Abstract
Near-infrared (NIR) fluorescence cancer imaging is a growing field for both preclinical and clinical application to the clinical management for cancer patients due to its advantageous features, including a high spatial resolution, portability, real-time display and detailed molecular profiling with the multiplexed use of fluorescent probes. In this review, we present a basic concept of NIR fluorescence imaging and overview its potential clinical applications for in vivo cancer imaging, including cancer detection/characterization, lymphatic imaging (sentinel lymph node detection) and surgical/endoscopic guidance. NIR fluorescence imaging can compensate some limitations of conventional imaging modalities, and thus it could play an important role for cancer imaging combined with other modalities in clinical practice.
Keywords: activatable probe, cancer, fluorescence imaging, lymphatic imaging, near infrared
Imaging has become an integral part of clinical oncology, and its role has been growing over the past 10 years, and now includes imaging for cancer detection (e.g., screening, staging and so on), characterization (e.g., vascularity, receptor expression and so on), surgical guidance and therapy response assessment. There is a long history of using imaging in cancer, beginning with simple x-ray radiography, but now the applications and modalities have greatly expanded so that modalities such as ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI) and nuclear imaging each play a major and unique role [1]. Since each modality has advantages and disadvantages, the combined use of different modalities has become a standard of practice. Fluorescence imaging also has a long history of clinical use, but not specifically in cancer. Dyes such as fluorescein or indocyanine green (ICG) are widely used for chorioretinal fluorescence angiography [2,3]. However, the picture is changing. Fluorescence-based mammography, which utilizes the normal spectral pattern of light in tissue, so-called diffuse optical mammography, is being investigated in clinical trials that are currently under way [4–10]. A number of other potential clinical applications of fluorescence imaging with animal and human subjects have been proposed using the near-infrared (NIR) part of the spectrum, based on the better tissue penetration and lower autofluorescence at these wavelengths. In this review, we focus on current clinical and future applications of NIR fluorescence imaging in cancer. Although nonenhanced NIR imaging has been intensively evaluated for breast cancer detection [4–10], here we will focus on efforts to design injectable NIR agents and their potential clinical applications.
Near-infrared fluorescence imaging
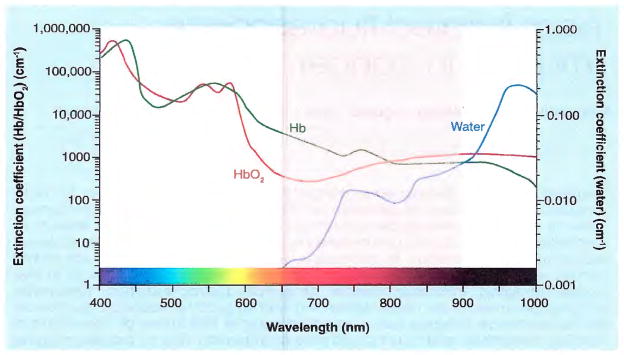
Fluorescence is the property of certain molecules to absorb light at one wavelength and to emit light at a longer wavelength [11]. Policard first reported in 1924 that the necrotic center of an experimental rat sarcoma showed a red fluorescence from endogenous porphyrins, when excited by ultraviolet light [12]. From this starting point, many new applications have emerged with different varieties of fluorophores and fluorescence proteins [13]. Light in the NIR range (wavelength: 650–900 nm) has several advantages over visible-range light, including deeper tissue penetration due to less absorption by hemoglobin and water (Figure 1), and less autofluorescence from surrounding tissues (low background). Since deep-tissue penetration and good signal-to-background ratio are desirable features for in vivo imaging, NIR fluorescence is generally preferred for in vivo fluorescence imaging.
Figure 1. Extinction coefficient of oxyhemoglobin, deoxyhemoglobin and water.
The ‘diagnostic window’ is in the near-infrared range (650–900 nm) (pink), where the extinction coefficients are at their minimum.
NIR fluorescence imaging can be categorized into one of two types: fluorescence reflectance imaging (FRI) and tomographic fluorescence imaging (e.g., diffuse optical tomography [DOT] and fluorescent molecular tomography [FMT]) [14,15]. FRI is a simple, two-dimensional (planar) imaging method that captures emitted fluorescence through an appropriate filter after excitation. General features of FRI are:
High spatial resolution
Fast, real-time display
Relatively low cost
Portability
Ability to multiplex several colors at the same time
Since the main limitation of this method is its poor tissue penetration (<1 cm), it is currently only used for superficial imaging or for surgical guidance.
In contrast, tomographic fluorescence imaging is a three-dimensional imaging technique, which requires reconstruction with sophisticated image processing algorithms, that can create tomographic images akin to traditional tomographic images, such as single photon emission computed tomography (SPECT), CT and MRI. Additionally, this method shows better depth sensitivity (<10 cm) compared with FRI, while temporal and spatial resolution is generally lower. Diffuse optical tomography is currently under testing for the detection of breast cancer in clinical trials.
Potential clinical applications
Cancer detection & characterization
The ability to detect cancers while they are small and curable has implications for screening. Once the tumor has grown larger, imaging can provide characterization and clinical staging and can be used to detect recurrence in previously treated patients. For in vivo fluorescence-mediated cancer detection, nonspecific agents and cancer-targeted agents are currently available for research. A nonspecific agent accumulates within a tumor depending on its vascularity and/or impaired tumor capillary permeability, and emits a particular fluorescence from target tissue (i.e., cancer). ICG (Figure 2) is one example of this type of NIR fluorescent agent, which has been approved by the US FDA for the cardiac/hepatic function test and chorioretinal fluorescence angiography [16]. ICG has a moderate-affinity binding site on human serum albumin (HSA), and thus noncovalently forms a complex (70 kDa) with HSA after intravenous injection [17]. Since ICG–HSA typically stays in vessels, it can be used as a nonspecific blood flow tracer. Ntziachristos et al. applied diffuse optical tomography to clinical patients with various breast diseases after administration of ICG, and demonstrated its ability to detect breast lesions by NIR fluorescence, which were also detected in Gd-DTPA-enhanced MRI [18]. More recently, a diffuse optical mammography scanner (Phillips Medical Systems, Amsterdam, The Netherlands) has been developed [10], and a clinical trial has already been carried out in combination with a novel indocyanine-based contrast agent (Omocianine, Bayer Schering, Berlin-Wedding, Germany). Such NIR-labeled nonspecific agents can be expected to increase cancer detection; however, such agents simply reflect increased tumor vascularity and/or impaired tumor capillary permeability, and are therefore not specific for cancer.
