Abstract
Acute inflammation triggers the innate immune response of neutrophils that efficiently traffic from the bloodstream to concentrate at high numbers at the site of tissue infection or wounding. A gatekeeper in this process is activation of β2 integrins, which form bond clusters with ICAM-1 on the endothelial surface. These bond clusters serve dual functions of providing adhesive strength to anchor neutrophils under the shear forces of blood flow and directional guidance for cell polarization and subsequent transmigration on inflamed endothelium. We hypothesized that shear forces transmitted through high-affinity LFA-1 facilitates the cooperation with the calcium release-activated channel Orai1 in directing localized cytoskeletal activation and directed migration. By using vascular mimetic microfluidic channels, we observed neutrophil arrest on a substrate of either ICAM-1 or allosteric Abs that stabilize a high- or low-affinity conformation of LFA-1. Neutrophils captured via low-affinity LFA-1 did not exhibit intracellular calcium flux, F-actin polymerization, cell polarization, or directional migration under shear flow. In contrast, high-affinity LFA-1 provided orientation along a uropod–pseudopod axis that required calcium flux through Orai1. We demonstrate how the shear stress of blood flow can transduce distinct outside–in signals at focal sites of high-affinity LFA-1 that provide contact-mediated guidance for neutrophil emigration.
Hydrodynamic shear stress is an omnipresent and necessary component of the multistep process of leukocyte recruitment providing mechanical forces that act on leukocyte adhesion receptors following contact with the vessel wall. E-selectin and L-selectin support not only leukocyte rolling, but also transduce outside–in signals that activate integrins in a manner dependent on the magnitude of shear stress (1, 2). Tensile force acting on β2 integrin bonds also provides a signal that is synergistic with that from chemokine mediated G protein-coupled receptors in that it stabilizes high-affinity bond formation with endothelial expressed ICAM-1 (3–5) The physiological importance of shear-induced signaling via LFA-1 (CD11a/CD18) is evidenced by the absence of effective transendothelial migration of T cells under shear-free conditions even in the presence of appropriate chemokines (6). Polymorphonuclear leukocytes (PMNs) also use high-affinity LFA-1 for orienting the direction of the uropod–pseudopod major axis that guides transmigration across inflamed endothelium (4). Such guidance is evident in a mouse model of microvascular inflammation, wherein we recently reported that PMNs are biased to migrate perpendicular to the direction of blood flow (7). Precisely how mechanotransduction through LFA-1 bonds on PMNs alters calcium signaling and subsequent cytoskeletal activation to permit a migratory phenotype remains unknown.
Formation of focal clusters of high-affinity integrin is required for adhesion strengthening, and we have recently reported a mechanism for temporal synchronization that involves local influx of calcium via Orai1 (8). Calcium transients following integrin binding and clustering are associated with cytoskeletal coordination and spreading of an adherent PMN (9). G protein-coupled receptor (GPCR)-mediated calcium flux occurs mainly through extracellular channels (10), and calcium flux triggered by high-affinity integrin binding to ICAM-1 appears to involve cooperativity between phospholipase C (PLC)-mediated intracellular stores and store-operated calcium entry (SOCE) channels on the membrane. Orai1 has recently been demonstrated as the predominant calcium release-activated channel (CRAC) member in mediating PMN migratory events, cooperating with inositol 1,4,5-triphosphate (IP3)-gated channels internally to guide rapid transition of cell rolling to arrest (8). Studies have also shown elevation of intracellular calcium levels in endothelial cells exposed to high shear stress (11), although the precise mechanism by which shear force acts on high-affinity CD18 bonds to open up Orai1 channels and how this signal assembles cytoskeletal machinery to guide polarization in leukocytes remain unknown.
Allosterically locking LFA-1 into an intermediate- or low-affinity state using small molecule inhibitors such as lovastatin severely inhibits PMN arrest, polarization, and transmigration, and this evidence suggests the importance of allostery of LFA-1 in PMN recruitment (4). ICAM-1 is expressed predominantly as a homodimer on endothelial cells and in this conformation it supports dimeric bonds with LFA-1 that provide ~10-fold longer adhesive lifetimes and >100-fold amplification in mechanical strength as compared with monomeric bonds (12–14). These high-avidity focal sites of adhesion in turn catalyze local cytoskeletal assembly of F-actin and phosphorylated Src kinase on the cytoplasmic domains of this clustered LFA-1 (15). Supporting this are studies in the Vav-1 knockout mouse in which LFA-1 was implicated in the coordination of pseudopod formation, cell polarization, and guidance under shear flow (16). Cytoskeletal adaptor proteins that can associate with the cytoplasmic domain of LFA-1 and orient cell migration are Talin, Kindlin-3, and F-actin, which are activated to associate with the cytodomain of CD18 and function to stabilize the high-affinity integrin bond with ICAM-1 (17). A key unanswered question is how tensile force is transduced via LFA-1 to catalyze cytoskeletal assembly and spatial and temporal guidance of pseudopod formation and directional migration.
In the present study, we tested the hypothesis that the tensile force conducted through LFA-1/ICAM-1 bonds provides a distinct physiological cue that locally activates the cytoskeleton and serves to guide directional migration. We found that high-affinity LFA-1 colocalized with Orai1 within the plane of adhesive contact producing localized calcium influx and release of cytosolic stores, which in turn catalyzed F-actin polymerization necessary for polarization and transendothelial migration. Genetic deletion of Orai1 disrupted calcium signaling, LFA-1 redistribution, F-actin recruitment, and resulted in defective PMN polarization in shear flow. We conclude that tensile force acting on high-affinity LFA-1/ICAM-1 bonds facilitates formation of an active signaling complex that provides spatial cues for directed PMN migration.
Materials and Methods
Abs, small molecules, and other reagents
Abs were used at 5 μg/ml or per the manufacturer’s suggestion. Human and mouse ICAM-1-Fc and E-selectin-Fc were purchased from R&D Systems (Minneapolis, MN). Protein A/G was purchased from Pierce (Rockford, IL). 2-Aminoethoxydiphenyl borate (2-APB) was purchased from EMD Biosciences (San Diego, CA), resuspended in dry DMSO at a concentration of 100 mM, and stored at −80°C under dry N2. PLC inhibitor U73122 was purchased from EMD Biosciences and resuspended to 1 mM in dry DMSO and stored at −80°C under dry N2. Thapsigargin was purchased from Invitrogen (Carlsbad, CA), and resuspended to 1 mM in dry DMSO the same day as the experiment. Anti-CD18 (327C), anti–LFA-1 (TS2/4), and anti-CD18 (240Q) were obtained as a gift from ICOS (Bothell, WA; now an entity of Eli Lilly, Indianapolis, IN). Anti-CD18 (TS1/18) and anti-CD45 were purchased from BioLegend (San Diego, CA). Anti–Mac-1 (ICRF44) and anti–LFA-1 (TS1/22) obtained from BioLegend and Thermo Fischer Scientific (Rockford, IL), respectively. Anti-CD18 (IB4) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Small-molecule lovastatin was purchased from Calbiochem. Polyclonal anti-mouse Orai1 was purchased from Abcam (Cambridge, MA). Goat anti–rabbit-Alexa 488 polyclonal secondary Ab and normal goat serum were purchased from Invitrogen and Thermo Fisher Scientific (Waltham, MA), respectively. Phalloidin 488 was purchased from R&D Systems.
