Abstract
Treatment of skin disorders with short interfering RNA (siRNA)-based therapeutics requires the development of effective delivery methodologies that reach target cells in affected tissues. Successful delivery of functional siRNA to the epidermis requires (1) crossing the stratum corneum, (2) transfer across the keratinocyte membrane, followed by (3) incorporation into the RNA-induced silencing complex. We have previously demonstrated that treatment with microneedle arrays loaded with self-delivery siRNA (sd-siRNA) can achieve inhibition of reporter gene expression in a transgenic mouse model. Furthermore, treatment of human cultured epidermal equivalents with sd-siRNA resulted in inhibition of target gene expression. Here, we demonstrate inhibition of CD44, a gene that is uniformly expressed throughout the epidermis, by sd-siRNA both in vitro (cultured human epidermal skin equivalents) and in vivo (full-thickness human skin equivalents xenografted on immunocompromised mice). Treatment of human skin equivalents with CD44 sd-siRNA markedly decreased CD44 mRNA levels, which led to a reduction of the target protein as confirmed by immunodetection in epidermal equivalent sections with a CD44-specific antibody. Taken together, these results demonstrate that sd-siRNA, delivered by microneedle arrays, can reduce expression of a targeted endogenous gene in a human skin xenograft model.
Lara and colleagues use self-delivery short interfering RNA (sd-siRNA) to inhibit CD44 in both cultured human epidermal skin equivalents and full-thickness human skin equivalents xenografted onto immunocompromised mice. CD44 mRNA and protein levels were markedly decreased.
Introduction
The discovery of RNA interference (RNAi), coupled with the development and synthesis of short interfering RNAs (siRNAs) with minimal off-target and immunostimulatory activities, has resulted in intense efforts to develop this new class of nucleic acid-based therapeutics. siRNAs have entered clinical trials for a number of indications (for reviews see Vaishnaw et al., 2010; Burnett et al., 2011; Chen and Zhaori, 2011), including skin (Leachman et al., 2010). Skin represents an attractive target tissue for siRNA therapeutics because of its accessibility, the availability of rapid outcome measures, and the existence of a large number of dominant genodermatoses as well as skin cancers that could benefit from siRNA-based therapies (Pfutzner and Vogel, 2000; Khavari et al., 2002; Leachman et al., 2008; McLean and Moore, 2011; Ra et al., 2011; Leslie Pedrioli et al., 2012). However, difficulties in delivering the siRNA across the outermost stratum corneum barrier and inefficient cellular uptake have hampered translation to the clinic (Leachman et al., 2010). We have shown that a delivery method composed of dissolvable microneedle arrays loaded with self-delivery siRNA (sd-siRNA) cargo can largely overcome these barriers in a transgenic mouse model (Gonzalez-Gonzalez et al., 2010b) and that sd-siRNA can inhibit mutant gene expression in an organotypic human epidermal model in the absence of transfection reagents such as cationic liposomes (Hickerson et al., 2011a).
The CD44 family contains transmembrane proteins (Screaton et al., 1992) that colocalize with hyaluronan throughout the epidermis (Wang et al., 1992) and bind hyaluronan at the cell surface through a common amino-terminal domain (Aruffo et al., 1990). More than 80% of CD44 in epidermis and cultured keratinocytes is expressed as epican, a heparan/chondroitin sulfate proteoglycan (Zhou et al., 1999) that (1) uses an alternatively spliced CD44 core protein (Kugelman et al., 1992), (2) is specific for stratified squamous epithelia (L.M. Milstone and J. Zhou, unpublished), and (3) is expressed from the basal layer through the granular layer (Haggerty et al., 1992). The epidermis is a dynamic structure composed primarily of keratinocytes at various stages of differentiation with variable gene expression, making analysis of siRNA functional activity in all strata challenging for many if not most genes. The uniform distribution of CD44 within the live layers of the epidermis makes this an attractive target for studying siRNA skin delivery.
In the present study, we demonstrate that a combination of two siRNA delivery technologies results in selective reduction of CD44 gene expression in cultured human epidermal equivalents and in human skin xenografts. Dissolvable microneedle arrays allow direct penetration through the stratum corneum barrier (Gonzalez-Gonzalez et al., 2010b), while self-delivery modifications facilitate keratinocyte uptake (Hickerson et al., 2011a). This combination has been successfully used with engineered reporter genes (Kaspar et al., 2009) and here we demonstrate that this utility may be extended to delivery of siRNA targeting an endogenous human gene in human skin equivalents.
Materials and Methods
siRNAs
So-called “self delivery” (Accell proprietary modifications allow cellular uptake in the absence of traditional transfection reagents). Versions of five siRNAs targeting human CD44 mRNA (Supplementary Fig. S1; supplementary data are available online at www.liebertonline.com/hum) were designed and synthesized by Dharmacon Products/Thermo Fisher Scientific (Lafayette, CO); these siRNAs and this technology are available commercially from this source. The siRNA sense strand (antisense sequence not shown) sequences are as follows: CD44 sd-siRNA-2 and CD44 non-sd-siRNA-2 (referred to as CD44 sd-siRNA and CD44 non-sd-siRNA, respectively, hereafter), 5′-GGCGCAGAUCGAUUUGAAU; CD44 sd-siRNA-14, 5′-CUCUGAGCAUCGGAUUUGA; CD44 sd-siRNA-15, 5′-CCAUUCACCUUUAUGUUAU; CD44 sd-siRNA-16, 5′-CCUUUGAUCAGUAUAAUUU; CD44 sd-siRNA-17, 5′-CUGUUUUAUCAGAGGAGUA. As negative controls, two nonspecific sd-siRNAs were used: Accell nontargeting #1 sd-siRNA (sense strand, 5′-UGGUUUACAUGUCGACUAA; Thermo Fisher Scientific) and K6a_513a.12 Accell sd-siRNA (targets a keratin 6a mutation not present in the skin model systems used; Hickerson et al., 2008).
