Abstract
Alstonia boonei De Wild is a herbal medicinal plant of West African origin, popularly known as God's tree or “Onyame dua”. Within West Africa, it is considered as sacred in some forest communities; consequently the plant parts are not eaten. The plant parts have been traditionally used for its antimalarial, aphrodisiac, antidiabetic, antimicrobial, and antipyretic activities, which have also been proved scientifically. The plant parts are rich in various bioactive compounds such as echitamidine, Nα-formylechitamidine, boonein, loganin, lupeol, ursolic acid, and β-amyrin among which the alkaloids and triterpenoids form a major portion. The present paper aims at investigating the main research undertaken on the plant in order to provide sufficient baseline information for future work and for commercial exploitation.
1. Introduction
Many cultures throughout the world still rely on indigenous medicinal plants for their primary health care needs [1]. To date, 25% of modern medicines are derived from plants that have been used by traditional medical practitioners [2]. It is a fact that traditional systems of medicine have become a topic of global importance. Although modern medicine may be available in many developed countries, people are still turning to alternative or complementary therapies including medicinal herbs. Yet, few plant species that provide medicinal herbs have been scientifically evaluated for their possible medical applications. The safety and efficacy data are available for even fewer herbs, their extracts and active ingredients and the preparation containing them. Tropical and subtropical Africa contains between 40–45,000 species of plant with a potential for development and out of which 5,000 species are used medicinally [3]. Still there is a paradox, in spite of this huge potential and diversity, the African continent has only contributed 83 of the 1100 classic drugs globally [3]. African countries are at a stage where traditional medicine is considered more for its capacity to generate other medicine than for its own sake. In many cases research undertakings and the commercial use stemming from that research have always relied on information provided by the local communities and, in many instances, have hardly benefited from the research results [4]. In Africa, traditional healers and remedies made from plants play an important role in the health of millions of people. The relative ratios of traditional practitioners and university-trained doctors in relation to the whole population in African countries are revealing. In Ghana, for example, in the Kwahu district, there are 224 people for every traditional practitioner, compared to nearly 21,000 people for one university-trained doctor [4].
Typically, studies on the medicinal plants such as Alstonia boonei have focused on the bioactivity of its chemical constituents, ethnobotany, pharmacology, and taxonomy. However, a comprehensive or systematic review on the plant is lacking. Furthermore, in much of the older literature concerning West Africa, the name Alstonia congensis has been erroneously used for Alstonia boonei. Consequently, this paper synthesizes studies on Alstonia boonei (Figure 1). This is necessary to recapitulate the findings on the plant and thereby provide a comprehensive and current repository for references on the plant.
Figure 1.

Plate showing clockwise order from top right, the leaves, stem, branches, and whole plant of Alstonia boonei.
2. Classification, Cultivation, and Ethnobotanical Uses of Alstonia
2.1. Classification
Alstonia comprises about 40 species and has a pantropical distribution. There are about twelve species of the genus Alstonia. Alstonia boonei De Wild belongs to the family Apocynaceae. The species are scattered all over the world of which two are indigenous to Africa. The plant is known locally in Ghana as Onyame dua, Osen-nuru, or Sinduro in Twi, Onyame dua in Fante, Sinu or Adawura in Ga-Adangbe, Bakunin, Nyamenlebaka, Emenle, or Emie in Nzema, and Siaketekre, Nyemi dua, or Asi atoe in Ewe [5]. Elsewhere, Alstonia is known as Australian fever bush, Australian quinine, Devil tree, Dita bark, fever bark, or palimara [6].
2.2. Cultivation
Alstonia grows into a giant tree in most of the evergreen rain forests of tropical West Africa. The plant thrives very well in damp riverbanks. It is well known to all the traditional healers practicing along the west coast of Africa. It occurs in deciduous and fringing forest of Ghana [6]. Alstonia boonei De Wild is a deciduous tree up to 35 meters high (Figure 1). It buttresses deep-fluted high and narrow. Its white latexes are copious. The leaves are in whorls at nodes, oblanceolate, apex rounded to acuminate, lateral vein prominent almost at right angle to midrib. The flowers are white with lax terminal cymes. The fruits are paired with slender follicle up to 16 cm long with brown floss at each end.
2.3. Ethnobotanical Uses
The bark of Alstonia tree is one of the effective analgesic [7] herbs available in nature. All the parts of the plant are very useful but the thick bark cut from the matured tree is the part that is most commonly used for therapeutic purposes.The bark of the tree is highly effective when it is used in its fresh form; however, the dried one could equally be used. Therapeutically, the bark has been found to possess antirheumatic [7], anti-inflammatory [7], analgesic/pain-killing, antimalaria/antipyretic, antidiabetic (mild hypoglycaemic), antihelminthic, antimicrobial and antibiotic properties [8–10]. A decoction could be sweetened with pure honey and be taken up to 4 times daily as an effective painkiller for the following conditions.
Painful menstruation (dysmenorrhoea), when associated with uterine fibroid or ovarian cysts in women; lower abdominal and pelvic congestion associated with gynaecological problems such as pelvic inflammatory diseases; to relieve the painful urethritis common with gonococcus or other microbial infections in men. Alstonia decoction also exerts a mild antibacterial effect in this case, relieving the aches and pains associated with malaria fever. Alstonia is taken in the form of preparations that exhibits antipyrexia and anti-malaria effects, to combat rheumatic and arthritic pains. The decoction of Alstonia bark could be taken alone as an effective pain-killing agent. A cold infusion made from the fresh or dried bark of Alstonia taken orally two to three times daily exerts a mild hypoglycaemic effect on diabetic patients. The cold infusion is also administered orally for the purpose of expelling round worms, threadworms [7], and other intestinal parasites in children.