Neutrophil isolation
PMNs were isolated from whole human blood obtained from healthy adults by venipuncture into sterile syringes with heparin (10 U/ml blood; Elkins-Sinn, Cherry Hill, NJ) according to protocol no. 200311635 of the University of California at Davis Institutional Review Board. Whole blood was layered over PMN separation media (Thermo Fisher Scientific) as previously described (2). After centrifugation, PMNs were extracted from the appropriate density layer and washed with HEPES-buffered salt solution and were maintained at room temperature in a calcium-free HEPES buffer until use. Mice heterozygous for expression of Orai1 were a gift from the laboratory of Dr. Anjana Rao (Harvard Medical School) (18). Mice were genotyped from tail clippings by PCR, and PMNs were isolated from the bone marrow of littermate Orai1+/− and wild-type ICR strain mice as previously described (19, 20).
Substrate derivitization and in vitro migration assays
Human and mouse ICAM-1, E-selectin, and 240Q, TS1/18, IB4, and CD54 Abs were absorbed to coverslips cleaned with piranha solution (21) and coated with aminosilane. Abs 240Q and TS1/18 were derivitized on 5.5-μm amine beads and coated on protein A/G-coated coverslip. Relative binding sites were measured using fluorescent secondary Abs to 240Q and TS1/18. PMNs were perfused through custom-made microfluidic chambers over above substrates at 4 dynes/cm2 and imaged for calcium flux or fixed and labeled for other protein as previously described (8).
Real-time calcium imaging of PMNs in microchannels
PMNs (human and mouse) were suspended at a concentration of 2 × 106/ml in HEPES-buffered salt solution and labeled with fura 2-AM for 30 min at 37°C. Cells were then washed and resuspended in HEPES-buffered salt solution. Labeled cells were perfused into a microfluidic flow chambers and imaged as previously described (5, 22). Briefly, cells were drawn into the microfluidic channels of diameter 200 μm at a calculated shear stress of 4 dynes/cm2 (i.e., venular magnitude of shear stress) and sequentially imaged over time with alternating excitation at 340 and 380 nM via a mercury arc lamp using an electronic filter wheel with 0.1 s switch time. Images were acquired with an Orca-ER camera (Hamamatsu) coupled to a Nikon 1200 microscope running SimplePCI 5.3 software (Compix). Image sequences were analyzed for the ratio between emission at 340 and 380 nM using custom macros written for Image-Pro Plus 5.1. During analysis, the average intensity of each cell was identified in a confined area of interest around each cell for both the 340 and 380 nM exposure. This method of cell identification and local overlap accounted for moderate motion of rolling PMNs during image acquisition.
Total internal reflection fluorescence in-plane microscopy of integrin, F-actin, calcium, and CRAC expression
Human PMNs were perfused over either ICAM-1–, 240Q-, TS1/18-, or CD45-coated glass coverslips in a microfluidic flow chamber at a calculated shear stress of 4 dynes/cm2, fixed with 4% PFA, permeabilized with 0.1% Triton X-100, and labeled with primary and secondary Abs to specific proteins. PMNs were labeled with 327C-Alexa 488 and TS2/4-Alexa 546 for 20 min before perfusion through flow chamber, washed and fixed, and then labeled with anti-Orai1 primary and secondary Abs following fixation. For real-time calcium imaging, cells were labeled with 1 μM Fluo-5F for 30 min prior to perfusion through flow chambers. Cells were imaged via total internal reflection fluorescence (TIRF) microscopy, which excites fluorophores within a maximal focal depth of ~100 nm from the glass coverslip–cell membrane surface.
Flow cytometric analysis of PMN Ags
Expression of β2 integrin conformation was measured by expression of the high-affinity reporter 327C as previously described (12). High affinity was allosterically induced by exposure of PMNs to mAb 240Q, and low affinity was stabilized by TS1/18 Ab (23). PMNs were subsequently labeled with 10 μg/ml 327C-Alexa 488 in presence of the allosteric Abs for 10 min at 37°C. PMNs were stimulated with 1 μM fMLP for 10 min and were then immediately placed on ice. PMNs were washed twice with PBS and median fluorescence intensity was quantified using CellQuest Pro.
Statistical analyses
Data analysis was performed using GraphPad Prism version 5.0 software (GraphPad Software, San Diego, CA.). Differences between single pairs of conditions were analyzed for significance by a two-tailed unpaired Student t test, and comparisons were deemed significant for two-tailed p values <0.05. Colocalization between LFA-1 and Orai1 was measured using the Pearson coefficient with Costes randomization as well as the intensity correlation quotient in ImageJ. All error bars are means ± SEM based on the number of independent experiments indicated in the figure legends.
Results
High-affinity LFA-1 bound to ICAM-1 initiates outside–in signaling of calcium flux in adherent PMNs
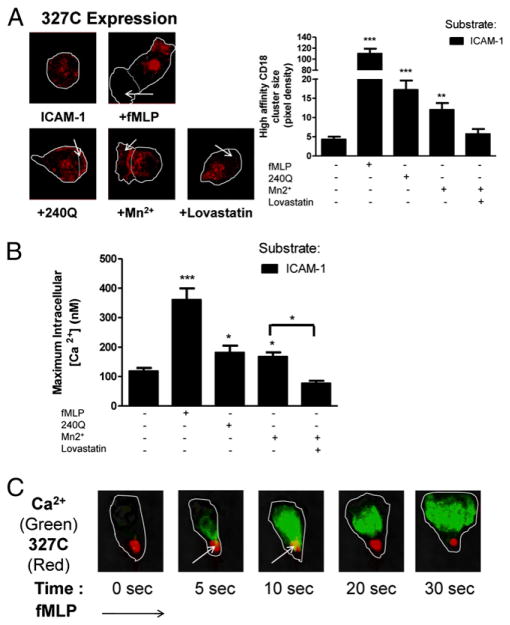
PMNs were infused into microchannels and allowed to settle and adhere on recombinant dimeric ICAM-1 to examine the contribution of soluble modulators of LFA-1 affinity on bond formation and intracellular signaling under shear stress. We first quantified expression and distribution of high-affinity CD18 on arrested PMNs. Immunofluorescence of 327C was imaged using TIRF optics, which excites Ab–fluorophore conjugates located within 100 nm of the plane of adhesive contact (Fig. 1A). PMN stimulation with the GPCR agonist fMLP elicited marked cell polarization accompanied by a 22-fold increase in high-affinity CD18 cluster size above baseline detected on untreated PMNs. Allosteric activation was achieved by infusion of mAb 240Q, or addition of the divalent cation manganese (Mn2+), which directly binds to the I domain of CD18 and stabilizes a high-affinity conformation (15). Allosteric agonists induced comparable upregulation and redistribution of 327C and elicited more modest PMN shape change compared with fMLP stimulation. The specificity of allosteric activation was confirmed by infusion of lovastatin, which stabilizes a low-affinity state in LFA-1 bound to ICAM-1 (15). This diminished 327C clustering and redistribution to baseline levels and PMNs adopted a more spherical morphology.
