Abstract
Objective
There is increasing evidence that adipocytokines may exert proinflammatory and destructive effects in rheumatoid arthritis (RA). Hence, the authors investigated the relationship between adipocytokines and several features associated with RA (inflammation, joint destruction and cardiovascular disease), as well as the effect of treatment with a tumour necrosis factor inhibitor or glucocorticoids (GCs) hereupon.
Methods
Serum levels of adiponectin, leptin, resistin, visfatin, vaspin and lipids were determined in a well-defined cohort of patients with RA before and after 16 weeks of adalimumab treatment (adalimumab cohort). The same parameters were analysed in two other cohorts of patients with RA before and after 2 weeks of high-dose prednisolone (high GC cohort) and before and after 22 weeks of treatment with a combination regimen with tapered high-dose prednisolone (COBRA -GC cohort). Radiographs of hands and feet (adalimumab and COBRA-GC cohorts) were assessed at baseline and after treatment.
Results
Treatment with adalimumab or GC showed opposing effects on vaspin and visfatin levels. Lipid levels improved after several months of adalimumab or GC treatment; in the adalimumab cohort, this was related to reduced visfatin levels, independent of C reactive protein levels. After long-term adalimumab or GC treatment, resistin levels declined, which was associated with a decrease in inflammation markers. In the adalimumab cohort, baseline resistin levels were predictive of baseline radiological damage, independent of anticitrullinated peptide antibodies status or C reactive protein levels.
Conclusion
Changes in serum adipocytokine levels were treatment specific, further strengthening the role of visfatin and resistin in several disease manifestations of RA.
Introduction
In rheumatoid arthritis (RA), synovitis may lead to progressive destruction of articular cartilage and subchondral bone.1 In addition, systemic inflammation, a hallmark of RA, is thought to play a key role in accelerated atherosclerosis, explaining the link between RA and an increased incidence of cardiovascular disease (CVD).2 White adipose tissue cells can influence immune functions and inflammatory processes in conditions like RA by secretion of adipocytokines as well as classic cytokines,3 4 and these mediators have provided a plausible link between obesity, inflammation and CVD.5 6 Increased serum adipocytokine levels in patients with active RA7 could perhaps be associated with the occurrence of accelerated atherosclerosis and CVD and are thought to play a role in the development of bone erosions.7–13
Tumour necrosis factor (TNF) blockade improves clinical signs and symptoms in RA14 and protects against progressive joint destruction15 and reduces the risk of first-ever CVD events.16 17 Of interest, we have recently shown that a high baseline body mass index (BMI) was related to less erosive disease at presentation as well as to a diminished clinical response to anti-TNF treatment with infliximab in established patients with RA.18 These data support the notion that fat tissue may play a role in RA pathogenesis. Glucocorticoids (GCs) effectively reduce synovitis.19–21 However, high-dose GC (≥7.5 mg daily) is known to be associated with CVD complications, such as atherosclerosis, in RA.22 23 Although both TNF inhibitors and GCs reduce synovitis, high doses of the latter do not reduce the risk of CVD, which could indicate that a different regulation of adipocytokines is at play.
To provide insight into the role of adipocytokines in RA, we investigated the adipocytokine serum levels in relationship to the acute phase response, radiological damage and lipid profile. In addition, we studied the effect of different antirheumatic treatments on serum adipocytokines in three different cohorts of patients with RA, who started treatment with either adalimumab or different regimens of GC treatment.
Patients and methods
Patients from all cohorts fulfilled the 1987 American College of Rheumatology classification criteria for RA24 and had active disease as defined by a disease activity score evaluated in 28 joints (DAS28) ≥3.2. The studies were performed according to the Declaration of Helsinki; all three cohorts were approved by the medical ethics committee, and all participants gave written informed consent.
Adalimumab cohort
Baseline demographic and clinical features of patients from the larger open-label, prospective, single-centre adalimumab cohort have been previously described.25 Forty-eight patients were included for the present analysis, based on the availability of serum at baseline and after 16 weeks combined with standardised follow-up data on the response to adalimumab treatment. All patients received 40 mg adalimumab subcutaneously every 2 weeks, in combination with a stable methotrexate dose for at least 16 weeks. Use of oral GCs (prednisone ≤10 mg/day) was allowed. Clinical response at 16 weeks was determined according to the European League Against Rheumatism (EULAR) response criteria.26
High-dose GC cohort
Nine patients from the active arm of a previously conducted, double-blind, randomised, placebo-controlled trial were treated with 60 mg of oral prednisolone daily for 1 week followed by 40 mg prednisolone daily during the second week;27 serum adipocytokine levels were measured at baseline and after 2 weeks. One patient of the original cohort was excluded due to an insufficient amount of stored serum. In this study, response was defined as a decrease in DAS28 ≥1.2 after 2 weeks of GC treatment.
COBRA-GC cohort
Twenty-one patients were treated according to the 40-week, intensified COBRA trial as described earlier.28 Serum was sampled at baseline and after 21 weeks of treatment and directly stored at −20°C. For the current study, samples of 19 patients were available and analysed for adipocytokine. Response was determined according to the EULAR response criteria.