Mice
Female 6- to 8-week-old severe combined immunodeficient (SCID) hairless mice (SHO; Charles River, Wilmington, MA) were used according to the Guide for the Care and Use of Laboratory Animals (National Research Council) and with strict adherence to a protocol approved by the TransDerm (Santa Cruz, CA) Institutional Animal Care and Use Committee.
Preparation and treatment of epidermal equivalents
Human primary epidermal keratinocyte progenitor (HPEKp) cells (CELLnTEC, Bern, Switzerland) were cultured and used to generate three-dimensional (3D) epidermal equivalents according to the manufacturer's instructions and as previously described (Hickerson et al., 2011a).
Grafting of human skin equivalent onto immune-deficient mice
Full-thickness 3D human skin equivalents (StrataTest) were obtained from a commercial source (Stratatech, Madison, WI) (Schurr et al., 2009). Grafting of 2×2 cm StrataTest skin equivalents was performed as previously described (Gonzalez-Gonzalez et al., 2011) (summarized in Supplementary Fig. S2) under sterile conditions. Mice were anesthetized by intraperitoneal injection of Avertin (2,2,2-tribromoethanol; Sigma-Aldrich, St. Louis, MO). A square region of mouse back skin (dorsal midline, approximately 1.8×1.8 cm) was removed and the StrataTest skin equivalent was placed over the defect, carefully aligning mouse and human skin equivalent edges. After waiting 5 min (to allow adherence of the graft to the mouse), the grafted area was covered with two layers of Vaseline gauze (Kendall, Mansfield, MA) precoated with Bacitracin cream (Perrigo Pharmaceuticals, Allegan, MI), a layer of Tegaderm (3M Health Care, St. Paul, MN), and two adhesive bandages (Derma Sciences, Princeton, NJ), and was finally wrapped with Coban 3M tape (Andover, Salisbury, MA) to hold the dressing in place for 14 days. Treatment with siRNA was initiated no sooner than 1 month after surgery. The human origin of the xenografts was confirmed by immunofluorescence using human-specific desmoglein-3 (DSG3) antibody as described subsequently.
In vivo skin imaging
Mice containing human skin equivalent xenografts were anesthetized with 2% isoflurane gas and evaluated 4 weeks after surgery with an intravital confocal microscope designed for skin imaging (Lucid VivaScope 2500 system; Lucid, Rochester, NY). The microscope uses a 630-nm laser and reflectance imaging as described (Gonzalez-Gonzalez et al., 2011). VivaStack (z-map) images of the border area of a mouse/human skin graft or the center of a human skin graft were obtained in reflectance mode (40 slices with a separation of 1.6 μm between slices). Image files were processed and 3D volumes and video files were later reconstructed using ImageJ 1.43u image-processing software.
Microneedle fabrication
Soluble protrusion array devices (PADs) were prepared as previously described (Gonzalez-Gonzalez et al., 2010b) with slight modifications. Briefly, a template pattern of projecting metal pins (2-mm spacing) was brought into momentary contact with a 350-μm-thick film of 20% polyvinyl alcohol polymer solution on a poly(methyl methacrylate) substrate, gradually withdrawn under uniform airflow to form fiber-like protrusions of elliptical cross-section, and then thoroughly dried. The protrusions were mechanically sheared at a 45-degree angle to normal across the wide axis to form an array of microneedles, each approximately 100 μm wide at the base, tapering to a sharp tip (radius, less than 5 μm). Each microneedle was loaded manually from a micropipet tip containing siRNA solution (200 mg/ml in phosphate-buffered saline [PBS]). Subsequent to loading, PADs were further processed by drying for 8 hr in a 50°C vacuum oven at −18 inHg reduced pressure to increase the rigidity of the polymer microneedles.
In vivo treatment of human skin equivalent xenografts
Two cohorts of mice harboring human skin equivalent xenografts were treated with microneedle arrays loaded with either CD44 or K6a_513a.12 sd-siRNA (all mice were anesthetized with isoflurane during treatment; Gonzalez-Gonzalez et al., 2010b). Microneedles (5×5 microneedles per unit dose array) were coated with 2 μg per needle of either control K6a_513a.12 or CD44 sd-siRNA and applied to xenografts, using a vacuum channel plate apparatus constructed for this purpose by modifying a syringe filter housing (part no. 431224; Corning, Corning, NY; Supplementary Fig. S2G). The two halves of the filter housing were separated and the filter disk was removed, providing a flat disk with integral channels leading to an exhaust port (the outlet of the original syringe filter). The exhaust port was attached to a vacuum line (vacuum pressure station, model 400-3910; Barnant, Barrington, IL) to provide a baseline (unobstructed) flow of 7.5 liters/min through the channel plate.
To apply an individual PAD to a graft, the PAD was positioned on top of the graft, and the vacuum channel plate was brought into contact with the graft area (Supplementary Fig. S2G). Because of reduced pressure at the plate surface, the skin quickly seated against the plate, and therefore also onto the PAD needles. The plate was left in place for an additional 1 min to accommodate elastic recovery of the skin to ensure that microneedles were fully embedded. Each inserted PAD was left in place for an additional 15 min after removal of the vacuum plate to allow sufficient time for the soluble needles to fully hydrate (Supplementary Fig. S2H). Postinsertion inspection of the array was performed to confirm penetration and microneedle tip deposition. Approximately 10 nl of a 200-ng/nl siRNA solution (2 μg) was loaded per microneedle tip (50 μg of siRNA per 5×5 microneedle array, with three arrays applied per day, to administer 150 μg of siRNA per mouse per day).
Delivery of microneedle siRNA payload
Postapplication inspection of the microneedle array found that typically 15 to 20 needles (of the 25-needle array) per application showed significant erosion consistent with penetration, hydration, and subsequent deposition of the needle tip to form a depot in subsurface skin. Observed erosion ranged from 30 to 50% of the needle length missing from the tip end. Approximating the loaded surface of needles as a cone before application, an average erosion of 40% of the length implies delivery of roughly 15% of the surface loading, and with approximately 70% of the needles penetrating, delivery is estimated as 10% of the loaded dose, or 15 μg of siRNA per mouse per day over 10 days, for a total dose of 150 μg/subject.
