Abstract
In patients with fibrotic idiopathic interstitial pneumonia (f-IIP), the diffusing capacity for carbon monoxide (DLCO) has been used to predict abnormal gas exchange in the lung. However, abnormal values for arterial blood gases during exercise are likely to be the most sensitive manifestations of lung disease. The aim of this study was to compare DLCO, resting PaO2, P(A-a)O2 at cardiopulmonary exercise testing peak, and oxygen desaturation during a 6-min walk test (6MWT). Results were obtained in 121 patients with idiopathic pulmonary fibrosis (IPF, n = 88) and fibrotic nonspecific interstitial pneumonias (NSIP, n = 33). All but 3 patients (97.5%) had low DLCO values (<LLN) whereas only 66.6% had low KCO; 42 patients (65%) exhibited resting hypoxemia (<75 mmHg); 112 patients (92.5%) exhibited a high P[(A-a)O2], peak (>35 mmHg) and 100 (83%) demonstrated significant oxygen desaturation during 6MWT (>4%). Interestingly 27 patients had low DLCO and normal P(A-a)O2, peak and/or no desaturation during the 6MWT. The 3 patients with normal DLCO also had normal PaO2, normal P(A-a)O2, peak, and normal oxygen saturation during 6MWT. Our results demonstrate that in fibrotic IIP, DLCO better defines impairment of pulmonary gas exchange than resting PaO2, exercise P(A-a)O2, peak, or 6MWT SpO2.
1. Introduction
According to the ATS/ERS statement, fibrotic interstitial idiopathic pneumonia (f-IIP) includes idiopathic pulmonary fibrosis (IPF) and fibrotic nonspecific interstitial pneumonia (f-NSIP) [1–4]. Although pathological abnormalities are quite different between these two diseases [5], alteration of gas exchange is a major abnormality which is thought to reflect the severity of fibrotic process [6].
Given the simplicity of pulmonary function testing, many investigators have examined the potential for simple resting physiologic measurements to stratify disease severity. The classic physiologic findings in the fibrotic IIP include a reduction in lung volumes (vital capacity; total lung capacity), a reduction in carbon monoxide diffusing capacity (DLCO), and hypoxemia that worsens with exercise [2]. Evaluation of gas exchange impairment can be performed in clinical practice by simple tests like DLCO, resting PaO2, and P(A-a)O2, measurement of SpO2 during a 6-min walk test (6MWT) or PaO2 and alveolar-arterial oxygen pressure difference P(A-a)O2 during cardiopulmonary exercise testing (CPET).
Whereas DLCO is a valuable tool in the assessment of the efficiency of pulmonary gas exchange, the P(A-a)O2, especially during exercise, is thought to better reflect the normality of respiratory gas exchange [8, 9]. In addition exercise-induced gas exchange can also be readily identified by simple testing such as the 6MWT [10].
To the best of our knowledge, comparison of all the various methods to detect pulmonary gas exchange abnormalities has never been performed. Previous studies compared DLCO and P(A-a)O2 in a small number of IPF patients but did not include 6MWT [9, 11] or analysed 6MWT oxygen desaturation but did not include analysis of exercise PaO2 or P(A-a)O2 [12, 13]. With this in mind we performed a retrospective analysis of resting and exercise tests in 138 consecutive patients with IPF or f-NSIP.
2. Patients and Methods
One hundred and thirty eight caucasian patients with a diagnosis of IPF or f-NSIP were consecutively referred for evaluation of dyspnea and CPET at the time of diagnosis or during followup, over a period of six years. Inclusion criteria consisted of diagnosis of IPF according to the American Thoracic Society/European Respiratory Society guidelines and/or histopathological evidence for usual interstitial pneumonia, or diagnosis of f-NSIP (radiographic or histopathological diagnosis) [1, 2]. Patients were not included if they had another pulmonary disease (including obstructive disease), left heart failure or a history of pulmonary embolism. Connective tissue diseases were ruled out. No acute exacerbation was observed in the three months preceding inclusion. Seventeen patients were excluded from the study because CPET was not performed (arthrosis). Therefore 121 patients (31 females, 90 males) were included. In 44 out of the 88 IPF patients and 20 out of the 33 patients with f-NSIP, diagnosis was confirmed by histopathological examination of lung biopsy. At the time of inclusion in the study, a majority of patients (76%) were not treated, 19 patients received corticosteroids, 12 patient received azathioprine, and 3 patients received mycophenolate mofetil. Clinical data and results of pulmonary function tests, 6MWT, and of CPET were collected. Only initial data were recorded when the patient was seen several times. Approval for the use of these data was provided by the Institutional Review Board of the French learned society for respiratory medicine (CEPRO 2011-039).
