Abstract
Purpose
Despite recent advances, multiple myeloma remains incurable and most patients eventually develop progressive disease. Allogeneic hematopoietic stem cell transplantation (allo HCT) offers a potentially curative option in 10–20% of patients with relapsed or refractory disease. We evaluated the outcome of patients undergoing allo HCT with reduced-intensity conditioning (RIC) for relapsed and/or refractory myeloma at our institution.
Methods
Fifty-one patients with heavily pretreated, relapsed myeloma, who received RIC allo HCT between 1996 and 2006, were included in this analysis.
Results
Median time from diagnosis to allo HCT was 34 months. Median follow-up in surviving patients was 27 months (3–98). Cumulative transplant-related mortality (TRM) at 1 year was 25%. Progression-free survival (PFS) and overall survival (OS) at 2 years were 19% and 32%, respectively. The incidence of grade II-IV acute or chronic graft-vs-host disease (GVHD) was 27% and 47%, respectively. At the time of this analysis, 12 patients (24%) were alive 7 of whom (14%) were in remission for up to 6 years after allo SCT. A lower β2 microglobulin (<3.3) and a prior autotransplant predicted a lower NRM, longer PFS and OS.
Conclusion
Allo HCT with RIC regimens is associated with acceptable toxicity and durable remission and survival in relapsed or refractory myeloma. Use of RIC allo HCT earlier in the course of the disease may offer greater benefit.
Introduction
Multiple myeloma represents approximately 10% of all hematologic malignancies. The treatment of newly diagnosed multiple myeloma has improved remarkably with the introduction of novel therapies such as thalidomide, lenalidomide and bortezomib 1–6. High dose therapy followed by single or tandem autologous hematopoietic stem cell transplantation (auto HCT) is a treatment option for patients less than 65 years old7, 8. Despite these advances, multiple myeloma remains an incurable disease and most patients develop progressive disease within 5 years of auto HCT 9.
Allogeneic hematopoietic stem cell transplantation (allo HCT) offers a potentially curative option in 10–20% of patients with relapsed or refractory disease 10–13. A tumor-free graft and graft-versus-myeloma (GVM) effect are two major reasons for this curative potential. The GVM effect has been well documented and is thought to be mediated by donor-derived T lymphocytes 10–12. However, the high treatment related mortality (TRM) of up to 55% with myeloablative (MA) conditioning regimen neutralizes any potential benefit in terms of progression free survival (PFS) and overall survival (OS), and limits the use of allo HCT in patients with advanced multiple myeloma 12, 14, 15.
RIC allo HCT offers the potential advantage of decreased TRM while preserving the GVM effect 16, 17. There is, however, limited information on the outcome of RIC regimen in multiple myeloma patients with advanced and heavily pretreated disease. In this retrospective single center study, we evaluated the outcomes of RIC allo HCT in 51 heavily pretreated patients with relapsed and/or refractory disease.
Patients and Methods
Patients
Fifty-one patients with multiple myeloma, who had relapsed disease and received an RIC allo HCT from a matched related or unrelated donor between 1996 and 2006, were included in this analysis. Patients were eligible for allo HCT if they were 18–70 years old, had a performance status of 0 or 1, adequate organ function, and no uncontrolled infection.
Hematopoietic Stem Cell Collection
Donor bone marrow or G-CSF-primed peripheral blood progenitor cells were collected using standard mobilization protocols and apheresis techniques. Bone marrow from unrelated donors was obtained through the National Marrow Donor Program according to standard guidelines. All patients signed written informed consent according to our institutional and the National Marrow Donor Program guidelines. The study was reviewed and approved by the Institutional Review Board at the University of Texas, MD Anderson Cancer Center (UT MDACC).
Preparative Regimen and Supportive care
The RIC regimen consisted of fludarabine (90–120 mg/m2) and melphalan (90–140 mg/m2). The definition of RIC conditioning was based on published guidelines and recommendations18. All Patients receiving unrelated donor progenitor cells also received antithymocyte globulin as part of their preparative regimen 19. GVHD prophylaxis consisted of a combination of tacrolimus and methotrexate. Patients received infection prophylaxis with levaquin or ciprofloxacin, voriconazole or fluconazole and acyclovir or valacyclovir. Filgrastim 5µg/kg was administered subcutaneously daily from 7 days after allo SCT until the recovery of absolute neutrophil count (ANC) to >1.5×109/L for 3 days. Blood products were irradiated and filtered to remove leukocytes prior to transfusion. After recovery of neutrophil count, patients received prophylaxis against pneumocystis jiroveci (formerly pneumocystis carini) infection with oral sulfamethoxazole-trimethoprim given twice weekly or intravenous pentamidine every 3 weeks.