Histology and immunohistochemistry
Deidentified human skin from abdominoplasty surgery (processed within 2 hr of the procedure) or xenografts from sacrificed mice were embedded in O.C.T. medium (Tissue-Tek, Torrance, CA), frozen and sectioned (10 μm), stained with hematoxylin and eosin (H&E; Sigma-Aldrich), and mounted with Histomount (National Diagnostics, Atlanta, GA) according to standard procedures.
For protein expression analysis, immunofluorescence detection was achieved with antibodies specific for keratin-5 (AE14; Santa Cruz Biotechnology, Santa Cruz, CA), keratin-10 (mouse monoclonal Ab-2, clone DE-K10; Lab Vision, Fremont, CA), DSG3 antibody (clone 5G11; Invitrogen, Carlsbad, CA), or CD44 (rabbit polyclonal ab41478; Abcam, San Francisco, CA). This CD44 antibody was generated from immunogen sequence DHTKQNQDWTQWNPSHSN (Abcam, personal communication), located within exon 8 of CD44 isoform 1 (NCBI Reference Sequence NM_000610.3; www.ncbi.nlm.gov). This epitope is present in CD44 epican, but not in CD44H or CD44E (see Supplementary Fig. S3). Skin sections from O.C.T. blocks were fixed in acetone at −20°C for 15 min; dried; blocked with 10% heat-inactivated goat serum, 1% bovine serum albumin (BSA), 0.025% Triton X-100 in PBS for 1 hr at 21°C; and incubated overnight in a 1:500 dilution of the primary antibody in the same solution used for blocking. Slides were rinsed in PBS containing 0.025% Triton X-100 and incubated for at least 1 hr with either Alexa Fluor 546-conjugated goat anti-rabbit or Alexa Fluor 488-conjugated goat anti-mouse secondary antibody in the same solution used for blocking (1:2000 dilution; Invitrogen). The slides were rinsed with PBS containing 0.025% Triton X-100 and mounted with Hydromount containing 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/ml) to allow visualization of nuclei. Sections were imaged with a Zeiss Axio Observer inverted fluorescence microscope equipped with fluorescein isothiocyanate (FITC), cyanine-3 (Cy3), and DAPI filter sets.
RNA isolation and RT-qPCR
Epidermal equivalents or Stratatech xenografts were homogenized in a FastPrep instrument (FastPrep-24, FP24; MP Biomedicals, Solon, OH) and the RNA was isolated, reverse-transcribed, amplified, and quantitated as previously described (Hickerson et al., 2011b). Target gene inhibition was measured using TaqMan gene expression assays specific for CD44 (Hs00153304_m1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Hs99999901_m1) (Applied Biosystems, Foster City, CA) with GAPDH serving as the reference gene. The CD44 amplicon spans the 17a/19 exon border, which is present in all CD44 isoforms (Tissue-Tek, Torrance, CA). Relative quantitation and statistics are reported as the mean of three replicate assays calculated by the ΔΔCT method and 7500 FAST sequence detection software (version 1.4; Applied Biosystems). Relative quantitation between mouse cohorts was calculated with the same software.
Results
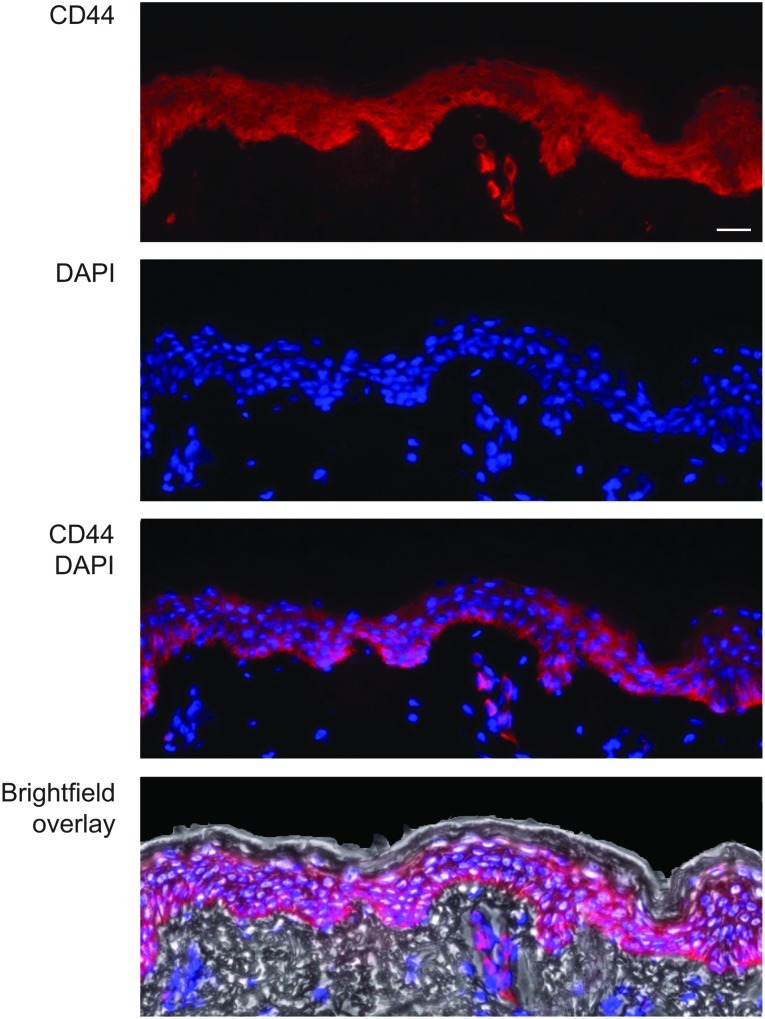
To confirm that CD44 expression is uniform throughout the epidermis and easily detected, skin sections were prepared from freshly obtained human abdominoplasty skin and analyzed by immunohistochemistry with an antibody that detects CD44 epican (see Materials and Methods). Figure 1 shows strong and uniform CD44 expression through the live strata with little or no detection in the stratum corneum or dermal skin compartments, confirming that CD44 may be an appropriate target gene for studying functional siRNA delivery to live epidermal strata. CD44 is similarly expressed in human epidermal skin equivalents (in vitro) and human skin xenografts (in vivo) (compare Fig. 1, Fig. 2C, and Supplementary Fig. 4A), suggesting these skin models may be useful intermediates to study functional CD44 siRNA delivery.