3. Pulmonary Function Tests
Forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and total lung capacity (TLC) were measured by spirometry and plethysmography with a Jaeger-Master lab cabin. Single-breath diffusing capacity of the lung for carbon monoxide (DLCO: mLCO·min−1·mmHg−1) and carbon monoxide transfer coefficient (KCO = DLCO/alveolar volume) were measured. DLCO was corrected for hemoglobin concentration in g·dL−1, according to Cotes' equation: corrected (Hb) DLCO = DLCO × (10.2 + Hb)/(1.7 × Hb). Values were expressed as percentages of the predicted normal values calculated according to gender, weight, and age. Reference equations for spirometry were taken from ERS for lung volumes and DLCO [14, 15]. Following ATS/ERS 2005 guidelines, the lower limits of normal (LLN) were set at the level of 5th percentile (or predicted minus 1.64 SD) of each reference population [16]. Results were conventionally expressed as percent predicted.
The 6MWT was performed in accordance with international recommendations [17] and was designed to ensure an accurate assessment of oxygen desaturation Patients were instructed as follows: “The object of this test is to walk as quickly as you can for 6 minutes to cover as much ground as possible. You may slow down if necessary. If you stop we wish you to continue the walk again as soon as possible. Your goal is to walk as fast and as far as you can in 6 minutes.” [18]. The pulse oximeter was lightweight, battery powered, and held in place by a “fanny pack” so that the patient does not have to hold or stabilize it. We evaluated the oxygen saturation at rest and the lowest saturation during the test. A desaturation ≥4% was considered as significant [2].
CPET was carried out using a standardized protocol as previously described [19] and consisted of a triangular test, carried out on an ergometric bicycle (Ergoline-Ergometrics 800). Briefly the expired gases were determined in each cycle with an Ergocard. During exercise, heart rate (HR) was monitored continually by 12-lead ECG and arterial oxygen saturation (SpO2) was measured by pulse oximetry with a Nellcor N-395 apparatus. Arterial blood samples were obtained from a small catheter placed in the radial artery under local anesthesia. Measurements of PaO2 and PaCO2 were performed on room air at rest and at peak exercise. Normal values for PaO2 were derived from Sorbini et al. [20]. The alveolar-arterial gradient in oxygen [P(A-a)O2] was calculated from the alveolar gas equation. According to ATS statement, [P(A-a)O2], peak >35 mmHg was considered as abnormal [21]. Exercise pulmonary gas exchange variables were either related or not related to the metabolic demand (VO2), that is, peak exercise-rest (Δ) [19, 22]. The modified Bohr equation was used to calculate dead space to tidal volume ratio (VD/VT). Predicted values were calculated from reference equations [22, 23]. Poor motivation was not a factor interfering with our analysis as suggested by the fact that all of the patients had one or more of the following criteria: breathing reserve less than 15%, peak HR more than 90% of predicted, peak lactate more than 7 mEq/L, peak exercise PaO2 less than 55 mmHg or peak VE/VO2 more than 35or RER >1.15 [24, 25].
4. Statistical Analysis
After certification of normal distribution, data are reported as mean ± SD. Student's t-test was used to determine differences between IPF and f-NSIP. Differences in proportions were assessed by χ2 tests. Correlations were analysed using Spearman's rank correlation test. All statistical analysises were carried out with GraphPad Prism 4.0 software (San Diego, Calif, USA). Values of P < 0.05 were considered significant.
5. Results
Characteristics of the population are summarized in Table 1. The overall population consisted of 90 men and 31 women with a mean age of 63.6 ± 8.4 years: 88 patients had a diagnostic of IPF and 33 of f-NSIP. Pulmonary function tests results are shown in Tables 1 and 2. As expected, we observed a reduction in TLC, VC, and FEV1, a reduced DLCO and KCO. DLCO was reduced to a greater extent than the lung volumes: 45 out of 121 patients (37%) showed a normal FVC and 24% a normal TLC despite a low DLCO.