Engraftment and Chimerism
Engraftment was defined as the first of 3 consecutive days with an ANC ≥ 0.5×109/L. Failure to engraft by day 30 was considered primary graft failure. Platelet engraftment was defined as the first of 7 consecutive days with a platelet of ≥ 20×109/L without transfusion support. Peripheral blood or bone marrow donor-recipient chimerism was performed on days 30 and 100 post transplantation, and as clinically indicated thereafter, by analysis of DNA microsatellite polymorphisms by polymerase chain reaction with D6S264, D3S1282, D18S62, and D3S1300 fluorescence-labeled primers, and by conventional cytogenetic analysis by G-banding or fluorescent in situ hybridization studies for Y chromosome in sex-mismatched cases.
Response and Outcome
Response, relapse and disease progression were defined based on the international uniform response criteria for multiple myeloma 20. Complete response (CR) was defined as negative immunofixation on the serum and urine, less than 5% plasma cells in bone marrow and absence of any plasmacytomas or soft tissue lesions. Very good partial response or major response (VGPR/MR) was serum and urine Monoclonal-protein (M-protein) detectable by immunofixation but not by electrophoresis, or at least 90% reduction in serum M-protein, and urine M-protein less than 100mg/24 hour. Partial response (PR) was defined as at least 50% reduction of serum M-protein, at least 50% reduction in the size of soft tissue plasmacytomas if present at baseline, and at least 90% reduction in 24hour-urinary M-protein. Response criteria had to be met on at least 2 assessments, at least six weeks apart. Progressive disease (PD) was defined as an increase in serum M-protein or urine light chains of 20% or more in patients with refractory or stable disease. Relapse was the reappearance of serum M-protein, urine light chains or bone marrow infiltration in patients in previous CR, or at least a 25% increase in any marker in patients in PR. Stable disease (SD) was not meeting the criteria for CR, VGPR, PR or PD.
Statistical Methods
Primary endpoints were Kaplan-Meier’s estimates of OS and PFS. Secondary endpoints were TRM, relapse, and incidence of acute and chronic GVHD. OS was measured from the day of allogeneic stem cell infusion (day 0) to death from any cause, with censoring performed at date of last contact. PFS was determined from the day of stem cell infusion to the day of documented relapse or progression. Death from any cause other than relapse was classified as TRM. GVHD occurring anytime after day 90 post transplant was termed chronic GVHD (cGVHD); otherwise it was acute GVHD (aGVHD). Standard criteria were used for the diagnosis and grading of acute and chronic GVHD21. The incidence of disease progression, TRM, acute and cGVHD was estimated using the cumulative incidence method accounting for competing risk. Statistical significance was determined at the 0.05 level. Analysis was performed using STATA (stataCorp.2001; Stata Statistical Software: Release 7.0.College Station, TX: Stata Corporation).
Results
Patients Characteristics
Between 1996 and 2006, 51 patients with relapsed or refractory multiple myeloma underwent allo HCT, using RIC regimens. Patient characteristics are summarized in Table 1. At the time of transplant, 55% of patients were in at least a partial remission (CR 4%, VGPR 6%, PR 45%). Median age was 51 years (range 32–65), and median time from diagnosis to transplant was 34 months (9–232). Thirty-six (70%) patients had a prior auto HCT, including 5 (10%) with 2 prior auto HCT. The median number of prior regimen was 5 (range 1–10). Peripheral blood (PB) stem cells were used in 41 (80%) patients and matched related donor stem cells in 40 (78%) patients. Results of chromosomal analyses were available for 40 of 51 patients prior to allo HCT. Twelve patients had cytogenetic abnormalities (including 3 patients with deletion 13p; 2 patients with 1q abnormality; and 2 patients with 17p deletion), while 28 patients had normal cytogenetics.
Table 1.