FIG. 1.
CD44 is uniformly distributed throughout the live epidermal strata of human skin. Frozen skin sections were prepared from freshly obtained abdominal skin and reacted with anti-CD44 antibody (red; see Materials and Methods). Blue: DAPI-stained nuclei. Scale bar: 50 μm.
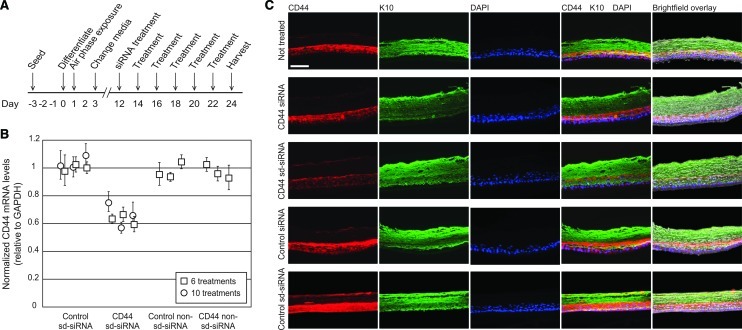
FIG. 2.
Treatment of human epidermal equivalents with self-delivery CD44 siRNA (sd-siRNA) results in CD44 target gene inhibition. (A) Schematic depicting experimental timeline. Epidermal equivalents prepared from primary human keratinocytes were treated 6 times (squares) or 10 times (circles) with 2 μM CD44 sd-siRNA. One day after the final treatment, the equivalents were harvested and subjected to (B) RT-qPCR analysis to determine relative mRNA levels or (C) immunofluorescence to visualize protein expression. The mRNA levels reported are relative to GAPDH (reference gene). The standard errors represent qPCR triplicates of each sample. CD44 protein levels were determined with antibodies specific to CD44 (red) or keratin-10 (green). Scale bar: 50 μm.
In vitro inhibition of CD44 gene expression in human epidermal equivalents using self-delivery siRNA
Five independent sd-siRNAs that target CD44 gene expression were prepared and comparatively evaluated in human HaCaT keratinocytes for their ability to inhibit CD44 expression in the absence of transfection reagents (Supplementary Fig. S1). CD44 sd-siRNA-2 (CD44 sd-siRNA) was chosen, based on its in vitro activity, for further investigation in the skin equivalent models. The target site for CD44 sd-siRNA spans the exon 1/exon 2 border (see Supplementary Fig. S3) of the CD44 isoform 1 coding region (NCBI Reference Sequence NM_000610.3; www.ncbi.nlm.gov). Both exon 1 and exon 2 are present in all CD44 isoforms (Naor et al., 1997); thus, this siRNA should target all CD44 mRNAs.
Epidermal equivalents were treated every other day with CD44 sd-siRNA over a span of 12 days, with treatments commencing on the twelfth day after initiation of keratinocyte stratification (Fig. 2A). The epidermal equivalents were harvested 24 hr after the last treatment; half of each epidermal equivalent was collected and processed for RNA analysis (RT-qPCR) and the other half for detection of CD44 via immunohistochemistry. Untreated epidermal equivalents, or those treated with nonspecific (K6a_513a.12) or non-self delivery (CD44) siRNAs, served as negative controls. CD44 sd-siRNA treatment of epidermal equivalents resulted in reduction of CD44 mRNA levels by 37±7% (p<0.001) compared with controls treated with nonspecific sd-siRNA (Fig. 2B) and 45±7% when compared with untreated controls. Levels of CD44 mRNA in tissues treated with nonspecific control sd-siRNA samples were not different from those in untreated samples (p>0.05). Furthermore, no statistically significant differences in keratin-10 mRNA levels (nontargeted mRNA) were observed with the various treatments analyzed (data not shown). Similar results were obtained when the skin equivalents were treated 10 times, every other day over a 20-day period (36±9% reduction compared with controls similarly treated with nonspecific sd-siRNA; Fig. 2B).
After siRNA treatment, representative samples of human epidermal equivalents from each treatment group were cryosectioned and analyzed for CD44 protein expression. Consistent with the RT-qPCR results, epidermal equivalents treated with CD44 sd-siRNA exhibited decreased CD44 immunofluorescence after 6 and 10 treatments. Representative images are shown in Fig. 2C for the epidermal equivalents receiving six treatments. Reductions in CD44 protein levels were not observed in epidermal equivalents treated with control sd-siRNAs and were similar to untreated tissue sections (Fig. 2C). Importantly, siRNA treatment did not noticeably alter keratin-10 (K10), K5, or K6 expression patterns (see Fig. 2C and Supplementary Fig. S4 for representative images), which localized as expected to the basal (K5) or suprabasal (K10 and K6) layers.