Table 1.
Pulmonary function tests results.
| All patients | IPF | f-NSIP | |
|---|---|---|---|
| n = 121 | n = 88 | n = 33 | |
| Age | 63.6 ± 8.4 | 64.3 ± 8.3 | 61.6 ± 8.5 |
| BMI | 28.4 ± 4.4 | 28.2 ± 4.1 | 29.1 ± 5.1 |
| TLC (L) | 4.35 ± 1 | 4.39 ± 1.01 | 4.23 ± 1 |
| TLC (%) | 70 ± 14.5 | 69.4 ± 14.6 | 73 ± 13.9 |
| % with low TLC | 76 | 82 | 62 |
| FVC (L) | 2.72 ± 0.74 | 2.77 ± 0.72 | 2.61 ± 0.8 |
| FVC (%) | 76 ± 16 | 75.5 ± 16.9 | 77.2 ± 13.6 |
| % with low FVC | 61 | 58 | 65 |
| FEV1 (L/sec) | 2.24 ± 0.58 | 2.26 ± 0.57 | 2.12 ± 0.6 |
| FEV1 (%) | 78.5 ± 16.4 | 78.4 ± 17.2 | 78.8 ± 14.1 |
| % with low FEV1 | 55 | 52 | 62 |
| DLCO (mLCO·min−1·mmHg−1) | 11.2 ± 4 | 11.17 ± 4.4 | 11.3 ± 3.2 |
| DLCO (%) | 42.9 ± 12.3 | 41.9 ± 12.5 | 45.6 ± 11.6 |
| % with low DLCO | 98 | 98 | 97 |
| KCO (mLCO·min−1·mmHg−1/L) | 2.99 ± 0.76 | 2.92 ± 0.77 | 3.2 ± 0.7 |
| KCO (%) | 71.3 ± 17 | 70.3 ± 17.7 | 74.2 ± 3.7 |
| % with low KCO | 66.6 | 70 | 56 |
| PaO2, rest (mmHg) | 76.8 ± 12.6 | 75.9 ± 12.8 | 79.4 ± 12 |
| % with low PaO2 | 42 | 46 | 31 |
| P(A-a) O2, rest (mmHg) | 30.8 ± 12.5 | 31.4 ± 12.6 | 29.2 ± 12.2 |
| % with low P(A-a) O2, rest | 26 | 30 | 16 |
Table 2.
Cardiopulmonary exercise testing and walking test results.
| All patients | IPF | f-NSIP | |
|---|---|---|---|
| n = 121 | n = 88 | n = 33 | |
| Workload, peak (Watts) | 81.4 ± 24 | 82.5 ± 23.7 | 78.3 ± 25.9 |
| Workload, peak (%) | 71.3 ± 9.9 | 61.2 ± 18.8 | 67.2 ± 24.8 |
| VO2, peak (mL/Kg/min) | 15.9 ± 3.9 | 15.9 ± 3.6 | 15.9 ± 4.6 |
| VO2, peak (%) | 66.5 ± 15.7 | 66 ± 15 | 67.8 ± 17.7 |
| % low VO2, peak (%) | 84 | 87 | 80 |
| PaO2, peak (mmHg) | 57.9 ± 13 | 56.6 ± 12.9 | 61.6 ± 12.6 |
| ΔPaO2 (mmHg) | 18.9 ± 8.3 | 19.4 ± 8.5 | 17.6 ± 7.8 |
| P(A-a)O2, peak (mmHg) | 58.1 ± 13 | 58.9 ± 13.2 | 61.7 ± 12.7 |
| % with high P(A-a)O2, peak | 92.5 | 95 | 84 |
| ΔP(A-a)O2/ΔVO2 (mmHg/L) | 34.2 ± 16.9 | 34.4 ± 17.1 | 33.6 ± 16.7 |
| % with high ΔP(A-a)O2/ΔVO2 | 83 | 82 | 84 |
| VD/VT, peak | 0.43 ± 0.09 | 0.44 ± 0.09 | 0.39 ± 0.08∗ |
| Walk test, distance (m) | 388 ± 102 | 393 ± 98 | 375 ± 114 |
| Walt test, nadir SaO2 (%) | 86 ± 5.7 | 85.6 ± 6 | 88.3 ± 4.6 |
| Walk test, ΔSaO2 (%) | 9.2 ± 4.7 | 9.7 ± 5 | 7.9 ± 3.7 |
| % with ΔSaO2 ≥4% | 83 | 83 | 84 |
∗Significantly different from IPF group (P = 0.01).
