Abstract
Our novel proposal is that TNFα exerts a direct effect on mitochondrial respiratory function in the heart, independently of its cell surface receptors. TNFα-induced cardioprotection is known to involve reactive oxygen species (ROS) and sphingolipids. We therefore further propose that this direct mitochondrial effect is mediated via ROS and sphingolipids. The protective concentration of TNFα (0.5 ng/ml) was added to isolated heart mitochondria from black 6 × 129 mice (WT) and double TNF receptor knockout mice (TNFR1&2−/−). Respiratory parameters and inner mitochondrial membrane potential were analyzed in the presence/absence of two antioxidants, N-acetyl-l-cysteine or N-tert-butyl-α-(2-sulfophenyl)nitrone or two antagonists of the sphingolipid pathway, N-oleoylethanolamine (NOE) or imipramine. In WT, TNFα reduced State 3 respiration from 279.3 ± 3 to 119.3 ± 2 (nmol O2/mg protein/min), increased proton leak from 15.7 ± 0.6% (control) to 36.6 ± 4.4%, and decreased membrane potential by 20.5 ± 3.1% compared to control groups. In TNFR1&2−/− mice, TNFα reduced State 3 respiration from 205.2 ± 4 to 75.7 ± 1 (p < 0.05 vs. respective control). In WT mice, both antioxidants added with TNFα restored State 3 respiration to 269.2 ± 2 and 257.6 ± 2, respectively. Imipramine and NOE also restored State 3 respiration to 248.4 ± 2 and 249.0 ± 2, respectively (p < 0.01 vs. TNFα alone). Similarly, both antioxidant and inhibitors of the sphingolipid pathway restored the proton leak to pre-TNF values. TNFα-treated mitochondria or isolated cardiac muscle fibers showed an increase in respiration after anoxia–reoxygenation, but this effect was lost in the presence of an antioxidant or NOE. Similar data were obtained in TNFR1&2−/− mice. TNFα exerts a protective effect on respiratory function in isolated mitochondria subjected to an anoxia–reoxygenation insult. This effect appears to be independent of its cell surface receptors, but is likely to be mediated by ROS and sphingolipids.
Keywords: Cytokines, Mitochondria, Oxygen consumption, Oxygen radicals, Sphingolipids
Introduction
It is now well established that inflammatory cytokines, including tumor necrosis factor alpha (TNFα), are implicated in the consequences of various cardiovascular events including ischemia/reperfusion (see review [31]). The cardiac effects of TNFα are bidirectional, including both negative inotropic and cardioprotective effects [43]. Recent studies suggest that this dual role of TNFα is dependent on which of its receptors are activated [18, 51]. TNFR1 (p55) and TNFR2 (p75) are the two distinct cell surface receptors mediating the effects of TNFα, both of which are present in cardiomyocytes [48]. We have previously demonstrated that a low concentration of TNFα confers protection against ischemia–reperfusion injury in the heart [22]. Moreover, the genetic depletion of TNFα precludes the capacity to evoke the cardioprotective program of ischemic preconditioning [45] or postconditioning [18]. Although we have begun to dissect out the signaling networks governing this cardioprotection, the cellular effectors are unknown [17, 20, 21, 23]. Interestingly, data are emerging which support a direct effect of TNFα on mitochondrial respiratory function. This includes studies showing that TNFα can inhibit mitochondrial electron transfer [19] and promote mitochondrial uncoupling [6] while TNFα, via an intracellular trafficking pathway, appears to bind to the outer mitochondrial membrane [24, 25].
We therefore reasoned that a novel mechanism by which TNFα could confer its cardioprotection might, at least in part, be via the direct modulation of mitochondrial respiratory function. In this study, we show that administration of TNFα to a cardiac mitochondrial preparation or saponinpermeabilized cardiac muscle fibers from wild type and TNFR1&2−/− mice has selective effects on mitochondrial respiratory function independent of its binding to cell surface receptors. These effects include: a reduction in the State 3 respiration, uncoupling protein (UCP) dependent induction of a proton leak, a decrease in membrane potential, inhibition of glutamate-dependent respiration, and an enhanced rate of respiratory recovery after anoxia–reoxygenation injury. In addition, we suggest that this effect is mediated via reactive oxygen species and the sphingolipid pathway, both known to be present in mitochondria and possibly activated in the presence of TNFα [7, 22, 36].
Materials and methods
All experiments were performed using adult male black 6 × 129 mice (WT) and double TNF receptor knock out mice (TNFR1&2−/−) at 10–12 weeks of age (weighing 20–25 g) in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health [NIH Publication No. 85 (23), revised 1996]. All procedures were approved by the Faculty of Health Sciences Animal Ethics Committee, University of Cape Town.
Isolation of mitochondria and permeabilized fibers
Mice were anesthetized with 50 mg/kg intraperitoneal sodium pentobarbitone and 25 IU heparin. Mitochondria and permeabilized fibers were extracted from the heart tissue as previously described [28, 46, 49], and mitochondrial protein content was determined by a modified method of Lowry [37]. All respiratory parameters and membrane potential were corrected per mg of protein for isolated mitochondria and per mg of dry weight for permeabilized fibers (the content of the respirometer chamber was removed at the end of each experiment and dried out in a 60°C oven before being weighed).
Respiration studies
Mitochondrial respiratory parameters were evaluated using an Oxytherm respirometer equipped with a Clark type electrode and a Peltier temperature control unit (Hansatech, Norfolk, UK) with all experiments performed at 25°C as previously described [28]. A TNFα dose–response curve was performed on isolated mitochondria at final concentrations ranging from 0 to 20 ng/ml. Glutamate (10 mM) and malate (5 mM) were used as substrates to stimulate electron transport activity at Complex I and examine the integrity of oxidative phosphorylation and the TCA cycle. To stimulate electron transport activity at Complex II, succinate was used. ADP given at a final concentration of 350 µM was used to trigger State 3 respiration. Oxidative phosphorylation-independent proton kinetics were evaluated with the addition of oligomycin (1 µg/ml) to prevent ATP synthesis [34]. The rate of respiration after the addition of oligomycin is termed State 3b, and the proton leak is calculated as a percentage of the ratio of State 3b/State 3. Inducible proton leak measurements were performed in the presence of succinate and oligomycin. Guanosine diphosphate (GDP 500 µM, final concentration) was then added, with succinate as the substrate, to assess the response of the mitochondria to this uncoupling protein inhibitor, and the percentage proton leak calculated as for the baseline proton kinetics. In a separate set of experiments, isolated mitochondria or permeabilized fibers were subjected to 25 min anoxia/5 min reoxygenation. State 3 respiration was assessed, and percentage respiration recovery was determined by calculating the ratio of State 3 respiration preand post-anoxia as described previously [30, 42]. Where appropriate, the antioxidants, N-tert-butyl-α-(2-sulfophenyl) nitrone (2-SPBN) and N-acetyl-l-cysteine (NAC) or the inhibitors of sphingosine metabolism, N-oleoylethanolamine (NOE) and imipramine (IMI) were added to the buffer 1 min before the addition of isolated mitochondria.