The fresh bark of Alstonia could be used in preparing herbal tinctures; it is particularly useful as an effective antidote against snake, rat, or scorpion poison. It is also useful in expelling retained products of conception and afterbirth when given to women. Asthma can be treated with a drink prepared from parts of Trema orientalis and decoction of the bark of Alstonia boonei mixed with the roots and bark of cola and fruits of Xylopia parviflora with hard potash [7]. The bark decoction of Alstonia boonei is used with other preparations in the treatment of fractures or dislocation [7], jaundice, and for inducing breast milk. Its latex is taken as a purgative. The hardened latex is used for the treatment of yaws. Alstonia boonei De Wild is regarded as one of few herbs with potential anti-HIV indicators. In some African countries Alstonia boonei is considered a sacred tree and worshiped in the forest and hence human beings in those countries do not eat its parts.
3. Chemical Composition
A wide array of chemical compounds has been isolated from Alstonia boonei. These include alkaloids, tannins, iridoids, and triterpenoids [5]. Chromatography of bark extracts of Alstonia boonei on silica gel plates with the solvent system AcOEt-MeOH-H2O (150 : 26 : 19) produced 6 separate spots with alkaloid reactions and the alkaloids isolated from the plant include echitamine (1) and echitamidine (Scheme 1), voacangine and akuammidine, Nα-formylechitamidine, and Nα-formyl-12-methoxyechitamidine [5, 11–13]. Echitamine (1), which is also isolated from the bark of Alstonia scholaris, Alstonia cogenesis, and Alstonia neriifolia, has been assigned the nomenclature [(3β, 16R)-3,17-Dihydroxy-16-(methoxycarbbonyl)-4-methyl-2-4(14)-cyclo-3, 4-secoakuammilinium. Its structure is as shown in Scheme 1.
Scheme 1.
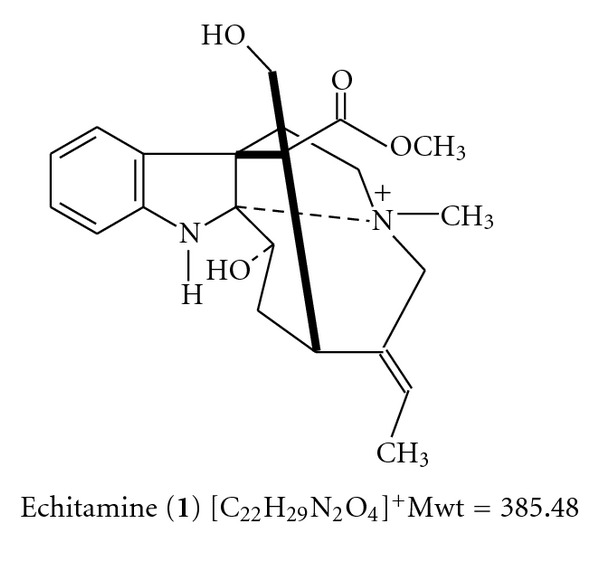
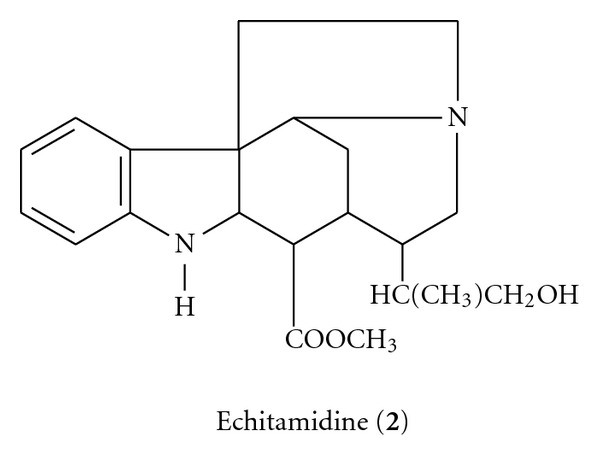
Echitamidine (2), on the other hand, has molecular formula of C20H22O3N2, with a melting point of 244°C and molecular weight of 338 g/mol. Its structure is as shown in Scheme 2.
Scheme 2.
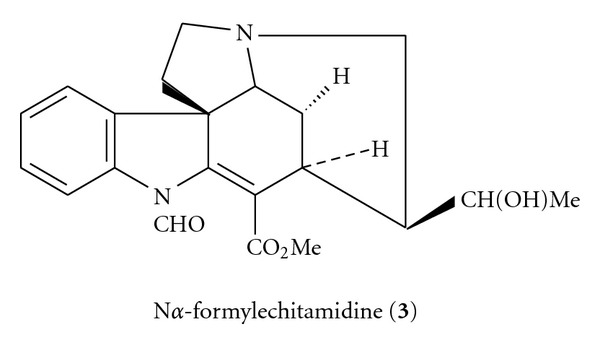
The structure of N-formylechitamidine (3) is as shown in Scheme 3.
Scheme 3.
These alkaloids, especially echitamine (1), possess a battery of pharmacological and autonomic activities [14, 15] including anticancer activities [16–23]. Alkaloids and related compounds isolated from other Alstonia species include the glycosides of venoterpene [24], nareline [25, 26], lagunamine [27], 19-epischolaricine [28], butamine, dobutamine [29, 30], alschomine [31], Nα-methylbutylamine, isoaslchomine[32], picrinine [33], strictamine [34, 35], rhazimanine [36], vallesamine [37], (20S)-19,20 dihydrocondylocarpine [38], scholarine[39], pseudoakuammigine[40], tetrahydroalstonine[41], akuammicine[42], picralinal[43], rhazine[44], scorlarine-N(4)- oxide, scholaricine [45, 46], and flavone glycosides [47, 48].
Iridoids isolated from Alstonia boonei include boonein and loganin. Loganin (Scheme 4) is a key intermediate in the biosynthesis of indole alkaloids. It is a crystal of melting point of 222-223°C [α]D20 −82.1 (water). It is freely soluble in water, less soluble in 96%, alcohol and sparingly soluble in absolute alcohol. It is practically insoluble in ether, pet. Ether, ligroin, ethyl acetate, and chloroform.
Scheme 4.

Boonein (5) is a C-9 monoterpenoid α-lactone, isolated from the bark of A. boonei (Apocynaceae). The structure was established by chemical and spectroscopic methods and by X-ray analysis. Its structure is as shown in Scheme 5. It is a possible precursor in the indole alkaloid biogenesis [49].