Adipocytokine assays
Serum adiponectin, leptin and resistin were analysed using a multiplex immunoassay for human adipocytokine profiling, as previously described.29 Commercially available, quantitative sandwich ELISAs were used to determine serum levels of visfatin (Biovision, Mountain View, California, USA) and vaspin (AdipoGen, San Diego, California, USA). Visfatin ELISA results from three patients were excluded from further analysis, because they were outside of assay range. To avoid possible confounding effects of rheumatoid factor (RF), we preincubated all samples for the multiplex immunoassay with protein L (Pierce, Rockford, Illinois, USA), and residual immunoglobulins were blocked with equal volume of 10% (v/v) normal rat and mouse serum (Rockland, Gilbertsville, Pennsylvania, USA), as previously described.30 For the vaspin and visfatin ELISAs, three randomly selected samples of RF-negative patients with RA were spiked with freeze-dried RF (200 IU/ml) in a 1:1 or 1:3 ratio (SKML, Nijmegen, The Netherlands). Samples demonstrated high recovery across the concentration range, and we concluded that serum RF did not interfere with vaspin or visfatin assessments (data not shown).
Lipid profiles and assessment of radiological damage
Blood was drawn from patients while fasting at the specified time points as described above. Lipid levels were measured using standard laboratory techniques. Radiographs of the hands and feet were obtained at baseline and after 1 year (adalimumab cohort) or 40 weeks (COBRA cohort) of treatment and were scored using the Sharp/van der Heijde score (SHS).31 No x-rays were available for the high-GC cohort.
Statistical analysis
Continuous data were described as mean (SD), if normally distributed, or median (IQR), if not normally distributed. The unpaired Student t test or, where appropriate, Mann–Whitney U test was used to compare responders and non-responders. Categorical data were represented as n (%) and analysed using the χ2 or Fisher's exact test. Correlations were assessed with the Pearson product–moment or Spearman rank-order correlation coefficients. A paired Student t test or Wilcoxon signed rank test was used to determine significant changes from baseline within groups. Linear regression analysis was used to test if levels of the different adipocytokines were related to SHS; SHS was log-transformed to fit this model. Multivariate linear regression analysis was performed to study the influence of other variables at baseline on baseline SHS. In a linear regression analysis, visfatin (or change in visfatin) and C reactive protein (CRP) (or change in CRP) were used as independent variables to assess their influence on, respectively, total cholesterol (TC)/high-density lipoprotein (HDL), HDL/low-density lipoprotein (LDL), or apo B/A-I ratios (or change in these above-mentioned ratios). All statistical analyses were performed with SPSS v16.0 for Windows (SPSS, Chicago, Illinois, USA). A p value of ≤0.05 was considered statistically significant.
Results
Baseline patient characteristics and clinical response to treatment
The baseline patient characteristics of the adalimumab cohort are summarised in table 1. Sixteen weeks after initiation of treatment, mean (SD) DAS28 decreased from 5.5 (1.1) at baseline to 3.6 (1.2) (p<0.001). All baseline patient characteristics were tested for differences between EULAR responders and non-responders; no significant differences were found (table 1). For the high-GC and COBRA-GC cohorts, baseline patient characteristics are shown in table 2. In the high-GC cohort, the mean (SD) DAS28 decreased from 6.2 (1.0) to 3.6 (1.5) after 2 weeks (p=0.008). In the COBRA-GC cohort, there was a mean (SD) reduction in DAS28 from 5.3 (0.9) to 2.1 (1.3) after 21 weeks (p<0.001, compared with baseline).
Table 1.
Baseline patient characteristics for EULAR good/moderate responders and non-responders after 16 weeks of adalimumab treatment
| All patients (n=48) | EULAR g/m (n=38) | EULAR non (n=10) | p Value | |
|---|---|---|---|---|
| Age (years) | 50 (13) | 51 (13) | 48 (17) | 0.49 |
| Female, n (%) | 39 (81) | 30 (79) | 9 (90) | 0.43 |
| DAS28 | 5.5 (1.1) | 5.6 (1.1) | 5.0 (0.8) | 0.11 |
| Erosive disease, n (%) | 32 (67) | 24 (63) | 7 (70) | 0.84 |
| RF, n (%) | 31 (65) | 28 (74) | 5 (50) | 0.82 |
| ACPA, n (%) | 33 (69) | 27 (71) | 6 (60) | 0.50 |
| ESR (mm/h) | 20 (11–35) | 20 (11–36) | 19 (15–21) | 0.72 |
| CRP (mg/dl) | 8 (5–19) | 7 (4–20) | 9 (6–21) | 0.74 |
| Disease duration (months) | 58 (31–143) | 60 (28–143) | 58 (36–201) | 0.55 |
| MTX dose (mg/week) | 19 (6.8) | 19 (6.5) | 19 (8.3) | 0.85 |
| Concomitant oral GC, n (%) | 13 (27) | 8 (22) | 4 (40) | 0.18 |
| BMI | 27.0 (6.1) | 27.4 (6.3) | 24.4 (4.6) | 0.17 |
| Resistin (ng/ml) | 24 (18–30) | 23 (18–29) | 26 (18–46) | 0.48 |
| Adiponectin (mg/ml) | 9.6 (7.7–14.0) | 9.4 (7.4–12.7) | 9.8 (8.4–17.9) | 0.31 |
| Vaspin (ng/ml) | 0.46 (0.26–1.03) | 0.42 (0.26–0.97) | 0.65 (0.26–1.34) | 0.51 |
| Visfatin (ng/ml) | 2.6 (1.5–3.3) | 2.5 (1.8–3.3) | 2.6 (1.0–3.2) | 0.49 |
| Leptin (ng/ml) | 57 (31–86) | 57 (35–83) | 59 (17–101) | 0.95 |
Data are represented as mean (SD), median (IQR) or n (%), as appropriate. Baseline characteristics of patients with RA treated with adalimumab (40 mg subcutaneously every 2 weeks), in combination with a stable MTX dose for at least 16 weeks, are described. Patients were compared, based on clinical response—according to the EULAR response criteria—at 16 weeks resulting in good/moderate responders (g/m) and non-responders (non), with a χ2 test, unpaired Student t test or Mann–Whitney U test, as appropriate. Presence of erosive joint disease was determined by x-ray. Presence of immunoglobulin M-RF was defined as serum levels ≥12.5 IU/ml and presence of ACPA was defined as serum levels ≥25 IU/ml.
