Abstract
A previously healthy 9-year-old girl presented to an emergency department (ED) with headache, dizziness, blurry vision, and abnormal visual perceptions. She was diagnosed with migraine, treated symptomatically, and discharged. Over the course of days, she became progressively somnolent, and returned to the ED, where she was found to have a right inferior quadrantanopsia and sixth nerve palsy. Magnetic resonance imaging (MRI) of the brain showed gyral swelling of the left parieto-occipital lobe. Continuous electroencephalogram (EEG) monitoring revealed focal non-convulsive status epilepticus (NCSE) in the left occipital region. Cerebrospinal fluid (CSF) was positive for antibodies directed against the N-methyl-d-aspartate receptor (NMDAR). This case is the first report of anti-NMDAR encephalitis presenting with focal non-convulsive status epilepticus (NCSE).
Keywords: status epilepticus, encephalitis, encephalitis, NMDA, non-convulsive status epilepticus
Introduction
Encephalitis mediated by antibodies against the NMDA receptor (anti-NMDAR encephalitis) is an increasingly described clinical entity in children and adults [4–7]. This syndrome typically includes psychiatric symptoms, cognitive impairment, language dysfunction, abnormal movements, seizures, autonomic dysfunction, and coma. In the pediatric population, behavioral changes, seizures, movement disorders, and autonomic instability are prominent [5, 7]. Seizures are present in approximately three-fourths of all patients [3, 5, 7], with a few reports of status epilepticus (SE) in adults, including 2 with generalized NCSE [9, 10]. Here we report the case of a 9-year-old female with a presentation of anti-NMDAR encephalitis that included focal NCSE.
Case Report
A previously healthy 9-year-old female presented to the ED with 2 days of headache, dizziness, blurry vision, and occasional perception of “flashing lights.” Her mother noted that she was “bumping into walls”. The patient had a family history significant only for migraine headache. On exam, she had a right inferior quadrantanopsia and a slight right pronator drift. Serological testing (including complete blood count, basic metabolic panel, and liver function tests), and a computed tomography (CT) scan of the head were normal. MRI of the brain showed elevated arterial spin labeled (ASL) perfusion in the left occipital lobe consistent with possible migraine [13], but was otherwise normal. Her headache improved with intravenous fluids, ketorolac, and metoclopramide, but her neurological deficits persisted; she was diagnosed with complicated migraine and was discharged home with a plan for short-interval outpatient follow-up.
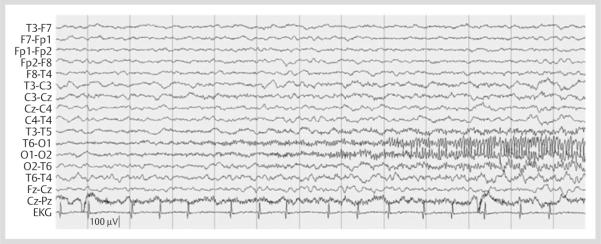
The patient returned to the ED 3 days later with continued headache accompanied by nausea and emesis. This evolved to somnolence over the next several hours. She was minimally arousable, non-verbal, with a right hemianopsia, partial right sixth nerve palsy, and increased tone in her right arm. The patient was admitted to the pediatric intensive care unit. Continuous EEG monitoring demonstrated nearly continuous seizures consisting of 12–13 Hertz (Hz), sharply contoured activity emanating from the left occipito-temporal region during stage II sleep (Fig. 1). The electrographic seizure location was consistent with the patient's right hemianopsia and location of prior MRI abnormalities. However, there were no associated abnormal movements apparent on physical examination or on video review. The patient was on no sedative or paralytic agents during this recording. NCSE was refractory to lorazepam 2 mg, 2 successive doses of levetiracetam 20 mg/kg (used off-label with parental consent), and an initial dose of fosphenytoin 20 mg/kg. An additional 10 mg/kg of fosphenytoin was administered (to a serum drug level of 28.4 μg/mL) which was followed by cessation of NCSE. Isolated electrographic seizures with similar electrographic appearance continued during wakefulness and sleep for an additional 24 h; however, SE did not recur. Focal polymorphic delta activity continued in the left posterior quadrant between seizures. The patient was then loaded with phenobarbital 20 mg/kg (to a serum drug level of 31.3 μg/mL), with ultimate cessation of seizures.
Fig. 1.
Left occipito-temporal seizure on continuous EEG monitoring.
Initial CSF studies revealed a mild pleocytosis with 7 white blood cells per high-powered field (WBCs per hpf; 97 % lymphocytes). CSF was negative for the following: herpes simplex virus, Lyme, parechovirus, enterovirus, and Epstein-Barr virus; CSF bacterial culture was negative. Repeat MRI showed interval development of gyral swelling (Fig. 2), now with hypoperfusion of the medial left parieto-occipital lobe. Mental status improved significantly following cessation of seizures. The patient was discharged on home 10 days after admission with continued moderate expressive aphasia and right hand dystonia, on oxcarbazepine for seizure prophylaxis and clonazepam for dystonia. The presumptive diagnosis of focal NCSE of unclear etiology with a prolonged post-ictal state was made.