Patient Characteristics
| Reduced intensity Conditioning (n=51) | |
|---|---|
| Age, median (range) | 51 (32–65) |
| Gender F/M | 24/27 |
|
Immunoglobulin Class (%) IgG IgA IgM Light chain only Non secretory Unknown |
29 (57) 8 (16) 1 10 (20) 2 1 |
|
Stage at initial diagnosis (%) I II III unknown |
5 14 (27) 30 (59) 2 |
|
Disease Status at transplantation (%) CR VGPR PR SD PD Unknown/not evaluated |
2 (4) 3 (6) 23 (45) 14 (27) 8 (16) 1 (2) |
| Median prior regimens (range) | 5 (1–10) |
|
Prior Auto HCT (%) 1 2 |
36 (70) 31 5 |
|
Median time from Diagnosis to allo HCT, months (range) |
34.4 (9.8 – 232.2) |
|
HCT source (%) Peripheral blood (PB) Bone marrow (BM) |
41 (80) 10 (20) |
|
DonorType (%) Related donor Unrelated donor |
40 (78) 11(22) |
|
Year of allo HCT (%) 1996–2000 Beyond 2000 |
15 (29) 36 (71) |
| # DLI (%) | 12 (22) |
Auto HCT stands for autologous hematopoietic stem cell transplant; allo HCT allogeneic hematopoietic stem cell transplant; CR complete response; VGPR very good partial response; PR partial response; SD stable disease; PD progressive disease; DLI donor lymphocyte infusion. Staging done according to Durie-salmon staging system.
Engraftment
All 51 patients (100%) achieved engraftment. Median times to neutrophil and platelet engraftment were 13 (9–25) and 15 days (10–28), respectively. Median percentage of donor cells at day 30 post transplant was 100% (95–100).
Response
Overall, 12 patients (23%) achieved a CR and 26 patients (51%) achieved a PR, with an overall response rate of 74% post RIC allo HCT. Three patients (6%) had minimal response (<50%) and 4 patients (8%) had stable disease. Two out of the 3 patients with a VGPR prior to transplant achieved a CR post RIC allo HCT. Of the 23 patients in PR prior to allo HCT, 4 (17%) achieved a CR, 1 developed progressive disease (PD) and the rest remained in PR; and of the 14 patients in SD prior to allo HCT, 3(21%) achieved a CR and 7(50%) achieved a PR/VGPR. Out of 8 patients with PD at allo HCT, 1 achieved a CR and 3 achieved a PR, with an overall response of 50% in this group. Seven patients, who received allo HCT from a matched related donor, received a total of twelve donor lymphocyte infusions (DLI) for persistent disease, progressive disease (PD), or relapse after allo HCT. One patient with persistent disease obtained a CR, and one with PD achieved VGPR after one DLI, with the rest having no response. The cell dose, interval between HCT and DLI, interval between DLI, and response are listed in Table 2.
Table 2.
Donor Lymphocyte Infusion and Outcome
| Patient # |
# DLI |
Cell Doses (CD3×107/kg) |
Reason for DLI Post HCT |
Interval from HCT to DLI (Days) |
Interval Between DLI (Days) |
Response Status Post DLI |
|---|---|---|---|---|---|---|
| 1 | 2 | 1.0; 3.85 | Persistent dis.; PD |
391 | 478 | PD |
| 2 | 4 | Unk; 0.3; 3.0, Unk |
Persistent dis.; PD |
97 | 197 | PD |
| 3 | 1 | Unk | Persistent dis. |
132 | - | CR |
| 4 | 2 | Unk | Relapse | 338 | 77 | PD |
| 5 | 1 | Unk | VGPR | 109 | - | VGPR |
| 6 | 1 | 3.3 | PD | 198 | - | PD |
| 7 | 1 | 1.4 | PD | 450 | - | VGPR |
DLI stands for donor lymphocyte infusion; HCT hematopoietic stem cell transplant; Persistent dis: persistent disease; PD progressive disease; VGPR very good partial response; Unk unknown
Graft versus Host Disease
Cumulative Incidence of grade II-IV aGVHD was 27%, (Table 3). Grade II aGVHD was seen in 16%, while grade III-IV aGVHD was seen in 11%. Cumulative incidence of cGVHD was 47%, with limited cGVHD in 23% of patients. The use of unrelated donor or peripheral blood stem cells as the graft source did not increase the incidence of acute or chronic GVHD. That may be due to a small number of patients with unrelated donors (11) and bone marrow stem cells (10).
Table 3.