ACPA, anticitrullinated peptide antibody; BMI, body mass index; CRP, C reactive protein; DAS28, disease activity score evaluated in 28 joints; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; GC, glucocorticoid; MTX, methotrexate; RF, IgM rheumatoid factor.
Table 2.
Baseline patient characteristics of GC and COBRA cohorts
| GC cohort (n=9) | COBRA cohort (n=19) | |
|---|---|---|
| Age (years) | 52 (8) | 51 (14) |
| Female, n (%) | 5 (56) | 12 (63) |
| DAS28 | 6.3 (1.0) | 5.3 (0.9) |
| Erosive disease, n (%)* | 8 (89) | 10 (67) |
| RF, n (%) | 5 (71) | 13 (72) |
| ACPA, n (%) | 4 (57) | 13 (75) |
| ESR (mm/h) | 45 (18–80) | 34 (26) |
| CRP (mg/dl) | 15 (4–60) | 16 (8–30) |
| Disease duration (months) | 10 (6–29) | 3.2 (4.4) |
| Leptin (ng/ml) | 39 (13–120) | 12 (10–37) |
| Resistin (ng/ml) | 31 (24–39) | 16.9 (13.5–22.0) |
| Adiponectin (mg/ml) | 72 (43–91) | 15.7 (15.2–16.3) |
| Visfatin (ng/ml) | 5.6 (4.3–7.7) | 1.4 (1.1–3.7) |
| Vaspin (ng/ml) | 0.30 (0.22–0.64) | 0.51 (0.16–0.72) |
Data are represented as mean (SD), median (IQR) or n (%), as appropriate. Baseline values of patients with RA treated with an oral GC (60 mg prednisolone daily for 1 week followed by 40 mg prednisolone daily for 1 week; GC cohort), or combination of oral dosages of hydroxychloroquine (400 mg/day), sulfasalazine (2 g/day), methotrexate (10 mg/week) and step-down high-dose prednisolone (tapered in 6 weeks from 60 to 7.5 mg/day (thereafter until end of trial); COBRA cohort). Presence of erosive joint disease was determined by x-ray. Presence of IgM-RF was defined as serum levels ≥12.5 IU/ml for the GC cohort and ≥30 IU/ml for the COBRA cohort.
ACPA, anticitrullinated peptide antibody; CRP, C reactive protein; DAS28, disease activity score evaluated in 28 joints; ESR, erythrocyte sedimentation rate; GC, glucocorticoid; RF, IgM rheumatoid factor.
Pretreatment adipocytokine serum levels are not associated with DAS28
In the adalimumab cohort, serum levels of the different adipocytokines were not correlated with CRP, erythrocyte sedimentation rate (ESR) or DAS28 at baseline with the exception of adiponectin. Adiponectin was inversely associated with CRP (r=−0.31, p=0.04), ESR (r=−0.33, p=0.02) and DAS28 (r=−0.26, p=0.08) at baseline. Only leptin, and none of the other four adipocytokines, was positively correlated with BMI (r=0.47, p=0.001). No differences or correlations in serum adipocytokine levels were found between anticitrullinated peptide antibodies (ACPAs) and/or RF-positive patients with RA and age. In the high-GC cohort and the COBRA-GC cohort, adipocytokines were not related to age, DAS28, CRP or ESR or ACPA at baseline. In the COBRA-GC cohort, RF was positively correlated to visfatin (r=0.58, p=0.01) and leptin (r=0.58, p=0.014). In the high-GC cohort, a negative association was found between RF and visfatin (r=−0.775, p=0.04).