Figure 2.
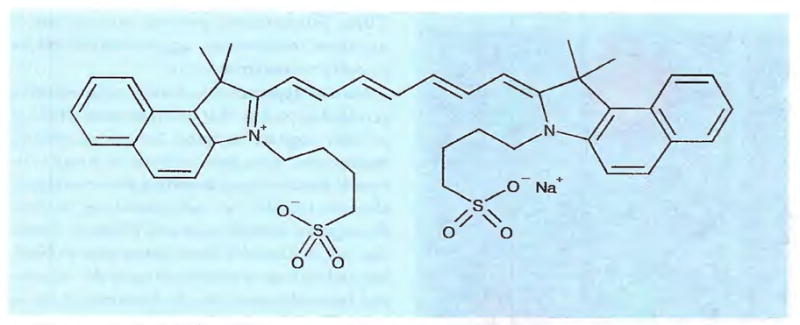
Indocyanine green (ICG).
In contrast to nonspecific agents, targeted NIR fluorescent agents, which consist of a specific targeting ligand or monoclonal antibody conjugated to NIR fluorophores, can provide molecular-specific information on cancer, as well as accelerate fluorophore accumulation into targeted tumors. This strategy has been developed for many years with nuclear imaging, and now many targeted NIR agents have been demonstrated to target a wide range of potentially important cell receptors, including somatostatin receptor [19–22], vasoactive intestinal peptide (VIP) [23,24], carcinoembryonic antigen (CEA) [25], and EGF receptor (EGFR) [26–29], among others [30]. Owing to its specificity for particular cell-surface molecules expressed in cancer, these agents can be used not only for cancer detection, but also for cancer characterization. For example, Barrett et al. demonstrated that fluorescently labeled monoclonal antibody could be used to diagnose EGFR expression in different tumor xenografts [26]. In this study, two different cancer cell lines (A431: overexpressing HER1 and NIH3T3/HER2: overexpressing HER2) were inoculated on the back of the same mouse, and then a cocktail of Cy5.5-labeled cetuximab (anti-HERl) and Cy7-labeled trastuzumab (anti-HER2) was intravenously administrated. After administration, each tumor could be depicted with different wavelength fluorescence (Figure 3), and investigators were able to correctly diagnose the type of EGFR expression’ in all tumors. This could be clinically relevant because EGFR expression status directly corresponds to a therapeutic outcome of anti-EGFR monoclonal antibody therapy, which has already been approved for human use. Currently, EGFR expression in breast cancer is determined by immunohistochemical analysis of an excised tissue sample. However, since receptor expression is often inhomogeneous, a sample may not be representative of the entire tumor. Thus, NIR imaging has the potential to determine the type of EGFR expression (e.g., HER1 and HER2) noninvasively and simultaneously with high spatial resolution. Although these targeted NIR fluorescent agents have not yet been applied to humans, there is interest in developing them into human imaging tools.
Figure 3. EGFR typing by multicolor NIR fluorescence imaging.
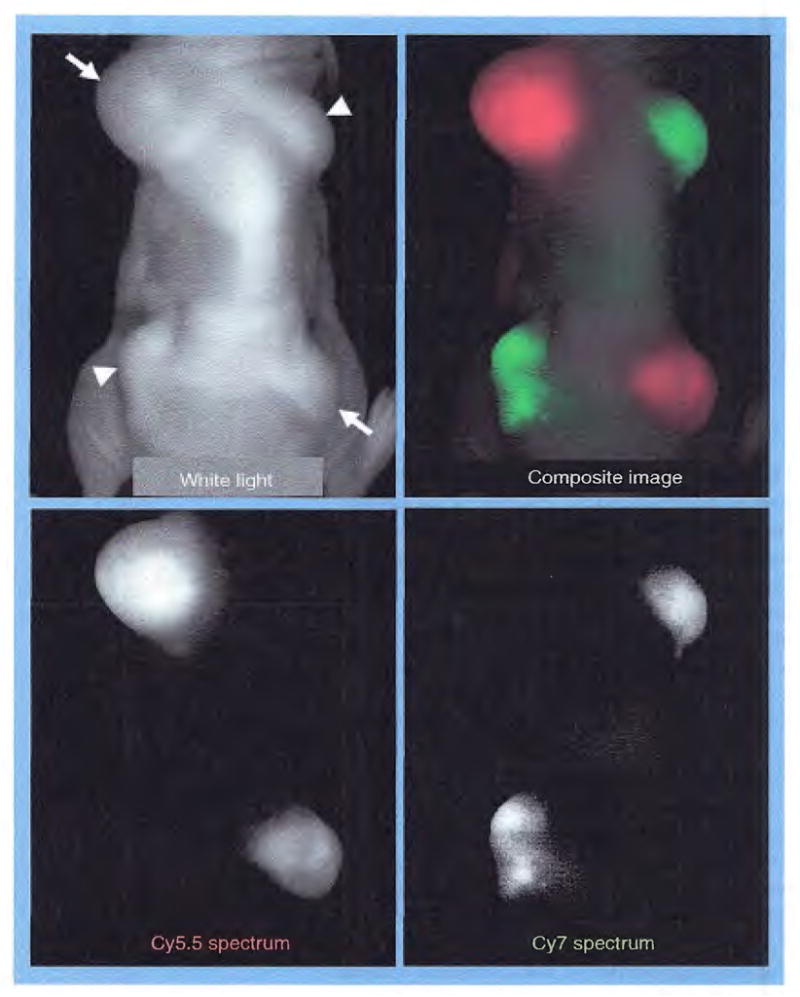
Mouse bearing two different types of EGF receptor (EGFR)-expressed tumors, A431 overexpressing HER1 (arrows) and NIH3T3/HER2 overexpressing HER2 (arrow heads), intravenously received a cocktail of Cy5.5-labeled cetuximab (anti-HER1) and Cy7-labeled trastuzumab (anti-HER2) 24 h prior to imaging. All tumors are depicted by different spectra (absorption and emission wavelengths: 675 nm and 694 nm for Cy5.5, and 743 nm and 767 nm for Cy7, respectively). EGFR receptor expressions can be noninvasively diagnosed by its distinct colors. Note that background fluorescence (mixture of autofluorescence, unbound Cy5.5-, and Cy7-fluorescence) are eliminated by spectral unmixing algorithm [26].