Scheme 5.

The triterpenoids isolated from Alstonia boonei include lupeol (6), ursolic acid (7), and β-amyrin (8).
Lupeol is also known as (3β)-Lup-20(29)-en-3-ol, monogynol B, β-viscol, or fagarasterol. Its structure is as shown in Scheme 6. It has a melting point of 215°C and a molecular weight of 426.73 with a molecular formular of C30H50O. The percentage composition of the various elements is, C = 84.44, H = 11.81, and O = 3.75. Lupeol forms needlelike crystals from alcohol or acetone. It is freely soluble in ether, benzene, pet. ether, and in warm alcohol. It is practically insoluble in water, dilute acids, and alkalis. Its acetate C32H52O2 forms needlelike crystals from acetone with melting point of 218°C and [α]D20+47.3.
Scheme 6.

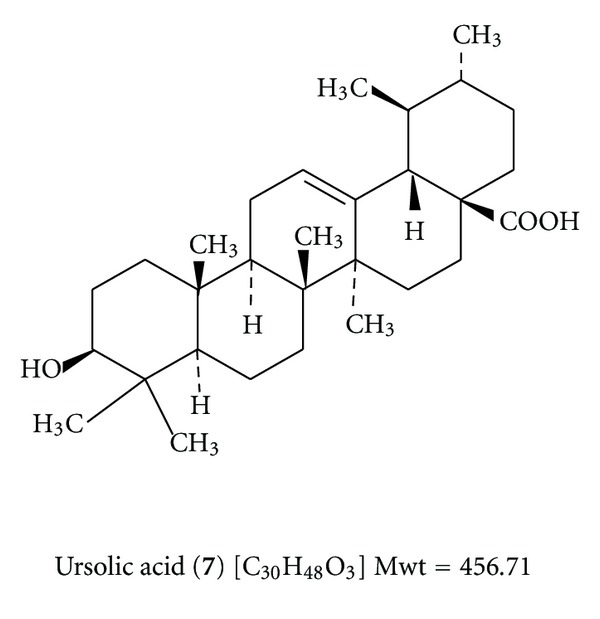
Ursolic acid is also known as urson, prunol micromerol, or malol. Its systematic name is (3β)-3-hydroxyurs-12-en-28-oic acid. Its structure is as shown in Scheme 7. Its melting point range is 285°C–288°C. One part dissolves in 88 parts of methanol, 178 alcohol (35 boiling alcohol), 140 ether, and 388 chloroform, 1675 carbon disulfide. It is moderately soluble in acetone and soluble in hot glacial acetic acid and in 2% alcoholic NaOH. It is insoluble in water and pet. ether its acetate has a melting point of 289-290°C. Ursolic acid is used as an emulsifying agent in pharmaceuticals and foods.
Scheme 7.
β-amyrin (8) has a molecular formula of C30H50O with a molecular weight of 426.73. Its structure is as shown in Scheme 8. Its melting point is 197–197.5°C. It forms needle-like crystals from pet. ether or alcohol.
Scheme 8.

For example, five compounds, which are triterpenes and sterols, were isolated from the hexane fraction of the alcohol extract of the leaves of Alstonia scholaris R. Br. The compounds are identified as 4α,14α,24-trimethyl-9β,19-cyclo-5α-cholest-24(29)-en-3β-ol, stigmasterol, betulin, betulinic acid, and α-amyrin acetate [50]. The structures of the isolated compounds were principally deduced by physiological and chromatographic characters as well as by spectroscopic analyses. The isolated compounds were reported for the first time.
The flowers of Alstonia scholaris contain n-hexacosane, lupeol, β-amyrin, palmitic acid, and ursolic acid. The components were extracted with petroleum ether at 60–80°C and separated by chromatographic methods. Mass spectra and spectrographic methods were used for identification. The root and root bark of Alstonia scholaris contain α-amyrin, α-amyrin acetate, lupeol acetate, stigmasterol, β-sitosterol, and campesterol and its isomer [50]. The stem bark of Alstonia scholaris contains α-amyrin acetate, lupeol acetate, and β-sitosterol [71, 72]. The isolation of α-amyrin acetate and lupeol acetate was done with 96% ethanol on the air-dried bark. The concentrated extract was stored for 2 weeks and gave a solid, which (on several crystallizations from EtOH) gave colorless needles of melting point of 160°C. This product was chromatographed through a column of alumina (4 × 32 cm) and eluted with pet. ether (b.p. 40–60°C). Rechromatography of the fractions gave 2 products. The first one forms colourless plates in (EtOH-Et2O) with mpt of 224–5°C, [α]29D 80° (all rotations detected in CHCl3), with one acetyl group and no active H. The second product colourless needles in (EtOH-Et2O) with mpt of 215–16°C, [α]28D 40°, containing an acetyl group and no active H. On hydrolysis of the first product with methanolic KOH, a substance with mpt of 184°C, [α]28D 84°, identical with α-amyrin was obtained. This on benzoylation gave α-amyrin benzoate which forms prisms in (C6H6-EtOH) with mpt of 198°C, [α]28D 92°. Similarly, on hydrolysis the second product gave a substance with mpt 212–13°C, [α]27D 22.6°, identical with lupeol. Benzoylation gave lupeol benzoate which forms colourless plates in (C6H6-EtOH) with mpt of 259–60°C, [α]27D 60.4°.
