The potential for understanding the mechanism of immune system damage by HIV-1 received an enormous boost last year, when it was discovered that several specific chemokine receptors are used by HIV-1 as coreceptors for entry into target cells (1–5). It had been known for close to a decade that the expression of human CD4, the high-affinity receptor for HIV-1, was necessary but not sufficient for viral entry and that a species-specific cofactor might also be required (6–8). The long-awaited identification of the cofactors fused the seemingly disparate areas of chemokine and HIV research and has fueled extraordinary progress in a short period of time. The recent discoveries not only provide insight into the mechanism of viral entry, but also hold the promise to explain the mechanism by which early viremia progresses to immunodeficiency. At the heart of this problem lies the paradox of why strains of virus involved in transmission of infection differ in their specificity for chemokine receptors from strains found late in disease progression. In this issue of the Proceedings, Bleul et al. (9) report on the expression patterns of the two principal HIV-1 coreceptors on T cell subsets and thus provide a potential clue toward resolving the mystery of the evolving cellular tropism of HIV-1 at different stages of infection.
The recent work follows up on studies performed during the last 6 years that had demonstrated the existence of distinct cellular tropisms of different strains of HIV-1. Most primary isolates of HIV-1, present throughout the course of infection, are able to infect macrophages and primary T cells, but not transformed T cell lines (designated as M-tropic strains). In contrast, strains of HIV-1 that have been adapted to grow in transformed T cell lines show a similar tropism to those that emerge in many HIV-1-infected individuals in the later course of infection and can infect primary T cells but not monocytes or macrophages (T-tropic strains; refs. 10–13). The difference in cellular tropism by different HIV-1 isolates correlates with differences in the viral envelope glycoproteins, in particular the third variable (V3) region of the gp120 subunit (14, 15).
Early last year, a G protein-coupled receptor CXCR4 (also termed fusin or Lestr) was shown to allow the entry of T-tropic strains of HIV-1 into CD4+ target cells. (1). Shortly thereafter, the natural ligand for CXCR4 was identified as the CXC family chemokine SDF-1 (16, 17). The discovery of CXCR4, together with a previous report demonstrating that CC family chemokines (RANTES, MIP-1α, MIP-1β) inhibited HIV-1 replication (18), led to the subsequent identification of another chemokine receptor, CCR5, as the major coreceptor for M-tropic HIV-1 entry (2–5). The focus of this research then rapidly shifted to studies of individuals who are repeatedly exposed to HIV, but nevertheless remain free from infection. Paxton et al. (19) had found that the PBMC from some highly exposed, uninfected subjects were resistant to infection with prototypical M-tropic viruses, but were readily infectable with T-tropic viruses. The discoveries of HIV coreceptors led Liu et al. (20) to quickly determine the defect as a homozygous mutation corresponding to a 32-bp deletion in the M-tropic receptor CCR5 (20). This remarkable finding was followed by studies revealing that although ≈20% of western European Caucasians are heterozygous and ≈1% are homozygous for this mutation, no homozygous mutants were found among >2000 HIV-1-infected cohorts (21, 22). This compelling evidence that a mutation in CCR5 renders individuals apparently resistant to infection with HIV-1 argues strongly that entry of M-tropic strains into susceptible target cells is critically important for establishing the infection. These results also correlate with earlier findings that only M-tropic strains could be cultured from individuals shortly after acquisition of infection.
Why the absence of one HIV-1 coreceptor confers resistance remains unclear. The apparently resistant individuals have intact CXCR4 and are likely to be exposed to both M-tropic and T-tropic viruses. The results of Bleul et al. (9) provide a framework for a hypothesis based on differential expression of chemokine/HIV receptors CCR5 and CXCR4 on human T cell subsets. They demonstrate that the expression pattern of these chemokine receptors on naive or memory T cells is largely mutually exclusive. CXCR4 is predominantly expressed on the unactivated naive subset of T cells, whereas CCR5 is almost exclusively expressed on the activated or memory subset of human T cells (9). In this perspective, we provide a model that may explain why only M-tropic virus can transmit HIV infection.