Targeted imaging agents improve tumor accumulation of agents, but unbound agents can be a source of background signal and will result in a reduction in tumor-to-background ratio (TBR). High TBR is crucial not only for cancer detection, but also for other cancer imaging applications. For instance, nuclear imaging with a radiolabeled antibody must allow a few days to elapse after administration of the agent to allow clearance of the background, permitting depiction of a targeted tumor. Background signals from unbound agents in the blood pool hamper depiction of targeted tumor until the agent begins to be cleared. This is also true for fluorescence imaging that must be unmixed from background autofluorescence [31]. However, fluorescence imaging differs from nuclear imaging in an important way; activatable probes, in which fluorescence can be quenched and dequenched, that is, turned on or off, can be designed. Targeted activation enables high TBR in in vivo cancer imaging. Weissleder and colleagues have developed a series of such activatable NIR probes that can be activated by proteases, such as cathepsin D and matrix metalloproteinases-2 [32–34]. These agents are minimally fluorescent in the steady state due to the interaction of the fluorophores, which result in self-quenching, but are held by a polymeric peptide or dextran backbone or bridge with an enzymatic cleavage site between fluorophores. Upon protyleatic cleavage, the fluorophores can separate from each other, resulting in fluorescence activation. Although these agents are not targeting a particular molecule in cancer cells, they are cleaved by proteases that are found in relatively higher abundance in pathological tissues, including cancer. This results in high TBRs. Furthermore, protease activity relates to cancer invasiveness, aggressiveness and its capacity to metastasize [35].
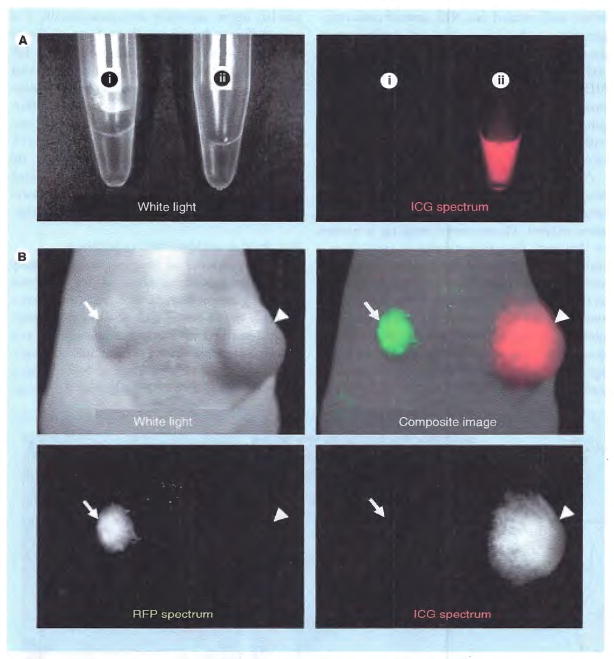
Another approach is to develop target-specific activatable probes that activate upon binding to their cognate receptor. Several quenching mechanisms have been utilized to create activatable fluoroescence, including photon-induced electron transfer [36], self-quenching [31,37,38], fluorophore quenchers [39] and H-dimer formation [40], to diminish fluorescence prior to binding and activate it after binding to the receptor and internalization into the lysosome of tumor cells. The lysosome has very unique properties that create a specific area of the cell where very low pH levels and high enzymatic activity can be maintained. For example, Ogawa et al. covalently conjugated ICG (ICG-sulfo-Osu, Dojindo Molecular Technologies, MD, USA) to a US FDA-approved monoclonal antibody for in vivo cancer imaging [38]. ICG loses its fluorescence once it is covalently bound to a protein, probably due to self-quenching; however, fluorescence can be recovered from conjugates by releasing the ICG from the protein, such as occurs after internalization and uptake into the lysosomes of cancer cells. Using this ‘activatable switch’, several different antigen-expressing tumor nodules were depicted by ICG-conjugated antibodies with minimal background signals (Figure 4). Because the antibody–ICG conjugate is composed of FDA-approved agents, there is improved likelihood of clinical translation with this agent.
Figure 4. In vivo cancer imaging with activatable ICG–antibody conjugates.
Activatable ICG–antibody conjugate (A) is not fluorescent in phosphate buffered saline (i), while it becomes fluorescent in SDS and 2-ME added condition (ii), which can dissociate and disrupt ICG and antibody interaction. The mouse bearing ATAC4 tumor (CD25+, arrow head) and A431/RFP (CD25−, arrow) intravenously received ICG-conjugated daclizumab (anti-CD25 monoclonal antibody) (B). The target tumor (ATAC4) is visualized at ICG spectrum (absorption and emission wavelengths: 780 and 820 nm, respectively), while nontarget tumor (A431/RFP) shows no fluorescence at ICG spectrum due to the quenching capability of conjugates.
ICG: Indocyanine green; RFP: Red fluorescent protein; SDS: Sodium dodecyl sulfate.