In vivo inhibition of CD44 in human skin equivalent xenografts
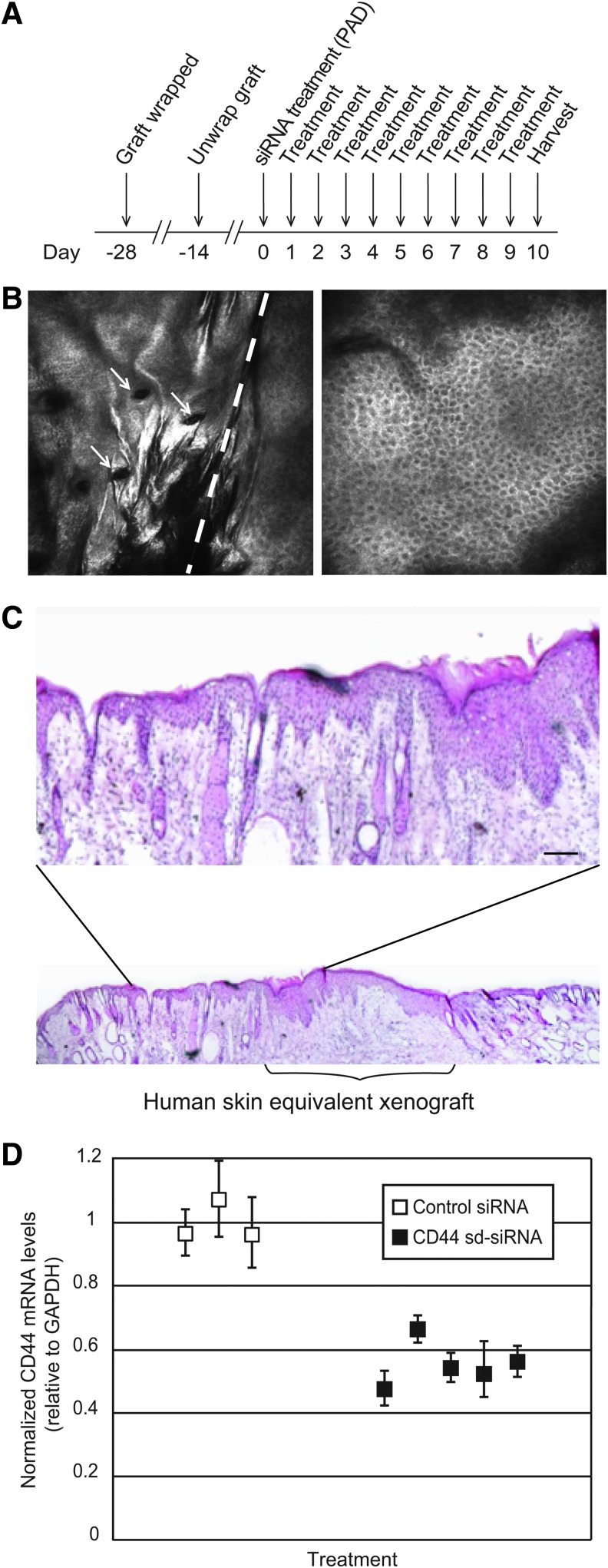
To analyze the ability of sd-siRNA to target an epidermal endogenous gene in vivo, full-thickness 3D human skin equivalents were grafted on immunocompromised mice and treated with sd-siRNA delivered with microneedle arrays as outlined in Fig. 3A. Four weeks after the xenograft procedure (before siRNA treatment), the nature and quality of the human skin equivalent grafts were assessed by intravital imaging (Fig. 3B and Supplementary Videos S1 and S2). The reflectance images revealed well-defined cellular structure at various depths for both human skin equivalent xenografts and mouse skin; these images were consistent with healthy human or mouse skin morphology (Gonzalez and Gilaberte-Calzada, 2008; Nehal et al., 2008; Gonzalez-Gonzalez et al., 2011). Figure 3B (left) shows the border of the recipient mouse skin (left of dashed line) with the xenograft (right of dashed line). Note the uniformity of cells and the absence of hair follicles in the xenograft (compare with Fig. 3B, right, showing human skin).
FIG. 3.
Inhibition of CD44 gene expression in human skin xenografts by CD44 sd-siRNA administered by microneedle arrays. (A) Schematic depicting experimental design. (B) In vivo images (see Materials and Methods) from full z-stacks (see Supplementary Videos S1 and S2 for the complete data set) of a human skin equivalent xenograft 4 weeks after grafting. Left: Mouse/human equivalent skin (left/right) border at a depth of 27 μm. Arrows indicate hair follicles present only in mouse skin. Right: Central image in human skin equivalent at 35-μm depth. Scale bar: 100 μm. (C) Hematoxylin–eosin (H&E) staining of a frozen section prepared from a human skin equivalent grafted on an immunocompromised mouse, 38 days postgrafting. Magnification shows mouse/human skin border. Scale bar: 50 μm. (D) CD44 siRNA-mediated inhibition of CD44 mRNA expression after siRNA administration by protrusion array devices (PADs). Human skin equivalents, grafted on immunocompromised mice, were treated with CD44 sd-siRNA or nonspecific control K6a_513a.12 sd-siRNA daily for 10 days by PAD administration. One day after the final treatment, the xenografts were harvested and subjected to RT-qPCR analysis to determine relative mRNA levels, using GAPDH as the reference gene. The standard errors represent qPCR triplicates of each sample.
The human skin equivalent grafts were treated with sd-siRNA-loaded PADs (three PADs per day) for 10 consecutive days (Fig. 3A). Daily treatment consisted of application of three 5×5 microneedle arrays loaded with CD44 (n=5 mice) or nonspecific control K6a_513a.12 (n=3) sd-siRNA. As an additional negative control, a mouse cohort (n=3) was left untreated. A final mouse cohort (n=2) was treated by intradermal injection of CD44 sd-siRNA (50 μg in 20 μl of PBS) every other day (total of five injections) and used as a positive control for delivery across the stratum corneum barrier. One day (24 hr) after the last treatment, the mice were killed and half of each skin graft was collected and processed for RT-qPCR and the other half was used for immunofluorescence (Supplementary Fig. S4) and H&E staining (Fig. 3C). Xenografts treated with CD44 sd-siRNA PADs showed a 45±6% reduction in CD44 mRNA levels compared with nonspecific sd-siRNA (Fig. 3D). A 52±6% reduction in CD44 mRNA levels in CD44 sd-siRNA-treated skin was observed compared with nontreated skin equivalent xenografts. Conventional intradermal injection of CD44 sd-siRNA resulted in similar levels of reduction (41% after five injections) compared with nonspecific siRNA-treated control or untreated grafts. It should be noted that an additional nonspecific control sd-siRNA (Accell nontargeting #1 siRNA) similarly had no significant effect on CD44 mRNA levels when administered by microneedles (data not shown).
