Abstract
Objective. To determine whether genetic variants suggested by the literature to be associated with physiology and fitness phenotypes predicted differential physiological and subjective responses to a bout of aerobic exercise among inactive but otherwise healthy adults. Method. Participants completed a 30-minute submaximal aerobic exercise session. Measures of physiological and subjective responding were taken before, during, and after exercise. 14 single nucleotide polymorphisms (SNPs) that have been previously associated with various exercise phenotypes were tested for associations with physiological and subjective response to exercise phenotypes. Results. We found that two SNPs in the FTO gene (rs8044769 and rs3751812) were related to positive affect change during exercise. Two SNPs in the CREB1 gene (rs2253206 and 2360969) were related to change in temperature during exercise and with maximal oxygen capacity (VO2 max). The SLIT2 SNP rs1379659 and the FAM5C SNP rs1935881 were associated with norepinephrine change during exercise. Finally, the OPRM1 SNP rs1799971 was related to changes in norepinephrine, lactate, and rate of perceived exertion (RPE) during exercise. Conclusion. Genetic factors influence both physiological and subjective responses to exercise. A better understanding of genetic factors underlying physiological and subjective responses to aerobic exercise has implications for development and potential tailoring of exercise interventions.
1. Introduction
In the United States, insufficient participation in leisure time physical activity constitutes a major threat to public health. Recent estimates suggest that 25% of Americans do not engage in any physical activity at all [1]. Even those engaging in physical activity are usually not doing so at recommended levels. In order to promote and maintain health, the American College of Sports Medicine (ACSM) recommends a minimum of 30 minutes of moderate intensity aerobic physical activity five days a week or a minimum of 20 minutes of vigorous intensity aerobic physical activity three days a week [2]. Despite these widely disseminated guidelines, the Centers for Disease Control (CDC) report that Americans have made “no substantial progress towards achieving recommended levels of physical activity” with the proportion of 18–29 year olds meeting guidelines hovering around 35% and the proportion of adults 65 and older meeting guidelines at about 20% [1].
These numbers are troubling, as aerobic exercise has been convincingly linked to the prevention of myriad negative health outcomes, including several forms of cancer. Numerous studies conducted over the past two decades have explored the association between physical activity participation and cancer prevention, consistently implicating strong or probable evidence for reduced risk of colon, breast, and endometrial cancers when physical activity recommendations are followed [3–6]. Likely mechanisms through which physical activity is believed to have an influence on cancer prevention include reduction in adiposity and changes to levels of circulating metabolic hormones and growth factors (e.g., estrogen, testosterone, and insulin-like growth factors) [7–9] as well as influences on DNA methylation [10, 11]. In respect to prostate cancer, because physical activity activates gut motility, gastrointestinal transit time for food wastes is lessened and thus, exposure to carcinogens is attenuated [7]. There is also research to suggest that immune function changes may mediate the relationship between physical activity and cancer development [9].
The promising body of literature regarding the relationship between physical activity and cancer has led the National Cancer Institute (NCI) to regard behavioral primary prevention of cancer (e.g., physical activity) as a top priority [12]. Unfortunately, interventions designed to change behavior are typically met with only modest success, even when grounded in empirically supported theory [13], and behavioral adherence is reported to be a principal challenge faced by exercise promotion programs [14]. Indeed, only 50% of individuals who adopt an exercise program stay with it for more than six months [14, 15]. Researchers devoted to the goal of improved physical activity participation have suggested that one likely determinant of physical activity behavior is the way in which individuals subjectively experience exercise [16].
In previous work, we organized genetic, physiological, subjective, and motivational factors that may contribute to the initiation and maintenance of physical activity into a conceptual transdisciplinary framework [16, 17]. This framework has received support among both active [16] and inactive [17] samples, and provides the basis for the selection of phenotypes in the current study. Briefly, we proposed that genetic factors influence how an individual physiologically (e.g., body-temperature regulation) and subjectively (e.g., affective response, perceived exertion) responds to the experience of exercise. Physiological response influences how one subjectively responds to the experience of exercise (e.g., increased lactate during exercise may increase perceived exertion) and these subjective responses influence motivation to exercise (e.g., exercise self-efficacy, exercise intentions). Exercise motivation then influences the likelihood of engaging in exercise. Moreover, exercise behavior itself influences both how a person physiologically responds to the experience of exercise and gene expression [18], thereby recapitulating the framework. Importantly, this framework is meant to be dynamic such that the factors selected to represent physiological response, subjective response, and/or motivation can vary depending on the goals of each individual research study.
The relationship between physiological changes induced by aerobic exercise (e.g., regulation of body temperature, heart rate, or blood pressure during exercise) and subjective responses to aerobic exercise (e.g., changes in affect during or immediately after exercise, ratings of perceived exertion or pain during an exercise bout) is one that has a clear influence on individual differences in exercise behavior. Bryan and colleagues [16] found that physiological factors such as heart rate were related to mood response to exercise, and that mood response was a significant correlate of both motivation to exercise in the future and of current exercise behavior. Additionally, subjective experiences during exercise may be influenced by interpretations of exercise-induced physiological responses. For instance, increases in lactate levels during aerobic exercise may be perceived as painful to varying degrees across individuals, and this perceived pain will in turn influence subjective exercise experiences and potentially impact motivation to engage in exercise behavior in the future. Understanding potential influences on subjective response to exercise is especially important, given that affective responses to acute exercise have been found to predict long-term exercise behavior [19, 20].
Although the heritability of exercise participation in adults has been shown in twin studies to be approximately 50% (with peak heritability of 85%, occurring at age 19-20) [21, 22], there is a surprising lack of research regarding the role played by genetic factors for determining physiological and affective responses to exercise. These responses may serve as promising intermediate phenotypes for the linkage of genes to broader exercise participation phenotypes [23]. Also important is the notion of a “gene by exercise interaction,” explained by de Geus and de Moor [21] as the genetic variance causing differential responses to exercise training, given that the effects of exercise on health and fitness gains appear not to be uniform across individuals [24, 25]. One type of gene-by-exercise interaction that is relevant to the present study is the role of exercise in reducing the phenotypic effects of some detrimental genetic variants. For example, Phares et al., [26] showed that sedentary individuals who possess two particular polymorphisms of the ADR gene have unfavorable body composition. However, these individuals experience greater loss in percent body fat after 24 weeks of aerobic training in comparison with all other genotypes. It follows that weight loss interventions for individuals with this particular genotype would likely be successful if they focused on aerobic training. Thus, identifying particular genetic markers that are related to exercise behavior and physiological and affective responses to exercise may have clear implications for matching individuals to tailored exercise intervention programs.
The goal of the current study is to determine whether genotypes predicted subjective physiological and affective responses to a 30-minute bout of aerobic exercise among sedentary individuals. Based on the literature and on our prior analysis of the relationships among a range of exercise response phenotypes (see [17] for analysis and detailed information on the rationale for selection of phenotypes), the variables from the physiological responses to exercise domain included in our analysis were temperature, heart rate, systolic blood pressure, lactate, and norepinephrine, all measured as change scores from immediately prior to a bout of exercise to 30-minutes into the bout (just before the end of the bout). Genetic associations with VO2 max were also examined, as cardiovascular fitness is highly heritable [27–30], and evidence exists for a strong genetic influence on athletic performance [31]. Additionally, genetically influenced cardiovascular fitness traits play a role in determining individual experience of exercise intensity and perception of exertion during exercise [32]. The variables selected from the subjective experience of exercise domain were affect (i.e., positive affect and affective valence), perceived pain, and rate of perceived exertion (RPE) [17], which were also change scores measured from prior to the bout to just before the end of the bout.
