4:23–32. doi: 10.1089/153036604773082960. [DOI] [PubMed] [Google Scholar]
- Jouda F. Perret JL. Gern L. Ixodes ricinus density, and distribution and prevalence of Borrelia burgdorferi sensu lato infection along an altitudinal gradient. J Med Entomol. 2004a;41:162–170. doi: 10.1603/0022-2585-41.2.162. [DOI] [PubMed] [Google Scholar]
- Kurtenbach K. Dizij A. Seitz HM, et al. Differential immune responses to Borrelia burgdorferi in European wild rodent species influence spirochete transmission to Ixodes ricinus L. (Acari: Ixodidae) Infect Immunol. 1994;62:5344–5352. doi: 10.1128/iai.62.12.5344-5352.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach K. Hanincová K. Tsao JI, et al. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nature Rev Microbiol. 2006;4:660–669. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- Kurtenbach K. Kampen H. Dizij A, et al. Infestation of rodents with larval Ixodes ricinus (Acari: Ixodidae) is an important factor in the transmission cycle of Borrelia burgdorferi s. l. in German woodlands. J Med Entomol. 1995;32:807–817. doi: 10.1093/jmedent/32.6.807. [DOI] [PubMed] [Google Scholar]
- Kurtenbach K. Sewell HS. Ogden HO, et al. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun. 1998;66:1248–1251. doi: 10.1128/iai.66.3.1248-1251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuda M. Jones LD. Williams T, et al. Efficient transmission of tick-home encephalitis virus between co-feeding ticks. J Med Entomol. 1992;30:295–299. doi: 10.1093/jmedent/30.1.295. [DOI] [PubMed] [Google Scholar]
- Margos G. Vollmer SA. Cornet M, et al. A new Borrelia species defined by multi-locus sequence analysis of housekeeping genes. Appl Environ Microbiol. 2009;75:5410–5416. doi: 10.1128/AEM.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalik J. Skotarczak B. Skoracki M. Wodecka B, et al. Borrelia burgdorferi sensu stricto in yellow-necked mice and feeding Ixodes ricinus ticks in a forest habitat of west central Poland. J Med Entomol. 2005;42:850–856. doi: 10.1093/jmedent/42.5.850. [DOI] [PubMed] [Google Scholar]
- Morán Cadenas F. Rais O. Humair PF, et al. Identification of host bloodmeal source and Borrelia burgdorferi sensu lato in field-collected Ixodes ricinus ticks in Chaumont (Switzerland) J Med Entomol. 2007b;44:1109–1117. doi: 10.1603/0022-2585(2007)44[1109:iohbsa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Morán Cadenas F. Rais O. Jouda F, et al. Phenology of the tick Ixodes ricinus and infection with Borrelia burgdorferi sensu lato along a north- and south-facing altitudinal gradient on Chaumont Mountain, Switzerland. J Med Entomol. 2007a;44:683–693. doi: 10.1603/0022-2585(2007)44[683:poirai]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Olsén B. Jaenson TG. Bergström S. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl Environ Microbiol. 1995;6:3082–3087. doi: 10.1128/aem.61.8.3082-3087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret JL. Guigoz E. Rais O, et al. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland) Parasitol Res. 2000;86:554–557. doi: 10.1007/s004360000209. [DOI] [PubMed] [Google Scholar]
- Perret JL. Rais O. Gern L. Influence of climate on the proportion of Ixodes ricinus nymphs and adults questing in a tick population. J Med Entomol. 2004;41:361–365. doi: 10.1603/0022-2585-41.3.361. [DOI] [PubMed] [Google Scholar]
- Pérez D. Kneubühler Y. Rais O, et al. Borrelia afzelii ospC genotype diversity in Ixodes ricinus questing ticks and ticks from rodents in two Lyme borreliosis endemic areas: contribution of co-feeding ticks. Ticks Tick-Borne Dis. 2011;2:137–142. doi: 10.1016/j.ttbdis.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Piesman J. Gern L. Lyme borreliosis in Europe and North America. Parasitology. 2004;129:191–220. doi: 10.1017/s0031182003004694. [DOI] [PubMed] [Google Scholar]
- Postic D. Assous MV. Grimont PA, et al. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- Randolph SE. Green RM. Hoodless AN, et al. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Intern J Parasitol. 2002;32:979–989. doi: 10.1016/s0020-7519(02)00030-9. [DOI] [PubMed] [Google Scholar]
- Randolph SE. Miklisova D. Lysy J, et al. Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis. Parasitology. 1999;118:177–186. doi: 10.1017/s0031182098003643. [DOI] [PubMed] [Google Scholar]
- Randolph SE. Storey K. Impact of microclimate on immature tick-rodent host interactions (Acari: Ixodidae): Implications for parasite transmission. J Med Entomol. 1999;36:741–748. doi: 10.1093/jmedent/36.6.741. [DOI] [PubMed] [Google Scholar]
- Randolph SE. Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology. 2004;129:S37–S65. doi: 10.1017/s0031182004004925. [DOI] [PubMed] [Google Scholar]
- Rauter C. Hartung T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl Environ Microbiol. 2005;71:7203–7216. doi: 10.1128/AEM.71.11.7203-7216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. Endepols S. Ohlenbusch A, et al. Genospecies diversity of Lyme disease spirochetes in rodent reservoirs. Emerg Inf Dis. 1999;5:291–296. doi: 10.3201/eid0502.990218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. Postic D. Sertour N, et al. Delineation of Borrelia burgdorferi sensu lato species by multilocus sequence analysis and confirmation of the delineation of Borrelia spielmanii sp. nov. Int J Syst Evol Microbiol. 2006;56:873–881. doi: 10.1099/ijs.0.64050-0. [DOI] [PubMed] [Google Scholar]
- Shaw DJ. Grenfell BT. Dobson AP. Patterns of macroparasite aggregation in wildlife host populations. Parasitology. 1998;117:597–610. doi: 10.1017/s0031182098003448. [DOI] [PubMed] [Google Scholar]
- Sinsky RJ. Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J Clin Microbiol. 1989;27:1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo M. Pichon B. Cabaret J, et al. Methodology for sampling questing nymphs of Ixodes ricinus (Acari: Ixodidae), the principal vector of Lyme disease in Europe. J Med Entomol. 1992;29:352–355. doi: 10.1093/jmedent/37.3.335. [DOI] [PubMed] [Google Scholar]
- Wyler R. Matile H. Die Zeckenenzephalitis in der Schweiz. 1. Klinik und Epidemiologie. 2. Diagnose und Immunprophylaxie. Schweiz Rundschau Med. 1984;73:601–619. [PubMed] [Google Scholar]