Mitochondrial inner membrane potential
JC-1 dye (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanide iodide) was employed to detect the change in mitochondrial membrane potential (Δψm). Isolated heart mitochondria were stained with JC-1 (5 µM; Molecular Probes) [27]. The suspension was kept at 37°C for 5 min, and then analyzed on a Becton-Dickson FACs Calibur flow cytometer. JC-1 aggregates at high Δψm and can be excited at 488 nm. JC-1 exhibits a membrane potential-dependent accumulation in the mitochondria, indicated by a fluorescence emission shift from green at 525 nm (FL-1) to red at 585 nm (FL-2) due to concentration-dependent formation of red-fluorescent J-aggregates [9, 38]. Consequently, mitochondrial depolarization is indicated by a decrease in the red/green fluorescence intensity ratio, which is dependent only on the membrane potential and not on other factors such as mitochondrial size, shape, and density. As a negative control, unstained mitochondria were analyzed. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP, 0.1 µM), a mitochondrial membrane disrupter, was used as a positive control.
ATP synthesis
The amount of ADP that was phosphorylated to ATP over a timed period was evaluated by a modified method of Budnikov [5] in which luciferin-luciferase luminometry is used to measure the rate of ATP synthesis. Briefly, isolated mitochondria were added to synthesis buffer (final concentrations: 150 mM sucrose, 75 mM KCl, 5 mM KH2PO4, 2.5 mM MgCl2, 3 µM rotenone) and warmed to 25°C. After 60 s of equilibration period, TNFα (0.5 ng/ml) was added to the buffer. ADP (350 µM) was then added to both control and TNFα-exposed mitochondria. A zero time sample was taken, and thereafter, a sample was taken every 10 s for 1 min. These samples were added directly into cold 2.5% trichloroacetic acid to arrest the reaction and release ATP. Tris base (1 M) was used to neutralize the reaction, and the samples were frozen at −80°C until analysis. Data were expressed as µM ATP/mg protein.
Chemicals and pharmacological agents
JC-1 was obtained from Molecular Probes, USA. JC-1 was dissolved in DMSO, with a final concentration of DMSO which was less than 0.02%. Murine recombinant TNFα was obtained from PeproTech Inc., Israel. Unless otherwise stated, all chemicals used were obtained from Merck, Darmstadt, Germany.
Statistical analysis
Data are presented as mean ± SEM. Comparisons between multiple groups were performed by one-way ANOVA followed by Tukey–Kramer multiple comparisons test (Instat, GraphPad Software Inc.). Comparison between animal strains was performed by two-way ANOVA (Prism, GraphPad Software Inc.). Comparison between two groups was performed by the Student’s t test. A value of p < 0.05 was considered statistically significant.
Results
Dose–response curve
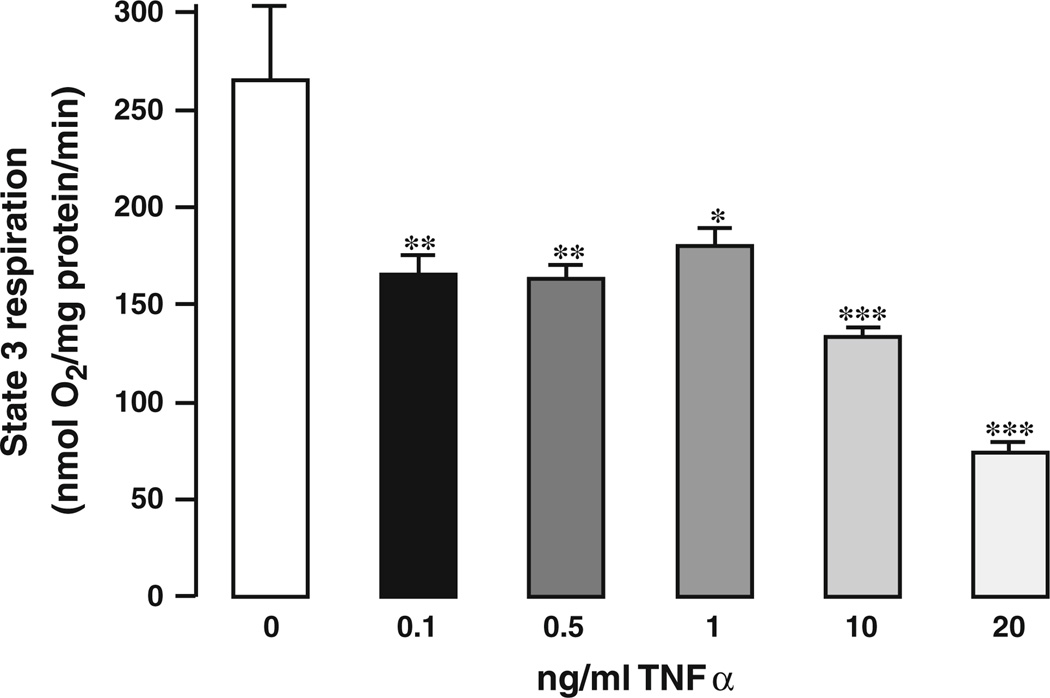
The rate of State 3 respiration in CTL mice was in agreement with data from previous studies [28, 41]. Addition of TNFα decreased State 3 respiration (nmol O2/mg protein/min) from 263 ± 5.6 in the CTL to 165.43 ± 6.2 for 1 ng/ml TNFα (p < 0.01) and to 163.5 ± 8.9 for 0.5 ng/ml TNFα (p < 0.05). Higher concentrations of TNFα (10–20 ng/ml) decreased State 3 respiration in a dose-dependent manner (Fig. 1a).
Fig. 1.