Lymphatic imaging
Lymphatic imaging, especially sentinel lymph node mapping, is currently performed with several imaging methods, such as dye-injection, nuclear imaging, CT and MRI [41]. However, these imaging methods each have their own limitation; the dye-injection method is less sensitive to subsurface tissue, lymphoscintigraphy has low spatial resolution and requires exposure to radioactivity, and CT/MRI requires a bulky imaging machine that prevents use during surgery. Advantages of NIR fluorescence imaging are that it can be performed without ionizing radiation with good spatial and temporal resolution. The clinical use of NIR fluorescence imaging for sentinel lymphatic mapping was first reported by Kitai et al. in 18 breast cancer patients [42]. They injected 25 mg of ICG near the areola of breast cancer patients, and successfully visualized the draining lymphatics in all patients and localized the sentinel lymph nodes in 17/18 patients. Following this study, additional clinical studies have confirmed the utility of NIR sentinel mapping in breast cancer [43] and melanoma [44]. Furthermore, Sevick-Muraca et al. demonstrated in human breast cancer that a microdose (<100 μg) of ICG was sufficient for lymphatic flow visualization and sentinel lymph node mapping [45]. In addition to superficial sentinel node mapping, intraoperative sentinel node mapping has also been reported by Soltesz et al. In anesthesized swine, the abdomen was opened and NIR quantum dots were administered into the subserosa of the stomach, jejunum and colon. These injected quantum dots clearly allowed visualization of the lymphatic channels and a single mesenteric/retrogastric lymph node (sentinel node) could be visualized within 1 min at all injection sites using a real-time intraoperative NIR fluorescence camera [46]. They also performed a similar study in the lungs of swine, and revealed that NIR sentinel node mapping was also feasible with injection of quantum dots into swine lungs [47]. Combined with recent technical developments involving intraoperative NIR fluorescence cameras [48] or portable NIR imaging devices [43], sentinel lymph node mapping is one of the most promising clinical applications for NIR fluorescence imaging in the field of oncology.
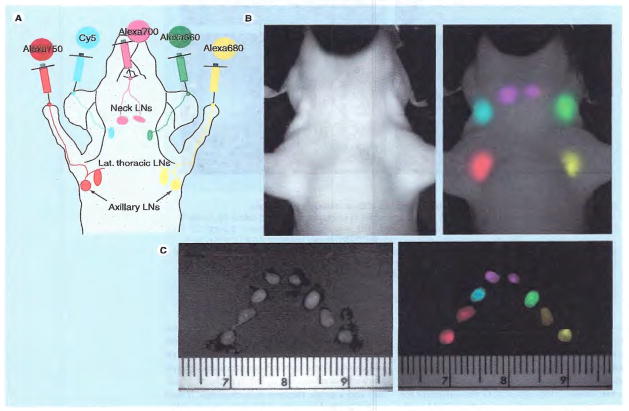
Another interesting application of NIR fluorescence for lymphatic imaging is multicolor imaging; multiplexed use of NIR probes in the same subject. Fluorescence imaging is unique in that each fluorophore emits its own characteristic spectral wavelength, thus permitting simultaneous depiction of multiple lymphatics at the same time. Hama et al. reported in vivo two-color lymphatic mapping using immunoglobulin G (IgG)-conjugated NIR probes or quantum dots in mice [49,50]. In the former study, IgG–Cy5.5 was injected into the left mammary pad and IgG–Cy7 into the middle phalanx of the left’ upper extremity intracutaneously. The two different lymphatic flows from different sites were simultaneously visualized using different fluorophores for each site [49]. Since this multicolor method of NIR lymphography enables simultaneous but separate visualization of different lymphatic basins, this may elucidate the complex human lymphatic system and reduce the risk of lymphedema after surgery. Furthermore, up to five colors have been simultaneously injected with dendrimer-conjugated organic fluorophores (Figure 5) [51] or nonorganic NIR quantum dots [52].
Figure 5. Sites of injection (A), and in vivo (B) and ex vivo (C) multicolor fluorescence lymphatic images.
Different lymph nodes are depicted by distinct colors.
LN: Lymph node.
Surgical & endoscopic guidance
Surgical guidance of imaging can be mainly subdivided into three areas: tumor detection (defining tumor margin and detecting tumor invisible to the naked eye), sentinel node mapping described in the previous section, and defining anatomy during surgical interventions. For these purposes, features of NIR fluorescence imaging, such as high spatial resolution, real-time capability, portability and no radiation exposure, are ideal as a means of providing an intraoperative imaging modality that is easily adapted to the operating room or endoscopy suite. Tumor detection with NIR fluorescence during a surgical procedure has been performed in several tumor types, including liver [53] and brain tumors [54] in humans, and colon [55], ovary [56], lung [57] and soft-tissue tumors [58] in orthotropic murine models. In these studies, a NIR probe was able to detect small cancers invisible to the naked eye, as well as define the cancer, thus enabling a more complete resection than would otherwise be possible. In addition to intraoperative tumor detection, several studies also demonstrated feasibility of an endoscopic system to detect tumors with NIR fluorescence during colonoscopy [55], thoracoscopy [57] and laparoscopy [56]. Since conventional endoscopy is already an ‘optical imaging instrument’, it can be retrofitted for fluorescence by using appropriate filters at both the excitation light source and at the camera. A visible-range fluorescence endoscope is currently under evaluation for its clinical utility [59,60], and it is likely that fluorescence endoscopy will be a useful tool in the future for oncologists [61,62].
Defining the anatomy of fine structures may assist surgeons in performing surgical interventions safely. Tanaka et al. demonstrated with a swine model that intravenous injection of NIR fluorophore (carboxyl form of IRDye 800CW, LI-COR, NE, USA) enabled visualization of the extrahepatic bile duct [63] or ureters [64] by NIR fluorescence. Such intraoperative visualization is currently performed under fluoroscopic guidance with iodine contrast materials, but they clearly demonstrated that it can be replaced with NIR fluorescence imaging that requires no ionizing radiation exposure. This clinical application may be useful not only for defining anatomy, but also for detecting stones inside ducts or for checking up on a leakage from ducts after surgical procedures.
Toxicity
Toxicity is an issue that constitutes a ‘go-no-go’ decision point in the development of novel imaging agents. Quantum dots are small crystals (2–10 nm diameter) made of inorganic semiconductor materials possessing several favorable fluorescent properties, such as high quantum yields (bright), tunable emission wavelength (visible to NIR) and photostablity. Accordingly, quantum dots are widely applied to in vitro and in vivo biomedical fluorescence imaging [65–67], including sentinel node mapping [68] and cancer-targeted imaging [69,70] in animal models. However, most of the current-generation quantum dots are based on heavy metal cores (Cd-Se, Cd-Te and so on), raising concerns over their toxicity when in their soluble form. The bare metal core of quantum dots has been reported to be cytotoxic, probably mediated by reactive oxygen species and the release of cadmium ions [71–73]. Derfus et al. have demonstrated that ion release is enhanced by oxidation by either air or UV irradiation, and encapsulating the core with appropriate shells, such as ZnS, can eliminate cytotoxicity due to oxidation [71], as well as prevent cadmium ion leakage from the core. Furthermore, this cytotoxicity has been partially overcome by coating quantum dots with polymers, poly(ethylene glycol) (PEG) or other inert molecules [72,74–76]. In addition, smaller quantum dots below 6 nm in diameter have been reported to be cleared through the kidney and rapidly excreted into the urine [77], and cadmium-free quantum dot formulations have been produced while maintaining their desirable fluorescence properties [78].