T Cell Subsets and HIV Infection
Following selection and maturation in the thymus, T lymphocytes migrate into the periphery to populate secondary lymphoid organs. These newly exported cells are considered to be immunologically naive in the sense that they have not encountered any foreign antigen. Once naive T cells are activated through triggering of the T cell receptor (TCR) via the major histocompatibility complex/peptide complexes on the surface of antigen-presenting cells, they undergo major phenotypic and functional changes as they proliferate. At this differentiation stage, cells are referred to as activated/effector cells. Some of these cells survive and revert to a resting state that is thought to represent the memory subset of T cells (23). Phenotypically naive and memory human T cells differ in their expression of CD45 isoforms. Naive cells are CD45RA+CD45RO− and are low in the expression of various activation/adhesion molecules, whereas memory cells are CD45RO+CD45RA− and express high levels of adhesion molecules, possibly reflecting their lower requirement for costimuli during TCR activation (24). Functionally, naive T cells, in contrast to memory cells, lack effector functions, such as cytokine production or B cell help, for immunoglobulin production (24, 25). These two subsets also differ in their migratory patterns. Naive lymphocytes traffic between secondary lymphoid organs, probably until they die or are activated by a specific antigen (26, 27). Memory and effector cells, on the other hand, display a broad range of migratory capacity to tertiary lymphoid organs, such as skin or intestinal lamina propria, and also to sites of inflammation (28).
So how would the expression pattern of chemokine/HIV receptors on these T cell subsets be relevant to HIV infection and perhaps AIDS pathogenesis? As mentioned above, the M-tropic isolates of HIV-1 appear critical in establishing persistent infection. Most primary isolates from infected individuals shortly after infection tend to use CCR5 as coreceptor even when the transmitting partner carries both M- and T-tropic viruses (29). Furthermore, even in individuals who are infected by HIV through direct inoculation of the virus, by intravenous drug use or injection of contaminated blood products, M-tropic viruses still predominate early in the course of infection (30, 31). Taken together, these results strongly suggest that, during viral transmission and early in the course of disease, entry of HIV occurs mainly through CCR5. It is likely that cells that are targets of initial infection lack sufficient levels of CXCR4 expression for T-tropic viruses to establish an infectious foothold.
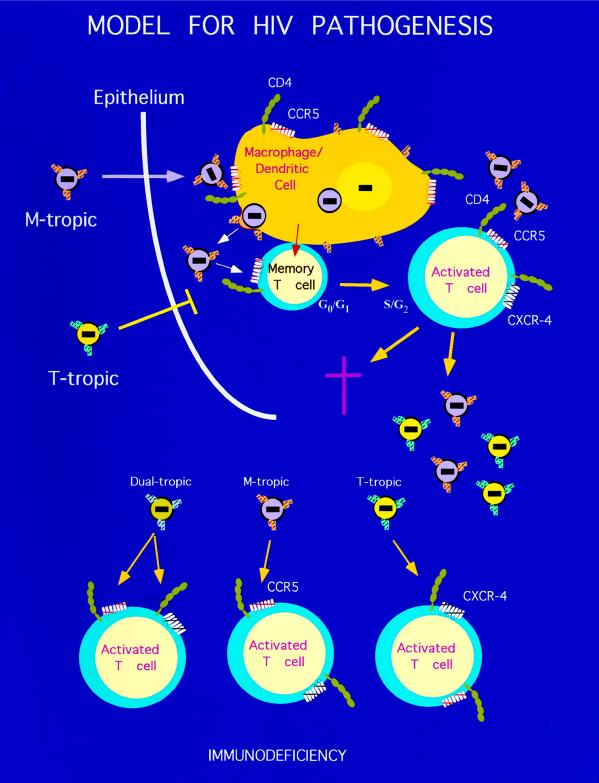
Macrophages and dendritic cells (DC) that line the mucosal surfaces are the most likely target cell candidates during viral transmission. DC exposed to HIV-1 are able to transmit the virus to CD4+ T cells (32). It is also well established that the main target cells of HIV are activated/memory CD4+ T cells. Indeed, memory CD4+ T cells are selectively infected and lost in HIV-1 infected individuals (33). The finding by Bleul et al. (9) that the memory subset of T cells expresses more CCR5 than CXCR4 brings forward a hypothesis as to why CCR5 may be the key receptor during viral transmission and early stages of infection (Fig. 1). A likely scenario would involve both macrophage/DC and memory T cells. Conjugates of DC and memory T cells are found in the skin and are likely to be present also at external linings of organs involved in sexual transmission of HIV-1. Such conjugates have been shown in vitro to facilitate productive infection of T cells with HIV-1 (34). Since activation of T cells is a prerequisite for productive infection with HIV (35, 36), infection of the DC may initiate a program for achieving T cell activation and subsequent infection. This could occur through the antigen-independent activation of neighboring memory T cells by infected and activated DC. Although DC seem to support and spread T-tropic viruses at low levels, productive infection with M-tropic strains that use CCR5 appears to be more effective within the conjugates (34). This may be due to higher levels of CCR5 expression compared with CXCR4 on these cells (9), or, alternatively, there may be a specific signal through CCR5 upon binding of the virus, which may cause cellular activation.