We chose the specific genetic factors for our analyses a priori based on evidence from the literature that they were linked to processes related to physiological and subjective responses during physical activity, general health and fitness traits, or because of evidence that they moderate responses to exercise interventions. A single nucleotide polymorphism (SNP) in the fat mass and obesity-associated protein gene (FTO; rs9930506) has been associated with obesity traits such as increased BMI and weight [33] and susceptibility to obesity [34]. Additionally, physical activity may slow down weight gain associated with the FTO risk-allele [35]. In addition, the μ-opioid receptor gene (OPRM1) may be associated with pain sensitivity such that individuals possessing the rare G allele have an increased pressure pain threshold [36]. Interestingly, this study also found gender differences in pain threshold among individuals with the G allele when heat pain was tested, such that women with this allele have lowered pain thresholds, and men exhibit higher pain thresholds. SNPs located within in the SLIT2 gene(rs1379659), FAM5C gene (rs1935881), KCNB2 gene (rs10505543), and rs10498091 (an SNP associated with left ventricle mass) have all been found to be associated with echocardiography traits (e.g., left ventricle diastolic dimension, diameter, and systolic dimension) in a genome-wide association study [37]. Another genome-wide analysis implicated CREB1 in the prediction of submaximal exercise heart rate in response to exercise training [38, 39]. Thus, each of these SNPs was investigated in the current study in order to determine potential relationships with phenotypes related to physiological and affective response to an acute bout of aerobic exercise.
2. Methods
2.1. Participants
Participants included in the present analysis were a subset of 238 individuals from a larger intervention study (COSTRIDE) [17, 40] in which participants were randomly assigned to the STRIDE exercise intervention (COSTRIDE) or a health-and-wellness contact control condition (HW). Participants were men and women (ages 18–45) who reported less than 90 minutes on average of at least moderate-intensity physical activity per week for the past three months. Individuals were excluded if they smoked cigarettes, were on a restricted diet, were taking psychotropic medications, were receiving treatment for any psychiatric disorder, were diabetic, had a history of cardiovascular or respiratory disease, had the flu or illness within the last month, or were pregnant (if female). All participants were required to be willing to be randomized to an intervention condition, to give informed consent, to be able to engage in moderate-intensity physical activity, to have a body mass index (BMI) between 18 and 37.5, and to have a regular menstrual cycle (if female). All participants were recruited from the Denver-metro area and the University of Colorado Boulder community [25]. The data reported herein are from assessments conducted prior to randomization, and the analysis and questions addressed are unique to this investigation.
As described in detail below, we used the Illumina Human 1M DuoV3 DNA Analysis BeadChip to genotype the DNA samples. The bead chips accommodate 4 samples each, and we ran a total of 50 bead chips. Thus, this experiment allowed for the genotyping of 200 individuals total. Due to limitations in funding, we were unable to genotype the remaining 38 individuals in the sample. Thus, individuals with the most complete baseline data (baseline DNA sample, self-report questionnaire assessments, VO2 max fitness assessment, and submaximal exercise session) were selected to be genotyped out of the full sample.
Statistical tests revealed no significant differences on demographic variables between participants who were included in genotyping procedures and those who were not included in genotyping procedures (details available from the first author). This reduced sample (N = 200) was comprised primarily of females (n = 160) and most participants identified as white (n = 137), followed by Hispanic/Latino (n = 22), Asian American (n = 22), African American (n = 9), Native American (n = 5), and mixed ethnicity (n = 5), The average age of participants at baseline was 28.68 (SD= 7.86) years old and mean body mass index (BMI; weight in kg/height in m2) was 25.18 (SD = 4.72). On average participants reported an average of 28.14 minutes of voluntary physical activity in the past week (SD= 50.95), and reached an average VO2 max peak of 34.06 mL/kg/min (SD= 8.11). Additional demographic characteristics are reported in Table 1.
Table 1.
Sample demographics.
| Characteristic | Frequencies | M |
|---|---|---|
| N | 200 | |
| Gender | ||
| Female | 160 | |
| Male | 40 | |
| Age | 28.68 (SD = 7.86) | |
| 18–25 | 78 | |
| 26–35 | 74 | |
| 36–45 | 48 | |
| BMI | 25.18 (SD = 4.72) | |
| Underweight (≤18.49) | 3 | |
| Normal weight (18.50–24.99) | 101 | |
| Overweight (25.00–29.99) | 57 | |
| Obese (30.00–34.99) | 29 | |
| Extreme obese (≥35.00) | 7 | |
| Ethnicity | ||
| White | 137 | |
| African American | 9 | |
| Asian | 22 | |
| Hispanic/Latino | 22 | |
| Native American | 5 | |
| Mixed ethnicity | 5 | |
| Other | 0 | |
| Number of years of education | 15.81 (SD = 2.64) | |
| ≤12 | 22 | |
| 13–16 | 117 | |
| 17–20 | 53 | |
| 21–24 | 5 | |
| 25-26 | 3 | |
| Average annual household income | ||
| $0–9,999 | 14 | |
| $10–29,999 | 39 | |
| $30,000–49,999 | 37 | |
| $50,000–69,999 | 40 | |
| $70,000–89,999 | 31 | |
| $90,000–109,999 | 20 | |
| ≥$110,000 | 14 |
Note: SD: standard deviation. BMI: body mass index. BMI is calculated as weight in kg/height in m2.
2.2. Procedure
Prior to randomization to intervention condition and after giving informed consent, participants completed three sessions: (1) an orientation (baseline) session in which self-report questionnaire assessments were completed, (2) a VO2 max cardiovascular fitness assessment, and (3) a submaximal exercise session. Prior to exercise sessions, each participant was instructed to eat a meal comprised of both carbohydrates and protein and to consume at least 300 calories two hours before coming into the lab (e.g., If a participant is scheduled to come into the lab at 12:00 p.m. a researcher instructed him/her to eat the 300 calorie meal at 10:00 a.m. and no later). Participants were also instructed to drink at least 17 oz. of water two hours prior to coming into the lab. Participants were instructed not to exercise on their own prior to the laboratory session, and not to consume alcohol during the 24 hours prior to testing. Further details regarding recruitment, selection of measures, and study procedures are available elsewhere [17]. This research was approved by all relevant institutional review boards.
2.2.1. Cardiorespiratory Fitness Test (VO2 Max)
Consistent with established procedures [41], maximal oxygen capacity (VO2 max) was assessed during a Balke protocol (a graded, incremental exercise test) on a motorized treadmill [42]. VO2 max was assessed with online computer-assisted open-circuit spirometry using the Medgraphics Cardi02/CP system. Prior to the fitness test, saliva samples (5 mL) were collected for DNA extraction and measurements of height and weight were taken for calculation of BMI.
2.2.2. Submaximal Exercise Session
Approximately one week after the fitness test, participants completed a standardized, short 30-minute bout of physical activity on the treadmill at 65% of their previously established VO2 max, calculated during the fitness test (VO2 max test session). Prior to beginning activity, an intravenous catheter was inserted by a nurse to collect blood samples during the bout. Intensity was maintained by measuring oxygen uptake and expired CO2 for two to three minutes at the beginning of exercise and at 10 and 20 minutes during exercise.
2.2.3. Physiological Phenotypes
Lactate concentration and catecholamine levels (epinephrine and norepinephrine) were collected via blood samples immediately before activity began (11.5 mL), and 10 (5.5 mL) and 30 (11.5 mL) minutes into activity. Tympanic temperature was measured by taking an average of 2-3 temperature readings at each measurement. Readings of temperature, blood pressure, and heart rate were taken before activity, at 10 minutes, 20 minutes, and 30 minutes (directly before completion) during activity.