Adalimumab treatment leads to a decrease in resistin and visfatin serum levels, but only resistin decline is associated with disease activity reduction
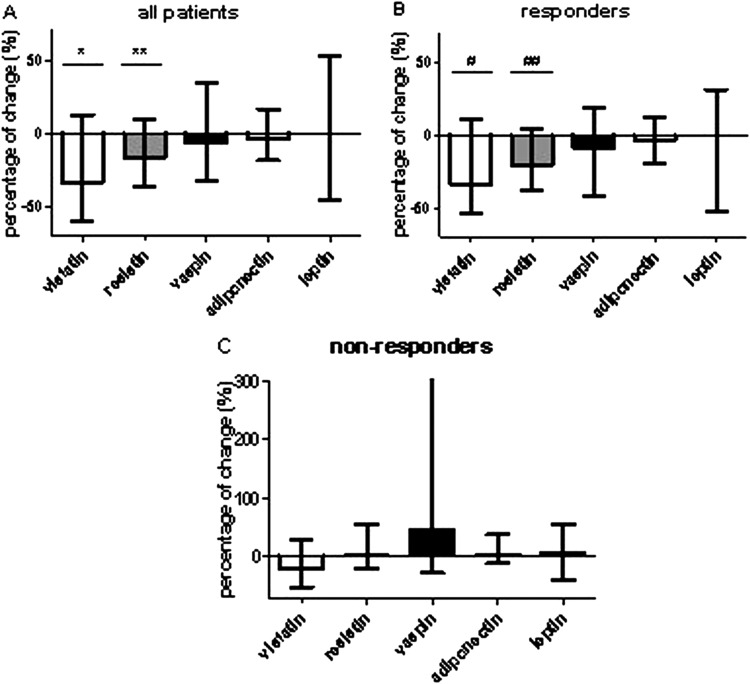
Median (IQR) levels of visfatin (2.6 (1.5–3.3) to 1.6 (1.0–2.7) ng/ml, p=0.004) and resistin (24 (18–30) to 20 (14–26) ng/ml, p=0.008) showed statistically significant decreases after 16 weeks of adalimumab treatment compared with baseline (figure 1A). Similar changes were observed after adalimumab treatment in the subgroup of 33 patients who did not use concomitant prednisolone (data not shown). Leptin, adiponectin and vaspin serum levels showed no statistically significant changes after 16 weeks of treatment with adalimumab (figure 1A). Visfatin and resistin decreased significantly only in EULAR responders (p=0.002 and p=0.004, respectively; figure 1B); in EULAR non-responders, none of the adipocytokines showed significant changes (figure 1C).
Figure 1.
Changes in adipocytokine serum levels after treatment with adalimumab. Serum levels of visfatin, resistin, vaspin, adiponectin and leptin from patients with RA were measured at baseline and after 16 weeks of treatment with adalimumab (40 mg subcutaneously every 2 weeks) in combination with methotrexate (stable dose for at least 16 weeks). Median (IQR) change (expressed as percentage) after treatment compared with baseline value is shown for all patients (A), EULAR good/moderate responders (B) and EULAR non-responders (C). Wilcoxon signed rank test was used to compare the changes for each adipocytokine. *p=0.004; **p=0.008; #p=0.004; ##p=0.002. EULAR, European League Against Rheumatism; RA, rheumatoid arthritis.
The decrease in resistin was significantly and positively correlated to the decrease in DAS28, CRP and ESR after 16 weeks (r=0.38, p=0.009; r=0.34, p=0.02; r=0.32, p=0.03, respectively), but not for visfatin (data not shown). None of the baseline adipocytokine levels predicted later clinical response to adalimumab, even after adjustment for gender and BMI (data not shown).
Vaspin serum levels increase and resistin levels decrease after GC treatment
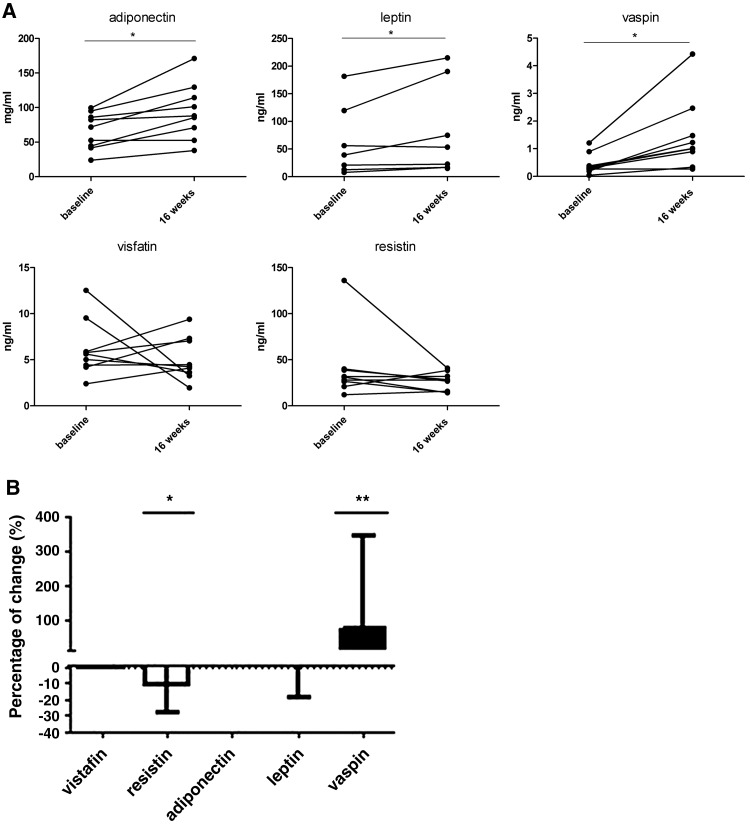
Short-term treatment with high-dose GC (high-GC cohort) resulted in significant median (IQR) increases of adiponectin, leptin and vaspin serum levels 2 weeks after start of treatment compared with baseline (adiponectin: 72 (43–91) to 88 (62–122) mg/ml, p=0.012; leptin: 39 (13–120) to 53 (17–190) ng/ml, p=0.025; vaspin: 0.30 (0.22–0.64) to 1.01 (0.61–2.00) ng/ml, p=0.008; figure 2A). Visfatin and resistin levels did not change significantly (figure 2A).