4. Pharmacological Properties
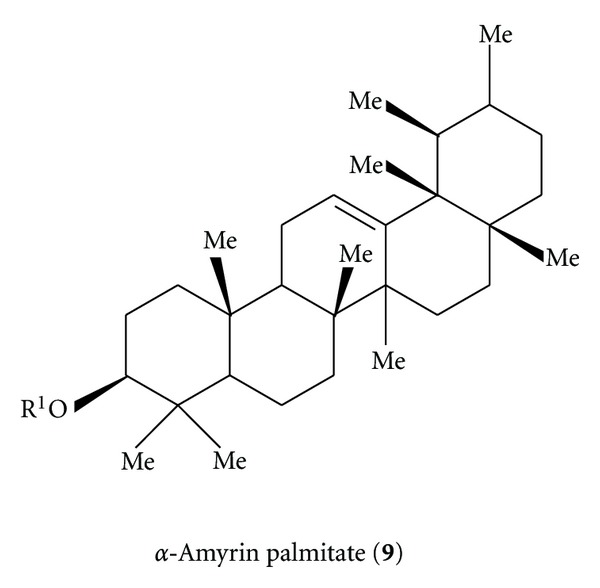
Triterpene compounds (R1 = H, C ≥ 10 fatty acid acyl) are useful as anti-inflammatory and antiarthritic agents [73]. α-Amyrin acetate isolated from petroleum ether extract of Alstonia boonei root bark was hydrolyzed with NaOH to α-amyrin followed by esterification with palmitoyl chloride to obtain α-amyrin palmitate (9). Rats were injected with 150 μL of complete Freund's adjuvant containing 10mg/mL Mycobacterium tuberculosis in the right hind footpad. Its structure is as shown in Scheme 9. From days 11–19 rats were fed orally with 56 mg I/kg in 1 mL of water. Regression analysis of the rate of the ankle diameter change from days 11–19, postadjuvant injection showed that the diameter of the ankle decreased by 31% in treated animals.
Scheme 9.
In a similar research work, the triterpenes-α-amyrin acetate, β-amyrin acetate, β-amyrin, and lupeol acetate isolated from the petroleum ether extract of Alstonia boonei De Wild, root barks were tested for their anti-inflammatory effects in CFA-induced arthritic rats [74]. When administered orally daily from days 11 to 19 after adjuvant, lupeol acetate and β-amyrin acetate were most effective in preventing further increases in ankle adjuvant swelling. All triterpenes abrogated the increases in WBC count, increased liver and/or kidney weights but only α-amyrin acetate significantly increased serum GOT levels. In the presence of β-amyrin, there was significant neutrophil degeneration. Triterpenes of Astonia boonei root barks were shown to be antiarthritic but the effects on liver and kidney weights raised the possibility of toxicity in antiarthritic therapy.
In another development, lupeol acetate isolated from the petroleum ether fraction of Alstonia boonei root barks was tested for its anti-arthritic effect in CFA-induced arthritic rats [75]. It was administered orally every 48 hrs (66 mg/kg body wt.) from days 32 to 40 after adjuvant and assessed on day 60. Lupeol acetate was able to return the increase in spleen weight and the reduction in serum alkyl phosphatase to nonarthritic control values.
The anti-inflammatory triterpenoids are also inhibitors of serine proteases [56]. The lupane triterpenoid lupeol, the ursane triterpenoid alpha-amyrin, and esters of these compounds present in the bark of roots of Alstonia boonei (Apocynaceae) have anti-inflammatory properties. Alpha-Amyrin is a competitive inhibitor of bovine trypsin and chymotrypsin (Ki values 29 microM and 18 microM, resp.). Lupeol linoleate, lupeol palmitate, and alpha-amyrin linoleate are noncompetitive inhibitors of trypsin (Ki values 7 microM, 10 microM, and 16 microM, resp.). Alpha-amyrin linoleate is also a non-competitive inhibitor of chymotrypsin (Ki value 28 microM). Lupeol is a competitive inhibitor of both trypsin and chymotrypsin (Ki values 22 and 8 microM, resp.). Alpha-amyrin palmitate is a potent non-competitive inhibitor of chymotrypsin (Ki 6 microM). Lupeol, alpha-amyrin, and the palmitic and linoleic acid esters of these compounds are ineffective or very weak as inhibitors of porcine pancreatic elastase and of Lucilia cuprina and Helicoverpa punctigera leucine aminopeptidases. These hydrophobic triterpenoids represent further examples of anti-inflammatory triterpenoids that are PKA inhibitors as well as being selective protease inhibitors.
When the methanol extract of the stem bark of Alstonia boonei was investigated for anti-inflammatory, analgesic, and antipyretic properties [76], it was found out that the extract caused a significant (P < 0.05) inhibition of the carrageenan-induced paw oedema, cotton pellet granuloma, and exhibited an anti-arthritic activity in rats. Vascular permeability induced by acetic acid in the peritoneum of mice was also inhibited. The extract also produced marked analgesic activity by reduction of writhing induced by acetic acid, as well as the early and late phases of paw licking in mice. A significant (P < 0.05) reduction in hyperpyrexia in mice was also produced by the extract. This study has established anti-inflammatory, analgesic, and antipyretic activities of the stem bark of Alstonia boonei.
The anti-inflammatory activity of a Ghanaian anti-arthritic herbal preparation was also investigated [76]. The herbal preparation is made of a boiling water extract from a powdered sample containing Alstonia boonei root bark (90%), Rauvolfia vomitoria root bark (5%), and Elaeis guineensis nut without pericarp (5%). The herbal preparation was tested intraperitoneally for its anti-inflammatory activity by measuring rat hind paw oedema induced by the subplantar injection of carrageenin in the presence or absence of arachidonic acid. Arachidonic acid increased swelling during the early phase of carrageenin oedema. The extract suppressed the late phase of carrageenin oedema and both phases in the presence of arachidonic acid. These preliminary results are consistent with a herbal preparation known to be used in the management of rheumatoid arthritis [77].
The extract was again tested for its anti-inflammatory activity by measuring over a period of 17 days the changes in rat ankle diameter caused by subplantar injection of complete Freund's adjuvant [76]. The extract fed in drinking water ad libitum reduced ipsilateral ankle adjuvant swelling by an average of 16% for the period of +4 to +17 days and improved weight gain.