2.2.4. Subjective Phenotypes
Subjective experiences during exercise were assessed at six points during the submaximal session: five minutes prior to activity, immediately before activity began, and 10, 20, and 30 minutes into activity (directly before completion of the session). The present study focuses only on change scores created from subtracting the values obtained immediately before the exercise bout began from the values obtained 30 minutes into the bout. For the time points that occurred 10, 20, and 30 minutes into the exercise bout, participants were assessed while they were exercising—the bout was not interrupted to make these assessments. While participants continued their session on the treadmill, a research assistant held up cards with the questionnaire items displayed on them. Participants indicated the number that they felt reflected their current subjective states, and their responses were manually recorded. Physiological measures were obtained at these time points using the IV catheter that was inserted prior to the bout. Positive affect was assessed using 3 items from the 12-item physical activity affect scale (PAAS) [43]. The positive affect subscale is computed by taking the average of the three items. Participants rated their current state for each item using a 5-point scale (0 = do not feel to 4 = feel very strongly). The adjectives assessed by the 3-item positive affect subscale were enthusiastic, energetic, and upbeat (α = .81). Affective valence during exercise was assessed using the 11-point single-item feeling scale (FS) [44], which ranges from −5 = very bad to +5 = very good. Perceived exertion was assessed using the 15-point single-item rating of perceived exertion (RPE) [45] that ranges from 6 to 20 (6 = no exertion at all, 20 = maximal exertion). Perceived pain was assessed using a single-item 12-point borg category ratio-10 scale (CR10) (0 = no pain at all, 10 = extremely intense pain) [45].
2.3. DNA Processing and SNP Selection
Genomic DNA was extracted from saliva samples of 200 participants. Samples were genotyped on the Illumina Infinitum Assay Platform using the Human 1M DuoV3 DNA Analysis BeadChip (Illumina, Inc., San Diego, CA, USA) following the manufacturer's protocol at the University of Colorado, Boulder. In this assay, the DNA undergoes whole genome amplification, followed by fragmentation and ethanol precipitation. The DNA is then resuspended in hybridization buffer and applied to the bead chip array for an overnight incubation. The amplified and fragmented DNA samples anneal to locus-specific 50-mers (covalently linked to one of over 1,000,000 bead types) during the hybridization step. Following hybridization, the arrays are washed to eliminate unhybridized and nonspecifically hybridized DNA. One bead type corresponds to each allele per SNP locus. The samples then undergo single-base extension and staining, followed by more washing. The arrays are allowed to dry, and then scanned using the Illumina iScan system. Genotype calls were made using Illumina's GenomeStudio software in conjunction with the Genome Studio genotyping module. We removed SNPs with a genotype call rate <98%. Additionally, we excluded SNPs with a minor allele frequency (MAF) of <10% and SNPs that showed significant deviation from Hardy-Weinberg equilibrium (P < 1 × 10−6). Following these quality control checks, a total of 842,777 SNPs remained available for analysis.
Although we used a genome-wide approach to genotyping, we only tested a total of 14 SNPs which were selected for analysis in this study based upon their potential association with aerobic exercise response phenotypes suggested by prior studies (see Table 2 for Hardy-Weinberg P values and minor allele frequencies for each SNP tested). Our search was conducted primarily using PubMed, and was focused on SNPs that were directly associated with specific phenotypes of interest and to traits that may be associated with those phenotypes. Analyses were run using the SNP and Variation Suite for Genetic Analysis (SVS) (version 7.5.6, Golden Helix Inc., Bozeman, MT).The 14 SNPs selected for inclusion based on our search were tested for associations with the phenotypes using a correlational trend test assuming additive effects of allele dosages for each SNP (i.e., homozygous for the minor allele = 0; heterozygous = 1; homozygous for the major allele = 2).
Table 2.
Summary of all SNPs tested for associations with exercise-induced physiological or subjective response-change phenotypes in the STRIDE sample.
| SNP | Chromosome | Position | Hardy-Weinberg equilibrium P | Minor allele frequency | Minor allele | Nearest gene locus |
|---|---|---|---|---|---|---|
| rs1935881 | 1 | 188333009 | 0.346 | 0.250 | G | FAM5C |
| rs2360969 | 2 | 208081241 | 0.706 | 0.373 | T | CREB1 |
| rs2253206 | 2 | 208100223 | 0.400 | 0.453 | A | CREB1 |
| rs10498091 | 2 | 221607688 | 0.154 | 0.135 | A | intergenic |
| rs1379659 | 4 | 20229781 | 0.068 | 0.165 | G | SLIT2 |
| rs1799971 | 6 | 154402490 | 0.529 | 0.178 | G | OPRM1 |
| rs8050136 | 16 | 52373776 | 0.277 | 0.350 | A | FTO |
| rs3751812 | 16 | 52375961 | 0.119 | 0.333 | T | FTO |
| rs11075989 | 16 | 52377378 | 0.172 | 0.358 | T | FTO |
| rs7202116 | 16 | 52379116 | 0.172 | 0.358 | G | FTO |
| rs7201850 | 16 | 52379363 | 0.228 | 0.371 | T | FTO |
| rs9941349 | 16 | 52382989 | 0.104 | 0.345 | T | FTO |
| rs7190492 | 16 | 52386253 | 0.768 | 0.400 | A | FTO |
| rs8044769 | 16 | 52396636 | 0.159 | 0.490 | T | FTO |
3. Results
These analyses focused on correlations between particular SNPs suggested by the relevant literature and exercise response phenotypes drawn from our previous work and the existant exercise literature. Due to the fact that both exercise phenotypes and candidate SNPs were selected a priori based on the literature as well as our transdisciplinary framework, critical alpha for all tests was maintained at the .05 level for all analyses. Additionally, given that the aim of this study was to examine changes in physiological and subjective responses to exercise over the course of the 30-minute exercise bout, it was not necessary to compare subjects cross-sectionally at the baseline or 30-minute time points. Rather, all phenotype values were determined using a change score created by subtracting each subject's baseline values from the values obtained by that subject 30 minutes after the exercise bout began.
3.1. Genotype Differences by Race
In order to determine whether allele frequencies for all SNPs examined in this study were significantly different across racial/ethnic groups, χ2 tests were performed on all SNPs based on racial categories. χ2 test statistics and corresponding P values are included in Table 3. Additionally, major and minor alleles, as well as minor allele frequencies (MAFs) for Caucasians, African Americans, Asians, and Hispanic/Latino participants are reported in Table 3. Significant differences in genotype across racial/ethnic groups were found for three SNPs, rs1935881 χ2 (10,200) = 19.25, P = .037, rs1799971 χ2 (10,200) = 38.13, P < .001, and rs8044769 χ2 (10,200) = 21.54, P = .018. For rs1935881, the MAF is lower among Asians. For rs1799971, an MAF of 0 was found in African Americans. For rs8044769, the MAF is also lowest in African Americans. Results of associations between SNPs and exercise response phenotypes presented below are uncorrected (no PCA correction applied).
Table 3.