All but 3 Patients (97.5%) had low DLCO values (<LLN, corresponding to a mean 73 ± 0.4% predicted) whereas only 66.6% had a low KCO; 42% patients exhibited resting hypoxemia (<75 mmHg) and 26% a high resting P(A-a)O2; 112 patients (92.5%) exhibited an increased P[(A-a)O2], peak, 83% a high ΔP(A-a)O2/ΔVO2 and 100 patients (83%) demonstrated significant O2 desaturation during 6MWT. There was no significant difference between IPF and f-NSIP for all parameters except VD/VT peak which was higher in IPF (P = 0.01).
DLCO was severely reduced in the 79 patients with normal resting PaO2. Interestingly 27 patients had low DLCO and normal P(A-a)O2, peak and/or no desaturation during the 6MWT. Nine patients had normal P(A-a)O2, peak: 6 out of 9 did not show significant desaturation during walk test. Conversely among the 21 patients with low DLCO and without significant desaturation at the 6MWT, all had abnormal P(A-a)O2, peak. The 3 patients with normal DLCO also had normal PaO2, normal P(A-a)O2, peak, and normal oxygen saturation during 6MWT.
We found a very good correlation between DLCO and lung volumes and other measures of gas exchange (Table 3 and Figure 1). Interestingly we also found a good correlation between DLCO and VD/VT.
Table 3.
Correlation between percent-predicted DLco and other measures.
| Variable | coefficient | P value |
|---|---|---|
| FVC | 0.56 | <0.0001 |
| TLC | 0.437 | <0.0001 |
| FEV1 | 0.508 | <0.0001 |
| Kco | 0.56 | <0.0001 |
| Resting PaO2 | 0.525 | <0.0001 |
| P(A-a)O2, peak (mmHg) | −0.465 | <0.0001 |
| ΔP(A-a)O2/ ΔVO2 (mmHg/L) | −0.534 | <0.0001 |
| 6MWT, nadir SpO2 (%) | 0.511 | <0.0001 |
| 6MWT, ΔSpO2 (%) | −0.47 | <0.0001 |
| VD/VT, peak | −0.404 | <0.0001 |
Figure 1.
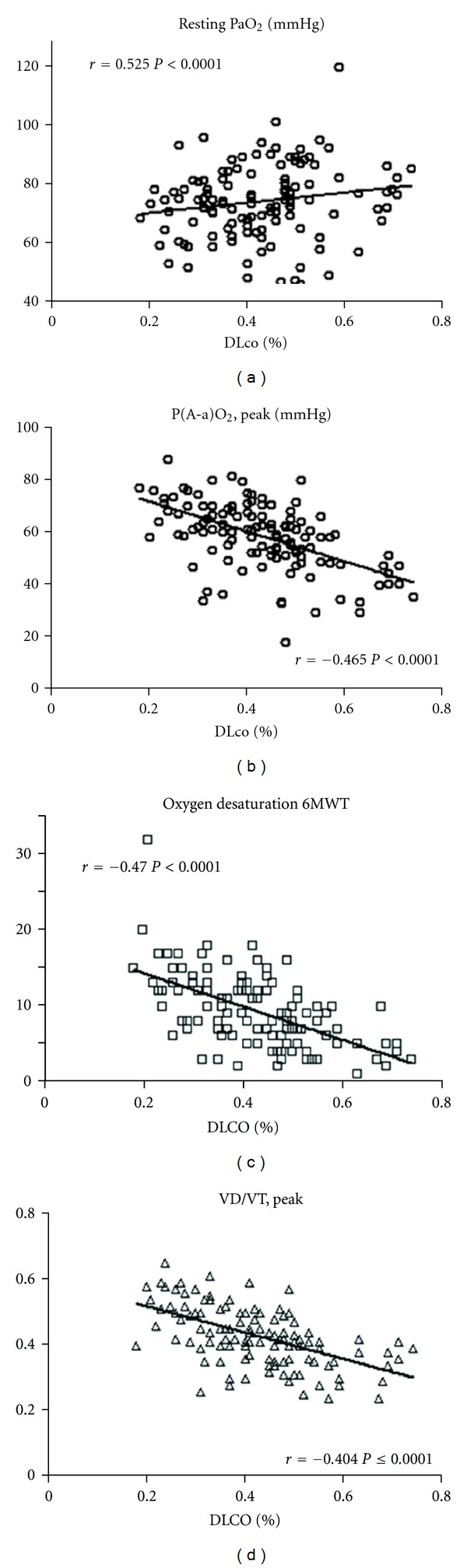
Correlation between DLCO (percent predicted) and resting PaO2, P(A-a)O2, peak, oxygen desaturation during 6MWT and VD/VT, peak in fibrotic IIP.