α-Amyrin palmitate, present in the Ghanaian antiarthritic herbal preparation of Alstonia boonei, Elates guineensis, and Rauvolfia vomitoria, was synthesized and tested on complete Freund's adjuvant-induced arthritic rats [57]. Administered orally at 56 mg/kg body wt (BW) daily for 8 days from days 11 to 18 after adjuvant (acute) or at 66 mg/kg BW every 48 hours for 5 days from days 32 to 40 (chronic), the drug returned the increases in serum hyaluronate and blood granulocytes towards nonarthritic levels and correct the moderate anemia of adjuvant arthritis. Histological examinations of the proximal interphalangeal foot joints showed reduced synovial proliferation and invasion of joints and reduced leukocyte infiltration of bone marrow and periarticular tissue in treated rats. The results suggest that α-amyrin palmitate contributes to the previously shown antiarthritic effect of the herbal preparation.
Odeku [78] reported the anti-malarial property of the stem bark of Alstonia boonei, which could be formulated in tablet form. Odugbemi et al. [79] studied the anti-malarial activities of Alstonia boonei. Other important pharmacological properties of the plant are shown in Table 1.
Table 1.
An overview of research work on pharmacological properties of Alstonia boonei.
| Plant part used | Activity | Reference |
|---|---|---|
| Stem bark | Antiplasmodial (anti-malarial/antipyretic) | Bello et al. [51]; Ebiloma et al. [52]; Abel and Busia [53] |
| Stem bark | Insecticidal | Abel and Busia, [53]; Oigiangbe et al. [54] |
| Root bark | Anti-inflammatory | Osadebe et al. [55]; Olajide et al. [56] |
| Stem bark | Analgesic, aphrodisiac, trypanocidal | Kweifio-Okai et al. [57]; Asuzu and Anaga [58] |
| Stem bark | Sedative | Adesina [59] |
| Stem bark | Anti-snake venom | Gabriel et al. [60]; Majekodunmi [61]; Olajide et al. [56] |
| Stem bark | insomnia | Asuzu and Anaga [58] |
| Leaves | Antidiabetic | Din et al. [62] |
| Stem bark | Antihelminthic | Wesche et al. [63] |
| Stem bark, leaves | Antimicrobial | Adomi [64]; Omoregbe et al. [65] |
| Stem bark | Rheumatic pains | Olajide et al. [56] |
| Stem bark | Antidiarrhoeal | Amole and Ilori [66] |
| Stem bark | Spasmolytic and hypotensive | Iwu, [67]; Forster et al. [68] |
| Stem bark | Abortifacient, astringent, immunostimulant potentials | Taiwo et al. [69] |
| Stem bark | Antipsychotic | Elisabetsky and Costa-campos [70] |
5. Toxicology, Contaminants, and Side Effects
Modern synthetic medicines have undergone various levels of experiments for safety and efficacy unlike some herbal preparations. Large proportion of public assumes that herbal remedies or complementary and alternative medicine (CAM) are inherently safe because it is natural. It is important to remember that the majority of powerful chemicals found in plant, which can be used to treat human diseases, have evolved to serve different purposes in the plant itself. For instance, the chemical is being used to protect the plant from insects. These natural insecticides will soon poison the bugs. In sufficient high doses, it can harm human too. The World Health Assembly has highlighted the need to develop herbal pharmacopoeias and to develop and apply scientific criteria and methods for proof of safety and efficacy of medicinal plant products. However, only few countries have developed national herbal pharmacopoeias; limited plant species that provide medicinal herbs have been scientifically evaluated for their possible medical applications; the safety and efficacy data are available for even fewer herbs. Without well-documented information on the safety, efficacy, and phytochemical characteristics of different compounds, it is difficult for external buyers to assess the likely utility or value of some new raw materials and extracts of African origin. In order to address these lacunae, the Association of African Medicinal Plants Standards is developing an African Herbal Pharmacopeia with trading standards which provide information and technical data on some 50 important medicinal plants.
6. Conclusion
Notwithstanding the potential pharmacological benefits of Alstonia boonei in particular and medicinal plants in general, herbal pharmacopeia are largely lacking. The main problem facing the use of traditional medicines is the proof requirement that the active components contained in medicinal plants are useful, safe, and effective. This is required to assure the medical field and the public regarding the use of medicinal plants as drug alternatives. The proofs of pharmacology activity that are available at present are mostly based on empirical experience. The scientific proof then becomes the most important thing, in order to eliminate the concern of using medicinal plants as drugs for alternative treatment. This study attempted to synthesize work on Alstonia boonei De Wild, a medicinal plant used in African alternative medicine for its anti-malarial, aphrodisiac, anti-diabetic, antimicrobial, and antipyretic activities. This study shows the potential of the plant that research on dose-dependent safety, side effects. and toxicological issues regarding the use of the plant in alternative medicine are essentially lacking; the situation might limit widespread commercial adoption.