Summary of minor allele frequency (MAF) of each SNP tested separately for each racial/ethnic group.
| SNP | Minor allele | Major allele | Caucasian | African American | Hispanic/Latino | Asian | χ 2 (10,200) | P value |
|---|---|---|---|---|---|---|---|---|
| MAF | MAF | MAF | MAF | |||||
| rs1935881 | G | A | 0.288 | 0.222 | 0.159 | 0.068 | 19.25 | .03 ∗ |
| rs2360969 | T | C | 0.401 | 0.111 | 0.432 | 0.227 | 18.22 | .051 |
| rs2253206 | A | G | 0.438 | 0.444 | 0.477 | 0.386 | 10.56 | .393 |
| rs10498091 | A | G | 0.157 | 0.111 | 0.068 | 0.045 | 10.6 | .389 |
| rs1379659 | G | A | 0.197 | 0.000 | 0.114 | 0.136 | 10.05 | .436 |
| rs1799971 | G | A | 0.153 | 0.000 | 0.136 | 0.432 | 38.13 | < .001 ∗ |
| rs8050136 | A | C | 0.354 | 0.500 | 0.318 | 0.273 | 11.37 | .329 |
| rs3751812 | T | G | 0.354 | 0.167 | 0.318 | 0.273 | 7.22 | .704 |
| rs11075989 | T | C | 0.354 | 0.444 | 0.364 | 0.273 | 9.342 | .5 |
| rs7202116 | G | A | 0.354 | 0.444 | 0.364 | 0.273 | 9.342 | .5 |
| rs7201850 | T | C | 0.358 | 0.444 | 0.386 | 0.318 | 12.05 | .676 |
| rs9941349 | T | C | 0.358 | 0.167 | 0.341 | 0.318 | 10.27 | .418 |
| rs7190492 | A | G | 0.431 | 0.222 | 0.455 | 0.205 | 15.02 | .131 |
| rs8044769 | T | C | 0.467 | 0.167 | 0.432 | 0.273 | 21.54 | .018 ∗ |
The ∗ indicates P values that are less than .05
3.2. Correlations between Phenotypes
A total of 10 different phenotypes were examined for association with genetic variants in this study. Given that many of these phenotypes may have common underlying physiological bases, we tested for associations between these phenotypes. In the following results, all phenotypes tested and reported (except for VO2 max) refer to a change score created by subtracting preexercise values from the values obtained 30 minutes into the exercise bout. Results of these analyses are reported in Table 4. VO2 max was significantly correlated with change in lactate (r = .177, <.05 ), heart-rate (r = .434, P < .01), systolic blood pressure (r = .193, P <.01), and rate of perceived exertion (r = .157, P < .05 ) from baseline to 30 minutes into the exercise bout. Lactate change was correlated with temperature change (r = .214, P < .01), heart rate change (r = .429, P < .01), systolic blood pressure change (r = .208, P < .01), change in affective valence (as measured by the feeling scale) (r = −.173, P < .05), and pain change (r = .215, P < .05). Norepinephrine change was significantly correlated with positive affect change (r = .174, P < .05) and pain change (r = −.165, P < .05). Temperature change was significantly correlated with heart-rate change (r = .172, P < .05) and affective valence change (r = .149, P < .05). Heart rate change was significantly correlated with systolic blood pressure change (r = .185, P < .05) and rate of perceived exertion change (r = .231, P < .05). Rate of perceived exertion change was significantly correlated with affective valence change (r = −.163, P < .05) and pain change (r = .316, P < .01). Finally, positive affect change was significantly correlated with affective valence (r = .455, P < .05).
Table 4.
Relationships between subjective response phenotypes (N = 200).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| (1) VO2 max | — | |||||||||
| (2) Lactate | .177 ∗ | — | ||||||||
| (3) Norepinephrine | .004 | −.040 | — | |||||||
| (4) Temperature | .033 | .214 ∗∗ | .047 | — | ||||||
| (5) HR | .434 ∗∗ | .429 ∗∗ | −.094 | .172 ∗ | — | |||||
| (6) SBP | .193 ∗∗ | .208 ∗∗ | .047 | .078 | .185 ∗ | — | ||||
| (7) RPE | .157 ∗ | .117 | −.039 | .014 | .231 ∗ | .048 | — | |||
| (8) PA | −.038 | −.125 | .174 ∗ | .060 | −.071 | −.024 | −.036 | — | ||
| (9) FS± | −.017 | − .173 ∗ | .086 | .149 ∗ | −.054 | −.071 | − .163 ∗ | .455 ∗ | — | |
| (10) Pain | −.023 | .215 ∗ | − .165 ∗ | .000 | .109 | .108 | .316 ∗∗ | −.028 | −.129 | — |
Note: *P < .05, **P < .01, ±Higher numbers indicate a more positive feeling state; All of the variables listed within this table are represented as change scores (VO2 max excepted). Change scores were created by subtracting baseline values from values recorded at 30 minutes into the exercise bout. PA: positive affect; FS: feeling scale (affective valence).
3.3. SNP Associations with Exercise-Response Phenotypes
Correlations between genotype and exercise-response phenotypes were calculated. Significant associations emerged for SNPs in five different genes. The CREB1 SNPs rs2360969 and rs2253206 were associated with temperature change during exercise (rs2360969, r = .17, P = .02; rs2253206, r = .17, P = .02) indicating that for rs2360969, individuals with the T allele had greater changes in temperature over the course of the exercise, and for rs2253206, individuals with the A allele had greater changes in temperature during exercise. These same SNPs were also significantly associated with VO2 max (rs2253206, r = −.17, P = .01; rs2360969, r = −.14 P = .049), such that for rs2253206, individuals with the G allele had higher VO2 max, and for rs2360969, individuals with the C allele had higher VO2 max. The OPRM1 SNP rs1799971 was significantly associated with lactate change during exercise (r = .17, P =.02), norepinephrine change during exercise (r = .16, P = .03), and change in RPE during exercise (r = .14, P = .048), indicating that individuals with the rare G allele had greater changes in lactate, norepinephrine, and rate of perceived exertion change over the course of exercise. The FTO SNP rs8044769 was related to change in positive affect during exercise (r = −.16, P = .03), and individuals with the C allele had greater change in positive affect over the course of the exercise. The FTO SNP rs3751812 was associated with positive affect change during exercise (r = .14, P = .04), such that individuals with the T allele experienced greater changes in positive affect. The FTO SNP rs9941349 was significantly related to change in systolic blood pressure during exercise (r = .15, P = .04), and individuals with the T allele experienced greater increases in systolic blood pressure during exercise. The FTO SNP rs7201850 was significantly related to change in systolic blood pressure during exercise (r = .17, P = .027), with individuals possessing the T allele experiencing greater increases in systolic blood pressure over the course of the exercise bout. The SLIT2 SNP rs1379659 was associated with norepinephrine change during exercise (r = .18, P = .01), with individuals with the G allele experiencing greater changes in norepinephrine during exercise. Finally, the FAM5C SNP rs1935881 was associated with change in norepinephrine during exercise (r = −.16, P = .03). Individuals with the G allele had greater changes in norepinephrine over the course of the exercise bout (All associations initially reported in the manuscript changed only slightly after applying the PCA correction. PCA corrected p-values for genotype-phenotype associations are as follows: rs1799971 and norepinephrine change (P = .104), rs1799971 and RPE change, (P = .038), rs8044769 and positive affect change (P = .038), rs3751812 and positive affect (P = .036), rs1935881 and norepinephrine change (P = .059), rs1379659 and norepinephrine change (P = .010), rs9941349 and systolic blood pressure change (P = .053), rs7201850 and systolic blood pressure change (P = .04998), rs2360969 and temperature change (P = .031), rs2253206 and temperature change (P = .066), rs1799971 and lactate change (P = .015), rs2253206 and VO2 max (P = .035), and rs2360969 and VO2 max (P = .032)).
Due to the fact that several of the variants that were associated with a particular phenotype were in the same gene, it is likely that these SNPs are in high-linkage disequilibrium with one another. These SNP sets within single genes are rs3751812 and rs8044769 in FTO, both significantly associated with positive affect change, rs2253206 and rs2360969 in CREB1, both significantly associated with temperature change as well as VO2 max, and rs7201850 and rs9941349, both in FTO, both significantly associated with systolic blood pressure change. To examine whether these SNPs were in LD, we ran correlations on each set of 2 SNPs in the same gene that were associated with the same phenotype. The correlation between rs2360969 and rs2253206 was .805 (P < .01), the correlation between rs3751812 and rs8044769 was −.676 (P < .01), and the correlation between rs7201850 and rs9941349 was .938 (P < .01).