Resting parameters and indexes of gas exchange were more severely altered according to disease severity as judged on alteration of DLCO (Table 4).
Table 4.
Rest and exercise parameters as a function of disease severity defined by DLco%: mild: DLco ≥ 60%, moderate: DLco < 60% and ≥40% and severe (advanced disease): DLco < 40% [7].
| Disease severity | Mild | Moderate | Severe |
|---|---|---|---|
| Parameter | Dlco ≥ 60% | 40% ≤ DLco < 60% | DLco < 40% |
| n = 10 | n = 65 | n = 46 | |
| FVC | 99 ± 20 | 77 ± 11∗ | 69 ± 16$∗∗ |
| FEV1 | 99 ± 19 | 81 ± 12∗ | 70 ± 16$£ |
| TLC | 89 ± 13 | 70 ± 11∗ | 66 ± 16$ |
| PaO2, rest (mmHg) | 89 ± 6.4 | 80 ± 9∗ | 69 ± 13.7$∗∗ |
| PaO2, peak (mmHg) | 78.7 ± 9 | 59 ± 10∗ | 51 ± 11$∗∗ |
| P(A-a)O2, rest (mmHg) | 22 ± 9 | 27 ± 9.8 | 38 ± 13$∗∗ |
| P(A-a)O2, peak (mmHg) | 40 ± 7 | 57 ± 12∗ | 64 ± 12$£ |
| ΔP(A-a)O2/ΔVO2 (mmHg/L) | 14.8 ± 11 | 31 ± 12.5∗ | 44 ± 18$∗∗ |
| Walt test, nadir SaO2 (%) | 92 ± 3 | 87 ± 4∗ | 83 ± 6$∗∗ |
| Walk test, ΔSaO2 (%) | 4.3 ± 2.4 | 8.4 ± 3.8∗ | 11.5 ± 5.2$∗∗ |
Significantly different from group moderate £ P < 0.001, ∗∗ P < 0.01.
Significantly different from group mild $ P < 0.001, ∗ P < 0.01.
6. Discussion
There were three main findings in this study: first, abnormal gas exchange is present in patients with normal lung volumes; second, a low DLCO was found in 97.5% patients with f-IIP whereas resting PaO2, 6MWT oxygen desaturation and P(A-a)O2, peak were abnormal, respectively, in 42%, 83%, and 92.5%; and third, no patient had normal DLCO and abnormal PaO2, 6MWT oxygen desaturation, or increased P(A-a)O2, peak. As a consequence, DLCO is more sensitive for demonstrating gas exchange abnormality in fibrotic IIP than resting PaO2, exercise P(A-a)O2, peak, or 6MWT SpO2.
Clearly, DLCO is reduced in a greater extent than lung volumes in f-IIP and therefore abnormal DLCO is a frequent finding in patients with normal lung volumes. This has been demonstrated in previous studies [26, 27], both in IPF and f-NSIP [28–39]. Along this line, Gaensler and coworkers noted a fair correlation between histologic severity and physiologic indices [38]. Crystal and colleagues reported a poor correlation with spirometry, lung volumes, DLCO, and resting gas exchange in IPF [39]. In 14 untreated patients with IPF, DLCO, and lung volumes correlated with the extent of fibrosis and cellular infiltration; both of these correlated more strongly than gas exchange with exercise [6].