Response Rate, TRM, Relapse and GVHD
| Reduced intensity Conditioning (n=51) |
|
|---|---|
| Time to neutrophil engraftment. Days (range) | 13 (9–25) |
| Time to platelet engraftment Days (range) | 15 (10–28) |
| 100 Day TRM (%) | 6 (12) |
| Cumulative 1 year TRM (%) | 13 (25) |
| Overall Response Rate | 74% (CR 23%, PR 51%) |
| Relapse at 2 years (%) | 25 (49) |
| Median PFS -months | 6.8 |
| 2 year PFS- % | 19 |
| Median OS-months | 13.9 |
| 2 year OS -% | 32 |
| Acute GVHD grade II-IV (%) | 14 (27) |
| Chronic GVHD grade Limited or extensive | 47% |
|
Number of death (%) Recurrence of disease Acute or chronic GVHD Infection Other |
39 (76) 22 (43) 10 (20) 3 (6) 4 (8) |
TRM stands for treatment related mortality; GVHD graft versus host disease; OS overall survival; PFS progression free survival
Transplant-related Mortality
One-hundred day TRM was 12% and 1-year TRM was 25%. At the time of this analysis, 12 patients (24%) were still alive, 7 of whom (14%) were in remission for up to 6 years post allo SCT. The most common causes of death were recurrent disease (22 patients; 43%), acute or chronic GVHD (10 patients; 20%) and opportunistic infections (3 patients; 6%; Table 3).
Survival and Prognostic Features
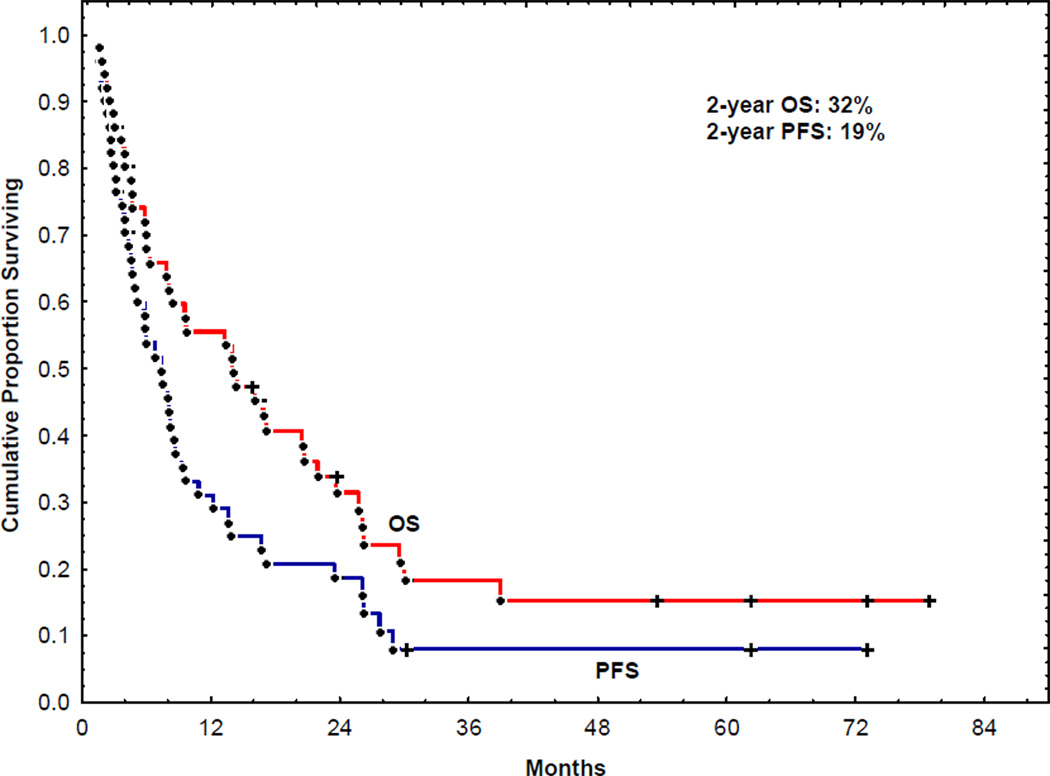
Median follow-up for surviving patients was 27 months (3–98). Twenty-five patients (49%) had relapsed at 2 years. Seven patients received a total of twelve DLI. The use of DLI did not contribute to an improvement in PFS and OS on multivariate and univariate analysis, perhaps due to the small number of patients who had DLI. The 2-year PFS and OS were 19% and 32%, respectively (Figure 1). On univariate analyses, a lower β–2 microglobulin (<3.3) and a prior auto SCT predicted longer PFS and OS (Table 4). These 2 factors also emerged as predictors of longer PFS and OS in a multivariate analysis (Table 5). Age, Immunoglobulin subtype (IG), serum lactate dehydrogenase (LDH), serum albumin, stem cell source, donor type, use of DLI, interval between diagnosis and allo SCT or interval between auto and allo SCT did not emerge as statistically significant predictors of outcome.