In order to further explore the direction of the relationship of genotype on exercise response, we graphed the adjusted means for each genotype of three SNPs which demonstrated particularly robust relationships with exercise response phenotypes. We graphed the relationship between rs2360969 and temperature 30 minutes into the exercise bout, between rs1799971 and RPE 30 minutes into the exercise bout, and between rs8044769 and positive affect score 30 minutes into the exercise bout. As can be seen in Figure 1, individuals with the TT genotype of rs2360969 showed the highest temperature after 30 minutes of aerobic exercise, controlling for baseline temperature. In Figure 2, we show that individuals with the AG/GG genotypes on rs1799971 show greater RPE after 30 minutes of aerobic exercise than individuals with the AA genotype, controlling for baseline RPE. In Figure 3, we show that individuals with the CC genotype in rs8044769 show the highest ratings of positive affect after 30 minutes of aerobic exercise, controlling for baseline positive affect.
Figure 1.
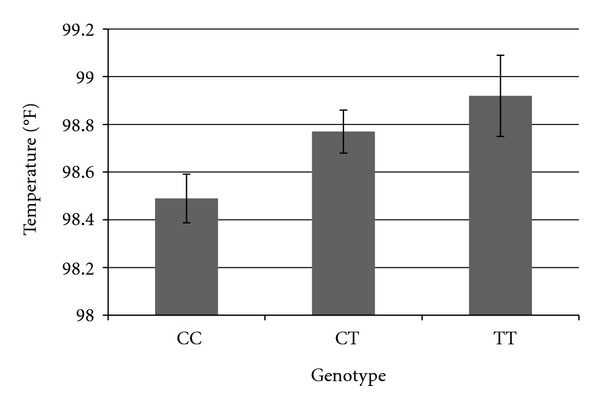
Adjusted mean temperature 30 minutes into the exercise bout, for individuals in each genotype of rs2360969 controlling for baseline temperature.
Figure 2.
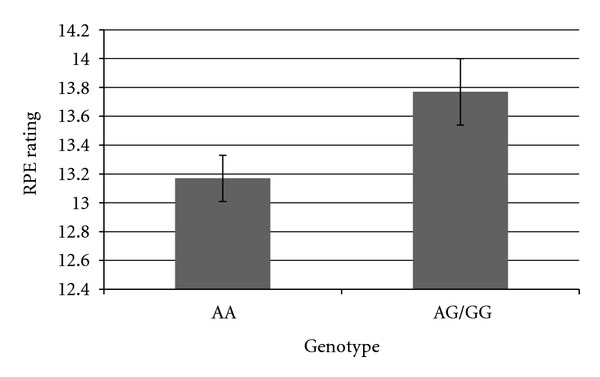
Adjusted mean rating of rate of perceived exertion 30 minutes into the exercise bout, for individuals in each genotype of rs1799971, controlling for baseline RPE rating. Individuals in the GG and AG group were combined, due to low n, for the GG group.
Figure 3.
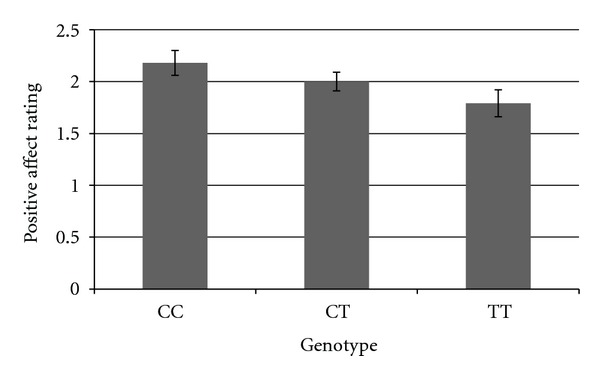
Adjusted mean rating of positive affect 30 minutes into the exercise bout, for individuals in each genotype of rs8044769, controlling for baseline positive affect rating.
4. Discussion
The present study replicated prior findings suggesting that SNPs in the CREB1, FTO, OPRM1, SLIT2, and FAM5C genes are all related to phenotypes encompassing various responses to exercise. Our study tested conceptually relevant phenotypes that to date had not been explored in this way inanyother exercise research. Given that the physiological response to aerobic exercise involves a complex interplay of metabolic, cardiovascular, musculoskeletal, ventilator, and hormonal functions [46], these genes and SNPs are likely to explain only a small portion of the variability in individual differences in response to aerobic exercise. Subjective responses to exercise may be yet more complex, involving sociocultural factors, effects of previous exercise experiences, and anticipated consequences/rewards of exercise [47]. Additionally, our findings suggest that individuals performing equivalent bouts of aerobic exercise may have vastly different subjective perceptions of this exercise (overall experiences which can range from negative to positive), and that these perceptions may be influenced by genotype. Giving sedentary individuals information about their propensity to respond to exercise in a particular way could provide useful insight, allowing these individuals to temper their expectations of what aerobic exercise “should” feel like for them or allowing intervention designers to incorporate external reinforcement contingencies (e.g., social interaction) for individuals who are less likely to experience intrinsic rewards from exercise.
Despite several inherent limitations, the present study's findings linking genetic variants to exercise responses among sedentary individuals presents promising initial evidence associating genes and exercise behavior. However, it is unlikely that variation at a single genetic locus could fully explain variation in physiological and subjective responses to exercise—more possibly, there are many genetic variables influencing this phenotype, each of which contributes only bya small fraction of the observed variation [48]. When combined into a genetic composite, these loci would likely correlate more strongly with phenotypic response. So, although the correlations between genotype and exercise response found in this study are not large, they represent a necessary first step in forming genetic composite scores that are likely to be more highly correlated and significantly predictive of exercise responses. In summary, linking SNPs to specific physiological and psychological mechanisms that contribute to exercise response will assist in informing individually tailored exercise programs, as well as deepen our understanding of the relationship between genetics, physiology, and psychology underlying health behaviors associated with cancer prevention.
4.1. FTO
Our study showed that for rs3751812, the presence of a T allele increased change in positive affect during exercise. This finding is somewhat at odds with previous work suggesting that TT individuals have higher BMI on average. Rs3751812 was found to have a strong association with BMI in African-derived populations, with the TT genotype predicting increased BMI [49]. However, the relationship between BMI and positive affective response to exercise is unclear because the Hassanein study [49] did not include information about exercise behavior of participants. It is possible that rs3751812 individuals are predisposed to have higher BMI, but if they engage in aerobic exercise, they are likely to have a more positive affective response. This is one example of how knowledge about the effect of a particular genotype could be used to prescribe tailored interventions—for overweight individuals with the TT genotype, exercise could be recommended as a more effective weight loss tool, given that these individuals have a more positive affective response to exercise.
We also found that for rs8044769, the TT individuals had greater changes in positive affect during exercise. In a Hispanic American sample, rs8044769 was found to be weakly associated with waist-to-hip ratio [50], and the C allele showed an association with variation in BMI [51]. Additionally, an association was demonstrated between rs8044769 and pediatric BMI [52]. Prior research suggests that the C allele of rs8044769 is associated with greater variation in BMI [51]. This SNP seems to be related to body fat mass, predisposition to obesity, and response to aerobic exercise—yet the nature of this relationship requires further exploration. Associations between this SNP and additional obesity-related phenotypes should be tested.