References
- 1.Farnsworth N, Akerele AO, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bulletin of the World Health Organization. 1985;63(6):965–981. [PMC free article] [PubMed] [Google Scholar]
- 2.Cragg G, Newman DJ. Biodiversity: a continuing source of novel drug leads. Pure and Applied Chemistry. 2005;77(1):7–24. [Google Scholar]
- 3.Van Wyk B-E. A broad review of commercially important Southern African medicinal plants. Journal of Ethnopharmacology. 2008;119(3):342–355. doi: 10.1016/j.jep.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 4.Rukangira E. Overview on medicinal plants and traditional medicine in Africa. Qualitative Health Research. 2004;14:675–690. [Google Scholar]
- 5.Ayiku MNB. Ghana Herbal Pharmacopoeia. Technology Transfer Centre; 1992. [Google Scholar]
- 6.Gosse BK, Bryson TA, Gokou T. Study of triterpenoids from Alstonia boonei. Société Ouest-Africaine de Chimie. 1999;5(8):123–128. [Google Scholar]
- 7.Abbiw D. Useful Plants of Ghana: West African Uses of Wild and Cultivated Plants. Kew, London: Intermediate Technology Publications, Royal Botanical Garden; 1990. [Google Scholar]
- 8.Hadi S, Bremner JB. Initial studies on alkaloids from Lombok medicinal plants. Molecules. 2001;6(2):117–129. [Google Scholar]
- 9.Fakae BB, Campbell AM, Barrett J, et al. Inhibition of glutathione S-transferases (GSTs) from parasitic nematodes by extracts from traditional Nigerian medicinal plants. Phytotherapy Research. 2000;14(8):630–634. doi: 10.1002/1099-1573(200012)14:8<630::aid-ptr773>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Kam TS, Nyeoh KT, Sim KM, Yoganathan K. Alkaloids from Alstonia scholaris. Phytochemistry. 1997;45(6):1303–1305. [Google Scholar]
- 11.Kucera M, Marquis VO, Kucerova H. Nigerian medicinal plants. I. TLC thin-layer chromatographic] separation and quantitative evaluation of Alstonia boonei alkaloids. Planta Medica. 1972;21(4):343–346. doi: 10.1055/s-0028-1099562. [DOI] [PubMed] [Google Scholar]
- 12.Croquelois G, Kunesch N, Debray M, Poisson J. Alstonia boonei alkaloids. Medicinal Plants and Phytotherapy. 1972;6(2):122–127. [Google Scholar]
- 13.Oguakwa JU. Nα-formylechitamidine, an alkaloid from Alstonia boonei. Phytochemistry. 1984;23(11):2708–2709. [Google Scholar]
- 14.Ojewole JAO. Studies on the pharmacology of echitamine, an alkaloid from the stem bark of Alstonia boonei L. (Apocynaceae) International Journal of Crude Drug Research. 1984;22(3):121–143. [Google Scholar]
- 15.Ojewole JAO. Autonomic pharmacology of echitamine, an alkaloid from Alstonia boonei De Wild. Fitoterapia. 1983;54(3):99–113. [Google Scholar]
- 16.Chandrasekaran B, Nagarajan B. Growth inhibitory effect of echitamine and plumbagin on fibrosarcoma in rats. Arogya. 1983;9(1):60–63. [Google Scholar]
- 17.Saraswathi V, Shyamala AC, Subramanian S, Govindasamy S. Studies on the effects of echitamine chloride on serum glycoproteins and lysosomal hydrolases of sarcoma-180 induced mice. Fitoterapia. 1998;69(1):73–75. [Google Scholar]
- 18.Saraswathi V, Ramamoorthy N, Subramaniam S, Mathuram V, Gunasekaran P, Govindasamy S. Inhibition of glycolysis and respiration of sarcoma-180 cells by echitamine chloride. Chemotherapy. 1998;44(3):198–205. doi: 10.1159/000007115. [DOI] [PubMed] [Google Scholar]
- 19.Saraswathi V, Mathuram V, Subramanian S, Govindasamy S. Modulation of the impaired drug metabolism in sarcoma-180 bearing mice by echitamine chloride. Cancer Biochemistry Biophysics. 1999;17(1-2):79–88. [PubMed] [Google Scholar]
- 20.Saraswathi V, Subramanian S, Ramamoorthy N, Mathuram V, Govindasamy S. In vitro cytotoxicity of echitamine chloride and adriamycin on Ehrlich ascites carcinoma cell cultures. Medical Science Research. 1997;25(3):167–170. [Google Scholar]
- 21.Viswanathan S, Ramamurthy N, Subramanian S, Mathuram V, Govindasamy S. Enhancement of the cytotoxic effects of Echitamine chloride by vitamin A: an in vitro study on Ehrlich ascites carcinoma cell culture. Indian Journal of Pharmacology. 1997;29(4):244–249. [Google Scholar]
- 22.Kamarajan P, Sekar N, Mathuram V, Govindasamy S. Antitumor effect of echitamine chloride on methylcholonthrene induced fibrosarcoma in rats. Biochemistry International. 1991;25(3):491–498. [PubMed] [Google Scholar]
- 23.Keawpradub N, Houghton PJ, Eno-Amooquaye E, Burke PJ. Activity of extracts and alkaloids of Thai Alstonia species against human lung cancer cell lines. Planta Medica. 1997;63(2):97–101. doi: 10.1055/s-2006-957621. [DOI] [PubMed] [Google Scholar]
- 24.Biswas GK, Saharia GS. A new alkaloidal glycoside from Alstonia scholaris. Indian Journal of Chemistry. 1978;1(1):66–67. [Google Scholar]
- 25.Kam TS, Nyeoh KT, Sim KM, Yoganathan K. Alkaloids from Alstonia scholaris. Phytochemistry. 1997;45(6):1303–1305. [Google Scholar]
- 26.Morita Y, Hesse M, Schmid H. Alstonia scholaris: the structure of the indole alkaloid nareline. Helvetica Chimica Acta. 1977;60(4):1419–1434. doi: 10.1002/hlca.19770600434. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi T, Abe F, Padolina WG, Dayrit FM. Alkaloids from leaves and bark of Alstonia scholaris in the Philippines. Phytochemistry. 1990;29(10):3321–3325. [Google Scholar]
- 28.Yamauchi T, Abe F, Chen RF, Nonaka GI, Santisuk T, Padolina WG. Alkaloids from the leaves of Alstonia scholaris in Taiwan, Thailand, Indonesia and the Philippines. Phytochemistry. 1990;29(11):3547–3552. [Google Scholar]