In patients with f-IIP, the DLCO has been widely used to predict abnormal gas exchange in the lung. Resting PaO2 correlates poorly with disease severity. In our studies, resting PaO2 was in the normal range in 58% cases. In contrast, abnormal values for arterial blood gases during exercise are more sensitive than resting PaO2. However our study in a large group of patients demonstrated that patients with abnormal gas exchange during exercise always exhibited abnormal DLCO and that, in contrast, abnormal DLCO was found in patients with normal gas exchange during exercise. A significant 6MWT oxygen desaturation and/or an increased P(A-a)O2 was never observed in f-IIP patients with normal DLCO whereas this has been previously reported in sarcoidosis [40, 41].
Our 6MWT results are in agreement with the results of Lama and coworkers [18] who reported 6MWT oxygen desaturation results in IPF and NSIP: in this study, 80% IPF patients and 64% NSIP patients exhibited an oxygen desaturation ≥4% during 6MWT. The 6MWT is a noninvasive, cheap, and simple field test to carry out and interpret. However despite these advantages, some variabilities in the results obtained are observed [42] and an increased ventilatory response during 6MWT might be responsible for higher PAO2 minimizing the decrease in SaO2.
Factors that contribute to reduction in DLCO include abnormal thickness of the alveolar capillary membrane and reduced pulmonary capillary blood volume. Thus, DLCO is highly dependent on pulmonary vascular blood volume. We recently reported in patients with f-IIP that the Vc component of the DLCO was significantly decreased in addition to the already lowered Dm, CO component as a consequence of the thickened membranes [43]. The correlation between DLCO and VD/VT, peak is in agreement with the findings by Agusti and coworkers [8] and supports the concept that the abnormalities of the pulmonary vasculature are important to modulate gas exchange in IPF during exercise.
It was not the scope of our study to evaluate the prognostic value of each test. Several studies found that distance or desaturation during a 6MWT was a strong predictor of mortality [18]. Mortality rate is higher among patients with DLCO <30% to 45% predicted [33, 44–46], but it is clear that the prognostic value of any pulmonary functional parameter at one point is limited.
In conclusion, DLCO appears as the best physiologic index to evaluate gas exchange abnormalities in fibrotic IIP and could take the place of formal exercise testing with arterial blood gas to evaluate the severity of gas exchange in patients with fibrotic IIP.
Author's Contribution
Benoit Wallaert and LidwineWemeau-Stervinou were responsible for Conception and design; Benoit Wallaert, LidwineWemeau-Stervinou, Julia Salleron, Isabelle Tillie-Leblond, and Thierry Perez were responsible for analysis and interpretation; BenoitWallaert, LidwineWemeau-Stervinou, Isabelle Tillie-Leblond, and Thierry Perez were responsible for drafting the paper for important intellectual content.
Conflict of Interests
For each author, no significant conflict of interest exist with any companies/organizations whose products or services may be discussed in this paper.
References
- 1.American Thoracic Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee. American Journal of Respiratory and Critical Care Medicine. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) American Journal of Respiratory and Critical Care Medicine. 2000;161(2, part 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 3.Bradley B, Branley HM, Egan JJ. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(5):v1–v58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Collard HR, Egan JJ, et al. An Official ATS/ERS/JRS/ALAT Statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American Journal of Respiratory and Critical Care Medicine. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katzenstein AL. Idiopathic interstitial pneumonia: classification and diagnosis. Monographs in Pathology. 1993:1–31. [PubMed] [Google Scholar]