Figure 2.
Changes in adipocytokine serum levels after short- or long-term GC treatment. Serum levels of visfatin, resistin, vaspin, adiponectin and leptin from patients with RA were measured at baseline and after treatment. Individual changes after 2-week treatment with an oral GC (60 mg prednisolone daily for 1 week followed by 40 mg prednisolone daily for 1 week; GC cohort) compared with baseline are shown (A). Wilcoxon signed rank test was used to compare the changes for each adipocytokine. Median (IQR) change (expressed as percentage) after 21 weeks of combination treatment with oral dosages of hydroxychloroquine (400 mg/day), sulfasalazine (2 g/day), methotrexate (10 mg/week) and step-down high-dose prednisolone (tapered in 6 weeks from 60 to 7.5 mg/day (thereafter until end of trial); COBRA cohort) compared with baseline value (B). Wilcoxon signed rank test was used. *p=0.03; **p=0.02. GC, glucocorticoid; RA, rheumatoid arthritis.
Similar to short-term usage, long-term GC treatment (COBRA-GC cohort) showed a significant increase in median (IQR) vaspin levels after 21 weeks compared with baseline (0.51 (0.16–0.72) to 0.83 (0.41–1.50), p=0.02) and no change in visfatin levels (figure 2B). However, adiponectin and leptin levels were not affected by long-term GC treatment (COBRA-GC cohort). Interestingly, the mean (SD) serum resistin level decreased from 16.9 (13.5–22.0) at baseline to 13.5 (9.9–19.0) ng/ml after 21 weeks (p=0.03); this 21-week decrease was associated with an ESR decline after 21 weeks (r=0.51, p=0.03). In both GC cohorts, no association was found between change in any of the adipocytokines and decrease in CRP, ESR or DAS28. None of the adipocytokines predicted response.
Decrease in visfatin serum levels is associated with improved lipid profiles after adalimumab treatment
In a previous analysis of the adalimumab cohort, we observed an improvement in the lipid profile.8 Visfatin levels at baseline were positively correlated to the TC/HDL (atherogenic index) and LDL/HDL ratios (r=0.30, p=0.05; r=0.35, p=0.02, respectively); these associations were independent of baseline CRP (data not shown). Furthermore, baseline adiponectin levels were positively correlated to HDL levels (r=0.62, p<0.001) and negatively correlated to the atherogenic index (r=−0.39, p=0.006) at baseline.
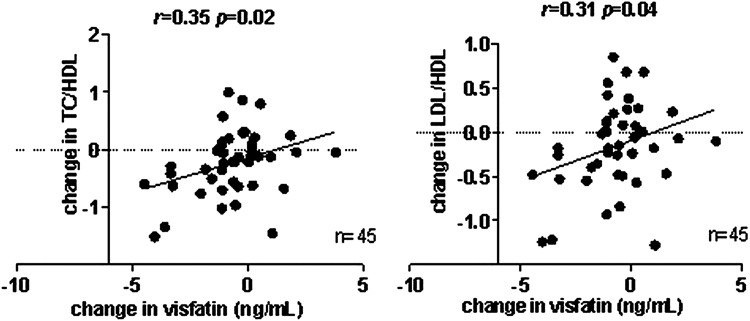
The decrease in serum visfatin levels was correlated with improved atherogenic index (r=0.35, p=0.02) and LDL/HDL (r=0.31, p=0.04) ratios after 16 weeks of adalimumab treatment (figure 3). In a multivariate linear regression analysis, these associations were independent of decrease in CRP levels (visfatin change: B=0.092, 95% CI 0.003 to 0.18; p=0.04). Serum resistin, leptin and vaspin were not associated with baseline lipid levels or changes in lipids after 16 weeks of treatment.
Figure 3.
Correlations between the atherogenic index (TC/HDL) or LDL/HDL ratio, and visfatin levels after treatment with adalimumab. Serum visfatin, TC, HDL and LDL cholesterol were measured in blood drawn from fasting patients with RA after 16 weeks of treatment with adalimumab (40 mg subcutaneously every 2 weeks). Change in visfatin levels compared with baseline, TC/HDL ratio (atherogenic index) and LDL/HDL ratio was calculated; Pearson's correlation coefficients are shown. These associations were independent of the decrease in CRP. CRP, C reactive protein; HDL, high-density lipoprotein; LCL, low-density lipoprotein; TC, total cholesterol.
Similar to the adalimumab cohort, there was an improvement in the mean (SD) lipid index in the COBRA-GC cohort from baseline to 21 weeks (3.3 (0.77) to 2.8 (0.60), p<0.001). In contrast to the adalimumab cohort, there were no associations between baseline (or changes in) lipid levels (TC, HDL and TC/HDL) and any of the baseline (or changes in) adipocytokine levels in the COBRA-GC cohort.