Dose–response curve of TNFα in isolated mitochondria. A range from 0 to 20 ng/ml TNFα was added directly to isolated mouse heart mitochondria, and the State 3 respiration rate was assessed; n = 6 for each concentration
Effect of TNFα in isolated heart mitochondria
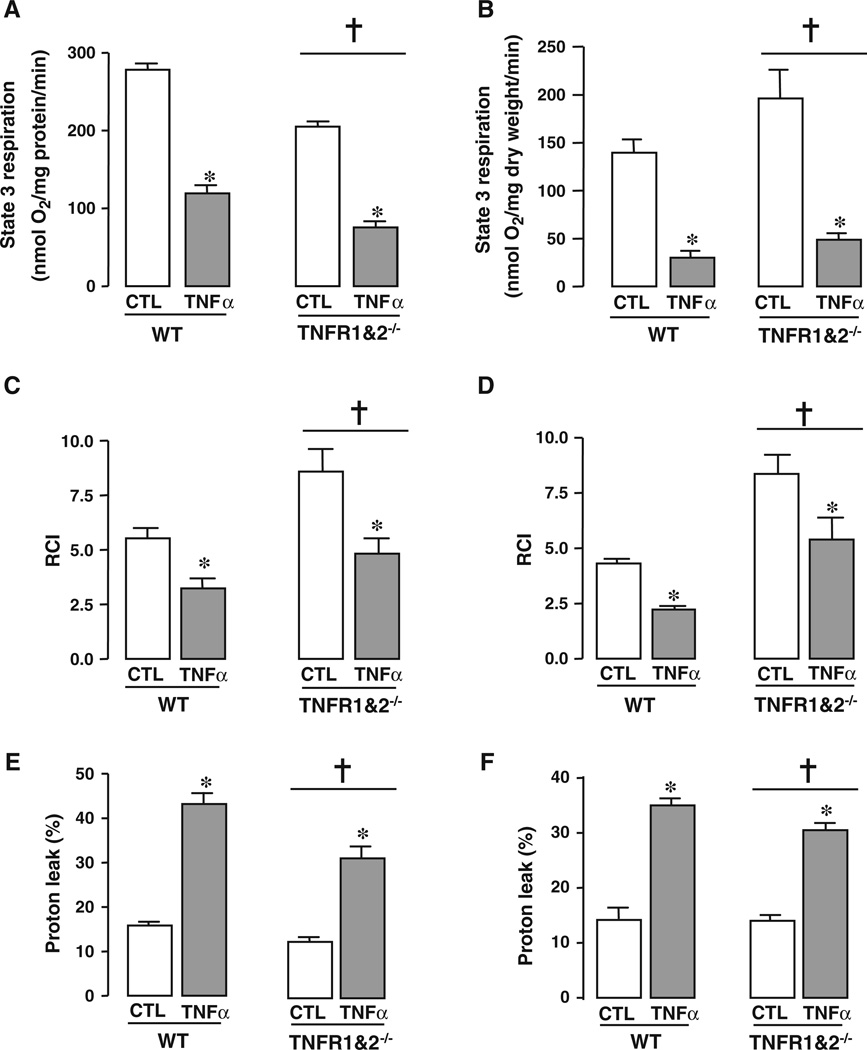
Addition of TNFα (0.5 ng/ml) to a suspension of isolated mitochondria decreased State 3 respiration (in nmol O2/mg protein/min) from 279.3 ± 3 (control) to 119.3 ± 2 (TNFα) in the WT hearts, p < 0.05 versus control and from 205.2 ± 4 (control) to 75.5 ± 1 (TNFα) in mitochondria isolated from TNFR1&2−/− hearts, p < 0.05 versus control (Fig. 2a). In permeabilized fibers, State 3 respiration (in nmol O2/mg protein/min) was also decreased by the addition of 0.5 ng/ml TNFα from 140 ± 13 (CTL) to 30 ± 2 (TNFα) in WT and from 196 ± 30 (CTL) to 49 ± 3 (TNFα) in TNFR1&2−/−, p < 0.001 for both groups (Fig. 2b). TNFα at 0.5 ng/ml reduced the RCI in WT mitochondria from 4.3 ± 0.1 (CTL) to 2.2 ± 0.1 (TNFα) and in the double receptor knock out from 8.4 ± 0.9 (CTL) to 5.4 ± 1.0 (TNFα), p < 0.05 for both groups (Fig. 2c). The RCI was decreased in a similar manner in permeabilized fibers in both WT and TNFR1&2−/− p < 0.05 for both groups (Fig. 2d). Similar State 3 levels have been previously reported in the literature [28, 41]. TNFα increased the proton leak in isolated WT mitochondria from 15.8 ± 0.6 to 43.2 ± 3% (p < 0.001 vs. control) and in TNFR1&2−/− mitochondria from 12.6 ± 0.9 to 31.5 ± 2.7, p < 0.001 versus control (Fig. 2e). Similarly, the proton leak was increased with the addition of TNFα in permeabilized fibers from 14.1 ± 0.5 to 35.0 ± 0.6 in WT, and from 13.6 ± 1.0 to 30.5 ± 0.3 in TNFR1&2−/− hearts, p < 0.001 (Fig. 2f). In addition, the degree of depolarization of the inner mitochondrial membrane was modestly decreased to 56% compared to the normalized control group (p < 0.05; Fig. S2A, supplementary data).
Fig. 2.
TNFα affects the respiration in isolated heart mitochondria and in permeabilized muscle fibers. TNFα (0.5 ng/ml) was added directly to isolated mouse heart mitochondria, or to saponinpermeabilized cardiac muscle fibers. State 3 respiration, RCI and proton leak were assessed. a State 3 respiration was decreased with TNFα in isolated mitochondria. b Addition of TNFα decreased State 3 respiration in permeabilized fibers. c RCI was decreased with TNFα in isolated mitochondria. d TNFα decreased RCI in permeabilized fibers. e The proton leak was increased with the addition of TNFα in isolated mitochondria. f The presence of TNFα increased the proton leak in permeabilized fibers. *p < 0.001 versus control group (CTL); †p < 0.001 TNFR1&2−/− versus WT. n ≥ 6. WT wild type; TNFR1&2−/− double TNF receptor knock out
The effect of TNFα on mitochondrial respiration is abrogated by antioxidants or sphingolipid pathway inhibitors
Addition of the antioxidants NAC and 2-SPBN in the presence of TNFα in WT cardiac mitochondria restored the State 3 respiration to 269.2 ± 2 nmol O2/mg protein/min for TNFα + NAC (p < 0.05 vs. TNFα) and 257.6 ± 2 nmol O2/mg protein/min for TNFα + 2-SPBN (p < 0.05 vs. TNFα) (Fig. 3a). Addition of NAC did not abolish the decrease in State 3 respiration induced by 20 ng/ml TNFα (data not shown). Addition of antioxidants without TNFα had no effect on State 3 respiration compared to control mitochondria (supplementary figure S1A).
Fig. 3.