4.2. CREB1
CREB1 is a key component of long-term cardiac memory formation (specific T-wave patterns on an electrocardiogram) [53], as well as long-term memory formation in the brain [54, 55]. Our results indicate that for the CREB1 SNP rs2253206, individuals with the A allele (AG genotypes, and to a greater extent AA genotypes) have a greater change in temperature during exercise. If greater temperature change while exercising translates into a more unpleasant subjective exercise experience, then our findings suggest that the AA individuals (and to a lesser extent AG individuals) may have less pleasant subjective experiences of exercise than GG individuals. rs2253206 was shown to be strongly associated with heart rate (HR) change in response to a 20-week endurance training program, with GG and AG genotypes and showing 57% and 20% better change in HR than the AA participants [38].
Our results make sense in the context of the Rankinen findings [38], as the AA individuals may have more unpleasant exercise experiences due to increased temperature, which could influence their ability to exercise effectively (and thus decrease the heart rate improvements they can obtain from an exercise intervention). We also found that this SNP was related to VO2 max (an indicator of cardiovascular fitness), such that the GG individuals had greater VO2 max than AG individuals, who had greater VO2 max than AA individuals. These results also coincide with our findings and the findings from previous research, as GG individuals may be more fit to begin with, and also more capable of gaining increased fitness through training, due to the fact that they experience exercise as less painful.
Additionally, we found that for rs2360969, TT individuals experienced greater change in temperature than did CT and CC individuals. rs2360969 has also been shown to be related to heart rate response endurance training [38, 39], however, these studies did not state direction of effect for this SNP.
4.3. OPRM1
In our analysis, rs1799971 (the A118G polymorphism) was related to RPE, as well as to lactate change during exercise and norepinephrine change during exercise. For all three of these phenotypes, individuals with the rare G allele showed greater change during the exercise bout. Previous research on this SNP has found that individuals with the G allele (genotypes of either AG or GG) demonstrated higher pressure pain thresholds than individuals with the AA genotype [36]. This study also found that when heat pain was tested, a sex by genotype interaction emerged, such that the G allele was associated with lower pain ratings among men but higher pain ratings among women. The A118G variant has greater binding affinity for β-endorphin (an exogenous opioid that activates the mu opioid receptor) [56], which is one possible mechanism by which this SNP could influence pain sensitivity.
The relationship between rs1799971 and subjective responses to pain may extend to the pain and exertion experienced during aerobic exercise. Given that our sample was 79.5% female, our findings of greater lactate, norepinephrine and RPE change over the course of exercise for the GG/AG group is in the same direction as the findings for females in the Fillingim [36] study. These results lend further support to the idea that individuals (and perhaps particularly women) with the AG/GG genotype have lowered pain threshold, and the present study suggests increases in lactate and norepinephrine as possible physiological explanations, at least in the context of aerobic exercise-induced pain.
4.4. FAM5C
Prior studies have shown that rs1379659 in FAM5C is associated with echocardiographic traits, and specifically left ventricular systolic dimension. The results of our study suggest that it is also associated with change in norepinephrine in response to exercise. To date, research has not examined the relationship between FAM5C and aerobic exercise response. Given the connection between this gene and cardiac function, examining the potential relationship between FAM5C and aerobic exercise would provide a logical next step for research in this area.
4.5. SLIT2
Previous research has demonstrated an association between the SLIT2 SNP rs1935881 and echocardiographic traits, specifically left ventricular diastolic dimension. The results of this study suggest that it is also related to norepinephrine change during exercise. Further research is needed to elucidate more specific relationships between SLIT2 and response to aerobic exercise.
The genes discussed above represent potential candidates for further explanation in terms of their relationship to exercise response phenotypes. More than a decade's worth of research on the psychophysiological responses associated with exercise has demonstrated that the subjective experience of exercise, how sensations are remembered, anticipated, and interpreted, is closely tied to subsequent exercise behavior [14, 19, 20, 47, 57]. A better understanding of the genetic basis for subjective responses to aerobic exercise may have the potential to lead to more effective and sophisticated intervention designs. Eventually, these advances in the basic science of exercise response could lead to the implementation of interventions tailored on the level of individual genetic variants.
Primary prevention of cancer through behavioral intervention is now a top priority of the NCI. This approach is intuitive given that approximately 30% of total cancer deaths are related to energy imbalance (e.g., excessive adiposity) [58, 59]. Physical inactivity is not the only contributing factor to energy imbalance, but it is a major contributing factor as trends clearly show that the least physically active regions of the country are also the most obese [60]. The hopeful perspective on behavioral intervention for physical activity is that even small increases in the total amount of participation accumulated per week stands to lead to meaningful differences in cancer risk. For example, [61] found evidence for a 3–8% reduction in risk for breast cancer with every additional 60 minutes of physical activity engaged in per week.
The link between physical activity participation and reduced risk for cancer, especially of the colon, breast, and endometrium is convincing, but also dependent upon good adherence [3–6, 61]. For this reason, it is imperative that researchers continue to search for ways to improve the likelihood of adherence to behavioral interventions. One way to achieve this goal may be through increasing the amount of focus that is placed on subjective response phenotypes and their underlying genetic variants. Developing a better understanding of the link between genes, exercise-relevant physiological mechanisms, and the resulting exercise-response phenotypes is a first step towards tailoring individualized exercise programs that would likely increase adherence and lead to improved health outcomes and decreased rates of cancer and other diseases.
4.6. Limitations
As with all research that involves genetic analyses, we cannot rule out the possibility that other genetic factors, including rare or common SNPs, insertions, deletions, or copy number variants, could play a role in determining the physiological responses to exercise that were measured in this study [48]. The phenotypes investigated in this study are likely to be polygenic traits, such that numerous genes and SNPs other than those examined in the present study may all contribute to these exercise response phenotypes. In contrast, the extent of the pleiotropic effects of the genes and SNPs investigated in this study are unknown. Thus, it is possible that the polymorphisms that influence exercise response may also be more strongly associated with other, possibly unrelated phenotypes that led to our findings [21].
Another limitation of note is the present study's lack of power to detect moderation effects of demographic variables. It is possible that variables such as age or ethnicity could moderate the associations between genetic variation and response to exercise. Further research should focus on testing these associations among different racial and age groups. Additionally, the results of this investigation are based on one single, standardized, bout of moderate-intensity exercise. For this reason, our results cannot be generalized to subjective exercise experiences that occur under less regulated circumstances (i.e., when type of activity, intensity, and duration are individually determined). Despite this limitation, there are many examples from the literature in which subjective responses to exercise are measured and analyzed based on a single bout of standardized exercise (e.g., [19, 20, 62–65]) and therefore, our procedures and analyses are in concert with the approach previously established by the field. Importantly, the purpose of the present investigation was to understand how genetic variants are associated with particular subjective responses to exercise when the parameters of the exercise experience are standardized across all individuals. In the present study, this level of standardization was achieved by having all participants perform the same activity (treadmill walking), for the same duration (30 minutes), at the same intensity (65% of each individual participant's previously established VO2 max). Further, efforts were made to standardize variables external to the exercise bout as well (i.e., instructions detailing recommended calorie and water consumption prior to the bout described in Section 2). It remains to be seen whether the SNPs and genes reported in this study to be related to exercise response phenotypes would show an association to these same phenotypes in other studies examining different types, duration, and intensity of exercise sessions. Further, there may be some effect of population substructure in these associations. However, as noted, the size of the associations changed negligibly after a PCA correction, suggesting that the population substructure did not play a major role. Overall, replication is needed in order to confirm findings from the present study, and to better understand the functional significance of these genes and SNPs in relation to physiological and subjective responses to aerobic exercise.