Organic fluorophores are accepted to be more biocompatible than inorganic fluorophores, because they can be metabolized in the human body. In fact, ICG and fluorescein have already shown excellent safety records in the clinic for over 20 years. Moreover, ICG encapsulated in polymeric nano-particulate has recently been revealed to enhance its chemical and photostability, which is also ideal for quantitative NIR imaging [79,80]. However, even with ICG or ICG derivatives, toxicity and pharmacokinetic profile may alter depending on modifications to the conjugated molecule, and thus the toxicity profile of each fluorophore or fluorophore conjugate needs to be revealed before human studies can be undertaken.
Conclusion
Several NIR fluorescence imaging applications have been proposed in clinical cancer imaging. These applications are not only alternatives to traditional methods, but also compensate for several limitations of traditional methods by employing the unique features of NIR fluorescence. Although fluorescence imaging has the limitation of poor tissue penetration, it also has a high spatial resolution, real-time display and can be multiplexed with other optical agents and other imaging modalities, which make this modality unique among imaging modalities, and thus it should continue to play an important role in the clinical imaging of cancer.
Future perspective
Some superficial NIR fluorescence imaging, such as sentinel node mapping and surgical/endoscopic guidance, as well as optical mammography with/without fluorescent agents, have already been applied to humans and showed reasonable results in the clinic. NIR fluorescence imaging is likely to be employed in routine clinical practice in the near future. In addition, multiplexed multicolor fluorescent agents could provide interesting applications for molecular imaging of human cancers in the clinic, since this enables one to simultaneously obtain multiple pieces of molecular information regarding targeted cancers. For this application, targeted NIR probes need to be approved for human use, and spectral fluorescence human imagers or endoscopes are also required. However, noninvasive NIR fluorescence imaging of human deep organs, such as liver, lung and brain, are currently unrealistic due to its limitation of tissue penetration, and it requires considerable improvement of sensitivity of the fluorescence imager and brightness (quantum efficiency) of fluorophores.
Executive Summary.
Near-infrared fluorescence imaging
Near-infrared (NIR) fluorescence is a light wavelength of 650–950 nm, and is generally preferred for in vivo fluorescence imaging because of its good tissue penetration and low autofluorescence from adjacent tissues.
NIR fluorescence imaging can be categorized into fluorescence reflectance imaging (FRI) and tomographic fluorescence imaging. Advantageous features of FRI are high spatial resolution, fast, real-time display, relatively low cost, portability and ability to multiplex several colors at the same time.
Cancer detection & characterization
For in vivo cancer detection, nonspecific and specific cancer-targeted fluorescent agents are currently available. A nonspecific blood flow tracer, indocyanine green (ICG), has already been applied to human studies for cancer detection.
Cancer-targeted reagents, which are designed to target specific cell-surface molecules expressed in cancer, can noninvasively diagnose molecular profiling of distinct cancers.
Activatable probes, which can have their fluorescent signal turned on or off depending on the environment, enable a target-specific signal activation, leading to high tumor-to-background ratio in in vivo cancer imaging.
Lymphatic imaging
ICG has been applied to the sentinel lymph node mapping and successfully visualized regional lymphatic flow from the cancer lesions and identified sentinel lymph nodes in humans.
Simultaneous but separate visualization of different lymphatic drainages (multicolor lymphatic imaging) is possible with a multiplexed fluorescence imaging by the use of fluorescent agents with multiple colors.
Surgical & endoscopic guidance
Tumor detection, sentinel node mapping and defining functional anatomy could be potential applications for surgical/endoscopic guidance with fluorescence.
An intraoperative NIR fluorescence imaging system, portable NIR imaging devices and fluorescence endoscope have been developed and used for clinical studies.
Toxicity
Toxicity of inorganic fluorophores may be overcome by surface coating or optimization of size, which can improve pharmacokinetics and lead to reduced toxicity. Organic fluorophores are generally more biocompatible than inorganic ones.
Footnotes
Financial & competing interests disclosure
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Nobuyuki Kosaka, Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, MD, USA.
Mikako Ogawa, Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, MD, USA.
Peter L Choyke, Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, MD, USA.