Fig. 2.
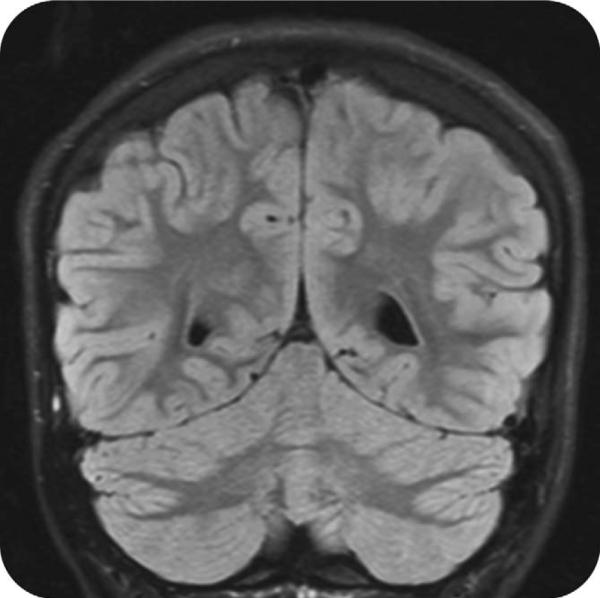
Coronal view of the brain obtained via T2 fluid-attenuated inversion recovery (FLAIR) sequence showing gyral swelling of the left medial parieto-occipital lobe.
The patient returned 3 days later with pain in her right arm and leg, weakness, and deteriorating mental status. Examination revealed expressive aphasia, right homonymous hemianopsia, a central right facial droop, mild right hemiparesis, right-sided (face, arm, and leg) dystonia and choreathetoid movements. EEG showed symmetric 2–4 Hz background slowing with poor organization, intermittent 3–4 Hz sharp-and-slow waves in the left occipital region, and no seizures. Repeat MRI of the brain showed interval resolution of the cortical swelling. CSF analysis was again unremarkable, with a WBC count of 1 per hpf, glucose of 79 mg/dL, and protein of < 10 mg/dL.
Anti-NMDAR encephalitis was suspected due to the combination of seizures, abnormal behavior/cognition, language dysfunction, and dyskinesias. 3 weeks after initial presentation, CSF sent during the patient's first admission tested strongly positive for anti-NMDAR antibodies. The presence of anti-NMDAR antibodies was assessed as previously described [3]. Briefly, anti-NMDA-R antibody reactivity was determined using patient CSF incubated with heterologously-expressed NMDA receptor NR1-NR2B heteromers in HEK293 cells, and assessed semi-quantitatively. CSF-specific oligoclonal bands were present, suggesting intrathecal production. Genetic testing for familial hemiplegic migraine, including for the voltage-gated calcium channel alpha-1A subunit (CACNA1A), adenosine triphosphate (ATP)-ase alpha-2 polypeptide (ATP1A2), and voltage-gated sodium channel type 1 alpha subunit (SCN1A) gene mutations sent early in the course of the illness was normal. The co-occurrence of anti-voltage gated potassium channel (VGKC) complex antibodies including leucine-rich glioma inactivated 1 protein (Lgi1) and contactin-associated protein-2 (Caspr2) was not assessed (see Discussion).
After the diagnosis of anti-NMDAR encephalitis was established, the patient was immediately re-admitted. The patient was initially treated with intravenous immunoglobulin (IVIg) and high-dose methylprednisolone. CT scans of the chest, abdomen, and pelvis, to screen for underlying tumor, were normal.
Repeat lumbar puncture performed during clinical remission 2 months after initial presentation showed that the patient's CSF remained weakly positive for anti-NMDAR antibodies. The patient experienced a relapse 1 month later, and received an additional course of IVIg and 2 courses of rituximab. 1 year after initial presentation, the patient had normal mental status, full visual fields, absence of dystonia, and residual but improving language impairment. Seizures have not recurred. A repeat routine EEG performed during her second remission remained diffusely slow but was without epileptiform discharges, and oxcarbazepine was discontinued after 6 months of seizure freedom.
Discussion
In children, anti-NMDAR encephalitis often presents with behavioral change, language dysfunction, seizures of various types, and dystonia/choreathetoid movements, as occurred in our patient. The likelihood of underlying tumor in anti-NMDAR encephalitis is low in children relative to adults and is approximately 10 % in children aged 7–12, but increases to 40 % in children aged 13–18 years [4]. No ovarian teratoma has been found in our patient, although she continues to undergo periodic screening screening.