Resistin serum levels are associated with more radiological damage at baseline in the adalimumab cohort
Higher resistin levels at baseline were associated with a higher baseline SHS in the adalimumab cohort (adjusted R2=12%, p=0.01). A multiple regression analysis with resistin, ACPA status and disease duration as independent variables showed that disease duration (p=0.002) and resistin (p=0.04) independently predicted radiological damage (adjusted R2=26%). ACPA status did not predict SHS at baseline in this cross-sectional analysis. When CRP was added to the model as a fourth variable, disease duration (p=0.008), CRP (p=0.01) and resistin (p=0.055) predicted radiological damage at baseline (adjusted R2=35%). The addition of BMI, concomitant low-dose prednisolone use at baseline or gender had no influence on this model. Baseline levels of the other adipocytokines did not predict joint destruction.
In the COBRA-GC cohort, a statistically significant mean (SD) SHS increase from 3.5 (0.5–10) to 5.3 (0.8–12.4) after 40 weeks was observed (p<0.005). Neither baseline nor change in serum adipocytokine levels was associated with baseline or change in SHS.
Discussion
We examined the levels of adipocytokines in relationship to inflammation, lipid profile and radiological damage in three cohorts of patients with RA initiating antirheumatic treatment. TNF blockade or GC showed opposing effects on vaspin and visfatin serum levels: GC treatment (both short term and long term) increased vaspin, but not visfatin, while adalimumab led to decreased visfatin levels and had no effect on vaspin. The lipid profile improved after adalimumab or long-term GC treatment; in the adalimumab cohort, this was related to a visfatin reduction independent of CRP levels. After several months of treatment with adalimumab or prednisolone, we observed a decline in resistin levels, associated with a decrease in DAS28, CRP and ESR, or ESR only, respectively. In the adalimumab cohort, serum resistin levels at baseline were predictive of radiological damage at baseline, independent of ACPA status or CRP.
As a main finding, we found a highly significant decrease in visfatin levels after adalimumab treatment, but not after GC treatment. In the adalimumab cohort, the baseline lipid profile and the subsequent improvement after treatment were related to the baseline serum visfatin level and decrease in visfatin concentrations, respectively. This latter relationship was independent of CRP levels, suggesting a role for visfatin specifically in the improvement of the lipid profile independent of a decrease in disease activity. Visfatin serum levels are also increased in patients with diabetes mellitus or hypertension and independently associated with increased CVD risk.32 The lipid profile improved after treatment with adalimumab and after treatment with GC in the COBRA-GC cohort. This effect has previously been ascribed to the decrease in inflammation after effective treatment.2
Another interesting finding in this study is the increase of vaspin levels in GC-treated patients, which were unaffected after adalimumab treatment. Increased vaspin levels are associated with decreased insulin sensitivity,33 34 which is commonly seen after GC treatment.35 Vaspin could, therefore, also be involved in the opposing effects of anti-TNF and GC treatments on CVD risk.
After several months of treatment with either adalimumab or prednisolone, there was a decline in resistin levels, associated with a decrease in DAS28, CRP and ESR, or ESR only, respectively. These results are consistent with previous reports showing that serum resistin levels are correlated with markers of inflammation in patients with RA.36 37 We are the first to report altered resistin levels after long-term treatment with GC. As resistin is mainly produced by macrophages8 38 and a decrease in the number of macrophages in synovium is consistently associated with improvement after effective treatment in RA,39 40 we hypothesise that the observed reduction in resistin levels may reflect the decreased number of synovial macrophages. Despite inducing low-grade inflammation, obesity is associated with reduced radiological damage,41 42 making adipocytokines, especially those related to obesity, likely regulators of this destructive process. We found that higher baseline resistin levels were associated with more radiological damage at baseline independent of inflammation. This is in accordance with two other cross-sectional studies.7 13 The role of resistin in the pathophysiology in the RA joint is supported by studies in mice showing that injection of resistin into the joint may induce arthritis associated with synovitis and pannus formation.8 Resistin has also been shown to have a role in osteoclast differentiation.43 We did not find an association between adiponectin levels and radiological damage. This is apparently in contrast with other studies showing an association between adiponectin levels and bone erosions.9–12 Of these studies, two were prospective.11 12 The average adiponectin levels over time correlated better with bone erosions than the baseline levels, which may help to reconcile in part the discrepancy with our study. Moreover, radiological damage was scored at later time points (after about 3 and 5 years, respectively) than in our study. 11 12 Consistent with our findings, most previous studies found no effect of TNF inhibition on circulating adiponectin levels in RA.37 44 45 Other studies have suggested an increase in adiponectin levels,46 47 in the absence of an effect on mRNA adiponectin levels in subcutaneous fat biopsies.47 Together, the effects of TNF inhibition on adiponectin levels are at present poorly understood. This study has some limitations. The number of patients in the GC cohorts is relatively small. In addition, there are differences in baseline characteristics like disease duration between the different cohorts. Of importance, however, all patients had active RA and were treated according to a stringent treatment protocol. Adipocytokine levels were measured at different time points in the three cohorts after start of treatment. Therefore, it is too early to draw definitive conclusions with regard to the kinetics of the effects of different therapeutic interventions on various adipocytokines.
In conclusion, we found opposing effects of adalimumab and GC treatments on vaspin and visfatin serum levels, possibly in part explaining the different CVD risk profile of both drugs. Moreover, resistin levels are associated with radiological damage, independent of CRP levels or ACPA status. These data further support an important pathophysiological role for resistin and visfatin in RA.