Hisataka Kobayashi, Email: kobayash@mail.nih.gov, Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Building 10, Room 1B40, MSC1088, Bethesda, MD 20892-1088, USA, Tel.: +1 301 451 4220, Fax: +1 301 402 3191.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1▪.Frangioni JV. New technologies for human cancer imaging. J Clin Oncol. 2008;26(24):4012–4021. doi: 10.1200/JCO.2007.14.3065. Excellent review of the recent technical advances for human cancer imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brancato R, Trabucchi G. Fluorescein and indocyanine green angiography in vascular chorioretinal diseases. Semin Ophthalmol. 1998;13(4):189–198. doi: 10.3109/08820539809056052. [DOI] [PubMed] [Google Scholar]
- 3.Flower RW, Hochheimer BF. Indocyanine green dye fluorescence and infrared absorption choroidal angiography performed simultaneously with fluorescein angiography. Johns Hopkins Med J. 1976;138(2):33–42. [PubMed] [Google Scholar]
- 4.Choe R, Corlu A, Lee K, et al. Diffuse optical tomography of breast cancer during neoadjuvant chemotherapy: a case study with comparison to MRI. Med Phys. 2005;32(4):1128–1139. doi: 10.1118/1.1869612. [DOI] [PubMed] [Google Scholar]
- 5.Demos SG, Gandour-Edwards R, Ramsamooj R, White R. Near-infrared autofluorescence imaging for detection of cancer. J Biomed Opt. 2004;9(3):587–592. doi: 10.1117/1.1688812. [DOI] [PubMed] [Google Scholar]
- 6.Intes X. Time-domain optical mammography SoftScan: initial results. Acad Radiol. 2005;12(8):934–947. doi: 10.1016/j.acra.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Poplack SP, Tosteson TD, Wells WA, et al. Electromagnetic breast imaging: results of a pilot study in women with abnormal mammograms. Radiology. 2007;243(2):350–359. doi: 10.1148/radiol.2432060286. [DOI] [PubMed] [Google Scholar]
- 8.Rinneberg H, Grosenick D, Moesta KT, et al. Scanning time-domain optical mammography: detection and characterization of breast tumors in vivo. Technol Cancer Res Treat. 2005;4(5):483–496. doi: 10.1177/153303460500400503. [DOI] [PubMed] [Google Scholar]
- 9.Tromberg BJ, Pogue BW, Paulsen KD, Yodh AG, Boas DA, Cerussi AE. Assessing the future of diffuse optical imaging technologies for breast cancer management. Med Phys. 2008;35(6):2443–2451. doi: 10.1118/1.2919078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Ven SM, Elias SG, Wiethoff AJ, et al. Diffuse optical tomography of the breast: preliminary findings of a new prototype and comparison with magnetic resonance imaging. Eur Radiol. 2009;19(5):1108–1113. doi: 10.1007/s00330-008-1268-3. [DOI] [PubMed] [Google Scholar]
- 11▪.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452(7187):580–589. doi: 10.1038/nature06917. Excellent review of the current status of molecular imaging, including near-infrared (NIR) fluorescence imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Policard A. Survey on the aspects offered by experimental tumors examined in the light of Wood. C R Séances Soc Biol Fil. 1924;91:1423–1424. [Google Scholar]
- 13.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer. 2005;5(10):796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 14.Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003;13(1):195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 15▪.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23(3):313–320. doi: 10.1038/nbt1074. Excellent review of the theoretical and instrumental advances in fluorescence imaging. [DOI] [PubMed] [Google Scholar]
- 16.Benson RC, Kues HA. Fluorescence properties of indocyanine green as related to angiography. Phys Med Biol. 1978;23(1):159–163. doi: 10.1088/0031-9155/23/1/017. [DOI] [PubMed] [Google Scholar]
- 17.Ohnishi S, Lomnes SJ, Laurence RG, Gogbashian A, Mariani G, Frangioni JV. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging. 2005;4(3):172–181. doi: 10.1162/15353500200505127. [DOI] [PubMed] [Google Scholar]
- 18▪.Ntziachristos V, Yodh AG, Schnall M, Chance B. Concurrent MRI and diffuse optical tomography of breast after indocyanine green enhancement. Proc Natl Acad Sci USA. 2000;97(6):2767–2772. doi: 10.1073/pnas.040570597. Original work for human breast cancer imaging using indocyanine green (ICG) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achilefu S, Dorshow RB, Bugaj JE, Rajagopalan R. Novel receptor-targeted fluorescent contrast agents for in vivo tumor imaging. Invest Radiol. 2000;35(8):479–485. doi: 10.1097/00004424-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Becker A, Hessenius C, Licha K, et al. Receptor-targeted optical imaging of tumors with near-infrared fluorescent ligands. Nat Biotechnol. 2001;19(4):327–331. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]
- 21.Bugaj JE, Achilefu S, Dorshow RB, Rajagopalan R. Novel fluorescent contrast agents for optical imaging of in vivo tumors based on a receptor-targeted dye-peptide conjugate platform. J Biomed Opt. 2001;6(2):122–133. doi: 10.1117/1.1352748. [DOI] [PubMed] [Google Scholar]
- 22.Licha K, Hessenius C, Becker A, et al. Synthesis, characterization, and biological properties of cyanine-labeled somatostatin analogues as receptor-targeted fluorescent probes. Bioconjug Chem. 2001;12(1):44–50. doi: 10.1021/bc000040s. [DOI] [PubMed] [Google Scholar]
- 23.Becker A, Hessenius C, Bhargava S, et al. Cyanine dye labeled vasoactive intestinal peptide and somatostatin analog for optical detection of gastroenteropancreatic tumors. Ann NY Acad Sci. 2000;921:275–278. doi: 10.1111/j.1749-6632.2000.tb06976.x. [DOI] [PubMed] [Google Scholar]
- 24.Bhargava S, Licha K, Knaute T, et al. A complete substitutional analysis of VIP for better tumor imaging properties. J Mol Recognit. 2002;15(3):145–153. doi: 10.1002/jmr.565. [DOI] [PubMed] [Google Scholar]
- 25.Folli S, Westermann P, Braichotte D, et al. Antibody-indocyanin conjugates for immunopbotodetection of human squamous cell carcinoma in nude mice. Cancer Res. 1994;54(10):2643–2649. [PubMed] [Google Scholar]
- 26▪▪.Barrett T, Koyama Y, Hama Y, et al. In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res. 2007;13(22 Pt 1):6639–6648. doi: 10.1158/1078-0432.CCR-07-1119. Original work for the in vivo EGFR typing by multicolor fluorescence imaging with cocktail injection of two cancer-targeted NIR probes. [DOI] [PubMed] [Google Scholar]
- 27.Ke S, Wen X, Gurfinkel M, et al. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 2003;63(22):7870–7875. [PubMed] [Google Scholar]