FIGURE 1.
High-affinity LFA-1 bound to ICAM-1 initiates calcium influx. Human PMNs were exposed to fMLP (1 μM), soluble 240Q (10 μg/ml), and Mn2+ (3 mM) added in bolus to the perfusate before and during infusion to the microchannels. In case of lovastatin, 10 μM lovastatin was added to stabilize LFA-1 at the low-affinity state followed by Mn2+ to allow arrest on substrate. A, PMNs were perfused in micro-channels coated with dimeric ICAM-1 at shear stress of 4 dynes/cm2. High-affinity CD18 was imaged by labeling cells with 327C and imaged with TIRF microscopy. Arrows indicate area of pseudopod formation, and dotted line indicates gelation front of F-actin. B, Intracellular calcium flux was measured by loading PMNs with fura 2-AM. C, PMNs were labeled with 1 μM Fluo-5F (calcium) and 10 μg/ml 327C (anti-CD18) and stimulated with fMLP during arrest on ICAM-1 while immunofluorescence was imaged using TIRF optics (original magnification ×60). Arrows indicates overlap of calcium and high-affinity CD18 signal. To ensure LFA-1–dependent adhesion, PMNs were pretreated with anti–Mac-1 ICRF44 for all experiments. Data shown are means ± SEM from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
We next quantified intracellular signaling following capture and arrest of PMNs in shear flow in the microchannels by measuring the peak in Ca2+ flux under defined conditions of allosteric and GPCR stimulation of high-affinity LFA-1 bond formation. PMNs were infused and allowed to settle on the ICAM-1 under a shear stress of 2 dynes/cm2 and subsequently exposed to a step increase to 4 dynes/cm2 to elicit a rapid increase in calcium flux (Fig. 1B). A baseline level of Ca2+ (~100 nM) was detected in adherent PMNs bound to ICAM-1, which remained spherical in shape. In response to fMLP infusion a 3-fold increase in Ca2+ influx was detected, which correlated with transition to a polarized shape. This level of Ca2+ influx was not different between static and high shear stress conditions. Allosteric activation of CD18 with 240Q or Mn2+ elicited equivalent levels of peak Ca2+ flux (~200 nM) that correlated with pseudopod formation (Fig. 1A, 1B). In contrast to inside–out signaling via GPCR, allosteric activation was significantly increased by shear stress compared with static (133% for Mn2+, 124% for 240Q) and was inhibited to baseline in the presence of lovastatin, suggesting a predominant role of high-affinity LFA-1 bonds in initiating Ca2+ influx and subsequent PMN shape change. Ca2+ influx in the plane of adhesive contact was initiated at sites of clustered high-affinity LFA-1 as detected using real-time two-color TIRF imaging (Fig. 1C, Supplemental Videos 1, 2). Furthermore, these focal clusters of high-affinity CD18 were enhanced by shear stress both for fMLP (85% increase over static) and allosteric activation (396% for Mn2+, 258% for 240Q).
Tension on high-affinity LFA-1 bonds mediates calcium flux
It has been reported that outside–in signaling via integrins accompanies a shift to high affinity as leukocytes or platelets adhere to inflamed endothelium (3, 5). We devised a system in which PMNs were captured on the substrate via a defined affinity state of CD18 or via the non-integrin control receptor CD45. ICAM-1 was replaced with Abs that were linked to the glass substrate of the microfluidic channels at equivalent site density. We first measured the effect of these Abs on CD18 affinity state based on 327C expression by analysis of PMNs in suspension using flow cytometry (Supplemental Fig. 1). Treatment with 240Q or fMLP alone elicited an equivalent 4-fold increase in binding of 327C. The combination of fMLP and 240Q was synergistic in boosting high affinity up to 8-fold, which was decreased in the presence of the allosteric antagonist mAb TS1/18 back down to the baseline level of the IgG isotype control. As an additional control we tested 327C expression in PMNs bound to anti-CD18 mAb IB4, which did not elicit a significant increase in 327C expression above baseline (Supplemental Fig. 1). We next measured Ca2+ flux in PMNs that were infused into the microchannels at low shear (~1 dynes/cm2) and allowed to sediment and capture on the Ab-presenting substrates and subsequently exposed to 4 dynes/cm2 shear stress. Capture of PMNs on 240Q under no shear conditions did not elicit significant calcium flux (~100 nM), whereas a rapid rise in Ca2+ that peaked at ~300 nM was detected under high shear. PMNs captured on substrates presenting TS1/18 (low-affinity CD18), IB4 (common CD18 epitope), or CD45 (control receptor) remained at a baseline level of ~100 nM (Fig. 2A). A further demonstration of the propensity for tension specifically on high-affinity LFA-1 bonds to elicit maximum Ca2+ flux was demonstrated for PMNs sheared on the LFA-1 I domain binding Ab TS1/22 (~180 nM), as compared with ICRF44 (~85 nM), which recognizes the I domain of Mac-1 (Supplemental Fig. 2). In a separate experiment it was confirmed that the increase in allosteric signaling for PMNs anchored via 240Q versus TS1/18 was not a function of tethering CD18 at different site densities. TIRF analysis revealed equivalent levels of LFA-1 fluorescence within the adhesive contact regions of PMNs bound to either mAb (Supplemental Fig. 3A). Moreover, PMNs adhered at equivalent strength under shearm which was also independent of Mac-1, as confirmed by a cell detachment assay with increasing shear stress (Supplemental Fig. 3B). Taken together, these data suggest that tensile forces acting on LFA-1/ICAM-1 bonds is dependent upon an allosteric shift to a high-affinity state that transduces outside–in signaling of Ca2+ flux that superposes with inside–out signaling via GPCR.
FIGURE 2.
Tension on high-affinity LFA-1 regulates calcium flux. A, PMNs labeled with fura 2-AM were perfused over 240Q, TS1/18, IB4, or anti-CD45 Ab substrates at 4 dynes/cm2 or allowed to settle on a 240Q substrate and intracellular calcium flux was measured upon arrest. B, A single site of adhesion was sufficient to induce calcium flux in PMN labeled with fura 2-AM and perfused over 5.5-μm beads coated with 240Q or TS1/18 Ab. Calcium flux was measured upon arrest at t = 0 s. Images are representative of n = 3 separate experiments. C, An average calcium flux of individual cells bound to 240Q- and TS1/18-coated beads is shown (n = 20–30 cells). D, PMNs were perfused at shear stress of 4 dynes/cm2 or allowed to settle under no shear on 5.5-μm beads coated with 240Q or TS1/18 Abs and intracellular calcium flux was measured on binding. Mac-1 was blocked with mAb ICRF44 in all experiments. Data shown are means ± SEM for n = 3 experiments. *p < 0.05, ***p < 0.001.