- 6.Fulmer JD, Roberts WC, von Gal ER, Crystal RG. Morphologic-physiologic correlates of the severity of fibrosis and degree of cellularity in idiopathic pulmonary fibrosis. Journal of Clinical Investigation. 1979;63(4):665–676. doi: 10.1172/JCI109349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan JJ, Martinez FJ, Wells AU, Williams T. Lung function estimates in idiopathic pulmonary fibrosis: the potential for a simple classification. Thorax. 2005;60(4):270–273. doi: 10.1136/thx.2004.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agusti AGN, Roca J, Gea J, Wagner PD, Xaubet A, Rodriguez-Roisin R. Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. American Review of Respiratory Disease. 1991;143(2):219–225. doi: 10.1164/ajrccm/143.2.219. [DOI] [PubMed] [Google Scholar]
- 9.Risk C, Epler GR, Gaensler EA. Exercise alveolar-arterial oxygen pressure difference in interstitial lung disease. Chest. 1984;85(1):69–74. doi: 10.1378/chest.85.1.69. [DOI] [PubMed] [Google Scholar]
- 10.Hallstrand TS, Boitano LJ, Johnson WC, Spada CA, Hayes JG, Raghu G. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. European Respiratory Journal. 2005;25(1):96–103. doi: 10.1183/09031936.04.00137203. [DOI] [PubMed] [Google Scholar]
- 11.Agusti C, Xaubet A, Agusti AGN, Roca J, Ramirez J, Rodriguez-Roisin R. Clinical and functional assessment of patients with idiopathic pulmonary fibrosis: results of a 3 year follow-up. European Respiratory Journal. 1994;7(4):643–650. doi: 10.1183/09031936.94.07040643. [DOI] [PubMed] [Google Scholar]
- 12.Cherniack RM, Colby TV, Flint A, et al. Correlation of structure and function in idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine. 1995;151(4):1180–1188. doi: 10.1164/ajrccm/151.4.1180. [DOI] [PubMed] [Google Scholar]
- 13.Xaubet A, Agustí C, Luburich P, et al. Pulmonary function tests and CT scan in the management of idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine. 1998;158(2):431–436. doi: 10.1164/ajrccm.158.2.9709008. [DOI] [PubMed] [Google Scholar]
- 14.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity: ATS Workshop on Lung Volume Measurements Official Statement of the European Respiratory Society. European Respiratory Journal. 1995;8(3):492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 15.Standardized lung function testing. Official statement of the European Respiratory Society. The European Respiratory Journal. Supplement. 1993;16:1–100. [PubMed] [Google Scholar]
- 16.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. European Respiratory Journal. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 17.ATS statement: guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine. 2002;1(166):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 18.Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6 minute walk test in idiopathic interstitial pneumonia. American Journal of Respiratory and Critical Care Medicine. 2003;168(9):1084–1090. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 19.Wallaert B, Talleu C, Wemeau-Stervinou L, Duhamel A, Robin S, Aguilaniu B. Reduction of maximal oxygen uptake in sarcoidosis: relationship with disease severity. Respiration. 2011;82:501–508. doi: 10.1159/000330050. [DOI] [PubMed] [Google Scholar]
- 20.Sorbini CA, Grassi V, Solinas E, Muiesan G. Arterial oxygen tension in relation to age in healthy subjects. Respiration. 1968;25(1):3–13. doi: 10.1159/000192549. [DOI] [PubMed] [Google Scholar]
- 21.ATS/ACCP Statement on cardiopulmonary exercise testing. American Journal of Respiratory and Critical Care Medicine. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 22.Cooper C, Storer T. Exercise Testing and Interpretation. A Practical Approach. Cambridge, UK: 2001. [Google Scholar]
- 23.Jones NL. Clinical Exercise Testing. Philadelphia, Pa, USA: WB Saunders; 1997. [Google Scholar]
- 24.Wasserman K, Hansen JE, Sue DY, Casaburi R, Whipp BJ. Principles of Exercise Testing and Interpretation. 3rd edition 1999. [Google Scholar]
- 25.Aguilaniu B, Richard R, Costes F, et al. Cardiopulmonary exercise testing. Revue des Maladies Respiratoires. 2007;24(3, part 2):2S111–2S160. [PubMed] [Google Scholar]
- 26.Dunn TL, Watters LC, Hendrix C, Cherniack RM, Schwarz MI, King TE. Gas exchange at a given degree of volume restriction is different in sarcoidosis and idiopathic pulmonary fibrosis. American Journal of Medicine. 1988;85(2):221–224. doi: 10.1016/s0002-9343(88)80347-4. [DOI] [PubMed] [Google Scholar]
- 27.Keogh BA, Lakatos E, Price D, Crystal RG. Importance of the lower respiratory tract in oxygen transfer. Exercise testing in patients with interstitial and destructive lung disease. American Review of Respiratory Disease. 1984;129(2):S76–S80. doi: 10.1164/arrd.1984.129.2P2.S76. [DOI] [PubMed] [Google Scholar]