Acknowledgments
The authors would like to thank Dr B M Lodde for editorial assistance.
Footnotes
Contributors: All authors meet the criteria for authorship and more specifically for contributorship statement: Berent Prakken and Wilco de Jager performed the adiponectin, resistin and leptin assays. Lilian van Tuyl and Mike Nurmohammed supplied the databases of the COBRA-GC cohort. Danielle Gerlag collected and supplied the data of the high-GC cohort. Carla Wijbrandts collected and supplied the data of the adalimumab cohort. Marieke Herenius and Ruth Klaasen performed the visfatin and vaspin assays. Ruth Klaasen and Marieke Herenius interpreted the data and wrote the article under the close supervision of Paul-Peter Tak and thereby take responsibility for this work. All other authors agreed to publish this work and critically reviewed the article. Conception and design of this work were discussed with all authors.
Funding: This study was supported by Abbott Laboratories.
Competing interests: None.
Ethics approval: This study was conducted with the approval of the medical ethical committees of the different universities.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Dear Dr Kvien, I hereby would like to resubmit our manuscript entitled ‘Treatment-specific changes in circulating adipocytokines: a comparison between tumour necrosis factor blockade and glucocorticoid treatment for rheumatoid arthritis' as an original contribution to Annals of the Rheumatic Diseases. We hope that you now find our article suitable for publication in Annals of the Rheumatic Diseases, on behalf of Professor Paul-Peter Tak, Ruth Klaasen.
References
- 1.Hulsmans HM, Jacobs JW, van der Heijde DM, et al. The course of radiologic damage during the first six years of rheumatoid arthritis. Arthritis Rheum 2000;43:1927–40 [DOI] [PubMed] [Google Scholar]
- 2.van Leuven SI, Franssen R, Kastelein JJ, et al. Systemic inflammation as a risk factor for atherothrombosis. Rheumatology (Oxford) 2008;47:3–7 [DOI] [PubMed] [Google Scholar]
- 3.Laurberg TB, Frystyk J, Ellingsen T, et al. Plasma adiponectin in patients with active, early, and chronic rheumatoid arthritis who are steroid- and disease-modifying antirheumatic drug-naive compared with patients with osteoarthritis and controls. J Rheumatol 2009;36:1885–91 [DOI] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772–83 [DOI] [PubMed] [Google Scholar]
- 5.Gualillo O, González-Juanatey JR, Lago F. The emerging role of adipokines as mediators of cardiovascular function: physiologic and clinical perspectives. Trends Cardiovasc Med 2007;17:275–83 [DOI] [PubMed] [Google Scholar]
- 6.Guzik TJ, Mangalat D, Korbut R. Adipocytokines—novel link between inflammation and vascular function? J Physiol Pharmacol 2006;57:505–28 [PubMed] [Google Scholar]
- 7.Rho YH, Solus J, Sokka T, et al. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum 2009;60:1906–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bokarewa M, Nagaev I, Dahlberg L, et al. Resistin, an adipokine with potent proinflammatory properties. J Immunol 2005;174:5789–95 [DOI] [PubMed] [Google Scholar]
- 9.Ebina K, Fukuhara A, Ando W, et al. Serum adiponectin concentrations correlate with severity of rheumatoid arthritis evaluated by extent of joint destruction. Clin Rheumatol 2009;28:445–51 [DOI] [PubMed] [Google Scholar]
- 10.Giles JT, Allison M, Bingham CO, 3rd, et al. Adiponectin is a mediator of the inverse association of adiposity with radiographic damage in rheumatoid arthritis. Arthritis Rheum 2009;61:1248–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giles JT, van der Heijde DM, Bathon JM. Association of circulating adiponectin levels with progression of radiographic joint destruction in rheumatoid arthritis. Ann Rheum Dis 2011;70:1562–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein-Wieringa IR, van der Linden MP, Knevel R, et al. Baseline serum adipokine levels predict radiographic progression in early rheumatoid arthritis. Arthritis Rheum 2011;63:2567–74 [DOI] [PubMed] [Google Scholar]
- 13.Forsblad dH, Pullerits R, Carlsten H, et al. Resistin in serum is associated with higher levels of IL-1Ra in post-menopausal women with rheumatoid arthritis. Rheumatology (Oxford) 2008;47:1082–7 [DOI] [PubMed] [Google Scholar]
- 14.Maini RN, Breedveld FC, Kalden JR, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum 2004;50:1051–65 [DOI] [PubMed] [Google Scholar]
- 15.Taylor PC. Anti-TNFalpha therapy for rheumatoid arthritis: an update. Intern Med 2003;42:15–20 [PubMed] [Google Scholar]
- 16.Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis 2011;70:576–82 [DOI] [PubMed] [Google Scholar]
- 17.Jacobsson LT, Turesson C, Gülfe A, et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J Rheumatol 2005;32:1213–8 [PubMed] [Google Scholar]
- 18.Klaasen R, Wijbrandts CA, Gerlag DM, et al. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum 2011;63:359–64 [DOI] [PubMed] [Google Scholar]
- 19.Gøtzsche PC, Johansen HK. Short-term low-dose corticosteroids versus placebo and non-steroidal anti-inflammatory drugs in rheumatoid arthritis. Cochrane Database Syst Rev 2004;3:CD000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirwan JR, Bijlsma JW, Boers M, et al. Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst Rev 2007;1:CD006356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boers M, Verhoeven AC, Markusse HM, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 1997;350:309–18 [DOI] [PubMed] [Google Scholar]
