To the editor
In a recent report published in the International Journal of Nanomedicine, Gulati et al1 have described the most innovative study addressing an important issue of the “blood–brain barrier,” which can act as a barrier to one of the fundamental goals of modern neurobiology that would have a direct impact on highly debated future therapeutics for both brain cancer and neurological disorders. Contrary to what has been the case with conventional therapy, the authors were able to completely bypass the blood–brain barrier (Figure 1) – a limiting factor for efficient drug delivery – by proposing a new, alternative approach using nanoengineered TNT/Ti implants for local delivery of chemotherapeutics such as doxorubicin into the brain. There must be millions of good drugs sitting in pharmaceutical company stores that cannot be delivered simply because they cannot get past the blood–brain barrier.2 This is an area that has been under-researched and its significance has not yet been recognized. Neuroscience textbooks bury this issue in the appendix, PhD programs give it a cursory treatment, and pharmaceutical companies have tried to ignore it. Despite the blood–brain barrier acting as a stubbornly real obstacle for potential drugs to be used against many disorders of the central nervous system, the field of drug delivery is advancing rapidly. Recently, for example, systemic injection with iRGD improved the therapeutic index of drugs of various compositions including doxorubicin, nanoparticles (nab-paclitaxel and doxorubicin liposomes), and a monoclonal antibody (trastuzumab).3
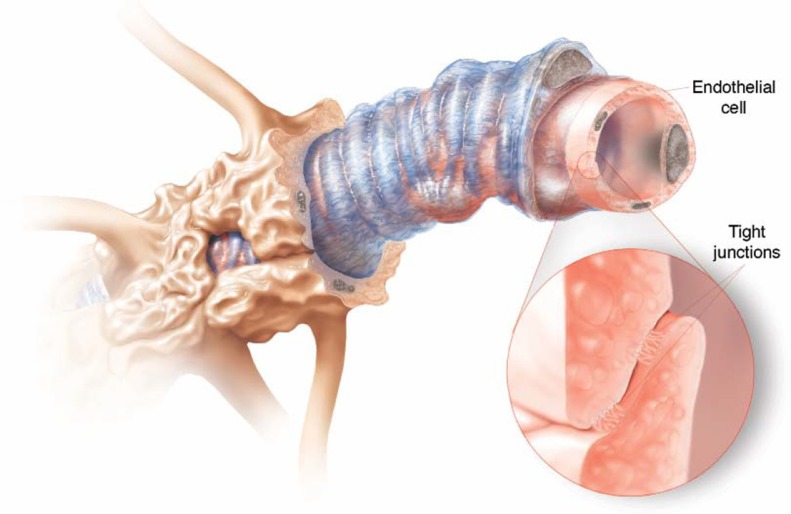
Figure 1.
Tight security. Brain capillaries are surrounded by different types of cells, but the real barriers are the tight junctions between endothelial cells in the capillary lining.2 From Greg Miller, SCIENCE 297:1116 (2002). Illustration: Cameron Slayden. Reprinted with permission from AAAS. Available from: http://www.sciencemag.org/content/297/5584/1116.summary.
Although the researchers were looking for another entryway into the brain, the study succeeded in providing evidence for controllable drug-releasing characteristics, a hallmark feature of modified Ti wires with several clinical and pharmacological advantages over currently existing methods that can prove to be very interesting. However, no evidence-based information about the possible limitations and the applicability of these implants in vivo is available.
In summary, regarding how to attain maximum patient benefits through any potential drug delivery, the use of nanoengineered TNT/Ti implants is obviously an invasive approach that requires microsurgery within the brain, which is a highly complicated part of the body. However, poor penetration of anticancer drugs into the blood–brain barrier is an important factor limiting the efficacy of these medications in reaching a number of tumors. Academic researchers have recently been compelled to search for alternative ways to overcome these limitations, yet safe and effective anticancer therapies are but a dream.
References
- 1.Gulati K, Aw MS, Losic D. Nanoengineered drug-releasing Ti wires as an alternative for delivery of chemotherapeutics in the brain. Int J Nanomedicine. 2012;7:2069–2076. doi: 10.2147/IJN.S29917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller G. Drug targeting. Breaking down barriers. Science. 2002;297(5584):1116–1118. doi: 10.1126/science.297.5584.1116. [DOI] [PubMed] [Google Scholar]
- 3.Sugahara KN, Teesalu T, Karmali PP, et al. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328(5981):1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]