To further ensure the quality and human origin of the xenografts, untreated mice with grafts were killed, skin sections from the xenografts were prepared, and protein expression was analyzed using a human-specific desmoglein-3 (DSG3) antibody (Supplementary Fig. S4B, compare with Supplementary Fig. S5A). Immunodetection with K5 and K10 antibodies (both cross-react with human and mouse proteins) were used to verify proper stratification (Supplementary Fig. S4C and D). As expected, the human xenograft, but not the adjacent mouse skin, stained positive for DSG3 in all cases. Furthermore, as expected, K5 localized mainly to the basal layer, while K6 and K10 (see representative images in Supplementary Fig. S4C and D; compare with Supplementary Fig. S5B and C) were expressed in the suprabasal layers in all xenografts. No gross changes in skin architecture were observed by H&E staining of frozen skin sections prepared from microneedle-treated xenografts as compared with controls nor when evaluated with the Lucid intravital imaging system (see Fig. 3C for a representative image).
Discussion
The ability to harness the RNAi pathway through synthetic siRNAs that directly enter the RNA-induced silencing complex (RISC) presents opportunities to develop novel therapeutics by targeting previously unassailable disease-causing genes including those affecting skin (Leachman et al., 2008, 2010; Geusens et al., 2009; McLean and Moore, 2011). Dominant negative genodermatoses or skin cancers resulting from overexpression of mutated or wild-type genes are particularly amenable to this approach. As a primary protective layer of the body, the stratum corneum barrier represents a formidable obstacle to entry of large and/or charged molecules such as siRNAs. Various approaches have been used to overcome this barrier including direct injection with hypodermic needles, topical formulations, and microneedle arrays (Prausnitz et al., 2004; Coulman et al., 2006; Kaspar et al., 2009, 2011; Shim and Kwon, 2010). Once the siRNA is delivered across the stratum corneum barrier, however, additional strategies are needed to facilitate cellular uptake, such as so-called “self-delivery” modifications, including the Accell technology used in this and previous studies (Gonzalez-Gonzalez et al., 2010b; Hickerson et al., 2011a).
Despite the success of earlier mouse and human epidermal model studies, no inhibition of an endogenous gene has been demonstrated in full-thickness human skin. To achieve this goal, we sought a suitable target, one that would be uniformly expressed at relatively high levels in the epidermis to allow efficient detection of functional siRNA activity. This is a nontrivial aspect of the study, because most genes are either nonuniformly expressed in the epidermal strata (following a differentiation program) or are transiently expressed as in the case of the inducible keratins K6, K16, and K17. As shown herein, CD44 meets the criteria for being a desirable target as it is uniformly expressed throughout the live layers of the epidermis under all conditions analyzed (Figs. 1 and 2C, and Supplementary Fig. S4A; see also Haggerty et al., 1992; Wang et al., 1992; Yasaka et al., 1995).
The present study demonstrates that sd-siRNA, added directly to keratinocytes in cultured epidermal equivalents or delivered by dissolvable microneedle arrays to keratinocytes in full-thickness human skin xenografts, can inhibit target CD44 gene expression without the need of traditional transfection reagents. The results obtained in human epidermal equivalents demonstrate inhibition of target gene expression (see Fig. 2), corroborating previously published results using the same epidermal organotypic model in which an sd-siRNA (Accell) selectively targeted mutant keratin-6a mRNA (responsible for the rare genodermatosis pachyonychia congenita [PC]) in the absence of transfection reagents (Hickerson et al., 2011a). The ability of self-delivery (Accell) modifications to facilitate siRNA uptake by keratinocytes in epidermal equivalents enabled the use of a microneedle array device to deliver siRNA to human skin equivalent xenografts, extending previous work demonstrating the ability of microneedle arrays to deliver functional self-delivery siRNA that blocks expression of reporter genes in transgenic mice (Gonzalez-Gonzalez et al., 2010b).
The CD44 inhibition (45%) observed after siRNA treatment of skin xenografts is consistent with the reduction in gene expression observed in other skin systems using intradermal injection, topical administration, or microneedle arrays (Gonzalez-Gonzalez et al., 2009, 2010b; Hsu and Mitragotri, 2011). The reason that more inhibition is not achieved is not clear, but similar results have been observed in nonskin tissues including eye (Huang et al., 2011), brain (Kuwahara et al., 2011), and heart (Guido et al., 2011), while higher levels of inhibition (up to 95%) have been reported in liver (Zimmermann et al., 2006; Frank-Kamenetsky et al., 2008; Tadin-Strapps et al., 2011). Although incomplete inhibition of gene expression was observed after treatment with sd-CD44 siRNA, complete silencing of a gene target may not be necessary to achieve a therapeutic effect. For example, in an inducible keratin-14 transgenic mouse model for the dominant disorder epidermolysis bullosa simplex (EBS), low-level expression of the mutant keratin-14 allele (1:2, mutant to wild type) produced no epidermal fragility phenotype (Cao et al., 2001). These observations suggest that strategies resulting in partial inhibition of the targeted gene may result in a therapeutic benefit for patients suffering from dominant keratin disorders (Chen and Roop, 2005). The intent of this study, therefore, was not to show a functional effect, but rather to demonstrate that sd-siRNA delivery via microneedle arrays can efficiently inhibit an endogenous gene that is uniformly distributed throughout the epidermal strata. Many, if not most, genes (including the genes involved in PC—KRT6a/b, KRT16, and KRT17) have altered epidermal expression patterns as keratinocytes undergo differentiation. In our preliminary experiments, as well as published studies, epidermal CD44 appeared to be quite uniformly expressed, making this the most appropriate epidermal target we could identify that is readily detected at both the protein and mRNA levels. The downside to the use of CD44 is that it is a large family of genes that may compensate for one another, masking potential phenotypic changes.