Figure 1.
Progression free survival and overall survival of patients receiving reduced-intensity conditioning regimen allogeneic HCT.
Table 4.
Univariate factors affecting NRM, PFS and OS in RIC Group
| NRM | p | OS: | p | PFS: | p | |
|---|---|---|---|---|---|---|
| Pretransplant factors | HR(95% CI) | HR (95% CI) | HR (95% CI) | |||
| Age at HCT | ||||||
| <=50 | ||||||
| >50 | 0.3 (0.1–1.6) | 0.2 | 0.8 (0.4–1.7) | 0.6 | 0.8 (0.4–1.5) | 0.4 |
| IgG | ||||||
| <=1600 | ||||||
| >1600 | 1.1 (0.2–5.5) | 0.9 | 1.4 (0.7–2.9) | 0.3 | 1.4 (0.7–2.8) | 0.3 |
| β2m | ||||||
| <=3.3 | ||||||
| >3.3 | 7 nrm | 0.01 | 3.1 (1.5–6.4) | 0.002 | 2.6 (1.3–5.1) | 0.005 |
| LDH | ||||||
| <=618 | ||||||
| >618 | 0.5 (0.1–4.6) | 0.6 | 0.7 (0.3–1.8) | 0.5 | 0.9 (0.4–1.9) | 0.8 |
| Albumin | ||||||
| <=3.5 | ||||||
| >3.5 | 0.6 (0.1–2.6) | 0.5 | 0.7 (0.3–1.4) | 0.3 | 0.7 (0.4–1.4) | 0.4 |
| Response prior to HCT | ||||||
| CR/PR | 0.7 (0.2–3.2) | 0.6 | 1.1 (0.6–2.2) | 0.7 | 1.1 (0.6–2.1) | 0.7 |
| Other | ||||||
| Cytogenetics pre HCT | ||||||
| High risk | 1.9 (0.4–11) | 0.4 | 2.1 (0.9–4.7) | 0.08 | 1.8 (0.8–3.9) | 0.1 |
| Other | ||||||
| Prior Auto HCT | ||||||
| No | 17 (2.0–142) | 0.009 | 2.2 (1.1–4.5) | 0.03 | 1.8 (0.9–3.4) | 0.08 |
| Yes | ||||||
| Donor Type | ||||||
| Related | 0.3 (0.1–1.5) | 0.1 | 0.7 (0.3–1.6) | 0.5 | 0.9 (0.4–1.9) | 0.9 |
| Unrelated | ||||||
| Cell Type | ||||||
| PB | 1.5 (0.2–12) | 0.7 | 0.9 (0.4–2.1) | 0.9 | 1.1 (0.5–2.3) | 0.9 |
| BM | ||||||
| Months DX to HCT | ||||||
| <=24 | ||||||
| >24 | 0.4 (0.1–1.8) | 0.2 | 0.5 (0.2–1.1) | 0.07 | 0.6 (0.3–1.1) | 0.1 |
| Months Auto to Allo HCT | ||||||
| <=24 | ||||||
| >24 | 0.9 (0.3–2.1) | 0.8 | 0.6 (0.3–1.4) | 0.2 |
Table 5.
Multivariate Factors affecting OS and PFS in RIC Group
| OS | p | PFS | p | |
|---|---|---|---|---|
| Factors | HR (95% CI) | HR (95% CI) | ||
| β2m | ||||
| <=3.3 | ||||
| >3.3 | 3.3 (1.6–6.9) | 0.002 | 2.7 (1.4–5.3) | 0.004 |
| Prior Auto HCT | ||||
| No | 2.8 (1.3–5.9) | 0.01 | 1.9 (0.9–3.9) | 0.06 |
| Yes |
NRM stands for non-relapse mortality; OS overall survival; PFS progression free survival; HR hazard ratio; CI confidence interval; HCT hematopoietic stem cell transplant; IgG immunoglobulin; β2m beta-2 microglobulin; LDH lactate dehydrogenase; Auto autologous; Allo allogeneic; Dx diagnosis.
The major differences between the long-term survivors (12 patients) and others were a lower β–2 microglobulin (median 2.45 versus 3.5, p=0.01) and more prior auto HCT (92% vs 64%, p=0.08) in the long-term survivor group (Table 6).
Table 6.