- 29.Yamauchi T, Abe F. Regional differences of indole alkaloids in Alstonia scholaris and Alstonia macrophylla. International Congress Series. 1998;1157:51–58. [Google Scholar]
- 30.Boonchuay W, Court WE. Minor alkaloids of Alstonia scholaris root. Phytochemistry. 1976;15(5):p. 821. doi: 10.1055/s-0028-1097681. [DOI] [PubMed] [Google Scholar]
- 31.Abe F, Chen RF, Yamauchi T, et al. Alkaloids From the Leaves of Alstonia scholaris. Vol. 31. Fukuoka, Japan: Fukuoka University; 1989. [Google Scholar]
- 32.Abe F, Chen RF, Yamauchi T, Marubayashi N, Ueda I. Studies on the constituents of Alstonia scholaris. Part I. Alschomine and isoalschomine, new alkaloids from the leaves of Alstonia scholaris. Chemical & Pharmaceutical Bulletin. 1989;37(4):887–890. [Google Scholar]
- 33.Ghosh R, Roychowdhury P, Chattopadhyay D, Iitaka Y. The structure of an alkaloid, picrinine, from Alstonia scholaris. Acta Crystallographica C. 1988;44(12):2151–2154. [Google Scholar]
- 34.Banerji J, Chakrabarti R. Constituents of Alstonia schorlaris R.Br:-conversion of picrinine into strictamine and to a pyrrolidinoindolenine system. Indian Journal of Chemistry B. 1984;23(5):453–454. [Google Scholar]
- 35.Bhattacharya SK, Bose R, Dutta SC, Ray AB, Guha SR. Neuropharmacological studies on strictamine isolated from Alstonia scholaris. Indian Journal of Experimental Biology. 1979;17(6):598–600. [PubMed] [Google Scholar]
- 36.Rahman A, Alvi KA. Indole alkaloids from Alstonia scholaris. Phytochemistry. 1987;26(7):2139–2142. [Google Scholar]
- 37.Rahman A, Alvi KA, Abbas SA, Voelter W. Isolation of 19,20-Z-vallesamine and 19,20-E-vallesamine from Alstonia scholaris. Heterocycles. 1987;26(2):413–419. [Google Scholar]
- 38.Rahman A, Alvi KA, Muzaffar A. Isolation and 1H/13C-NMR studies on 19,20-dihydrocondylocarpine: an alkaloid from the leaves of Ervatamia coronaria and Alstonia scholaris. Planta Medica. 1986;52(4):325–326. doi: 10.1055/s-2007-969167. [DOI] [PubMed] [Google Scholar]
- 39.Banerji A, Siddhanta AK. Scholarine: an indole alkaloid of Alstonia scholaris. Phytochemistry. 1981;20(3):540–542. [Google Scholar]
- 40.Banerji A, Banerji J. Isolation of pseudoakuammigine from the leaves of Alstonia scholaris R. Br. Indian Journal of Chemistry B. 1977;15(4):390–391. [Google Scholar]
- 41.Dutta SC, Bhattacharya SK, Ray AB. Flower alkaloids of Alstonia scholaris. Planta Medica. 1976;30(1):86–89. doi: 10.1055/s-0028-1097699. [DOI] [PubMed] [Google Scholar]
- 42.Boonchuay W, Court WE. Alkaloids of Alstonia scholaris from Thailand. Planta Medica. 1976;29(4):380–390. doi: 10.1055/s-0028-1097681. [DOI] [PubMed] [Google Scholar]
- 43.Rastogi RC, Kapil RS, Popli SP. Picralinal—A key alkaloid of picralima group from Alstonia scholaris. Experientia. 1970;26(10):p. 1056. doi: 10.1007/BF02112667. [DOI] [PubMed] [Google Scholar]
- 44.Chatterjee A, Mukherjee B, Ghosal S, Banerjee PK. Occurrence of rhazine in Alstonia scholaris: biogenetic and chemotaxonomic significance of the co-occurrence of several indole alkaloids having a common structural pattern. Journal of the Indian Chemical Society. 1969;46(7):635–638. [Google Scholar]
- 45.Rahman A, Asif M, Ghazala M, Fatima J, Alvi KA. Scholaricine, an alkaloid from Alstonia scholaris. Phytochemistry. 1985;24(11):2771–2773. [Google Scholar]
- 46.Banerji J, Mustafi R, Roy DJ. (-)-Scholarine and (+)-lochneridine—constituents of Alstonia scholaris R.Br. (Apocynaceae) Indian Journal of Chemistry B. 1984;23(5):p. 455. [Google Scholar]
- 47.Chauhan JS, Chaturvedi R, Kumar S. Isookanin-7-O-a-L-rhamnopyranoside, a new flavanone glycoside from the roots of Alstonia scholaris R. Br. Indian Journal of Chemistry B. 1985;24(2):p. 219. [Google Scholar]
- 48.Desoky EK. Flavonoidal contents of Alstonia scholaris R.Br. Bulletin of Pharmaceutical Sciences. 1999;22(2):117–121. [Google Scholar]
- 49.Marini-Bettolo GB, Nicoletti M, Messana I, et al. Research on African medicinal plants-IV1 1 Part III: see ref. 1. Boonein, a new C-9 terpenoid lactone from Alstonia boonei: a possible precursor in the indole alkaloid biogenesis. Tetrahedron. 1983;39(2):323–329. [Google Scholar]
- 50.Desoky EK, Kamel MS, Bishay DW. Sterols and triterpenes from Alstonia scholaris R.Br. Bulletin of Pharmaceutical Sciences. 2000;23(1):65–71. [Google Scholar]
- 51.Bello IS, Oduola T, Adeosun OG, Omisore NOA, Raheem GO, Ademosun AA. Evaluation of antimalarial activity of various fractions of Morinda lucida leaf extract and Alstonia boonei stem bark. Global Journal of Pharmacology. 2009;3:163–165. [Google Scholar]
- 52.Ebiloma G, Amlabu E, Atanu FO, Amlabu W, Aminu Rhoda O. Effect of the aqueous extracts of Alstonia boonei on the haematological profiles of mice experimentally infected with the chloroquine-sensitive strain of Plasmodium berghei NK-65. Hematologia. 2012;1:11–18. [Google Scholar]
- 53.Abel C, Busia K. An exploratory ethnobotanical study of the practice of herbal medicine by the Akan peoples of Ghana. Alternative Medicine Review. 2005;10(2):112–122. [PubMed] [Google Scholar]