5. Conclusion
The purpose of this paper was to explore the genetic underpinnings of individual physiological and subjective responses to aerobic exercise. One strength of this study was its focus on a sedentary population, a group that has been rarely tested in terms of associations between genetics and exercise phenotypes. The relationship between particular genetic variants and responses to exercise has important implications for the prevention of cancer via increasing exercise behavior in sedentary populations. Future studies designed to test genetic influences on a wide range of exercise response phenotypes would help to advance this goal, potentially leading to a panel of markers important for characterizing the physiological and subjective response to exercise. Moreover, giving feedback to sedentary individuals regarding the genetic basis for their strengths and weaknesses in fitness/exercise/sports activities could be a potentially useful motivational tool for increasing exercise behavior [13, 66]. In sum, expanding our understanding of the association between genetics and exercise response phenotypes has a myriad of implications for helping to increase exercise behavior in sedentary individuals, an outcome which is crucially important for the reduction of morbidity and mortality associated with cancer.
Conflict of Interests
The authors declared that no conflict of interests exist.
Acknowledgments
The research was supported by Grants awarded to Angela Bryan from the National Cancer Institute (RO1 CA109858), the General Clinical Research Center Program of the National Center for Research Resources, and National Institutes of Health (M01-RR00051) now the Colorado Clinical and Translational Sciences Institute (UL1-RR025780). The authors would like to thank Marilee Morgan at the Neurogenetics lab of the Mind Research Network, Albuquerque New Mexico, for her contribution to the DNA analyses for this project.
References
- 1.Health, United States, 2008: With Chartbook. Hyattsville, Md, USA: The National Center for Health Statistics; 2009. [Google Scholar]
- 2.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American college of sports medicine and the American heart association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 3.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. European Journal of Cancer. 2010;46(14):2593–2604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Friedenreich CM, Woolcott CG, McTiernan A, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. Journal of Clinical Oncology. 2010;28(9):1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results in Cancer Research. 2011;186:13–42. doi: 10.1007/978-3-642-04231-7_2. [DOI] [PubMed] [Google Scholar]
- 6.Wolin KY, Patel AV, Campbell PT, et al. Change in physical activity and colon cancer incidence and mortality. Cancer Epidemiology Biomarkers and Prevention. 2010;19(12):3000–3004. doi: 10.1158/1055-9965.EPI-10-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. Journal of Nutrition. 2002;132(supplement 11):3456S–3464S. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 8.Lee IM. Physical activity and cancer prevention—data from epidemiologic studies. Medicine and Science in Sports and Exercise. 2003;35(11):1823–1827. doi: 10.1249/01.MSS.0000093620.27893.23. [DOI] [PubMed] [Google Scholar]
- 9.Parkin DM. Cancers attributable to inadequate physical exercise in the UK in 2010. British Journal of Cancer. 2011;105(supplement 2):S38–S41. doi: 10.1038/bjc.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nature Reviews Genetics. 2007;8(4):286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nature Medicine. 2011;17(3):330–339. doi: 10.1038/nm.2305. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute. Fact Sheet: Physical Activity and Cancer, http://www.cancer.gov/cancertopics/factsheet/prevention/physicalactivity, 2009.
- 13.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychology. 2009;28(6):690–701. doi: 10.1037/a0016136. [DOI] [PubMed] [Google Scholar]
- 14.Ekkekakis P, Parfitt G, Petruzzello SJ. The pleasure and displeasure people feel when they exercise at different intensities: decennial update and progress towards a tripartite rationale for exercise intensity prescription. Sports Medicine. 2011;41(8):641–671. doi: 10.2165/11590680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Buckworth J, Dishman R. Exercise adherence. In: Eklund RC, editor. Handbook of Sport Psychology. Hoboken, NJ, USA: Wiley; 2007. pp. 509–536. [Google Scholar]
- 16.Bryan A, Hutchison KE, Seals DR, Allen DL. A transdisciplinary model integrating genetic, physiological, and psychological correlates of voluntary exercise. Health Psychology. 2007;26(1):30–39. doi: 10.1037/0278-6133.26.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryan AD, Hooper AEC, Ciccolo J, Marcus B, Hutchison KE, Magnan RE. Colorado Stride (COStride): genetic and physiological moderators of response to an intervention to increase physical activity. In press. [DOI] [PMC free article] [PubMed]
- 18.Booth F, Neufer PD. Exercise genomics and proteomics. In: Tipton CM, editor. ACSM’s Advanced Exercise Physiology. Baltimore, Md, USA: Lipincott, Williams & Wilkins; 2006. [Google Scholar]
- 19.Kwan BM, Bryan AD. Affective response to exercise as a component of exercise motivation: attitudes, norms, self-efficacy, and temporal stability of intentions. Psychology of Sport and Exercise. 2010;11(1):71–79. doi: 10.1016/j.psychsport.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DM, Dunsiger S, Ciccolo JT, Lewis BA, Albrecht AE, Marcus BH. Acute affective response to a moderate-intensity exercise stimulus predicts physical activity participation 6 and 12 months later. Psychology of Sport and Exercise. 2008;9(3):231–245. doi: 10.1016/j.psychsport.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Geus EJC, de Moor MHM. A genetic perspective on the association between exercise and mental health. Mental Health and Physical Activity. 2008;1(2):53–61. [Google Scholar]
- 22.Stubbe JH, Boomsma DI, Vink JM, et al. Genetic influences on exercise participation in 37.051 twin pairs from seven countries. PLoS ONE. 2006;1(1) doi: 10.1371/journal.pone.0000022.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annual Review of Clinical Psychology. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- 24.Bea JW, Lohman TG, Cussler EC, Going SB, Thompson PA. Lifestyle modifies the relationship between body composition and adrenergic receptor genetic polymorphisms, ADRB2, ADRB3 and ADRA2B: a secondary analysis of a randomized controlled trial of physical activity among postmenopausal women. Behavior Genetics. 2010;40(5):649–659. doi: 10.1007/s10519-010-9361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilpelainen TO, Laaksonen DE, Lakka TA, et al. The rs1800629 polymorphism in the TNF gene interacts with physical activity on the changes in C-reactive protein levels in the finnish diabetes prevention study. Experimental and Clinical Endocrinology and Diabetes. 2010;118(10):757–759. doi: 10.1055/s-0030-1249686. [DOI] [PubMed] [Google Scholar]
- 26.Phares DA, Halverstadt AA, Shuldiner AR, et al. Association between body fat response to exercise training and multilocus ADR genotypes. Obesity Research. 2004;12(5):807–815. doi: 10.1038/oby.2004.97. [DOI] [PubMed] [Google Scholar]
- 27.An P, Rice T, Gagnon J, et al. Familial aggregation of stroke volume and cardiac output during submaximal exercise: the HERITAGE family study. International Journal of Sports Medicine. 2000;21(8):566–572. doi: 10.1055/s-2000-12983. [DOI] [PubMed] [Google Scholar]
- 28.Bouchard C, Warwick Daw E, Rice T, et al. Familial resemblance for VO2(2max) in the sedentary state: the HERITAHE family study. Medicine and Science in Sports and Exercise. 1998;30(2):252–258. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Gaskill SE, Rice T, Bouchard C, et al. Familial resemblance in ventilatory threshold: the HERITAGE Family Study. Medicine and Science in Sports and Exercise. 2001;33(11):1832–1840. doi: 10.1097/00005768-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Perusse L, Gagnon J, Province MA, et al. Familial aggregation of submaximal aerobic performance in the heritage family study. Medicine and Science in Sports and Exercise. 2001;33(4):597–604. doi: 10.1097/00005768-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 31.MacArthur DG, North KN. Genes and human elite athletic performance. Human Genetics. 2005;116(5):331–339. doi: 10.1007/s00439-005-1261-8. [DOI] [PubMed] [Google Scholar]