To determine whether shear force-mediated bond tension at a single site of adhesive contact can initiate Ca2+ flux, PMNs were infused above 5.5-μm glass beads coated with 240Q or TS1/18 at equivalent site density and used as the substrate to capture fura 2-AM–labeled PMNs from the free stream of the microchannel (Fig. 2B, 2C). Following capture on the bead, intracellular Ca2+ ramped up to a maximum within 40 s, before reversing within minutes in the presence of continuous shear. As on the planar substrate, Ca2+ flux was observed for the high-affinity conformation induced by 240Q, which reached twice the level observed for TS1/18 Ab (Fig. 2C, 2D). Also required was the presence of high shear stress that deformed the PMNs on the bead, since under no shear conditions, PMNs were captured on the beads but did not release significant Ca2+ (Fig. 2D). Taken together, these data suggest that high affinity and tensile force on LFA-1 are necessary and sufficient to initiate calcium flux at a local site of adhesive contact.
High-affinity CD18 cooperates with CRAC channels to mediate calcium flux
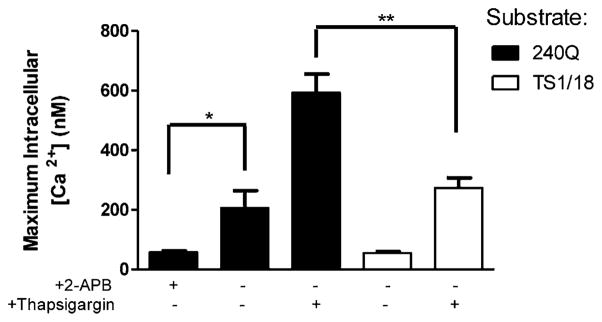
Bacterial peptides such as fMLP produce an elevation of intracellular calcium through the combination of GPCR-signaled PLC-dependent store release and SOCE. We recently reported that SOCE initiated within the plane of adhesive contact cooperates locally with IP3-gated channels downstream of PLC in activation of β2 integrins during the transition from PMNs rolling to arrest and shape polarization (8). We hypothesized that calcium influx initiated by tension on high-affinity LFA-1 bonds occurs primarily through CRAC. PMNs loaded with 1 μM fura 2-AM were infused and captured by either 240Q- or TS1/18-derivatized substrates to stabilize via high- or low-affinity CD18, respectively (Fig. 3A). Blocking SOCE with 2-APB revealed that the 3-fold increase in calcium flux induced by 240Q, as compared with TS1/18, was mediated by CRAC influx. To isolate the function of CD18 and SOCE in initiating Ca2+ influx, PMNs were labeled with fura 2-AM, and then calcium stores were depleted by incubation with 1 μM thapsigargin in Ca2+-free media. PMNs were then sheared over 240Q- or TS1/18-coated microchannels and allowed to capture before infusion of 1.5 mM calcium buffer to initiate CRAC-mediated influx. Extracellular calcium influx was 1-fold greater for PMNs bound to 240Q as compared with TS1/18. These data indicate that influx of Ca2+ through CRAC channels is significantly increased by linkage to high-affinity CD18 under shear flow.
FIGURE 3.
SOCE mediates influx of calcium via high-affinity CD18. A, PMNs were labeled with fura 2-AM and treated with 2-APB or thapsigargin plus EGTA. Cells were then perfused over 240Q or TS1/18 Ab substrate at shear stress of 4 dynes/cm2 and intracellular calcium flux was measured. For thapsigargin plus EGTA-treated PMNs, CaCl2 was perfused at 1 min to induce CRAC opening. Data shown are means ± SEM for n = 3 experiments. *p < 0.05, **p < 0.01.
Calcium influx through SOCE and Orai1 is regulated by high-affinity LFA-1
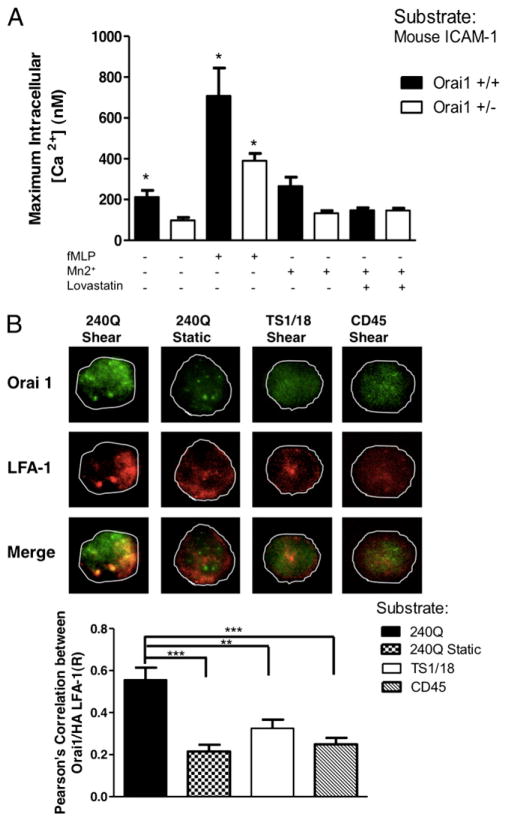
We hypothesized that Orai1 was the predominant CRAC channel mediating calcium influx in PMNs bound under shear via high0-affinity LFA-1. Heterozygous Orai1+/− mice were derived in a B6 strain background by homologous recombination and then back-crossed for six generations with outbred ICR mice to improve the survivability of knockouts (8). Bone marrow-isolated PMNs from wild-type Orai1+/+ and heterozygous Orai1+/− mice were sheared on recombinant ICAM-1 in the microchannels and found to capture at equivalent numbers (data not shown). Calcium flux was detected at a low baseline level of 100 nM in the Orai1+/− and at 200 nM in Orai1+/+ (Fig. 4A). Activation of PMNs by infusion of Mn2+ increased Ca2+ to 300 nM and stimulation with 10 μM fMLP increased the signal to 700 nM in Orai1+/+, which was ~1-fold above that of Orai1+/−. Pretreatment of PMNs with lovastatin before addition of Mn2+ did not further decrease the Ca2+ signal for Orai1+/−, but dropped it to below baseline in Orai1+/+, revealing the cooperativity between high-affinity LFA-1 and sufficient levels of Orai1 for influx. The predominant role of Orai1 in outside–in signaling was corroborated in an identical experiment employing PMNs from homozygous Orai1−/−, completely deficient in expression of Orai1. Ca2+ influx induced with Mn2+ was again absent, whereas stimulation with fMLP was equivalent to that observed for Orai1+/− PMNs (Supplemental Fig. 4). Taken together, these data lead to the conclusion that Orai1 plays a predominant role in initiating intracellular calcium flux in PMNs engaged by high-affinity LFA-1.
FIGURE 4.