Figure 1.
Model for transmission of HIV-1. In this model of HIV pathogenesis, the infection is restricted to M-tropic viruses, which use CCR5 and CD4 for entry into target cells. Infection of macrophages/DC and memory T cell conjugates activates macrophage/DC, which in turn stimulate memory T cells. These conjugates allow productive HIV replication of M-tropic strains that eventually evolve into T-tropic or dual-tropic (strains using both coreceptors) viruses.
Some T-tropic viruses do appear in early stages of the infection; however, M-tropic strains remain predominant until the late stages. The eventual transition to T-tropic strains usually coincides with the dramatic reduction in CD4+ T cell counts and the development of full-blown AIDS. It is, however, not clear whether the transition to T-tropic strains is the cause or the result of the sudden decline in the CD4+ T cell numbers. HIV-infected individuals have persistent T cell activation, possibly due to the cytokine-rich milieu in the secondary lymphoid organs. In fact, addition of recombinant interleukin 2 to peripheral blood mononuclear cell (PBMC) cultures allows very efficient replication of HIV-1 isolates (37). Interestingly, Bleul et al. (9) show that both chemokine receptors are up-regulated in response to IL-2 priming, an effect that was also observed with other chemokine receptors (38), and that mitogenic stimulation of T cells results in CXCR4 up-regulation. Thus it can be deduced that the expression levels of HIV coreceptors on the activated T cells within the lymphoid organs of HIV-infected individuals should not be a limiting factor for entry of both M- and T-tropic viruses. However, high levels of SDF-1 produced by stromal cells may initially counteract this by inhibiting the replication of T-tropic viruses, through occupation of the coreceptor (16, 17). The disruption of the stromal architecture of lymphoid tissue may remove this major inhibitor of T-tropic virus and enable the late outgrowth of T-tropic strains (39).
Chemokine Receptors and Lymphocyte Homing
The results of Bleul et al. (9) are also of major interest to those working in the field of chemoattractants and T cell migration patterns. The ability of cells to direct movement along a chemotactic gradient is critical for their migratory capacity. The trafficking of leukocytes into tissues requires bidirectional interactions with endothelial cells, mediated by selectins and integrins, and chemotaxis through a gradient of chemoattractants (40). Chemokines are a large family of small (8- to 10-kDa) proteins that signal through specific chemokine receptors. Different chemokines have been found to differentially attract distinct lymphocyte subpopulations. For example, the CC family chemokine RANTES is a chemoattractant for memory T cells in vitro (41). The selective chemoattractant activity of the chemokines for lymphocyte subsets makes them ideal molecules for directing the subsets of lymphocytes into different tissues. SDF-1 is the first chemokine to be identified that attracts naive/quiescent cells (42). The preferential expression of CXCR4 on naive T cells suggests that the ligand SDF-1 might be involved in lymph node homing of these lymphocytes (9). SDF-1 is a member of the CXC group of chemokines, which has a broad range of constitutive tissue expression. Recently, it was found that mice lacking SDF-1 died perinatally and that the numbers of B cell and myeloid progenitors were severely reduced in the bone marrow of the mutant embryos (43). It is not clear, however, whether all biological effects of SDF-1 are mediated through CXCR4 signaling. Analysis of mice with a targeted disruption of CXCR4 should clarify this issue. It will be important to examine whether preferential expression of CXCR4 on naive T cells has an important role in their migration. It is also conceivable that SDF-1 signals have other effects on these cells, such as survival or maturation in the periphery.
Understanding the physiological role and regulation of HIV coreceptors on different subsets of T cells will not only pave the way for elucidating the interplay between these receptors and HIV and thus their role in the pathogenesis of AIDS, but will also guide future strategies for deploying therapeutics that block entry of HIV into target cells.