Effect of antioxidants and sphingolipid inhibitors in TNFα-mediated uncoupling of wild-type mouse mitochondria. TNFα (0.5 ng/ml) was added directly to isolated mouse heart mitochondria or permeabilized fibers in the presence of NAC (N-acetyl-cysteine, 30 µM), 2-SPBN (N-tert-butyl-α-(2-sulfophenyl)nitrone sodium, 10 µM), IMI (imipramine, 10 µM) or NOE (N-oleoyl ethanolamine, 5 µM), and State 3 respiration, RCI, and proton leak were assessed. a The effect of TNFα on State 3 respiration was attenuated in the presence of antioxidants or sphingolipid pathway inhibitors in isolated mitochondria. b The effect of TNFα on State 3 respiration was reversed with the addition of NAC or NOE in permeabilized fibers. c Antioxidants and sphingolipid pathway inhibitors abolished the effect of TNFα on RCI in isolated mitochondria. d Antioxidants and sphingolipid pathway inhibitors abolished the effect of TNFα on RCI in permeabilized fibers. e The increase in proton leak observed with TNFα was abrogated in the presence of antioxidants or sphingolipid pathway inhibitors in isolated mitochondria. f NAC and NOE abolished the increase in proton leak observed with addition of TNFα in permeabilized fibers. *p < 0.05 versus CTL; ≠p < 0.05 versus TNFα; †p < 0.001 TNFR1&2−/− versus WT; n = 6
Similarly, in the presence of TNFα, imipramine and NOE, inhibitors of sphingolipid metabolism, restored State 3 respiration in isolated mitochondria to 248.4 ± 2 nmol O2/mg protein/min (p < 0.05 vs. control) (Fig. 3a) and 249.0 ± 2 nmol O2/mg protein/min (p < 0.05 vs. control) (Fig. 3a), respectively. Addition of NOE did not abolish the decrease in State 3 respiration induced by 20 ng/ml TNFα (data not shown). The inhibitors of sphingolipid metabolism had no effect on the State 3 respiration in the absence of TNFα, compared to control mitochondria (Supplementary figure S1). In permeabilized fibers from both the WT and TNFR1&2−/−, the presence of TNFα was associated with an increase in State 3 respiration (in nmol O2/mg dry weight/min) when challenged with NAC or NOE from 49.0 ± 3.4 to 88.2 ± 3.9 and 107.3 ± 2.5, respectively (p < 0.05 vs. TNFα) (Fig. 3b). Moreover, the decrease in RCI observed in the presence of TNFα was reversed by NAC and 2-SPBN to 4.4 ± 0.2 and 3.9 ± 0.6, respectively (p < 0.05 vs. TNFα) (Fig. 3c). Similarly, imipramine and NOE reversed the effect of TNFα on RCI in WT mitochondria (Fig. 3c). A similar trend was observed in permeabilized fibers, with TNFα decreasing the RCI in the WT from 4.3 ± 0.1 to 1.3 ± 0.1 (p < 0.05 vs. CTL) and in the TNFR1&2−/− from 8.4 ± 0.9 to 3.1 ± 0.3 (p < 0.05 vs. CTL) (Fig. 3d).
Antioxidants or sphingolipid pathway inhibitors abrogate direct effect of TNFα on proton leak
The proton leak was restored by the presence of the antioxidant, 2-SPBN in WT control mitochondria (16.2 ± 1.3%, p < 0.05 vs. TNFα; Fig. 3e). Similarly, NAC restored the proton leak to 15.0 ± 0.8% (p < 0.001 vs. TNFα; Fig. 3e). Both antioxidants given alone had no effect (Fig. S1B, supplementary data). In WT mitochondria, the inhibitors of sphingolipid metabolism, NOE or imipramine abolished the increase on the proton leak of TNFα, restoring the value to 16.6 ± 0.7 and 15 ± 1.6%, respectively (p < 0.05 vs. TNFα) (Fig. 3e). Both inhibitors of sphingolipid metabolism when given alone did not alter the proton leak versus control (Fig. S1B, supplementary data). Similar data were observed in permeabilized fibers when antioxidants and sphingolipid metabolism inhibitors were given with TNFα (Fig. 3f).
Effect of TNFα on respiratory recovery after anoxia–reoxygenation
TNFα (0.5 ng/ml) improved the recovery in the State 3 respiratory rate to 59.4 ± 6 and 75.1 ± 9% in WT animals and TNFR1&2−/−, respectively (p < 0.05 vs. their respective controls) (Fig. 4a). Addition of NAC or NOE reversed the effect of TNFα, returning the percentage recovery to that of the controls, while the addition of NAC or NOE alone demonstrated no difference versus the controls (Fig. 4b).
Fig. 4.
Effect of TNFα on respiratory recovery in the presence/absence of antioxidants, or sphingolipid pathway inhibitors TNFα (0.5 ng/ml) was added directly saponin-permeabilized cardiac muscle fibers. Following anoxia, the preparation was reoxygenated via pipetting air into the buffer, and the State 3 respiratory rate was assessed. The percentage recovery data from permeabilized fibers represents the percentage recovery of State 3 respiration after the anoxia/reoxygenation and is calculated as the ratio between State 3b (occurring after anoxia/reoxygenation) and State 3a (baseline respiration), *p < 0.05 versus CTL. The percentage recovery was reduced with TNFα in the presence of either NAC or NOE, *p < 0.05 versus CTL; n = 6 for all groups
ATP synthesis in isolated mitochondria
The ATP synthesis measured in isolated mitochondria was decreased from 0.53 ± 0.06 (control) to 0.23 ± 0.07 µM/mg protein (TNFα) in the presence of TNFα (0.5 ng/ml), p < 0.05 versus CTL, reinforcing the reduced State 3 respiration findings (Fig. 5).
Fig. 5.
TNFα decreases rate of ATP synthesis. Addition of 0.5 ng/ml TNFα to isolated mitochondria decreased the rate at which ATP was synthesized; *p < 0.05 versus CTL
Effect of closure of mPTP on respiratory parameters in permeabilized fibers
In permeabilized fibers from WT or TNFR1&2−/− animals, addition of 0.2 µM cyclosporine-A (CsA), a well-known inhibitor of the mitochondrial permeability transition pore (mPTP), did not alter State 3 respiration observed with 0.5 ng/ml or 20 ng/ml TNFα (Fig. 6a). Addition of CsA together with 0.5 ng/ml TNFα reversed the decrease in RCI from 4.3 ± 0.1(CTL) to 5.3 ± 1.1 in WT (p < 0.05) and to 7.7 ± 1.1 with 20 ng/ml TNFα (p < 0.01) (Fig. 6b). Similarly, CsA reversed the increase in proton leak in both WT and TNFR1&2−/− to 19.5 ± 8% (p < 0.001) in WT and in TNFR1&2−/− to 10 ± 2.0% (p < 0.001) (Fig. 6c). Surprisingly, when CsA was given with 20 ng/ml TNFα, further increase in the proton leak occurred in both WT and TNFR1&2−/− to 54.5 ± 3.3 and 47.8 ± 3.1%, respectively (Fig. 6c). The recovery of State 3 respiration after anoxia–reoxygenation that was observed with both concentrations of TNFα (0.5 ng/ml and 20 ng/ml) were not altered with the administration of CsA in both WT and TNFR1&2−/− when compared with TNFα alone (Fig. 6d).