Orai1 mediates influx by cooperating with high-affinity LFA-1. A, Bone marrow PMNs were isolated from wild-type Orai1+/+ and heterozygous Orai1+/− mice, labeled with fura 2-AM and were treated with Mn2+, fMLP, and lovastatin. Cells were perfused over an ICAM-1 substrate at shear stress of 4 dynes/cm2 and intracellular calcium flux was measured. B, Freshly isolated human PMNs were perfused over a 240Q, TS1/18, or CD45 Ab substrate at 4 dynes/cm2 shear or made to bind to Ab substrate under static conditions. Cells were fixed at 2 min and labeled for Orai1 and LFA-1 (TS2/4) (original magnification ×60). Mac-1 was inhibited in all experiments using blocking Mac-1 mAb ICRF44. Colocalization of Orai1 and LFA-1 at the contact sites was measured using the Pearson coefficient. Data shown and images are representative of n > 3 experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
To investigate whether force on high-affinity LFA-1 bonds is necessary and sufficient to recruit Orai1, PMNs were allowed to bind to 240Q, TS1/18, or CD45 substrates during shear in the microchannels as compared with static conditions. Following adhesion and Ca2+ flux, PMNs were fixed and labeled for Orai1 and LFA-1. TIRF was used to measure Orai1 and LFA-1 expression in the plane of adhesive contact (Fig. 4B). Spatial colocalization of these two proteins was analyzed using the Pearson coefficient to quantify the level of correlation between the fluorescence Ab reporting on high-affinity LFA-1 and Orai1 using TIRF optics. PMNs sheared on substrates coated with 240Q exhibited ~60% colocalization between LFA-1 and Orai1. In contrast, an ~30% baseline level of overlap was detected for PMNs bound at low affinity to TS1/18, the control anti-CD45, or bound to 240Q under no shear conditions. Data analyzed for the intensity correlation quotient further reinforced the high degree of colocalization of LFA-1 and Orai1 under conditions of PMNs bound to 240Q mAb under shear (intensity correlation quotient values of 0.2, 0.04, 0.12, and 0.06 for 240Q, 240Q static, TS1/18, and CD45, respectively). Thus, we conclude that tension on high-affinity LFA-1 specifically initiates its clustering and redistribution with Orai1.
Orai1 and LFA-1 cooperate in directing Ca2+ influx and cytoskeletal activation
To determine whether Orai1-mediated Ca2+ influx is cooperative with high-affinity LFA-1 in orienting PMN polarization and migration processes, we studied pseudopod formation in PMNs from Orai1+/+ and Orai1+/− mice under shear flow. PMNs were isolated from bone marrow, infused in the microchannels, and observed during capture, arrest, and polarization on a substrate of recombinant mouse E-selectin and ICAM-1. Within seconds of arrest, PMNs from Orai1+/+ mice adopted a polarized shape along a characteristic uropod–pseudopod axis (Fig. 5A). Calcium flux was initiated at the leading edge of pseudopod projection and increased in intensity during polarization. To image precisely where LFA-1 was expressed in comparison with F-actin formation, PMNs were stimulated with fMLP and then fixed with 2% paraformaldehyde and labeled for expression over the time course of pseudopod projection. TIRF optics revealed that LFA-1 was clustered at two distinct sites along the major uropod–pseudopod axis in PMNs from Orai1+/+ mice (Fig. 5B). Pseudopod formation at the leading edge followed a wave of Ca2+ that was initiated where LFA-1 bonds first coalesced (Fig. 5A, 5B). In contrast, the extent and distribution of LFA-1 was more diffuse in Orai1−/+ PMNs and this correlated with a 1-fold lower level of Ca2+ flux and total F-actin formation that was less organized in Orai1-deficient PMNs (Fig. 5A, 5B).
FIGURE 5.
Orai1 CRAC is required for directional PMN migration and F-actin formation. A, Orai1+/+ and Orai1+/− PMNs were labeled with 1 μM Fluo-5F, and local calcium flux was measured in real time on binding to ICAM-1/E-selectin–coated substrate. Arrows indicate locations of calcium flux initiation. B, Orai1+/+ and Orai1+/− mouse PMNs treated with 1 μM fMLP were perfused over ICAM-1/E-selectin substrate, fixed, and labeled for LFA-1 and F-actin distribution using Alexa Fluor 488-phalloidin (original magnification ×60). Arrows indicate location of high-density LFA-1 clusters and F-actin localization. Images are representative of n = 3 experiments. C, Orai1+/+ and Orai1+/− PMNs treated with 1 μM fMLP were perfused over ICAM-1/E-selectin substrate at shear stress of 2 dynes/cm2 and cell migration was tracked for 10 min. Data shown as deviation to flow and are representative of n = 3 experiments. *p < 0.05, ***p < 0.001 when comparing Orai1+/+ and Orai1+/−, respectively. MFI, mean fluorescence intensity.
Observation of the migration trajectory with respect to the direction of shear flow revealed that following arrest on E-selectin and ICAM-1 and in response to fMLP infusion, PMNs from Orai1+/+ mice tended to migrate in a perpendicular direction to shear flow as compared with Orai1+/− (Fig. 5C). Although there was only a small difference in total distance migrated, persistence of migration within 45° perpendicular to the direction of shear flow was 2-fold higher for Orai1+/+ as compared with Orai1+/− PMN. This impaired migratory behavior in Orai1+/− cells suggests a defect in integrin-mediated signaling following fMLP activation, as there was no difference observed in number of cells arrested or in the migration velocity between Orai1+/+ and Orai1+/− PMNs (~0.47 and 0.43 μm/s, respectively; p > 0.05). A more severe defect was observed for Orai1−/− knockout PMNs that failed to migrate effectively, as ~80% of cells remained stationary even in the presence of fMLP stimulation (data not shown). These data suggest that fluid shear stress transmitted to LFA-1 bond clusters at sites of adhesion cooperate with Orai1 to effectively localize Ca2+ influx and orient the direction of F-actin polymerization, pseudopod projection, and contact-guided migration perpendicular to flow direction.
Neutrophil mechanotaxis in response to direction of shear flow
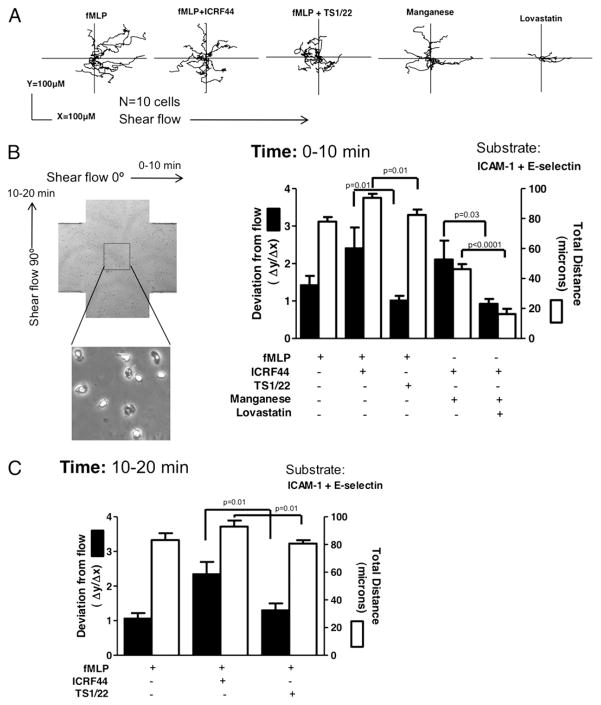
We have previously observed that PMNs crawling along an inflamed venule tend to migrate in a direction perpendicular to direction of blood flow until they reach an endothelial junction where they shift direction or transmigrate (7). We assessed whether CRAC and LFA-1 cooperate in dynamically orienting PMN migration relative to the direction of shear stress. A customized microfluidic cross-channel was fashioned with two entry and exit ports such that the direction of flow could be shifted by 90° within seconds. PMNs were again infused over ICAM-1– and E-selectin–coated substrates and activated with fMLP for 10 min before the flow was switched orthogonally for an additional 10 min. A record of PMN trajectory reveals principally downstream migration at a 45° angle to the flow, with little deviation in the Δy/Δx persistence coefficient observed over ~80 μm migration (Fig. 6A). Blocking Mac-1 with ICRF44 revealed that LFA-1–dependent migration tended to be significantly more perpendicular to the direction of shear flow. This bias was abolished in the presence of function blocking anti–LFA-1 Ab TS1/22. PMNs were allosterically activated with Mn2+ to shift LFA-1 to high affinity, and we observed a similar trend in that PMNs migrated in a more perpendicular direction to shear flow. Treatment with lovastatin abolished this bias and confirmed that this function was dependent on high-affinity LFA-1/ICAM-1 bonds (Fig. 6B). A rapid 90° shift in the direction of shear flow was achieved within seconds in the cross-channel and PMNs were tracked for an additional 10 min. Migration velocity and total distance were comparable to the initial 10 min migration, and PMNs rapidly sensed the shift in direction of shear flow and again tended to migrate perpendicular in a manner dependent on LFA-1 (Fig. 6C). This provides evidence that allosteric activation of LFA-1 provides a distinct outside–in signal that is dependent on the direction of shear flow, thereby providing contact-mediated mechanotaxis of PMNs (Fig. 7).