Characteristics of Long-Term Survivors Compared to Others
| Long term Survivors N=12 |
Others N=39 |
|
|---|---|---|
| Age, median (range) | 51 (32–55) | 52 (41–65) |
| Gender female:male | 6:6 | 18:21 |
|
Immunoglobulin Class (%) IgG IgA IgM Light chain only Non secretory Unknown |
6 (50) 2 (17) 1 3 (25) 0 0 |
23 (59) 6 (15) 0 7 (18) 2 1 |
|
Stage at initial diagnosis (%) I II III unknown |
0 4 (33) 7 (58) 1 |
5 (19) 10 (25) 23 (59) 1 |
|
Cytogenetics Normal Deletion 13p 1q abnormality 17p deletion Other unknown |
4 (33) 0 0 0 3 5 |
24 (62) 3 (8) 2 (5) 2 (5) 2 6 |
| β2M, median (range) | 2.45 (1.8–4.9) | 3.5 (1.0–8.2) |
|
Disease Status at transplantation (%) CR VGPR PR SD PD Unknown/not evaluated |
2 (17) 1 (8) 4 (33) 2 (17) 2 (17) 1 |
0 2 (5) 19 (49) 12 (31) 6 (15) 0 |
| Median prior regimens (range) | 5 (2–10) | 5 (1–10) |
|
Prior Auto HCT (%) 1 / 2 |
11 (92) 8 / 3 |
25 (64) 23 / 2 |
| Performance Status | 0–1 | 0–1 |
|
Median time from Diagnosis to allo HCT, months (range) |
40.2 (19.8–89.1) |
34.0 (9.8 – 232.2) |
|
HCT source (%) PB / BM |
9 (75) / 3 (25) |
32 (82) / 7 (18) |
|
DonorType (%) Related donor / Unrelated donor |
9 (75) / 3 (25) |
31 (79) / 8 (21) |
|
Year of allo HCT (%) 1996–2000 / Beyond 2000 |
2 (17) / 10 (83) |
13 (33) / 26 (66) |
| # DLI (%) | 2 (17) | 10 (25) |
β2M stands for β–2 microglobulin; Auto HCT autologous hematopoietic stem cell transplant; allo HCT allogeneic hematopoietic stem cell transplant; CR complete response; VGPR very good partial response; PR partial response; SD stable disease; PD progressive disease; DLI donor lymphocyte infusion; PB peripheral blood; BM bone marrow.
Discussion
Even with recent progress in the treatment of multiple myeloma, it remains an incurable disease. The response duration gets shorter with each successive relapse and salvage, reflecting acquired drug resistance. Prior to the novel therapies, event free survival (EFS) was 7 months for patients undergoing a second line regimen, and 3 months for patients receiving a 6th line regimen 22. Novel therapies have improved this dismal outcome. In 289 relapsed patients who had received thalidomide, lenalidomide or bortezomib at some point after their relapse, the median and 2-year OS were 23.9 months and 49% compared to 11.8 months and 24% for 98 patients who had not received these novel drugs at relapse6. Despite this improvement, the EFS in most patients with persistent or refractory disease is only 6–14 months 23–26. These numbers highlight the need for more effective therapy, especially in patients with good performance status.
Allografting is a potentially curative option for selected patients with relapsed or refractory disease, in part due to a tumor free graft and GVM effect 10–12, 27, 28. The increased TRM using myeloablative (MA) regimens offsets any improvement in EFS and OS12, 14, 15, 29. Randomized studies using RIC allo HCT in combination with autologous transplant (auto-RIC allo HCT) have mainly been performed in newly diagnosed patients with matched related donors30–32. These studies compared tandem auto HCT to auto-RIC allo HCT. Although there was a higher CR rate in the auto-RIC allo HCT groups, two out of the 3 studies found no difference in EFS and OS, mostly due to increased TRM 30, 32. The differences in results may be explained through the differences in the study designs. In the Spanish trial, only patients failing to achieve a near CR with the first auto HCT were included and the conditioning regimen was fludarabine and melphalan30. In the Italian study, all patients were included irrespective of the prognostic factors and disease status after the first auto HCT, and the conditioning regimen was 2Gy total body irradiation31. In the French study only patients with high-risk features (β–2 microglobulin >3mg/L and 13q-) were included and the conditioning regimen was busulfan, fludarabine and antithymocyte globulin32. The high TRM has been attributed to profound immunosuppression from the prior auto HCT, exacerbated further by opportunistic infections and GVHD due to RIC allo HCT. Because of the lack of an unequivocal benefit, the role of RIC allo HCT in front-line therapy is considered experimental and these transplants should only be performed as part of well-designed clinical trials in the highest-risk patients 33.