siRNA therapeutics have the potential to transform treatment of many skin disorders if delivery obstacles can be overcome. The present work demonstrates that administration of dissolvable microneedle arrays, loaded with sd-siRNA cargo, can inhibit expression of an endogenous gene in human skin models. The amount of sd-siRNA needed for microneedle administration in a clinical setting is potentially less than what was required for the initial phase 1b PC clinical trial (Leachman et al., 2010). In that study, no therapeutic effects were observed until doses of up to 17 mg of unmodified siRNA were delivered by intralesional injection (hypodermic needle). In contrast, only 150 μg (100-fold less) of sd-siRNA (delivered by the dissolvable microneedle arrays) would be required to treat a surface area of a size equivalent to the area (∼10 cm2) estimated to be reached by hypodermic needle injection. The successful translation of this delivery platform to the clinic will enable development of therapeutics for many heretofore untreatable skin disorders, particularly dominant genodermatoses, potentially transforming the way patients are treated. On the basis of this and other ongoing studies, microneedle array-mediated delivery of siRNA appears to be a viable and patient-friendly (i.e., involving little or no pain Gill et al., 2008) alternative to siRNA delivery by intradermal hypodermic needle injections, offering a more patient-acceptable path forward for future clinical applications.
Supplementary Material
Acknowledgments
The authors sincerely thank Dr. Christine Collin-Djangone (L'Oreal) for insightful discussions, suggesting the use of CD44 as a model endogenous target and identifying the CD44 target site sequence used for the siRNA experiments described herein. The authors thank Xiaomin Bao (Paul Khavari's laboratory, Stanford University) for providing the DSG3 human-specific antibody. The authors also thank Stella Chang, Manny Flores, and Andrea Burgon for technical support and the participants of the GO Delivery! Project for input and support. This project was supported by NIAMS/NIH grants RC2AR058955-02 (R.L.K., L.M.M., and C.H.C.) and R43AR059474 (R.L.K.).
Author Disclosure Statement
Roger Kaspar, Robyn Hickerson, Tycho Speaker, and Maria Fernanda Lara are employees of TransDerm, which has a patent pending for use of microneedle arrays to deliver nucleic acids. Devin Leake is an employee of Thermo Fisher Scientific.
References
- Aruffo A. Stamenkovic I. Melnick M., et al. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Burnett J.C. Rossi J.J. Tiemann K. Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol. J. 2011;6:1130–1146. doi: 10.1002/biot.201100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao T. Longley M.A. Wang X.J. Roop D.R. An inducible mouse model for epidermolysis bullosa simplex: implications for gene therapy. J. Cell Biol. 2001;152:651–656. doi: 10.1083/jcb.152.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Roop D.R. Mouse models in preclinical studies for pachyonychia congenita. J. Investig. Dermatol. Symp. Proc. 2005;10:37–46. doi: 10.1111/j.1087-0024.2005.10206.x. [DOI] [PubMed] [Google Scholar]
- Chen S.H. Zhaori G. Potential clinical applications of siRNA technique: benefits and limitations. Eur. J. Clin. Invest. 2011;41:221–232. doi: 10.1111/j.1365-2362.2010.02400.x. [DOI] [PubMed] [Google Scholar]
- Coulman S. Allender C. Birchall J. Microneedles and other physical methods for overcoming the stratum corneum barrier for cutaneous gene therapy. Crit. Rev. Ther. Drug Carrier Syst. 2006;23:205–258. doi: 10.1615/critrevtherdrugcarriersyst.v23.i3.20. [DOI] [PubMed] [Google Scholar]
- Frank-Kamenetsky M. Grefhorst A. Anderson N.N., et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geusens B. Sanders N. Prow T., et al. Cutaneous short-interfering RNA therapy. Expert Opin. Drug Deliv. 2009;6:1333–1349. doi: 10.1517/17425240903304032. [DOI] [PubMed] [Google Scholar]
- Gill H.S. Denson D.D. Burris B.A. Prausnitz M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S. Gilaberte-Calzada Y. In vivo reflectance-mode confocal microscopy in clinical dermatology and cosmetology. Int. J. Cosmet. Sci. 2008;30:1–17. doi: 10.1111/j.1468-2494.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez E. Ra H. Hickerson R.P., et al. siRNA silencing of keratinocyte-specific GFP expression in a transgenic mouse skin model. Gene Ther. 2009;16:963–972. doi: 10.1038/gt.2009.62. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez E. Speaker T.J. Hickerson R.P., et al. Silencing of reporter gene expression in skin using siRNAs and expression of plasmid DNA delivered by a soluble protrusion array device (PAD) Mol. Ther. 2010b;18:1667–1674. doi: 10.1038/mt.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez E. Kim Y.C. Speaker T.J., et al. Visualization of plasmid delivery to keratinocytes in mouse and human epidermis. Sci. Rep. 2011;1:158. doi: 10.1038/srep00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido M.C. Clemente C.F. Moretti A.I., et al. Small interfering RNA targeting focal adhesion kinase prevents cardiac dysfunction in endotoxemia. Shock. 2011;37:77–84. doi: 10.1097/SHK.0b013e31823532ec. [DOI] [PubMed] [Google Scholar]
- Haggerty J.G. Bretton R.H. Milstone L.M. Identification and characterization of a cell surface proteoglycan on keratinocytes. J. Invest. Dermatol. 1992;99:374–380. doi: 10.1111/1523-1747.ep12616087. [DOI] [PubMed] [Google Scholar]
- Hickerson R.P. Smith F.J. Reeves R.E., et al. Single-nucleotide specific siRNA targeting in a dominant-negative skin model. J. Invest. Dermatol. 2008;128:594–605. doi: 10.1038/sj.jid.5701060. [DOI] [PubMed] [Google Scholar]