FIGURE 6.
LFA-1 bonds bias and orient PMN migration perpendicular to direction of shear. Human PMNs were treated with Mn2+, soluble fMLP, and lovastatin; inhibited with Mac-1 or LFA-1 blocking Abs; and perfused over an ICAM-1/E-selectin substrate at shear stress of 2 dynes/cm2 in a custom-made microfluidic cross-channel. A, Cell migration was tracked for 10 min with respect to direction of shear flow. B, Deviation to shear flow under the above conditions is shown from video micrographs taken with a 20× objective magnification. C, Direction of shear flow was shifted by 90° through the cross-channel, and the same human PMNs treated with soluble fMLP while blocking either Mac-1 or LFA-1 were tracked for another 10 min. Data shown are from n = 3 experiments.
FIGURE 7.
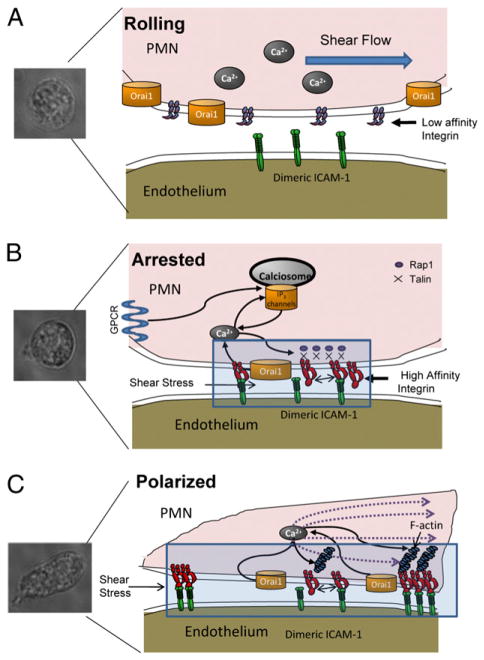
Schematic of signaling events governing PMN emigration. A, During PMN capture and rolling on inflamed endothelium, β2 integrins are distributed at low affinity and randomly throughout the membrane and a low baseline level of intracellular calcium is maintained. Neutrophil shapes were imaged for each state from video micrographs at 40× objective magnification. B, Transition to arrest is mediated through GPCR signaling that upshifts LFA-1 to a high-affinity state and binding of integrins with ICAM-1 on inflamed endothelium. As LFA-1/ICAM-1 bonds take up tensile forces they colocalize with Orai1 and facilitate cooperation with IP3-mediated calciosomes to activate calcium flux, which in turn catalyzes recruitment of Rap-1 GTPases and cytoskeletal elements such as Talin to LFA-1 cytodomains to direct pseudopod projection. C, Shape polarization is guided locally by the dynamic redistribution of LFA-1/ICAM-1 clusters, Orai1, and assembly of the F-actin cytoskeleton, which drives directional migration in a manner dependent on direction of shear stress.
Discussion
We document that shear stress acting on adherent PMNs transmits tensile forces to LFA-1/ICAM-1 adhesive bonds that function to dynamically orient PMNs in a direction perpendicular to the velocity vector of fluid flow. Application of TIRF optics and vascular mimetic microchannels allowed us to capture real-time image sequences of PMNs transitioning from a passive spherical to activated polarized state. We reported that: 1) a specific polar distribution of high-affinity LFA-1 clusters was associated with sites of Ca2+ influx; 2) the rise in cytosolic Ca2+ was initiated within the plane of adhesion and was synergistic with G protein-coupled activation and CRAC opening, provided that high-affinity clusters of LFA-1 were under tensile force; 3) this Ca2+ influx occurs primarily through Orai1, which colocalizes and promotes focal clustering of LFA-1 and F-actin formation at the leading edge of polarized PMNs; and 4) pseudopod projection correlated with the dynamic redistribution of high-affinity LFA-1 clusters and cytosolic Ca2+ that function to bias cell migration in a direction perpendicular to the flow vector. These data point to the potential significance of integrin mechanotransduction in providing spatial cues that guide PMN migration in response to vascular inflammation.
Allosteric control of LFA-1 and PMN cytoskeletal activity
A high-affinity extended conformation of LFA-1 initiates and sustains PMN arrest and cytoskeletal activation since abrogating this affinity state results in less stable PMN arrest and shape polarization under shear flow (4, 24). PMN activation in response to ligation of chemokine or chemotactic peptide to their cognate GPCRs elicits both an upshift in integrin affinity and redistribution of high-density CD18 clusters to the uropod and the base of nascent pseudopods, as detected by the high-affinity reporter mAb 327C. We observed a similar upshift in affinity in response to infusion of Mn2+; however, this mode of activation promoted comparatively smaller and more diffuse clusters of CD18. Inside–out signaling via fMLP-stimulated GPCR elicited ~6-fold more clustering of high-affinity CD18 and ~1.5-fold higher calcium signal than outside–in allosteric activation with mAb 240Q or Mn2+. It is also noteworthy that simultaneous activation via fMLP and 240Q was synergistic in upregulating high-affinity CD18. These data reveal a direct correlation between high-affinity LFA-1 clustering, the rise in cytosolic Ca2+, and the extent of PMN shape change. Replacing ICAM-1 with a substrate of allosteric Ab, we demonstrated that merely applying stress to PMNs captured on microspheres via LFA-1 was not sufficient to signal a calcium flux. This suggests that tensile force itself does not facilitate LFA-1 extension to a high-affinity state, nor opening of Ca2+ channels in PMNs. Rather, the transmission of force across the membrane via an extended LFA-1 heterodimer is required to initiate local calcium influx and cytoskeletal activation. There is documentation that amino acid residues on the cytoplasmic domain of CD11a are sites of recruitment of phosphokinase and adaptor proteins that catalyze a signaling cascade following bond formation (17). It has also been demonstrated in lymphocytes that shear force acting on high-affinity LFA-1 in the presence of endothelial chemokines can trigger adhesive and invasive filopodia to promote migration (25). However, our data are the first, to our knowledge, to link tensile force on LFA-1 to cytoskeletal activation and calcium-dependent pseudopod formation (3). A key question that remains is the identification of the force-sensing molecule that associates with the cytodomain of extended LFA-1 and subsequently translates the mechanical signal to Orai1 to facilitate channel opening.