Retrospective studies in relapsed or refractory multiple myeloma using RIC allo HCT have shown decrease in TRM while maintaining the GVM effect 16, 17, 34–37. However, most of these reports were based on a small number of patients, with the exception of the EBMT study16. In this study, 229 patients received RIC allo HCT, with 168 patients beyond first remission and 74% patients having received a prior auto HCT. The TRM at 100 days and 1 year was 11% and 22%, respectively. The response rate (CR+PR) was 73% and 3 yr OS and PFS were 41% and 21%, respectively. Ninety-three patients (41 %) relapsed or progressed and 80 of these patients went on to receive donor lymphocyte infusion (DLI) with 63% obtaining CR or PR post DLI 16. Other studies have been with much smaller sample size (19–45 patients), but with similar TRM and response outcomes. Poor prognostic factors for outcome included chemoresistant disease, disease beyond first remission, more than one previous auto HCT, and ≥1 year interval between diagnosis and HCT16, 34.
In our study, the 100 day and one year TRM were 12% and 25%, comparable to the EMBT series16. Response rate was 74% (CR23%, PR51%) similar to what was seen in the prior studies 16, 17, 34. The incidence of GVHD was not adversely affected by the use of unrelated donors or peripheral blood stem cells. This may be attributed to high resolution molecular HLA typing and the use of ATG for GVHD prophylaxis 19. The 2- year OS was 32%, comparable to earlier series although our patients in general had more advanced disease. We would like to underscore the fact that patients in our series were more heavily pretreated and their median time from diagnosis to allo HCT was 34 months, compared to18 months in the EBMT study. Age, IG subtype, disease status, serum LDH, serum albumin, stem cell source, donor type, use of DLI, interval between diagnosis and allo SCT or interval between the auto and allo SCT did not emerge as statistically significant predictors of outcome. We recognize that some of these analyses suffer from the known limitations of a retrospective analysis: a small sample size, heterogeneous patient population and missing data. The ongoing multicenter BMT-CTN trial comparing tandem auto HCT +/- maintenance therapy versus single auto HCT followed by RIC allo HCT in newly diagnosed multiple myeloma patients will help define the role of RIC allo HCT in this patient population.
In conclusion, allo HCT after RIC regimens in selected patients with advanced multiple myeloma is associated with durable PFS and OS, and a lower TRM as compared to allo HCT after MA regimens. RIC regimens in advanced multiple myeloma patients do not increase the risk of relapse or the incidence of acute and chronic GVHD. Utilization of RIC allo HCT earlier in the course of the disease may offer greater benefit in selected patients.
Footnotes
Disclaimers: none
References
- 1.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SVJS, Callender N, Fonseca R, Vesole D, Greipp P. Randomized phase 111 trial of lenalidomide plus High-dose dexamethasone vs lenalidomide plus low-dose dexamethasone in newly diagnosed multiple myeloma(E4A03): a trial coordinated by the Eastern Cooperative Oncology Group(abstract) Blood. 2006;108 [Google Scholar]
- 4.Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo A, Falco P, Corradini P, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA--Italian Multiple Myeloma Network. J Clin Oncol. 2007;25:4459–4465. doi: 10.1200/JCO.2007.12.3463. [DOI] [PubMed] [Google Scholar]
- 6.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koreth J, Cutler CS, Djulbegovic B, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13:183–196. doi: 10.1016/j.bbmt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Kharfan-Dabaja MA, Glasmacher A, Djulbegovic B. Tandem versus single autologous hematopoietic cell transplantation for the treatment of multiple myeloma: a systematic review and meta-analysis. J Natl Cancer Inst. 2009;101:100–106. doi: 10.1093/jnci/djn439. [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bensinger WI. Is there still a role for allogeneic stem-cell transplantation in multiple myeloma? Best Pract Res Clin Haematol. 2007;20:783–795. doi: 10.1016/j.beha.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Blanc R, Montminy-Metivier S, Belanger R, et al. Allogeneic transplantation for multiple myeloma: further evidence for a GVHD-associated graft-versus-myeloma effect. Bone Marrow Transplant. 2001;28:841–848. doi: 10.1038/sj.bmt.1703253. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds C, Ratanatharathorn V, Adams P, et al. Allogeneic stem cell transplantation reduces disease progression compared to autologous transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2001;27:801–807. doi: 10.1038/sj.bmt.1703006. [DOI] [PubMed] [Google Scholar]