- 32.Travlos AK, Marisi DQ. Perceived exertion during physical exercise among individuals high and low in fitness. Perceptual and Motor Skills. 1996;84(2):419–424. doi: 10.2466/pms.1996.82.2.419. [DOI] [PubMed] [Google Scholar]
- 33.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS genetics. 2007;3(7) doi: 10.1371/journal.pgen.0030115.e115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rankinen T, Rice T, Teran-Garcia M, Rao DC, Bouchard C. FTO genotype is associated with exercise training-induced changes in body composition. Obesity. 2010;18(2):322–326. doi: 10.1038/oby.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Speakman JR, Rance KA, Johnstone AM. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity. 2008;16(8):1961–1965. doi: 10.1038/oby.2008.318. [DOI] [PubMed] [Google Scholar]
- 36.Fillingim RB, Kaplan L, Staud R, et al. The A118G single nucleotide polymorphism of the μ-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. Journal of Pain. 2005;6(3):159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Vasan RS, Larson MG, Aragam J, et al. Genome-wide association of echocardiographic dimensions, brachial artery endothelial function and treadmill exercise responses in the Framingham Heart Study. BMC Medical Genetics. 2007;8(supplement 1):p. S2. doi: 10.1186/1471-2350-8-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rankinen T, Argyropoulos G, Rice T, Rao DC, Bouchard C. CREB1 is a strong genetic predictor of the variation in exercise heart rate response to regular exercise: the HERITAGE Family Study. Circulation. 2010;3(3):294–299. doi: 10.1161/CIRCGENETICS.109.925644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rankinen T, Sung YJ, Sarzynski MA, Rice TK, Rao DC, Bouchard C. Heritability of submaximal exercise heart rate response to exercise training is accounted for by nine SNPs. Journal of Applied Physiology. 2012;112(5):892–897. doi: 10.1152/japplphysiol.01287.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magnan RE, Ciccolo JT, Bryan AD, Marcus BH, Nilsson R. A transdisciplinary approach to the moderators of exercise behavior change. Journal of Behavioral Medicine. In press. [DOI] [PMC free article] [PubMed]
- 41.Christou DD, Gentile CL, DeSouza CA, Seals DR, Gates PE. Fatness is a better predictor of cardiovascular disease risk factor profile than aerobic fitness in healthy men. Circulation. 2005;111(15):1904–1914. doi: 10.1161/01.CIR.0000161818.28974.1A. [DOI] [PubMed] [Google Scholar]
- 42.American College of Sports. ACSM’s Guidelines for Exercise Testing and Prescription. New York, NY, USA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 43.Lox CL, Jackson S, Tuholski SW, Wasley D, Treasure DC. Revisiting the measurement of exercise-induced feeling states: the physical activity affect scale (PAAS) Measurement in Physical Education and Exercise Science. 2000;4(2):79–95. [Google Scholar]
- 44.Hardy WJ, Rejeski CJ. Not what, but how one feels: the measurement of affect during exercise. Journal of Sport & Exercise Psychology. 1989;11(3):304–317. [Google Scholar]
- 45.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, Ill, USA: Human Kinetics; 1998. [Google Scholar]
- 46.Burton DA, Stokes K, Hall GM. Physiological effects of exercise. Continuing education in anaesthesia. Critical Care & Pain. 2004;4(6):185–188. [Google Scholar]
- 47.Dunton GF, Vaughan E. Anticipated Affective Consequences of Physical Activity Adoption and Maintenance. Health Psychology. 2008;27(6):703–710. doi: 10.1037/0278-6133.27.6.703. [DOI] [PubMed] [Google Scholar]
- 48.Marian AJ. Molecular genetic studies of complex phenotypes. Translational Research. 2012;159(2):64–79. doi: 10.1016/j.trsl.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassanein MT, Lyon HN, Nguyen TT, et al. Fine mapping of the association with obesity at the FTO locus in African-derived populations. Human Molecular Genetics. 2010;19(14):2907–2916. doi: 10.1093/hmg/ddq178.ddq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wing MR, Ziegler J, Langefeld CD, et al. Analysis of FTO gene variants with measures of obesity and glucose homeostasis in the IRAS Family Study. Human Genetics. 2009;125(5-6):615–626. doi: 10.1007/s00439-009-0656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thorleifsson G, Walters GB, Gudbjartsson DF, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nature Genetics. 2009;41(1):18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 52.Zhao J, Bradfield JP, Li M, et al. The role of obesity-associated loci identified in genome-wide association studies in the determination of pediatric BMI. Obesity. 2009;17(12):2254–2257. doi: 10.1038/oby.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patberg KW, Shvilkin A, Plotnikov AN, Chandra P, Josephson ME, Rosen MR. Cardiac memory: mechanisms and clinical implications. Heart Rhythm. 2005;2(12):1376–1382. doi: 10.1016/j.hrthm.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 54.Rosen MR, Cohen IS. Cardiac memory ... new insights into molecular mechanisms. Journal of Physiology. 2006;570(2):209–218. doi: 10.1113/jphysiol.2005.097873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu H, Zhou Y, Xiong ZQ. Transducer of regulated CREB and late phase long-term synaptic potentiation. FEBS Journal. 2007;274(13):3218–3223. doi: 10.1111/j.1742-4658.2007.05891.x. [DOI] [PubMed] [Google Scholar]
- 56.Bond C, Laforge KS, Tian M, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: possible implications for opiate addiction. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(16):9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baumeister RF, Vohs KD, DeWall CN, Zhang L. How emotion shapes behavior: feedback, anticipation, and reflection, rather than direct causation. Personality and Social Psychology Review. 2007;11(2):167–203. doi: 10.1177/1088868307301033. [DOI] [PubMed] [Google Scholar]
- 58.Kaaks R, Lukanova A. Effects of weight control and physical activity in cancer prevention: role of endogenous hormone metabolism. Annals of the New York Academy of Sciences. 2002;963:268–281. doi: 10.1111/j.1749-6632.2002.tb04118.x. [DOI] [PubMed] [Google Scholar]
- 59.Society AC. Cancer Facts & Figures 2011. Atlanta, Ga, USA: American Cancer Society; 2011. [Google Scholar]
- 60.Levi LMSJ, St. Laurent R, Kohn D. F as in Fat: How Obesity Threatens America’s Future. Princeton, NJ, USA: Robert Wood Johnson Foundation and The Trust for America’s Health; 2011. [Google Scholar]
- 61.Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18(1):137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 62.Carels RA, Berger B, Darby L. The association between mood states and physical activity in postmenopausal, obese, sedentary women. Journal of Aging and Physical Activity. 2006;14(1):12–28. doi: 10.1123/japa.14.1.12. [DOI] [PubMed] [Google Scholar]
- 63.Ekkekakis P, Lind E. Exercise does not feel the same when you are overweight: the impact of self-selected and imposed intensity on affect and exertion. International Journal of Obesity. 2006;30(4):652–660. doi: 10.1038/sj.ijo.0803052. [DOI] [PubMed] [Google Scholar]
- 64.Ekkekakis P, Lind E, Vazou S. Affective responses to increasing levels of exercise intensity in normal-weight, overweight, and obese middle-aged women. Obesity. 2010;18(1):79–85. doi: 10.1038/oby.2009.204. [DOI] [PubMed] [Google Scholar]
- 65.Kwan BM, Bryan A. In-task and post-task affective response to exercise: translating exercise intentions into behaviour. British Journal of Health Psychology. 2010;15(1):115–131. doi: 10.1348/135910709X433267. [DOI] [PubMed] [Google Scholar]
- 66.Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality-social, clinical, and health psychology. Psychological Bulletin. 1982;92(1):111–135. [PubMed] [Google Scholar]