- 28.Erbes R, Schaberg T, Loddenkemper R. Lung function tests in patients with idiopathic pulmonary fibrosis: are they helpful for predicting outcome? Chest. 1997;111(1):51–57. doi: 10.1378/chest.111.1.51. [DOI] [PubMed] [Google Scholar]
- 29.Nagai S, Kitaichi M, Itoh H, Nishimura K, Izumi T, Colby TV. Idiopathic nonspecific interstitial pneumonia/fibrosis: comparison with idiopathic pulmonary fibrosis and BOOP. European Respiratory Journal. 1998;12(3):1010–1019. doi: 10.1183/09031936.98.12051010. [DOI] [PubMed] [Google Scholar]
- 30.Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine. 1998;157(1):199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 31.Daniil ZD, Gilchrist FC, Nicholson AG, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. American Journal of Respiratory and Critical Care Medicine. 1999;160(3):899–905. doi: 10.1164/ajrccm.160.3.9903021. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson AG, Colby TV, Dubois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. American Journal of Respiratory and Critical Care Medicine. 2000;162(6):2213–2217. doi: 10.1164/ajrccm.162.6.2003049. [DOI] [PubMed] [Google Scholar]
- 33.Mogulkoc N, Brutsche MH, Bishop PW, Greaves SM, Horrocks AW, Egan JJ. Pulmonary function in idiopathic pulmonary fibrosis and referral for lung transplantation. American Journal of Respiratory and Critical Care Medicine. 2001;164(1):103–108. doi: 10.1164/ajrccm.164.1.2007077. [DOI] [PubMed] [Google Scholar]
- 34.Latsi PI, Du Bois RM, Nicholson AG, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. American Journal of Respiratory and Critical Care Medicine. 2003;168(5):531–537. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 35.Collard HR, King TE, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine. 2003;168(5):538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 36.Flaherty KR, Mumford JA, Murray S, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. American Journal of Respiratory and Critical Care Medicine. 2003;168(5):543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 37.Jegal Y, Dong SK, Tae SS, et al. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. American Journal of Respiratory and Critical Care Medicine. 2005;171(6):639–644. doi: 10.1164/rccm.200403-331OC. [DOI] [PubMed] [Google Scholar]
- 38.Gaensler EA, Carrington CB. Open biopsy for chronic diffuse infiltrative lung disease: clinical, roentgenographic, and physiological correlations in 502 patients. The Annals of Thoracic Surgery. 1980;30(5):411–426. doi: 10.1016/s0003-4975(10)61291-x. [DOI] [PubMed] [Google Scholar]
- 39.Crystal RG, Fulmer JD, Roberts WC, Moss ML, Line BR, Reynolds HY. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Annals of Internal Medicine. 1976;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- 40.Miller A, Brown LK, Sloane MF, Bhuptani A, Teirstein AS. Cardiorespiratory responses to incremental exercise in sarcoidosis patients with normal spirometry. Chest. 1995;107(2):323–329. doi: 10.1378/chest.107.2.323. [DOI] [PubMed] [Google Scholar]
- 41.Karetzky M, McDonough M. Exercise and resting pulmonary function in sarcoidosis. Sarcoidosis Vasculitis and Diffuse Lung Disease. 1996;13(1):43–49. [PubMed] [Google Scholar]
- 42.Elpern EH, Stevens D, Kesten S. Variability in performance of timed walk tests in pulmonary rehabilitation programs. Chest. 2000;118(1):98–105. doi: 10.1378/chest.118.1.98. [DOI] [PubMed] [Google Scholar]
- 43.Wémeau-Stervinou L, Perez T, Murphy C, Polge A-S, Wallaert B. Lung capillary blood volume and membrane diffusion in idiopathic interstitial pneumonia. Respiratory Medicine. 2012;106:564–570. doi: 10.1016/j.rmed.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 44.King TE, Jr., Safrin S, Starko KM, et al. Analyses of efficacy end points in a controlled trial of interferon-γ1b for idiopathic pulmonary fibrosis. Chest. 2005;127(1):171–177. doi: 10.1378/chest.127.1.171. [DOI] [PubMed] [Google Scholar]
- 45.Lynch DA, Godwin JD, Safrin S, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. American Journal of Respiratory and Critical Care Medicine. 2005;172(4):488–493. doi: 10.1164/rccm.200412-1756OC. [DOI] [PubMed] [Google Scholar]
- 46.Raghu G, Brown KK, Bradford WZ, et al. A placebo-controlled trial of interferon Gamma-1b in patients with idiopathic pulmonary fibrosis. The New England Journal of Medicine. 2004;350(2):125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]