- 13.Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 14.Kuruvilla J, Shepherd JD, Sutherland HJ, et al. Long-term outcome of myeloablative allogeneic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2007;13:925–931. doi: 10.1016/j.bbmt.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Bjorkstrand BB, Ljungman P, Svensson H, et al. Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation. Blood. 1996;88:4711–4718. [PubMed] [Google Scholar]
- 16.Crawley C, Lalancette M, Szydlo R, et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors from the Chronic Leukaemia Working Party of the EBMT. Blood. 2005;105:4532–4539. doi: 10.1182/blood-2004-06-2387. [DOI] [PubMed] [Google Scholar]
- 17.Kroger N, Sayer HG, Schwerdtfeger R, et al. Unrelated stem cell transplantation in multiple myeloma after a reduced-intensity conditioning with pretransplantation antithymocyte globulin is highly effective with low transplantation-related mortality. Blood. 2002;100:3919–3924. doi: 10.1182/blood-2002-04-1150. [DOI] [PubMed] [Google Scholar]
- 18.Champlin R, Khouri I, Anderlini P, et al. Nonmyeloablative preparative regimens for allogeneic hematopoietic transplantation. Bone Marrow Transplant. 2001;27(Suppl 2):S13–S22. doi: 10.1038/sj.bmt.1702864. [DOI] [PubMed] [Google Scholar]
- 19.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12:560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 22.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 23.von Lilienfeld-Toal M, Hahn-Ast C, Furkert K, et al. A systematic review of phase II trials of thalidomide/dexamethasone combination therapy in patients with relapsed or refractory multiple myeloma. Eur J Haematol. 2008;81:247–252. doi: 10.1111/j.1600-0609.2008.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 25.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 26.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 27.Corradini P, Cavo M, Lokhorst H, et al. Molecular remission after myeloablative allogeneic stem cell transplantation predicts a better relapse-free survival in patients with multiple myeloma. Blood. 2003;102:1927–1929. doi: 10.1182/blood-2003-01-0189. [DOI] [PubMed] [Google Scholar]
- 28.Corradini P, Voena C, Tarella C, et al. Molecular and clinical remissions in multiple myeloma: role of autologous and allogeneic transplantation of hematopoietic cells. J Clin Oncol. 1999;17:208–215. doi: 10.1200/JCO.1999.17.1.208. [DOI] [PubMed] [Google Scholar]
- 29.Khaled Y, Mellacheruvu S, Reddy P, Peres E, Mineishi S. Long-term outcomes following myeloablative allogeneic transplantation for multiple myeloma compared to autologous transplantation and the impact of graft-versus-myeloma effect. Bone Marrow Transplant. 2009;44:325–326. doi: 10.1038/bmt.2009.14. [DOI] [PubMed] [Google Scholar]
- 30.Rosinol L, Perez-Simon JA, Sureda A, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112:3591–3593. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 31.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 32.Garban F, Attal M, Michallet M, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–3480. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 33.Stewart K. Reduced-Intensity allogeneic transplantation for myeloma: reality bites. blood. 2009;113:3135–3136. doi: 10.1182/blood-2008-12-173526. [DOI] [PubMed] [Google Scholar]
- 34.Lee CK, Badros A, Barlogie B, et al. Prognostic factors in allogeneic transplantation for patients with high-risk multiple myeloma after reduced intensity conditioning. Exp Hematol. 2003;31:73–80. doi: 10.1016/s0301-472x(02)01010-x. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Simon JA, Martino R, Alegre A, et al. Chronic but not acute graft-versus-host disease improves outcome in multiple myeloma patients after non-myeloablative allogeneic transplantation. Br J Haematol. 2003;121:104–108. doi: 10.1046/j.1365-2141.2003.04237.x. [DOI] [PubMed] [Google Scholar]
- 36.Badros A, Barlogie B, Siegel E, et al. Improved outcome of allogeneic transplantation in high-risk multiple myeloma patients after nonmyeloablative conditioning. J Clin Oncol. 2002;20:1295–1303. doi: 10.1200/JCO.2002.20.5.1295. [DOI] [PubMed] [Google Scholar]
- 37.de Lavallade H, El-Cheikh J, Faucher C, et al. Reduced-intensity conditioning allogeneic SCT as salvage treatment for relapsed multiple myeloma. Bone Marrow Transplant. 2008;41:953–960. doi: 10.1038/bmt.2008.22. [DOI] [PubMed] [Google Scholar]