- Hickerson R.P. Flores M.A. Leake D., et al. Use of self-delivery siRNAs to inhibit gene expression in an organotypic pachyonychia congenita model. J. Invest. Dermatol. 2011a;131:1037–1044. doi: 10.1038/jid.2010.426. [DOI] [PubMed] [Google Scholar]
- Hickerson R.P. Leachman S.A. Pho L.N., et al. Development of quantitative molecular clinical end points for siRNA clinical trials. J. Invest. Dermatol. 2011b;131:1029–1036. doi: 10.1038/jid.2010.372. [DOI] [PubMed] [Google Scholar]
- Hsu T. Mitragotri S. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. Proc. Natl. Acad. Sci. U.S.A. 2011;108:15816–15821. doi: 10.1073/pnas.1016152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.R. Fan X.X. Tang X. SiRNA targeting EGFR effectively prevents posterior capsular opacification after cataract surgery. Mol. Vis. 2011;17:2349–2355. [PMC free article] [PubMed] [Google Scholar]
- Kaspar R.L. McLean W.H. Schwartz M.E. Achieving successful delivery of nucleic acids to skin: 6th Annual Meeting of the International Pachyonychia Congenita Consortium. J. Invest. Dermatol. 2009;129:2085–2087. doi: 10.1038/jid.2009.220. [DOI] [PubMed] [Google Scholar]
- Kaspar R.L. Leachman S.A. McLean W.H. Schwartz M.E. Toward a treatment for pachyonychia congenita: Report on the 7th Annual International Pachyonychia Congenita Consortium meeting. J. Invest. Dermatol. 2011;131:1011–1014. doi: 10.1038/jid.2011.44. [DOI] [PubMed] [Google Scholar]
- Khavari P.A. Rollman O. Vahlquist A. Cutaneous gene transfer for skin and systemic diseases. J. Intern. Med. 2002;252:1–10. doi: 10.1046/j.1365-2796.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- Kugelman L.C. Ganguly S. Haggerty J.G., et al. The core protein of epican, a heparan sulfate proteoglycan on keratinocytes, is an alternative form of CD44. J. Invest. Dermatol. 1992;99:886–891. doi: 10.1111/1523-1747.ep12614896. [DOI] [PubMed] [Google Scholar]
- Kuwahara H. Nishina K. Yoshida K., et al. Efficient in vivo delivery of siRNA into brain capillary endothelial cells along with endogenous lipoprotein. Mol. Ther. 2011;19:2213–2221. doi: 10.1038/mt.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leachman S.A. Hickerson R.P. Hull P.R., et al. Therapeutic siRNAs for dominant genetic skin disorders including pachyonychia congenita. J. Dermatol. Sci. 2008;51:151–157. doi: 10.1016/j.jdermsci.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leachman S.A. Hickerson R.P. Schwartz M.E., et al. First-in-human mutation-targeted siRNA phase Ib trial of an inherited skin disorder. Mol. Ther. 2010;18:442–446. doi: 10.1038/mt.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie Pedrioli D.M. Fu D.J. Gonzalez-Gonzalez E., et al. Generic and personalized RNAi therapeutics for a dominant-negative epidermal fragility disorder. J. Invest. Dermatol. 2012 doi: 10.1038/jid.2012.28. (in press). [DOI] [PubMed] [Google Scholar]
- McLean W.H. Moore C.B. Keratin disorders: From gene to therapy. Hum. Mol. Genet. 2011;20:R189–R197. doi: 10.1093/hmg/ddr379. [DOI] [PubMed] [Google Scholar]
- Naor D. Sionov R.V. Ish-Shalom D. CD44: Structure, function, and association with the malignant process. Adv. Cancer Res. 1997;71:241–319. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- Nehal K.S. Gareau D. Rajadhyaksha M. Skin imaging with reflectance confocal microscopy. Semin. Cutan. Med. Surg. 2008;27:37–43. doi: 10.1016/j.sder.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Pfutzner W. Vogel J.C. Advances in skin gene therapy. Expert Opin. Investig. Drugs. 2000;9:2069–2083. doi: 10.1517/13543784.9.9.2069. [DOI] [PubMed] [Google Scholar]
- Prausnit M.R. Mitragotri S. Langer R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004;3:115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- Ra H. Piyawattanametha W. Gonzalez-Gonzalez E., et al. In vivo imaging of human and mouse skin with a handheld dual-axis confocal fluorescence microscope. J. Invest. Dermatol. 2011;131:1061–1066. doi: 10.1038/jid.2010.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr M.J. Foster K.N. Centanni J.M., et al. Phase I/II clinical evaluation of StrataGraft: A consistent, pathogen-free human skin substitute. J. Trauma. 2009;66:866–873. doi: 10.1097/TA.0b013e31819849d6. discussion 873–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screaton G.R. Bell M.V. Jackson D.G., et al. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc. Natl. Acad. Sci. U.S.A. 1992;89:12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim M.S. Kwon Y.J. Efficient and targeted delivery of siRNA in vivo. FEBS J. 2010;277:4814–4827. doi: 10.1111/j.1742-4658.2010.07904.x. [DOI] [PubMed] [Google Scholar]
- Tadin-Strapps M. Peterson L.B. Cumiskey A.M., et al. siRNA-induced liver ApoB knockdown lowers serum LDL-cholesterol in a mouse model with human-like serum lipids. J. Lipid Res. 2011;52:1084–1097. doi: 10.1194/jlr.M012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnaw A.K. Gollob J. Gamba-Vitalo C., et al. A status report on RNAi therapeutics. Silence. 2010;1:14. doi: 10.1186/1758-907X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Tammi M. Tammi R. Distribution of hyaluronan and its CD44 receptor in the epithelia of human skin appendages. Histochemistry. 1992;98:105–112. doi: 10.1007/BF00717001. [DOI] [PubMed] [Google Scholar]
- Yasaka N. Furue M. Tamaki K. CD44 expression in normal human skin and skin tumors. J. Dermatol. 1995;22:88–94. doi: 10.1111/j.1346-8138.1995.tb03349.x. [DOI] [PubMed] [Google Scholar]
- Zhou J. Haggerty J.G. Milstone L.M. Growth and differentiation regulate CD44 expression on human keratinocytes. In Vitro Cell Dev. Biol. Anim. 1999;35:228–235. doi: 10.1007/s11626-999-0031-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann T.S. Lee A.C. Akinc A., et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.