Regulation of local calcium flux and cytoskeletal activation
Data suggest that the vast majority of PMNs traveling in the circulation maintain LFA-1 in a low-affinity conformation and low levels of cytosolic calcium (i.e., <100 nM) (5, 10, 24) (Fig. 7A). We propose that an early event in the multistep process of PMN recruitment is application of tensile force to microclusters of high-affinity LFA-1 as they bind to ICAM-1 upregulated by endothelium during inflammation. This facilitates colocalization of LFA-1 and Orai1 and a local rise in cytosolic Ca2+, which in turn recruits proximal high-affinity LFA-1 to bind ICAM-1 (Fig. 7B). We observed that the SOCE channel blocker 2-APB abrogated Ca2+ influx for PMNs bound via high-affinity LFA-1. Furthermore, depletion of internal stores of calcium by treatment with thapsigargin revealed that the predominant source of the rapid rise in the calcium signal was via entry through CRAC and specifically Orai1. Two-color fluorescence imaging of Abs to LFA-1 and Orai1 revealed a close physical proximity between these receptors that increased in response to activation of LFA-1 and application of shear stress. It was recently shown that SCID patients with the Orai1 defective gene suffer from severe immunodeficiencies and that Orai1-deficient mice display impaired cytokine production, cell proliferation, integrin activation, and immune cell recruitment to inflammatory sites (18, 26). In this study, we show that PMNs isolated from Orai1+/− or Orai1−/− mice exhibited one-half the level of calcium influx upon stimulation with fMLP, suggesting that Orai1 cooperates with other transient receptor potential channels in mediating calcium flux in response to GPCR stimulation (i.e., TRPC1, 3, 4, and 6) (27–29). A more complete defect in Ca2+ influx for PMNs from Orai1+/− or Orai1−/− was noted in response to allosteric activation via Mn2+. This suggests that Orai1 is unique among CRAC by functioning more specifically in LFA-1–mediated outside–in signaling.
A model for mechanorheotaxis regulated by integrin activation and local calcium influx
Following PMN arrest, focal clusters of Orai1 and LFA-1 under membrane stress mediate influx of calcium, which in turn catalyzes the association of CalDAG-GEFI and Rap-1 to the cytodomain of LFA-1 (Fig. 7B). GPCR inside–out signaling is known to activate diacylglycerol and this is contemporaneous with integrin engagement and a rapid increase in calcium upon PMN arrest. Integrin binding may enhance store release as Orai1 cooperates with IP3-gated channels on calciosomes to refill depleted stores and produce the robust calcium release that is observed during both fMLP stimulation and engagement of LFA-1. We have recently reported that this process facilitates synchronization of the transition from PMNs rolling to arrest and cell polarization (8). Talin is an essential component of focal adhesions that may function to couple the cytodomains of LFA-1 to F-actin and provide a scaffold for additional signaling proteins such as Src kinase (15). Supporting the importance of this macromolecular complex in adhesion strengthening and migration is the report of defective Rap1 activity in leukocyte adhesion deficiency-III patients who lack the Rap-1 activator CalDAG-GEFI and possess impaired Kindlin-3 expression. Leukocyte adhesion deficiency-III patients present clinically with severe defects in leukocyte and platelet integrin activation and are thus immunocompromised (30, 31).
Previous studies indicate that shear forces together with a chemokine gradient conspire to produce a “biased random walk” of neutrophils as they navigate their migratory route to the proximal endothelial cell junction (32, 33). A conceptual model for how biological and mechanical events direct pseudopod projection is depicted in Fig. 7C. Shear stress acts over the cell body and repulsive forces are opposed by tension taken up by CD18/ICAM-1 adhesive bonds. A defect in the capacity to build up sufficient cytoskeletal associations and anchorage to integrin sites can result in impaired PMN migration under shear as observed in Vav GEF-deficient mouse PMNs (7). As in our model, PMNs from Vav-deficient mice arrest are unable to migrate in a perpendicular orientation to the direction of shear flow even though they migrate at comparable velocity at high shear flow. Also supporting a role for force acting on the cytodomain of LFA-1 in orienting the cytoskeleton for optimum leukocyte function is the report that F-actin bundling and polymerization are required for LFA-1 adhesion strengthening in T cells and PMNs (15, 34). Orai1 CRAC together with STIM1 have been implicated in the assembly and disassembly of focal adhesions in tumor cells where they promote tumor cell migration (35), suggesting a major role for Orai1 in the integrin-mediated signaling to facilitate migration. Orai1 and STIM1 are also dynamically recruited to the immunological synapse and up-regulate calcium influx upon Ag exposure, and depletion of Orai1 abrogates this response (36). We found that Orai1-defective PMNs are unable to induce LFA-1–mediated outside–in calcium flux, recruit F-actin in a polarized manner, and migrate in a perpendicular-biased direction to shear flow. Orai1+/− PMNs retained the capacity to migrate but were biased in the direction of shear flow in a manner similar to that observed for Vav-1−/− PMNs (7). The amount of calcium fluxed locally at the contact site was dependent on the number and distribution of stressed LFA-1/Orai1 complexes, which also correlated with the extent of F-actin formation. The precise mechanism of how this migratory process is fine-tuned through interplay between these signaling molecules and controlled in a dynamic cyclic manner with each pseudopod projection is an active area of research. Future studies will be focused on identifying the molecules that directly link force-mediated changes in the cytodomain of LFA-1 to calcium influx via Orai1 and thereby facilitate assembly of a macromolecular complex required for localized cytoskeletal force generation.
In summary, the process of high-affinity bond formation between LFA-1 and ICAM-1 serves to catalyze downstream signaling events that regulate PMN adhesion strengthening, cytoskeletal activation, and subsequent directional migration relative to the direction of shear flow. Orai1 is the predominant CRAC channel regulating the local intracellular calcium concentration that in turn catalyzes the association of adaptor GTPase molecules and cytoskeletal proteins to the cytodomain of LFA-1. We propose that this process may be a key adaptive mechanism for directing pseudopod projection and migratory traction that supports the homing of PMNs proximal to the site of vascular inflammation.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant A1472294 (to S.I.S.).
We are grateful to Dr. Anjana Rao for supplying the Orai1+/− mice and to Dr. Donald Staunton of CisThera, Inc. for the development of Abs.