- 54.Oigiangbe ON, Igbinosa IB, Tamo M. Insecti cidal activity of the medicinal plant, Alstonia boonei De Wild, against Sesamia calamistis Hampson. Journal of Zhejiang University Science B. 2007;8(10):752–755. doi: 10.1631/jzus.2007.B0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osadebe PO, Okide GB, Akabogu IC. Study on anti-diabetic activities of crude methanolic extracts of Loranthus micranthus (Linn.) sourced from five different host trees. Journal of Ethnopharmacology. 2004;95(2-3):133–138. doi: 10.1016/j.jep.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 56.Olajide OA, Awe SO, Makinde JM, et al. Studies on the anti-inflammatory, antipyretic and analgesic properties of Alstonia boonei stem bark. Journal of Ethnopharmacology. 2000;71(1-2):179–186. doi: 10.1016/s0378-8741(99)00200-7. [DOI] [PubMed] [Google Scholar]
- 57.Kweifio-Okai G, Bird D, Field B, et al. Antiinflammatory activity of a Ghanaian antiarthritic herbal preparation: III. Journal of Ethnopharmacology. 1995;46(1):7–15. doi: 10.1016/0378-8741(95)01222-y. [DOI] [PubMed] [Google Scholar]
- 58.Asuzu U, Anaga AO. Pharmacological screening of the aqueous extract of Alstonia boonei bark. Fitoterapia. 1991;63(5):411–417. [Google Scholar]
- 59.Adesina SK. Studies on a Nigerian herbal anticonvulsant recipe. International Journal of Crude Drug Research. 1982;20:93–100. [Google Scholar]
- 60.Gabriel O, Harrision N, Okey O, Ukoha A. Changes in lipid and haematological profile of aqueous ethanolic extract of Alstonia boonei in rats. The Internet Journal of Hematology. 2008;4(1):1–5. [Google Scholar]
- 61.Majekodunmi SO. Formulation of the extract of the stem bark of Alstonia booneias tablet dosage form. Tropical Journal of Pharmaceutical Research. 2008;7(2):987–994. [Google Scholar]
- 62.Din N, Dibong SD, Mpondo Mpondo E, Priso RJ, Kwin NF, Ngoye A. Inventory and identification of plants used in the treatment of diabetes in Douala town (Cameroon) European Journal of Medicinal Plants. 2011;1(3):60–73. [Google Scholar]
- 63.Wesche D, Black D, Milhous WK. Proceedings of the 39th Annual Meeting of the American Society of Tropical Medicine and Hygiene; 1990; New Orleans, LA, USA. [Google Scholar]
- 64.Adomi PO. Antibacterial activity of aqueous and ethanol extracts of the stembark of Alstonia boonei and M. Iucida. Scientific Research and Essays. 2006;1(2):50–53. [Google Scholar]
- 65.Omoregbe RE, Ikuebe OM, Ihimire IG. Antimicrobial activity of some medicinal plants extracts on Escherichia coli, Salmonella paratyphi and Shigella dysenteriae. African Journal of Medicine and Medical Sciences. 1996;25(4):373–375. [PubMed] [Google Scholar]
- 66.Amole OO, Ilori OO. Antimicrobial activity of the aqueous and ethanolic extracts of the stem bark of Alstonia boonei. International Journal of Phytopharmacology. 2010;1(2):119–123. [Google Scholar]
- 67.Iwu MM. Handbook of African Medicinal Plants. London, UK: CRC Press; 1993. [Google Scholar]
- 68.Forster H, Niklas H, Lutz S. Antispasmodic effects of some medicinal plants. Planta Medica. 1980;40(4):309–319. doi: 10.1055/s-2008-1074977. [DOI] [PubMed] [Google Scholar]
- 69.Taiwo OB, Kroes BH, Beukelman CJ, Horsten STAJ, Makinde JM, Labadie RP. Activity of the stem bark extract of Alstonia boonei de wild (apocynaceae) on human complement and polymorphonuclear leukocytes. Journal of Ethnopharmacology. 1998;17:13–15. [Google Scholar]
- 70.Elisabetsky E, Costa-Campos L. The alkaloid alstonine: a review of its pharmacological properties. Evidence-Based Complementary and Alternative Medicine. 2006;3(1):39–48. doi: 10.1093/ecam/nek011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khaleque A, Ismail KM, Shafiullah M. Chemical investigations on Alstonia scholaris. Part I: isolation of lupeol acetate, β-sitosterol, one alkaloid and three other non-alkaloidal compounds from the bark. Bangladesh Journal of Scientific & Industrial Research. 1991;26(1–4):1–7. [Google Scholar]
- 72.Kweifio-Okai G. Triterpene Compounds Having Antiinflammatory and Antiarthritic Activity. PCT International; 1993. [Google Scholar]
- 73.Kweifio-Okai G, Carroll G. Antiarthritic effect of Alstonia boonei triterpenes. Recent Advances in Toxinology Research. 1992;10(3):19–28. [Google Scholar]
- 74.Kweifio-Okai G, Carroll AR. Antiarthritic effect of lupeol acetate. Phytotherapy Research. 1993;7(2):213–215. [Google Scholar]
- 75.Rajic A, Kweifio-Okai G, Macrides T, Sandeman RM, Chandler DS, Polya GM. Inhibition of serine proteases by anti-inflammatory triterpenoids. Planta Medica. 2000;66(3):206–210. doi: 10.1055/s-2000-8657. [DOI] [PubMed] [Google Scholar]
- 76.Kweifio-Okai G. Antiinflammatory activity of a Ghanaian antiarthritic herbal preparation: I. Journal of Ethnopharmacology. 1991;33(3):263–267. doi: 10.1016/0378-8741(91)90087-t. [DOI] [PubMed] [Google Scholar]
- 77.Kweifio-Okai G. Antiinflammatory activity of a Ghanaian antiarthritic herbal preparation: II. Journal of Ethnopharmacology. 1991;33(1-2):129–133. doi: 10.1016/0378-8741(91)90171-9. [DOI] [PubMed] [Google Scholar]
- 78.Odeku A. Formulation of the extract of the stem bark of Alstonia boonei. Tropical Journal of Pharmaceutical Research. 2008;7(2):987–994. [Google Scholar]
- 79.Odugbemi TO, Akinsulire OR, Aibinu IE, Fabeku PO. Medicinal plants useful for malaria therapy in Okeigbo, Ondo State, Southwest Nigeria. African Journal of Traditional, Complementary and Alternative Medicines. 2007;4(2):191–198. doi: 10.4314/ajtcam.v4i2.31207. [DOI] [PMC free article] [PubMed] [Google Scholar]