- 28.Koyama Y, Barrett T, Hama Y, Ravizzini G, Choyke PL, Kobayashi H. In vivo molecular imaging to diagnose and subtype tumors through receptor-targeted optically labeled monoclonal antibodies. Neoplasia. 2007;9(12):1021–1029. doi: 10.1593/neo.07787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soukos NS, Hamblin MR, Keel S, Fabian RL, Deutsch TF, Hasan T. Epidermal growth factor receptor-targeted immunophotodiagnosis and photoimmunotherapy of oral precancer in vivo. Cancer Res. 2001;61(11):4490–4496. [PMC free article] [PubMed] [Google Scholar]
- 30▪.Achilefu S. Lighting up tumors with receptor-specific optical molecular probes. Technol Cancer Res Treat. 2004;3(4):393–409. doi: 10.1177/153303460400300410. Excellent review of the receptor-targeted NIR fluorescence probes. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa M, Regino CA, Choyke PL, Kobayashi H. In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol Cancer Ther. 2009;8(1):232–239. doi: 10.1158/1535-7163.MCT-08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bremer C, Bredow S, Mahmood U, Weissleder R, Tung CH. Optical imaging of matrix metalloproteinase-2 activity in tumors feasibility study in a mouse model. Radiology. 2001;221(2):523–529. doi: 10.1148/radiol.2212010368. [DOI] [PubMed] [Google Scholar]
- 33.Mahmood U, Weissleder R. Near-infrared optical imaging of proteases in cancer. Mol Cancer Ther. 2003;2(5):489–496. [PubMed] [Google Scholar]
- 34▪▪.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17(4):375–378. doi: 10.1038/7933. Original work for the protease-activated NIR fluorescence probes. [DOI] [PubMed] [Google Scholar]
- 35.Bremer C, Tung CH, Bogdanov A, Jr, Weissleder R. Imaging of differential protease expression in breast cancers for detection of aggressive tumor phenotypes. Radiology. 2002;222(3):814–818. doi: 10.1148/radiol.2223010812. [DOI] [PubMed] [Google Scholar]
- 36▪▪.Urano Y, Asanuma D, Hama Y, et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009;15(1):104–109. doi: 10.1038/nm.1854. Original work for the reversible pH-activatable fluorescence probes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37▪.Hama Y, Urano Y, Koyama Y, Gunn AJ, Choyke PL, Kobayashi H. A self-quenched galactosamine-serum albumin-rhodamineX conjugate: a ‘smart’ fluorescent molecular imaging probe synthesized with clinically applicable material for detecting peritoneal ovarian cancer metastases. Clin Cancer Res. 2007;13(21):6335–6343. doi: 10.1158/1078-0432.CCR-07-1004. Original work with the target-cell-specific activatable fluorescence imaging probe for cancer detection. [DOI] [PubMed] [Google Scholar]
- 38▪▪.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 2009;69(4):1268–1272. doi: 10.1158/0008-5472.CAN-08-3116. Original work for the activatable ICG and monoclonal antibody conjugates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogawa M, Kosaka N, Longmire MR, Urano Y, Choyke PL, Kobayashi H. Fluorophore-quencher based activatable targeted optical probes for detecting in vivo cancer metastases. Mol Pharm. 2009;6(2):386–395. doi: 10.1021/mp800115t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. H-type dimer formation of fluorophores: a mechanism for activatable, in vivo optical molecular imaging. ACS Chem Biol. 2009;4(7):535–546. doi: 10.1021/cb900089j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett T, Choyke PL, Kobayashi H. Imaging of the lymphatic system: new horizons. Contrast Media Mol Imaging. 2006;1(6):230–245. doi: 10.1002/cmmi.116. [DOI] [PubMed] [Google Scholar]
- 42▪.Kitai T, Inomoto T, Miwa M, Shikayama T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12(3):211–215. doi: 10.2325/jbcs.12.211. Original work for the sentinel lymph node mapping with ICG in breast cancer patients. [DOI] [PubMed] [Google Scholar]
- 43.Tagaya N, Yamazaki R, Nakagawa A, et al. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg. 2008;195(6):850–853. doi: 10.1016/j.amjsurg.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 44.Fujiwara M, Mizukami T, Suzuki A, Fukamizu H. Sentinel lymph node detection in skin cancer patients using real-time fluorescence navigation with indocyanine green: preliminary experience. J Plast Reconstr Aesthet Surg. 2009;62(10):E373–E378. doi: 10.1016/j.bjps.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 45▪.Sevick-Muraca EM, Sharma R, Rasmussen JC, et al. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology. 2008;246(3):734–741. doi: 10.1148/radiol.2463070962. Original work with human lymphatic imaging with a microdose of ICG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soltesz EG, Kim S, Kim SW, et al. Sentinel lymph node mapping of the gastrointestinal tract by using invisible light. Ann Surg Oncol. 2006;13(3):386–396. doi: 10.1245/ASO.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 47.Soltesz EG, Kim S, Laurence RG, et al. Intraoperative sentinel lymph node mapping of the lung using near-infrared fluorescent quantum dots. Ann Thorac Surg. 2005;79(1):269–277. doi: 10.1016/j.athoracsur.2004.06.055. discussion 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka E, Choi HS, Fujii H, Bawendi MG, Frangioni JV. Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann Surg Oncol. 2006;13(12):1671–1681. doi: 10.1245/s10434-006-9194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪▪.Hama Y, Koyama Y, Urano Y, Choyke PL, Kobayashi H. Two-color lymphatic mapping using Ig-conjugated near infrared optical probes. J Invest Dermatol. 2007;127(10):2351–2356. doi: 10.1038/sj.jid.5700892. Original work with multicolor lymphatic mapping with organic and inorganic NIR probes. [DOI] [PubMed] [Google Scholar]
- 50▪▪.Hama Y, Koyama Y, Urano Y, Choyke PL, Kobayashi H. Simultaneous two-color spectral fluorescence lymphangiography with near infrared quantum dots to map two lymphatic flows from the breast and the upper extremity. Breast Cancer Res Treat. 2007;103(1):23–28. doi: 10.1007/s10549-006-9347-0. Original work with multicolor lymphatic mapping with organic and inorganic NIR probes. [DOI] [PubMed] [Google Scholar]
- 51▪▪.Kobayashi H, Koyama Y, Barrett T, et al. Multimodal nanoprobes for radionuclide and five-color near-infrared optical lymphatic imaging. ACS Nano. 2007;1(4):258–264. doi: 10.1021/nn700062z. Original work with multicolor lymphatic mapping with organic and inorganic NIR probes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52▪▪.Kobayashi H, Hama Y, Koyama Y, et al. Simultaneous multicolor imaging of five different lymphatic basins using quantum dots. Nano Lett. 2007;7(6):1711–1716. doi: 10.1021/nl0707003. Original work with multicolor lymphatic mapping with organic and inorganic NIR probes. [DOI] [PubMed] [Google Scholar]