Abbreviations used in this article
- 2-ABP
2-aminoethoxydiphenyl borate
- CRAC
calcium release-activated channel
- GPCR
G protein-coupled receptor
- IP3
inositol 1,4,5-triphosphate
- PLC
phospholipase C
- PMN
polymorphonuclear leukocyte
- SOCE
store-operated calcium entry
- TIRF
total internal reflection fluorescence
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Evans E, Leung A, Hammer D, Simon S. Chemically distinct transition states govern rapid dissociation of single L-selectin bonds under force. Proc Natl Acad Sci USA. 2001;98:3784–3789. doi: 10.1073/pnas.061324998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates β2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164:4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- 3.Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26:17–27. doi: 10.1016/j.immuni.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Green CE, Schaff UY, Sarantos MR, Lum AF, Staunton DE, Simon SI. Dynamic shifts in LFA-1 affinity regulate neutrophil rolling, arrest, and transmigration on inflamed endothelium. Blood. 2006;107:2101–2111. doi: 10.1182/blood-2005-06-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaff UY, Yamayoshi I, Tse T, Griffin D, Kibathi L, Simon SI. Calcium flux in neutrophils synchronizes β2 integrin adhesive and signaling events that guide inflammatory recruitment. Ann Biomed Eng. 2008;36:632–646. doi: 10.1007/s10439-008-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber TH, Shinder V, Cain DW, Alon R, Sackstein R. Shear flow-dependent integration of apical and subendothelial chemokines in T-cell transmigration: implications for locomotion and the multistep paradigm. Blood. 2007;109:1381–1386. doi: 10.1182/blood-2006-07-032995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillipson M, Heit B, Parsons SA, Petri B, Mullaly SC, Colarusso P, Gower RM, Neely G, Simon SI, Kubes P. Vav1 is essential for mechanotactic crawling and migration of neutrophils out of the inflamed microvasculature. J Immunol. 2009;182:6870–6878. doi: 10.4049/jimmunol.0803414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaff UY, Dixit N, Procyk E, Yamayoshi I, Tse T, Simon SI. Orai1 regulates intracellular calcium, arrest, and shape polarization during neutrophil recruitment in shear flow. Blood. 2010;115:657–666. doi: 10.1182/blood-2009-05-224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjaastad MD, Nelson WJ. Integrin-mediated calcium signaling and regulation of cell adhesion by intracellular calcium. Bioessays. 1997;19:47–55. doi: 10.1002/bies.950190109. [DOI] [PubMed] [Google Scholar]
- 10.Krause KH, Campbell KP, Welsh MJ, Lew DP. The calcium signal and neutrophil activation. Clin Biochem. 1990;23:159–166. doi: 10.1016/0009-9120(90)80030-m. [DOI] [PubMed] [Google Scholar]
- 11.Shen J, Luscinskas FW, Connolly A, Dewey CF, Jr, Gimbrone MA., Jr Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am J Physiol. 1992;262:C384–C390. doi: 10.1152/ajpcell.1992.262.2.C384. [DOI] [PubMed] [Google Scholar]
- 12.Sarantos MR, Raychaudhuri S, Lum AF, Staunton DE, Simon SI. Leukocyte function-associated antigen 1-mediated adhesion stability is dynamically regulated through affinity and valency during bond formation with intercellular adhesion molecule-1. J Biol Chem. 2005;280:28290–28298. doi: 10.1074/jbc.M501662200. [DOI] [PubMed] [Google Scholar]
- 13.Jun CD, Shimaoka M, Carman CV, Takagi J, Springer TA. Dimerization and the effectiveness of ICAM-1 in mediating LFA-1-dependent adhesion. Proc Natl Acad Sci USA. 2001;98:6830–6835. doi: 10.1073/pnas.121186998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller J, Knorr R, Ferrone M, Houdei R, Carron CP, Dustin ML. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J Exp Med. 1995;182:1231–1241. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarantos MR, Zhang H, Schaff UY, Dixit N, Hayenga HN, Lowell CA, Simon SI. Transmigration of neutrophils across inflamed endothelium is signaled through LFA-1 and Src family kinase. J Immunol. 2008;181:8660–8669. doi: 10.4049/jimmunol.181.12.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gakidis MA, Cullere X, Olson T, Wilsbacher JL, Zhang B, Moores SL, Ley K, Swat W, Mayadas T, Brugge JS. Vav GEFs are required for β2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166:273–282. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J Cell Sci. 2009;122:179–186. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 19.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchon LN, White JM. Growth and analysis of octadecylsiloxane monolayers on Al2O3 (0001) Langmuir. 2006;22:6549–6554. doi: 10.1021/la0600189. [DOI] [PubMed] [Google Scholar]
- 22.Schaff UY, Xing MM, Lin KK, Pan N, Jeon NL, Simon SI. Vascular mimetics based on microfluidics for imaging the leukocyte: endothelial inflammatory response. Lab Chip. 2007;7:448–456. doi: 10.1039/b617915k. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Lu C, Springer TA. Folding of the conserved domain but not of flanking regions in the integrin β2 subunit requires association with the α subunit. Proc Natl Acad Sci USA. 1997;94:3156–3161. doi: 10.1073/pnas.94.7.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lum AF, Green CE, Lee GR, Staunton DE, Simon SI. Dynamic regulation of LFA-1 activation and neutrophil arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow. J Biol Chem. 2002;277:20660–20670. doi: 10.1074/jbc.M202223200. [DOI] [PubMed] [Google Scholar]
- 25.Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, Montresor A, Bolomini-Vittori M, Feigelson SW, Kirchhausen T, et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30:384–396. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bréchard S, Melchior C, Plançon S, Schenten V, Tschirhart EJ. Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium. 2008;44:492–506. doi: 10.1016/j.ceca.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 28.McMeekin SR, Dransfield I, Rossi AG, Haslett C, Walker TR. E-selectin permits communication between PAF receptors and TRPC channels in human neutrophils. Blood. 2006;107:4938–4945. doi: 10.1182/blood-2005-09-3803. [DOI] [PubMed] [Google Scholar]
- 29.Heiner I, Eisfeld J, Halaszovich CR, Wehage E, Jüngling E, Zitt C, Lückhoff A. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J. 2003;371:1045–1053. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasvolsky R, Feigelson SW, Kilic SS, Simon AJ, Tal-Lapidot G, Grabovsky V, Crittenden JR, Amariglio N, Safran M, Graybiel AM, et al. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204:1571–1582. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mory A, Feigelson SW, Yarali N, Kilic SS, Bayhan GI, Gershoni-Baruch R, Etzioni A, Alon R. Kindlin-3: a new gene involved in the pathogenesis of LAD-III. Blood. 2008;112:2591. doi: 10.1182/blood-2008-06-163162. [DOI] [PubMed] [Google Scholar]
- 32.Kitayama J, Hidemura A, Saito H, Nagawa H. Shear stress affects migration behavior of polymorphonuclear cells arrested on endothelium. Cell Immunol. 2000;203:39–46. doi: 10.1006/cimm.2000.1671. [DOI] [PubMed] [Google Scholar]
- 33.Ambravaneswaran V, I, Wong Y, Aranyosi AJ, Toner M, Irimia D. Directional decisions during neutrophil chemotaxis inside bifurcating channels. Integr Biol (Camb) 2010;2:639–647. doi: 10.1039/c0ib00011f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter JC, Bracke M, Smith A, Davies D, Hogg N. Signaling through integrin LFA-1 leads to filamentous actin polymerization and remodeling, resulting in enhanced T cell adhesion. J Immunol. 2002;168:6330–6335. doi: 10.4049/jimmunol.168.12.6330. [DOI] [PubMed] [Google Scholar]
- 35.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci USA. 2008;105:2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.