Fig. 6.
Effect of TNFα on respiratory parameters in presence of cyclosporine-A (CSA). a The effect of 0.5 ng/ml or 20 ng/ml TNFα on State 3 respiration remained unchanged with addition of CSA in permeabilized fibers of WT and TNFR1&2−/− hearts. b Addition of CSA reversed the decrease in RCI observed with TNFα in WT mice. Although TNFα decreased RCI in TNFR1&2−/−, this effect was lost in the presence of CSA. c Proton leak was significantly increased with addition of TNFα. This effect was lost in the presence of CSA with TNFα 0.5 ng/ml only. d Addition of CSA did not significantly affect the percentage of recovery after anoxia–reoxygenation in fibers treated with 0.5 ng/ml or 20 ng/ml TNFα in WT and TNFR1&2−/−. *p < 0.05 versus no TNFα; ≠p < 0.05 versus TNFα alone; n = 6
Addition of TNFα to isolated mitochondria induces a GDP-sensitive proton leak
Addition of GDP to TNFα-treated mitochondria decreased the respiration by 29 ± 2 versus 6 ± 2% in the CTL (p < 0.05 vs. control; Table 1), showing that the TNFα treatment promoted a higher rate of GDP-sensitive uncoupled respiration.
Table 1.
GDP-sensitive proton leak in isolated wild type mouse mitochondria
| Succinate + oligomycin (nmol O2/mg protein/min) |
Succinate + oligomycin + GDP (nmol O2/mg protein/min) | GDP-induced ▲ in respiration (%) | |
|---|---|---|---|
| Control | 22 ±2 | 10 ±2 | 6± 2 |
| +0.5 ng/ml TNFα | 21 ±1 | 15 ±1 | 29 ± 2* |
Succinate and oligomycin respiration represents steady-state oxygen consumption in nmol O2/mg protein/min. Addition of GDP reflects the respiratory rate after blockade of UCP-sensitive oxygen consumption. Values are reflected as mean ± SE (n = 6)
p < 0.05 versus control
Antioxidants and sphingolipid pathway inhibitors reverse the effect of TNFα (0.5 ng/ml) on mitochondrial inner membrane potential
TNFα decreased the inner mitochondrial membrane potential of isolated mitochondria to 44 ± 2% versus the normalized control (p < 0.001). This effect was reversed with the addition of either of the antioxidants, NAC and 2-SPBN or the inhibitors of sphingolipid metabolism, NOE and imipramine. The membrane potential was increased by the putative mitochondrial potassium channel (mKATP) antagonist, 5-HD and the mPTP inhibitor, CsA to 172.5 ± 15.9 AU and 210.8 ± 28.9 AU, respectively (p < 0.05 vs. TNFα) (Fig. S2A, supplementary data). Antioxidants and sphingolipid metabolism inhibitors given alone did not influence the membrane potential (Fig. S2B, supplementary data).
Discussion
Our data demonstrate that TNFα, at a concentration of 0.5 ng/ml, exerts an effect on murine mitochondrial respiratory function and on mitochondrial tolerance to an anoxia–reoxygenation stress. This effect is independent of TNFα binding to its cell surface receptors, TNFR1 or TNFR2. In addition, our data strongly suggest that TNFα modulates mitochondrial respiration via ROS and sphingolipids. The main data leading to these conclusions are as follows: (1) isolated cardiac mitochondria or isolated cardiac muscle fibers challenged with a low concentration of TNFα had a decreased State 3 respiration, a reduced inner mitochondrial membrane potential, and a corresponding increase in proton leak; (2) similar data were obtained in isolated preparations from TNFR1&2−/− mice; (3) addition of TNFα improved the rate of State 3 respiration in mitochondria subjected to a stress induced by anoxia–reoxygenation in both WT or TNFR1&2−/− mice; (4) this protective effect of TNFα was lost in the presence of antioxidants or inhibitors of the sphingolipids metabolism.
Selection of TNFα concentration
Our group has previously reported that a concentration of 0.5 ng/ml, which corresponds to a physiological range of circulating TNFα levels in rats subjected to an ischemia–reperfusion insult [12], was the optimal concentration to protect the isolated heart [17] or isolated cells against ischemia–reperfusion [13]. Our dose–response curve obtained on isolated mitochondria shows a protective effect at similar concentrations of TNFα (Fig. 1). Cardiomyocytes produce and release TNFα [11], and immuno-electron microscopy studies suggest that TNF is localized between myofibrils and mitochondria [16]. Although the amount of TNFα released from isolated cardiomyocytes can attain 1 ng/ml [35, 52], the physiological concentration range of this cytokine that may surround the mitochondria is unknown. We must, therefore, acknowledge that our dose–response performed in isolated mitochondria may not correlate to physiological conditions and, as such, poses a critical limitation to our study.
Our previous work demonstrated that this low concentration of TNFα reduces the respiratory function and the inner mitochondrial membrane potential in cell suspension, and that this effect was associated with cellular protection prior to a simulated ischemic insult [17]. In earlier studies, where the cytotoxic effects of TNFα were being explored in isolated cardiac mitochondria and mouse fibrosarcoma cell lines, it was noted that at cytotoxic concentrations ranging from 2.5 ng/ml to 10 ng/ml, TNFα inhibits various complexes of the electron transport chain, 3–6 h prior to the onset of cell death [19, 40]. Utilizing a complex I initiating electron transport chain substrate (glutamate and malate), our study shows that the protective concentration of 0.5 ng/ml TNFα improves post-anoxic recovery of respiration in isolated cardiac mitochondria and those within permeabilized fibers. The mechanism, whereby this low concentration of TNFα modifies Complex I activity, remains to be elucidated.
The effects of TNFα on mitochondrial respiration
The reduction in State 3 respiration and inner mitochondrial membrane potential, with an accompanying increase in proton leak in response to mitochondrial exposure to TNFα, is indicative of mild mitochondrial uncoupling. A similar decrease in respiratory parameter occurred in the TNFα-treated mitochondria from TNFR1&2−/− mice. Addition of 0.5 ng/ml TNFα to isolated WT mitochondria decreased ATP synthesis and increased the GDP-induced respiration. Taken together with the respiratory data, our results suggest the involvement of uncoupling proteins in oxidative phosphorylation uncoupling with TNFα. This modulation of oxidative phosphorylation has previously been shown to be operational in the cardiac tolerant phenotype in response to ischemic preconditioning [6, 29, 32, 33]. In cardiomyocytes, two different populations of mitochondria exist: subsarcolemmal (SSM) which are directly beneath the plasma membrane and interfibrillar (IFM) mitochondria which are situated between the myofibrils [4], and these two populations differ in their respiratory capacity and calcium retention. In a study by Boengler et al. [44], they found that there were differences in State 3 respiration between SSM and IFM populations, with ADP being converted to ATP at a faster rate by the IFM. In our saponin-permeabilized cardiac muscle fibers, the population of mitochondria would be the IFM, whereas in our isolated mitochondria these would be the SSM, and this may explain the differences seen in the baseline respiratory parameters. In addition to the different populations of mitochondria, the mitochondria in permeabilized fibers are in their endogenous milieu, which may also contribute to the baseline differences. However, overall, isolated mitochondria and permeabilized fibers gave similar results.