- 22.del Rincón I, O'Leary DH, Haas RW, et al. Effect of glucocorticoids on the arteries in rheumatoid arthritis. Arthritis Rheum 2004;50:3813–22 [DOI] [PubMed] [Google Scholar]
- 23.Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med 2004;141:764–70 [DOI] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24 [DOI] [PubMed] [Google Scholar]
- 25.Wijbrandts CA, van Leuven SI, Boom HD, et al. Sustained changes in lipid profile and macrophage migration inhibitory factor levels after anti-tumour necrosis factor therapy in rheumatoid arthritis. Ann Rheum Dis 2009;68:1316–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Gestel AM, Anderson JJ, van Riel PL, et al. ACR and EULAR improvement criteria have comparable validity in rheumatoid arthritis trials. American College of Rheumatology European League of Associations for Rheumatology. J Rheumatol 1999;26:705–11 [PubMed] [Google Scholar]
- 27.Gerlag DM, Haringman JJ, Smeets TJ, et al. Effects of oral prednisolone on biomarkers in synovial tissue and clinical improvement in rheumatoid arthritis. Arthritis Rheum 2004;50:3783–91 [DOI] [PubMed] [Google Scholar]
- 28.van Tuyl LH, Lems WF, Voskuyl AE, et al. Tight control and intensified COBRA combination treatment in early rheumatoid arthritis: 90% remission in a pilot trial. Ann Rheum Dis 2008;67:1574–7 [DOI] [PubMed] [Google Scholar]
- 29.Schipper HS, de Jager W, van Dijk ME, et al. A multiplex immunoassay for human adipokine profiling. Clin Chem 2010;56:1320–8 [DOI] [PubMed] [Google Scholar]
- 30.de Jager W, Prakken BJ, Bijlsma JW, et al. Improved multiplex immunoassay performance in human plasma and synovial fluid following removal of interfering heterophilic antibodies. J Immunol Methods 2005;300:124–35 [DOI] [PubMed] [Google Scholar]
- 31.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261–3 [PubMed] [Google Scholar]
- 32.Chang YH, Chang DM, Lin KC, et al. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev 2011;27:515–27 [DOI] [PubMed] [Google Scholar]
- 33.Li K, Li L, Yang M, et al. Short-term continuous subcutaneous insulin infusion decreases the plasma vaspin levels in patients with type 2 diabetes mellitus concomitant with improvement in insulin sensitivity. Eur J Endocrinol 2011;164:905–10 [DOI] [PubMed] [Google Scholar]
- 34.Tan BK, Heutling D, Chen J, et al. Metformin decreases the adipokine vaspin in overweight women with polycystic ovary syndrome concomitant with improvement in insulin sensitivity and a decrease in insulin resistance. Diabetes 2008;57:1501–7 [DOI] [PubMed] [Google Scholar]
- 35.van Raalte DH, Brands M, van der Zijl NJ, et al. Low-dose glucocorticoid treatment affects multiple aspects of intermediary metabolism in healthy humans: a randomised controlled trial. Diabetologia 2011;54:2103–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Gay MA, Garcia-Unzueta MT, Gonzalez-Juanatey C, et al. Anti-TNF-alpha therapy modulates resistin in patients with rheumatoid arthritis. Clin Exp Rheumatol 2008;26:311–6 [PubMed] [Google Scholar]
- 37.Karmiris K, Koutroubakis IE, Xidakis C, et al. The effect of infliximab on circulating levels of leptin, adiponectin and resistin in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2007;19:789–94 [DOI] [PubMed] [Google Scholar]
- 38.Lehrke M, Reilly MP, Millington SC, et al. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med 2004;1:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamilton JA, Tak PP. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum 2009;60:1210–21 [DOI] [PubMed] [Google Scholar]
- 40.Haringman JJ, Gerlag DM, Zwinderman AH, et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2005;64:834–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Helm-van Mil AH, van der Kooij SM, Allaart CF, et al. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis 2008;67:769–74 [DOI] [PubMed] [Google Scholar]
- 42.Westhoff G, Rau R, Zink A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum 2007;56:3575–82 [DOI] [PubMed] [Google Scholar]
- 43.Thommesen L, Stunes AK, Monjo M, et al. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J Cell Biochem 2006;99:824–34 [DOI] [PubMed] [Google Scholar]
- 44.Härle P, Sarzi-Puttini P, Cutolo M, et al. No change of serum levels of leptin and adiponectin during anti-tumour necrosis factor antibody treatment with adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis 2006;65:970–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters MJ, Watt P, Cherry L, et al. Lack of effect of TNFalpha blockade therapy on circulating adiponectin levels in patients with autoimmune disease: results from two independent prospective studies. Ann Rheum Dis 2010;69:1687–90 [DOI] [PubMed] [Google Scholar]
- 46.Ehling A, Schäffler A, Herfarth H, et al. The potential of adiponectin in driving arthritis. J Immunol 2006;176:4468–78 [DOI] [PubMed] [Google Scholar]
- 47.Stanley TL, Zanni MV, Johnsen S, et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab 2011;96:E146–50 [DOI] [PMC free article] [PubMed] [Google Scholar]