EEG tracings in anti-NMDAR encephalitis are typically diffusely slow and disorganized, and seizures (of various types) are common. SE in the setting of anti-NMDAR encephalitis is unusual but described, and 2 patients with anti-NMDAR encephalitis and refractory SE have died [4]. Non-convulsive seizures and NCSE describe electrographic seizures with either no clinical change or a subtle clinical change [8] and are common in critically ill children with acute encephalopathy [1]. There are 2 reported cases in the literature of NCSE in adults with anti-NMDAR encephalitis, including a 25-year-old with refractory generalized NCSE and underlying ovarian teratoma [9] and a 19-year-old with generalized NCSE and mediastinal teratoma [10]. This case is unique in that the patient presented with focal NCSE, which was accompanied by focal signs (right hemianopsia) and focal swelling and arterial spin labeled (ASL) perfusion abnormalities on MRI that was likely due to ongoing seizures.
Limbic encephalitis mediated by anti-voltage gated potassium channel (VGKC) antibodies presents with encephalopathy, seizures, and cognitive and behavioral alterations [11, 14], similar to anti-NMDAR encephalitis. We did not test for anti-VGKC complex antibodies. However, encephalitis mediated by anti-VGKC complex antibodies is thought to be unusual in children; furthermore, in the case series by Suleiman et al. [14], children with anti-VGKC complex antibody-mediated limbic encephalitis presented with convulsive SE, and most had fever; seizure onset was either generalized or focal temporal. The patients described in Suleiman et al. [14] all experienced poor outcomes, although none of the patients received immunotherapy during the acute phase of the illness. While it is extremely unlikely that our patient had anti-NMDAR and anti-VGKC complex antibodies, this co-occurrence has been described in children [2] and adults [12].
This case reports a unique finding in anti-NMDAR encephalitis and further illustrates the need to consider the diagnosis of anti-NMDAR encephalitis in children and adults with unexplained SE in the appropriate clinical context. Furthermore, EEG monitoring may be useful in cases of suspected anti-NMDAR encephalitis even in the absence of overt seizures, given the possibility of NCSE.
Acknowledgements
We thank Josep Dalmau, Nicole Ryan, Hisham Dahmoush, and David Bearden, for valuable discussions. Dr. Goldberg receives research support from the Epilepsy Foundation. Dr. Kessler and Dr. Abend receive research support from the NINDS Neurological Sciences Academic Development Award (NSADA) K12 NS049453.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose. All co-authors have been substantially involved in the study and preparation of the manuscript; all authors have read and approved the submitted manuscript. Information and images herein are presented with appropriate consent obtained and with details removed that might potentially reveal the identity of the patient.
References
- 1.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Non-convulsive seizures are common in critically ill children. Neurology. 2011;76:1071–1077. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dale RC, Irani SR, Birlot F, et al. N -Methyl- d -aspartate receptor antibodies in pediatric dyskinetic encephalitis lethargica. Ann Neurol. 2009;66:704–709. doi: 10.1002/ana.21807. [DOI] [PubMed] [Google Scholar]
- 3.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J, Lancaster E, Martinez-Hernandez M, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–71. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florance NR, Davis RL, Lam C, et al. Anti- N -methyl- d -aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Florance-Ryan NR, Dalmau J. Update on anti- N -methyl- d -aspartate receptor encephalitis in children and adolescents. Curr Opin Pediatr. 2010;22:739–44. doi: 10.1097/MOP.0b013e3283402d2f. [DOI] [PubMed] [Google Scholar]
- 7.Irani SR, Bera K, Waters P, et al. N -Methyl- d -aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jirsch J, Hirsch LJ. Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophys. 2007;118:1660–1670. doi: 10.1016/j.clinph.2006.11.312. [DOI] [PubMed] [Google Scholar]
- 9.Johnson N, Henry C, Fessler AJ, et al. Anti-NMDA receptor encephalitis causing prolonged nonconvulsive status epilepticus. Neurology. 2010;75:1480–1482. doi: 10.1212/WNL.0b013e3181f8831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirpatrick MP, Clarke CD, Sonmezturk HH, et al. Rhythmic delta activity represents a form of nonconvulsive status epilepticus in anti-NMDA receptor antibody encephalitis. Epilepsy and Behavior. 2011;20:392–394. doi: 10.1016/j.yebeh.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Lai M, Huijbers MGM, Lancaster E, et al. Investivation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellkofer HL, Kuempfel T, Jacobson L, et al. Non-paraneoplastic limbic encephalitis associated with NMDAR and VGKC antibodies. J Neurol Neurosurg Psychiatry. 2010;81:1407–1408. doi: 10.1136/jnnp.2009.186494. [DOI] [PubMed] [Google Scholar]
- 13.Pollock JM, Deibler AR, Burdette JH, et al. Migraine associated cerebral hyperperfusion with arterial spin-labeled MR imaging. AJNR Am J Neuroradiol. 29:1494–1497. doi: 10.3174/ajnr.A1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suleiman J, Brenner T, Gill D, et al. VGKC antibodies in pediatric encephalitis presenting with status epilepticus. Neurology. 2011;76:1252–1255. doi: 10.1212/WNL.0b013e3182143552. [DOI] [PubMed] [Google Scholar]