TNFα receptors
In addition to the well-known TNFα cell surface receptors (TNFR1 and TNFR2), a mitochondrial-binding protein for TNFα has been described as a 60-kD protein in the inner mitochondrial membrane [24]. In our study, we used the mitochondria isolated from both wild type and TNFR1& 2-deficient mice to measure State 3 respiration. Mitochondria from the hearts in which the cell surface receptors are absent responded to exogenous TNFα in a similar manner to that observed in the wild type, therefore indicating that the effect of TNFα on respiratory function does not require its cell surface receptors. Our data also support the presence of a mitochondrial TNF-binding protein.
Interestingly, there was a difference in baseline State 3 respiration between the WT and TNFR1&2−/− mice. State 3 baseline respiration was higher in TNFR1&2−/− compared with WT, therefore suggesting that endogenous TNFα may contribute to mitochondrial respiration partly via its cell surface receptors. However, addition of TNFα in WT or TNFR1&2−/− exhibited a similar trend in the decrease of State 3 respiration.
Schulz et al. [39] have referred to the ambivalent role of both TNFα and its receptors in myocardial infarction. Our present study reveals that with no cell surface receptors present, TNFα can still exert an effect on the mitochondria, and this may partly account for the disappointing results obtained in clinical trials where the individual receptors were inhibited.
TNFα activation of ROS and sphingolipids
In isolated hearts or in cells, 0.5 ng/ml of TNFα exerts its effect via the activation of the sphingolipid, ceramide [22], and reactive oxygen species [17, 23]. There is an existing mitochondrial pool of ceramide in physiological conditions [3], and TNFα can increase intramitochondrial levels of ceramide through activation of at least two pathways: one mediated by sphingomyelinase and the other involving de novo synthesis of ceramide [8, 47]. Imipramine, which we have used in our study, exerts its effect by indirectly inhibiting the sphingomyelinase, thus preventing the conversion of sphingomyelin to ceramide. NOE exerts its antagonistic effect by inhibiting ceramide synthase, thus preventing the conversion of ceramide to sphingosine. The exact role of ceramide within the mitochondria still remains to be elucidated, but our study provides firm evidence that it can mediate the effect of TNFα on the mitochondrial respiratory status, possibly via sphingosine formation as previous studies have demonstrated that a low concentration of sphingosine is also cardioprotective [14, 15, 50].
Interestingly, in the presence of 20 ng/ml TNFα, addition of NAC or NOE to mitochondria did not abolish the significant decrease in State 3 respiration, whereas at the low, protective concentration of 0.5 ng/ml TNFα the decrease in State 3 respiration was abrogated. These results suggest that the higher concentration of TNFα may exert its deleterious effects either by (1) excessive production of ROS and sphingolipids that cannot be inhibited with our current concentration of inhibitors or (2) independently of ROS formation and the sphingolipid pathway.
Most intracellular oxidative stress originates in the mitochondria [10], and it is now well established that both TNFα and ceramide can activate ROS formation. A previous study demonstrated that ceramide could activate ROS originating from the mitochondria [7]. There are at least nine known mitochondrial sites that are capable of producing superoxide anion (reviewed [1]). Complex I and III of the electron transport chain (ETC) have been accepted as major sites of mitochondrial ROS production [26]. TNFα and ceramide are known to induce their effect via the generation of ROS from complex III, but the exact origin of ROS generation in our experimental conditions remains to be elucidated.
TNFα and anoxia–reoxygenation
Our data demonstrating the improved respiratory recovery rate in permeabilized fibers subjected to anoxia–reoxygenation stress in the presence of TNFα suggests that TNFα activates a cardioprotective signaling cascade leading to an increase in State 3 respiration compared to that observed under normoxic conditions. The concept of the existence of a TNFα-binding protein in the inner mitochondrial membrane is strengthened by the fact that this increase in percentage recovery also occurs in the double TNF receptor knockout animals.
Addition of TNFα to permeabilized fibers under normoxic conditions decreased State 3 respiration, and this effect was abolished in the presence of NAC or NOE. However, the improved percentage recovery observed initially after anoxia/reoxygenation in the presence of TNFα was lost with addition of NAC or NOE, therefore suggesting that ROS and sphingolipids are involved in the cardioprotective signaling cascade initiated by TNFα subjected to anoxia–reoxygenation.
CsA inhibits TNFα-induced decrease in membrane potential
Inhibition of the mitochondrial permeability transition pore (mPTP) has been perceived as the “holy grail” in cardio-protection [13]. Prevention of mPTP opening during the first seconds of reperfusion represents a powerful tool to protect the heart against ischemia–reperfusion injury [2]. Our data demonstrate that the inner mitochondrial membrane potential was significantly decreased with the addition of 0.5 ng/ml TNFα, and this effect was lost in the presence of the mPTP inhibitor, CsA, therefore suggesting that TNFα may mediate its effect via transient opening of the mPTP.
Reservations
One concern was the purity of our mitochondrial preparation as a percoll-gradient centrifugation is routinely used to isolate relatively pure mitochondria. This technique, however, was not suitable in our hands for adequate mitochondrial respiration in a murine preparation. To confirm that cell surface membrane remnants were not present in our mitochondrial preparation from wild-type mice, we also isolated heart mitochondria from TNFR1&2−/− mice in which both TNF receptors are absent. The data obtained after addition of TNFα to these mitochondria gave similar results to wild-type mice, therefore strongly suggesting that this effect is not related to the presence of TNF receptors. Indeed, these results support the presence of a mitochondrial TNF-binding protein although it should also be mentioned that characterization of this binding protein has not yet been accomplished, and its existence remains somewhat controversial.
Another concern was that isolated mitochondria removed from their natural environment may behave differently. To address this concern, we repeated our experiments on respiratory functions, proton leak and anoxia–reoxygenation experiments with saponin-permeabilized cardiac muscle fibers in which the mitochondria are situated in their endogenous milieu. The results from permeabilized were similar to that obtained from isolated mitochondria, thus validating the isolated mitochondrial preparation.