Acknowledgments
We are grateful to Vineet KewalRamani, Craig Davis, Ed Skolnik, and Mark Hill for helpful comments on this manuscript.
References
- 1.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 2.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 3.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 4.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 5.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 6.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 7.Ashorn P A, Berger E A, Moss B. J Virol. 1990;64:2149–2156. doi: 10.1128/jvi.64.5.2149-2156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page K A, Landau N R, Littman D R. J Virol. 1990;64:5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 11.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 12.Fisher A G, Ensoli B, Looney D, Rose A, Gallo R C, Saag M S, Shaw G M, Hahn B H, Wong-Staal F. Nature (London) 1988;334:444–447. doi: 10.1038/334444a0. [DOI] [PubMed] [Google Scholar]
- 13.Cheng-Mayer C, Weiss C, Seto D, Levy J A. Proc Natl Acad Sci USA. 1989;86:8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien W A, Koyanagi Y, Namazie A, Zhao J Q, Diagne A, Idler K, Zack J A, Chen I S. Nature (London) 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z Q, Wood C, Levy J A, Cheng-Mayer C. J Virol. 1990;64:6148–6153. doi: 10.1128/jvi.64.12.6148-6153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. Nature (London) 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 17.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. Nature (London) 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 18.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 19.Paxton W A, Martin S R, Tse D, O’Brien T R, Skurnick J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, Koup R A. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 20.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 21.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O’Brien S J. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau N R, Phair J, Ho D D, Koup R A. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 23.Sprent J, Tough D F. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 24.Beverley P. Curr Opin Immunol. 1991;3:355–360. doi: 10.1016/0952-7915(91)90038-3. [DOI] [PubMed] [Google Scholar]
- 25.Akbar A N, Salmon M, Janossy G. Immunol Today. 1991;12:184–188. doi: 10.1016/0167-5699(91)90050-4. [DOI] [PubMed] [Google Scholar]
- 26.Butcher E C, Picker L J. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 27.Mackay C R. Adv Immunol. 1993;53:217–265. doi: 10.1016/s0065-2776(08)60501-5. [DOI] [PubMed] [Google Scholar]
- 28.Mackay C R. Semin Immunol. 1992;4:51–58. [PubMed] [Google Scholar]
- 29.Cornelissen M, Mulder-Kampinga G, Veenstra J, Zorgdrager F, Kuiken C, Hartman S, Dekker J, van der Hoek L, Sol C, Coutinho R, Goudsmit J. J Virol. 1995;69:1810–1818. doi: 10.1128/jvi.69.3.1810-1818.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spijkerman I J, Koot M, Prins M, Keet I P, van den Hoek A J, Miedema F, Coutinho R A. AIDS. 1995;9:1085–1092. doi: 10.1097/00002030-199509000-00016. [DOI] [PubMed] [Google Scholar]
- 31.van’t Wout A B, Kootstra N A, Mulder-Kampinga G A, Albrecht-van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. J Clin Invest. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 33.Schnittman S M, Lane H C, Greenhouse J, Justement J S, Baseler M, Fauci A S. Proc Natl Acad Sci USA. 1990;87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pope M, Betjes M G, Romani N, Hirmand H, Cameron P U, Hoffman L, Gezelter S, Schuler G, Steinman R M. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 35.Siekevitz M, Josephs S F, Dukovich M, Peffer N, Wong-Staal F, Greene W C. Science. 1987;238:1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- 36.Tong-Starkesen S E, Luciw P A, Peterlin B M. J Immunol. 1989;142:702–707. [PubMed] [Google Scholar]
- 37.Kinter A L, Poli G, Fox L, Hardy E, Fauci A S. J Immunol. 1995;154:2448–2459. [PubMed] [Google Scholar]
- 38.Loetscher P, Seitz M, Baggiolini M, Moser B. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fauci A S. Nature (London) 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 40.Schall T J, Bacon K B. Curr Opin Immunol. 1994;6:865–873. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 41.Schall T J, Bacon K, Toy K J, Goeddel D V. Nature (London) 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 42.Bleul C C, Fuhlbrigge R C, Casasnovas J M, Aiuti A, Springer T A. J Exp Med. 1996;184:1101–1110. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Nature (London) 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]