- 53.Gotoh K, Yamada T, Ishikawa O, et al. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J Surg Oncol. 2009;100(1):75–79. doi: 10.1002/jso.21272. [DOI] [PubMed] [Google Scholar]
- 54.Haglund MM, Berger MS, Hochman DW. Enhanced optical imaging of human gliomas and tumor margins. Neurosurgery. 1996;38(2):308–317. doi: 10.1097/00006123-199602000-00015. [DOI] [PubMed] [Google Scholar]
- 55.Alencar H, Funovics MA, Figueiredo J, Sawaya H, Weissleder R, Mahmood U. Colonic adenocarcinomas: near-infrared microcatheter imaging of smart probes for early detection – study in mice. Radiology. 2007;244(1):232–238. doi: 10.1148/radiol.2441052114. [DOI] [PubMed] [Google Scholar]
- 56.Sheth RA, Upadhyay R, Stangenberg L, Sheth R, Weissleder R, Mahmood U. Improved detection of ovarian cancer metastases by intraoperative quantitative fluorescence protease imaging in a pre-clinical model. Gynecol Oncol. 2009;112(3):616–622. doi: 10.1016/j.ygyno.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Figueiredo JL, Alencar H, Weissleder R, Mahmood U. Near infrared thoracoscopy of tumoral protease activity for improved detection of peripheral lung cancer. Int J Cancer. 2006;118(11):2672–2677. doi: 10.1002/ijc.21713. [DOI] [PubMed] [Google Scholar]
- 58.Kirsch DG, Dinulescu DM, Miller JB, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13(8):992–997. doi: 10.1038/nm1602. [DOI] [PubMed] [Google Scholar]
- 59.El-Bayoumi E, Silvestri GA. Bronchoscopy for the diagnosis and staging of lung cancer. Semin Respir Crit Care Med. 2008;29(3):261–270. doi: 10.1055/s-2008-1076746. [DOI] [PubMed] [Google Scholar]
- 60.Spiess PE, Grossman HB. Fluorescence cystoscopy: is it ready for use in routine clinical practice? Curr Opin Urol. 2006;16(5):372–376. doi: 10.1097/01.mou.0000240312.16324.9a. [DOI] [PubMed] [Google Scholar]
- 61.Ito S, Muguruma N, Kimura T, et al. Principle and clinical usefulness of the infrared fluorescence endoscopy. J Med Invest. 2006;53(1–2):1–8. doi: 10.2152/jmi.53.1. [DOI] [PubMed] [Google Scholar]
- 62.Ito S, Muguruma N, Kusaka Y, et al. Detection of human gastric cancer in resected specimens using a novel infrared fluorescent anti-human carcinoembryonic antigen antibody with an infrared fluorescence endoscope in vitro. Endoscopy. 2001;33(10):849–853. doi: 10.1055/s-2001-17328. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka E, Choi HS, Humblet V, Ohnishi S, Laurence RG, Frangioni JV. Real-time intraoperative assessment of the extrahepatic bile ducts in rats and pigs using invisible near-infrared fluorescent light. Surgery. 2008;144(1):39–48. doi: 10.1016/j.surg.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanaka E, Ohnishi S, Laurence RG, Choi HS, Humblet V, Frangioni JV. Real-time intraoperative ureteral guidance using invisible near-infrared fluorescence. J Urol. 2007;178(5):2197–2202. doi: 10.1016/j.juro.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iga AM, Robertson JH, Winslet MC, Seifalian AM. Clinical potential of quantum dots. J Biomed Biotechnol. 2007;2007(10):76087. doi: 10.1155/2007/76087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bentolila LA, Ebenstein Y, Weiss S. Quantum dots for in vivo small-animal imaging. J Nucl Med. 2009;50(4):493–496. doi: 10.2967/jnumed.108.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinaud F, Michalet X, Bentolila LA, et al. Advances in fluorescence imaging with quantum dot bio-probes. Biomaterials. 2006;27(9):1679–1687. doi: 10.1016/j.biomaterials.2005.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68▪▪.Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22(1):93–97. doi: 10.1038/nbt920. Original work with sentinel lymph node mapping using quantum dots. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai W, Shin DW, Chen K, et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6(4):669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 70.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22(8):969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 71.Derfus A, Chan W, Bhatia S. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4(1):11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirchner C, Liedl T, Kudera S, et al. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005;5(2):331–338. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- 73.Lovric J, Cho SJ, Winnik FM, Maysinger D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem Biol. 2005;12(11):1227–1234. doi: 10.1016/j.chembiol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Ballou B. Quantum dot surfaces for use in vivo and in vitro. Cur Top Dev Biol. 2005;70:103–120. doi: 10.1016/S0070-2153(05)70005-3. [DOI] [PubMed] [Google Scholar]
- 75.Hoshino A, Fujioka K, Oku T, et al. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004;4(11):2163–2169. [Google Scholar]
- 76.Zhang T, Stilwell JL, Gerion D, et al. Cellular effect of high doses of silica-coated quantum dot profiled with high throughput gene expression analysis and high content cellomics measurements. Nano Lett. 2006;6(4):800–808. doi: 10.1021/nl0603350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77▪▪.Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–1170. doi: 10.1038/nbt1340. Original work with renal clearance of quantum dots with small sizes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim SW, Zimmer JP, Ohnishi S, Tracy JB, Frangioni JV, Bawendi MG. Engineering InAs(x)P(1−x)/InP/ZnSe III–V alloyed core/shell quantum dots for the near-infrared. J Am Chem Soc. 2005;127(30):10526–10532. doi: 10.1021/ja0434331. [DOI] [PubMed] [Google Scholar]
- 79.Saxena V, Sadoqi M, Shao J. Enhanced photo-stability, thermal-stability and aqueous-stability of indocyanine green in polymeric nanoparticulate systems. J Photochem Photobiol B, Biol. 2004;74(1):29–38. doi: 10.1016/j.jphotobiol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Xu RX, Huang J, Xu JS, et al. Fabrication of indocyanine green encapsulated biodegradable microbubbles for structural and functional imaging of cancer. J Biomed Opt. 2009;14(3):034020. doi: 10.1117/1.3147424. [DOI] [PubMed] [Google Scholar]