With the isolation technique used in our laboratory to obtain a sufficient number of viable, respiring mitochondria from mouse hearts, we cannot guarantee a totally pure mitochondrial suspension. As a consequence, we cannot exclude the possibility that TNFα-induced sphingolipid production could occur outside of the mitochondria. However, our experiments conducted in TNFR1&2−/− mice demonstrate that, should TNFα-induced sphingolipid production occur outside of the mitochondria, this production is still independent of the cell surface receptors.
The contribution of these many mitochondrial perturbations to the protective action of TNFα and the mechanisms involved remain to be delineated.
In the future, the technology required to measure exact amounts of TNFα produced by cardiomyocytes may become available. In that case, future studies will be required to define whether the concentration used in our system corresponds to physiological values.
Conclusion
Finally, these novel results demonstrate that a low concentration of TNFα exerts an effect on cardiac mitochondrial respiration function, either by partial inhibition of electron flux through Complex I, or by modest mitochondrial uncoupling. Most importantly, this effect occurs independently of its cell surface receptors, but requires the presence of ROS and sphingolipids and, speculatively, the activation of mitochondrial uncoupling proteins, as demonstrated by the greater inducible proton leak in mitochondria exposed to TNFα and the decrease in ATP synthesis. The recovery after anoxia–reoxygenation suggests that addition of TNFα “preconditions” the mitochondria so that they are capable to withstand the stress of anoxia by decreasing their rate of respiration and synthesis of ATP. Our data open a novel approach mitochondrial-centered approach to TNFα-induced protection against ischemia–reperfusion injury.
Supplementary Material
Acknowledgments
This work was supported in part by a CRIG grant from the Wellcome, the Interuniversity Cape Heart Group of the South African Medical Research Council and the National Research Foundation. S.L. was supported by a Servier Senior Fellowship for Research in Heart Failure and a Medical Research Council career award. L.L. by the Wellcome Trust and the Medical Research Council of South Africa.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00395-010-0113-4) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Lydia Lacerda, Email: lydia.lacerda@uct.ac.za, Cardioprotection Group, Hatter Cardiovascular Research Institute, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory 7925, Cape Town, South Africa.
Joy McCarthy, Cardioprotection Group, Hatter Cardiovascular Research Institute, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory 7925, Cape Town, South Africa.
Shazia F. K. Mungly, Cardioprotection Group, Hatter Cardiovascular Research Institute, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory 7925, Cape Town, South Africa
Edward G. Lynn, Translational Medicine Branch, NHLBI, National Institute of Health, Bethesda, MD, USA
Michael N. Sack, Translational Medicine Branch, NHLBI, National Institute of Health, Bethesda, MD, USA
Lionel H. Opie, Cardioprotection Group, Hatter Cardiovascular Research Institute, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory 7925, Cape Town, South Africa
Sandrine Lecour, Cardioprotection Group, Hatter Cardiovascular Research Institute, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory 7925, Cape Town, South Africa.
References
- 1.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry. 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 2.Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol. 2009;104:181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boengler K, Stahlhofen S, van de Sand A, Gres P, Ruiz-Meana M, Garcia-Dorado D, Heusch G, Schulz R. Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res Cardiol. 2009;104:141–147. doi: 10.1007/s00395-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 5.Budnikov E, Postnov A, Doroshchuk AD, Afanasjeva GV, Postnov Iu V. Decreased ATP-synthesis ability of liver mitochondria in spontaneously hypertensive rats (SHR): role of calcium overload of the mitochondria. Kardiologiia. 2002;42:47–50. [PubMed] [Google Scholar]
- 6.Busquets S, Aranda X, Ribas-Carbo M, Azcon-Bieto J, Lopez-Soriano FJ, Argiles JM. Tumour necrosis factor-alpha uncouples respiration in isolated rat mitochondria. Cytokine. 2003;22:1–4. doi: 10.1016/s1043-4666(03)00098-x. [DOI] [PubMed] [Google Scholar]
- 7.Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 8.Dbaibo GS, El-Assaad W, Krikorian A, Liu B, Diab K, Idriss NZ, El-Sabban M, Driscoll TA, Perry DK, Hannun YA. Ceramide generation by two distinct pathways in tumor necrosis factor alpha-induced cell death. FEBS Lett. 2001;503:7–12. doi: 10.1016/s0014-5793(01)02625-4. [DOI] [PubMed] [Google Scholar]
- 9.Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995;486(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66(Shc) and monoamine oxidase. Basic Res Cardiol. 2009;104:131–139. doi: 10.1007/s00395-009-0008-4. [DOI] [PubMed] [Google Scholar]
- 11.Dorge H, Schulz R, Belesjorow S, Post H, van de Sand A, Konietzka I, Frede S, Hartung T, Vinten-Johansen J, Youker A, Entman ML, Erbel R, Heusch G. Coronary microembolization: the role of TNFα in contractile dysfunction. J Mol Cell Cardiol. 2002;34:51–62. doi: 10.1006/jmcc.2001.1489. [DOI] [PubMed] [Google Scholar]
- 12.Gurevitch J, Frolkis I, Yuhas Y, Paz Y, Matsa M, Mohr R, Yakirevich V. Tumor necrosis factor-alpha is released from the isolated heart undergoing ischemia and reperfusion. J Am Coll Cardiol. 1996;28:247–252. doi: 10.1016/0735-1097(96)00105-2. [DOI] [PubMed] [Google Scholar]
- 13.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol. 2010;105:151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 14.Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–1989. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- 15.Karliner JS. Sphingosine kinase regulation and cardioprotection. Cardiovasc Res. 2009;82:184–192. doi: 10.1093/cvr/cvn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura H, Shintani-Ishida K, Nakajima M, Liu S, Matsumoto K, Yoshida K. Ischemic preconditioning or p38 MAP kinase inhibition attenuates myocardial TNF alpha production and mitochondria damage in brief myocardial ischemia. Life Sci. 2006;78:1901–1910. doi: 10.1016/j.lfs.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Lacerda L, Smith RM, Opie L, Lecour S. TNFalpha-induced cytoprotection requires the production of free radicals within mitochondria in C2C12 myotubes. Life Sci. 2006;79:2194–2201. doi: 10.1016/j.lfs.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 19.Lancaster JR, Jr, Laster SM, Gooding LR. Inhibition of target cell mitochondrial electron transfer by tumor necrosis factor. FEBS Lett. 1989;248:169–174. doi: 10.1016/0014-5793(89)80454-5. [DOI] [PubMed] [Google Scholar]
- 20.Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Lecour S, Owira P, Opie LH. Ceramide-induced preconditioning involves reactive oxygen species. Life Sci. 2006;78:1702–1706. doi: 10.1016/j.lfs.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNF alpha and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34:509–518. doi: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]
- 23.Lecour S, Suleman N, Deuchar GA, Somers S, Lacerda L, Huisamen B, Opie LH. Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase) Circulation. 2005;112:3911–3918. doi: 10.1161/CIRCULATIONAHA.105.581058. [DOI] [PubMed] [Google Scholar]
- 24.Ledgerwood EC, Prins JB, Bright NA, Johnson DR, Wolfreys K, Pober JS, O’Rahilly S, Bradley JR. Tumor necrosis factor is delivered to mitochondria where a tumor necrosis factor-binding protein is localized. Lab Invest. 1998;78:1583–1589. [PubMed] [Google Scholar]
- 25.Ledgerwood EC, Prins JB, Bright NA, Johnson DR, Wolfreys K, Pober JS, O’Rahilly S, Bradley JR. Tumour necrosis factor is trafficked to a mitochondrial tumour necrosis factor binding protein. Biochem Soc Trans. 1998;26:S316. doi: 10.1042/bst026s316. [DOI] [PubMed] [Google Scholar]
- 26.Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–164. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- 27.Macouillard-Poulletier de G, Belaud-Rotureau MA, Voisin P, Leducq N, Belloc F, Canioni P, Diolez P. Flow cytometric analysis of mitochondrial activity in situ: application to acetylceramide-induced mitochondrial swelling and apoptosis. Cytometry. 1998;33:333–339. [PubMed] [Google Scholar]
- 28.McCarthy J, McLeod CJ, Minners J, Essop MF, Ping P, Sack MN. PKCepsilon activation augments cardiac mitochondrial respiratory post-anoxic reserve—a putative mechanism in PKCepsilon cardioprotection. J Mol Cell Cardiol. 2005;38:697–700. doi: 10.1016/j.yjmcc.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 29.McLeod CJ, Aziz A, Hoyt RF, Jr, McCoy JP, Jr, Sack MN. Uncoupling proteins 2 and 3 function in concert to augment tolerance to cardiac ischemia. J Biol Chem. 2005;280:33470–33476. doi: 10.1074/jbc.M505258200. [DOI] [PubMed] [Google Scholar]
- 30.McLeod CJ, Jeyabalan AP, Minners JO, Clevenger R, Hoyt RF, Jr, Sack MN. Delayed ischemic preconditioning activates nuclear-encoded electron-transfer-chain gene expression in parallel with enhanced postanoxic mitochondrial respiratory recovery. Circulation. 2004;110:534–539. doi: 10.1161/01.CIR.0000136997.53612.6C. [DOI] [PubMed] [Google Scholar]
- 31.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 32.Minners J, Lacerda L, McCarthy J, Meiring JJ, Yellon DM, Sack MN. Ischemic and pharmacological preconditioning in Girardi cells and C2C12 myotubes induce mitochondrial uncoupling. Circ Res. 2001;89:787–792. doi: 10.1161/hh2101.098372. [DOI] [PubMed] [Google Scholar]
- 33.Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardioprotection. Biochem J. 2006;395:611–618. doi: 10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nogueira V, Rigoulet M, Piquet MA, Devin A, Fontaine E, Leverve XM. Mitochondrial respiratory chain adjustment to cellular energy demand. J Biol Chem. 2001;276:46104–46110. doi: 10.1074/jbc.M107425200. [DOI] [PubMed] [Google Scholar]
- 35.Pellieux C, Montessuit C, Papageorgiou I, Lerch R. Angiotensin II downregulates the fatty acid oxidation pathway in adult rat cardiomyocytes via release of tumour necrosis factor-alpha. Cardiovasc Res. 2009;82:341–350. doi: 10.1093/cvr/cvp004. [DOI] [PubMed] [Google Scholar]
- 36.Perry G, Castellani RJ, Hirai K, Smith MA. Reactive oxygen species mediate cellular damage in Alzheimer disease. J Alzheimers Dis. 1998;1:45–55. doi: 10.3233/jad-1998-1103. [DOI] [PubMed] [Google Scholar]
- 37.Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 38.Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 39.Schulz R, Heusch G. Tumor necrosis factor-alpha and its receptors 1 and 2: Yin and Yang in myocardial infarction? Circulation. 2009;119:1355–1357. doi: 10.1161/CIRCULATIONAHA.108.846105. [DOI] [PubMed] [Google Scholar]
- 40.Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- 41.Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 42.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res. 2007;100:140–146. doi: 10.1161/01.RES.0000255031.15793.86. [DOI] [PubMed] [Google Scholar]
- 44.Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 45.Smith RM, Suleman N, McCarthy J, Sack MN. Classic ischemic but not pharmacologic preconditioning is abrogated following genetic ablation of the TNFalpha gene. Cardiovasc Res. 2002;55:553–560. doi: 10.1016/s0008-6363(02)00283-3. [DOI] [PubMed] [Google Scholar]
- 46.Sordahl LA, Besch HR, Jr, Allen JC, Crow C, Lindenmayer GE, Schwartz A. Enzymatic aspects of the cardiac muscle cell: mitochondria, sarcoplasmic reticulum and nonovalent cation active transport system. Methods Achiev Exp Pathol. 1971;5:287–346. [PubMed] [Google Scholar]
- 47.Thielmann M, Dorge H, Martin C, Belosjorow S, Schwanke U, van de Sand A, Konietska I, Buchert A, Kruger A, Schulz R, Heusch G. Myocardial dysfunction with coronary microembolization. Circ Res. 2002;90(7):807–813. doi: 10.1161/01.res.0000014451.75415.36. [DOI] [PubMed] [Google Scholar]
- 48.Torre-Amione G, Kapadia S, Lee J, Bies RD, Lebovitz R, Mann DL. Expression and functional significance of tumor necrosis factor receptors in human myocardium. Circulation. 1995;92:1487–1493. doi: 10.1161/01.cir.92.6.1487. [DOI] [PubMed] [Google Scholar]
- 49.Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta. 1987;892:191–196. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]
- 50.Vessey DA, Li L, Kelley M, Karliner JS. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008;375:425–429. doi: 10.1016/j.bbrc.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Tumor necrosis factor receptor 1 signaling resistance in the female myocardium during ischemia. Circulation. 2006;114:I282–I289. doi: 10.1161/CIRCULATIONAHA.105.001164. [DOI] [PubMed] [Google Scholar]
- 52.Wei L, Sun D, Yin Z, Yuan Y, Hwang A, Zhang Y, Si R, Zhang R, Guo W, Cao F, Wang H. A PKC-beta inhibitor protects against cardiac microvascular ischemia reperfusion injury in diabetic rats. Apoptosis. 2010;15:488–498. doi: 10.1007/s10495-009-0439-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.