Abstract
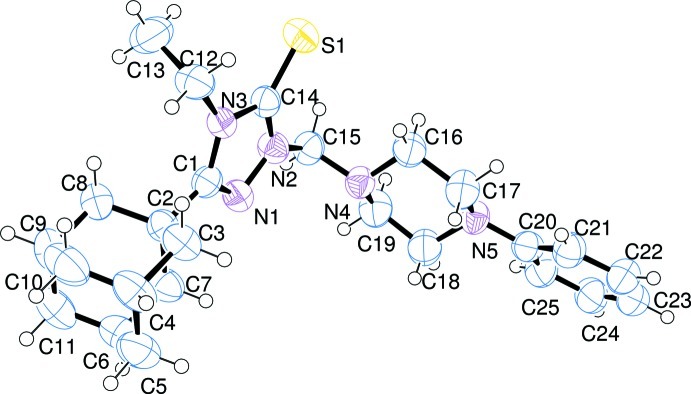
The title compound, C25H35N5S, has an approximately C-shaped conformation. The dihedral angle between the triazole and phenyl planes is 79.5 (2)°. The crystal structure consists of infinite chains parallel to the b axis, constructed by C—H⋯S hydrogen bonds between translation-related molecules. Adjacent chains are linked via weak C—H⋯C interactions between the adamantyl and phenyl groups.
Related literature
For the biological activity of adamantane derivatives and adamantyl-1,2,4-triazoles, see: Vernier et al. (1969 ▶); Al-Deeb et al. (2006 ▶); Al-Omar et al. (2010 ▶); El-Emam & Ibrahim (1991 ▶); El-Emam et al. (2004 ▶); Kadi et al. (2007 ▶, 2010 ▶). For related adamantyl-1,2,4-triazole structures, see: Al-Tamimi et al. (2010 ▶); Al-Abdullah et al. (2012 ▶); El-Emam et al. (2012 ▶); Lahsasni et al. (2012 ▶).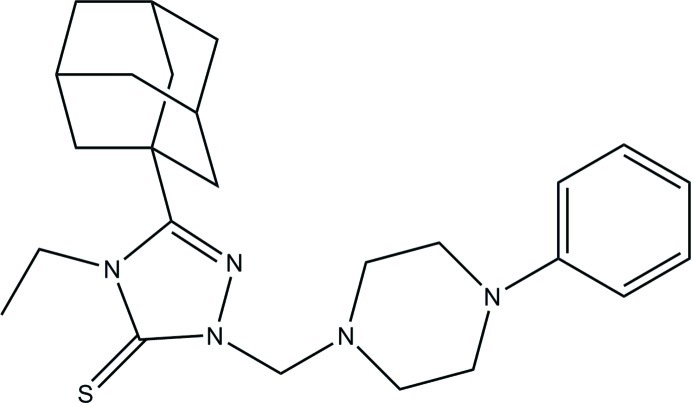
Experimental
Crystal data
C25H35N5S
M r = 437.65
Orthorhombic,
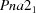
a = 27.382 (4) Å
b = 6.5083 (7) Å
c = 13.369 (2) Å
V = 2382.4 (5) Å3
Z = 4
Cu Kα radiation
μ = 1.36 mm−1
T = 293 K
0.16 × 0.06 × 0.02 mm
Data collection
Oxford Diffraction Xcalibur Gemini R diffractometer
Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2010 ▶) T min = 0.919, T max = 1.000
5844 measured reflections
3016 independent reflections
1828 reflections with I > 2σ(I)
R int = 0.086
Refinement
R[F 2 > 2σ(F 2)] = 0.057
wR(F 2) = 0.128
S = 1.00
3016 reflections
282 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.17 e Å−3
Δρmin = −0.18 e Å−3
Absolute structure: Flack (1983 ▶), 632 Friedel pairs
Flack parameter: 0.00 (4)
Data collection: CrysAlis CCD (Oxford Diffraction, 2010 ▶); cell refinement: CrysAlis RED (Oxford Diffraction, 2010 ▶); data reduction: CrysAlis RED; program(s) used to solve structure: SIR92 (Altomare et al., 1994 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶) and publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S160053681202990X/fy2061sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681202990X/fy2061Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681202990X/fy2061Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C15—H15A⋯S1i | 0.97 | 2.90 | 3.836 (5) | 162 |
| C5—H5A⋯C20ii | 0.97 | 2.80 | 3.750 (6) | 167 |
Symmetry codes: (i) 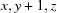 ; (ii)
; (ii) 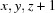 .
.
Acknowledgments
The financial support of the Deanship of Scientific Research and the Research Center for Female Scientific and Medical Colleges, King Saud University, is greatly appreciated. The authors are grateful for financial support from the Spanish Ministerio de Economía y Competitividad (MAT2010-15094, MAT2006-01997, Factoría de Cristalización – Consolider Ingenio 2010, and FPI grant BES-2011-046948 to MSM-A.) and FEDER.
supplementary crystallographic information
Comment
Adamantane derivatives were early recognized for their diverse biological activities including antiviral activity against the influenza (Vernier et al., 1969) and HIV viruses (El-Emam et al., 2004). In addition, adamantane derivatives were reported to exhibit marked antibacterial (Kadi et al., 2007, 2010) and anti-inflammatory (El-Emam & Ibrahim, 1991) activities. In continuation to our interest in the chemical and pharmacological properties of adamantane derivatives, we synthesized the title compound (I) as a potential bioactive agent. The structure consists of infinite chains parallel to the b axis, constructed by translations of a single molecule. The molecules in the the same chain are connected through C—H···S interactions with a H···S distance of 2.90 Å. Moreover, chains are linked via the weak C5—H5B···C20 interaction with a bond distance of 2.80 Å. The plane of the 1,2,4-triazole ring includes the S,C(ethyl group), C (adamantyl group) and C15 substituent atoms with deviations from the L.S. plane (in Å) of 0.0582, -0.1062, 0.0568 and -0.0964, respectively. The phenyl ring plane includes atom N5 with a deviation of 0.0668 Å. The angle between these two planes is 79.5 (2)°.
Experimental
A mixture of 527 mg (2 mmol) of 3-(1-adamantyl)-4-ethyl-4H-1,2,4-triazole-5-thiol (El-Emam & Ibrahim, 1991), 1-phenylpiperazine (325 mg, 2 mmol) and 37% formaldehyde solution (1 ml), in ethanol (8 ml), was heated under reflux for 15 min when a clear solution was obtained. Stirring was continued for 12 h at room temperature and the mixture was allowed to stand overnight. Cold water (5 ml) was slowly added and the mixture was stirred for 20 min. The precipitated crude product was filtered, washed with water, dried, and crystallized from ethanol to yield 770 mg (88%) of the title compound (C25H35N5S) as colorless needle crystals. M.P.: 139–141°C. Single crystals suitable for X-ray analysis were obtained by slow evaporation of CHCl3:EtOH solution (1:1; 5 ml) at room temperature. 1H NMR (CDCl3, 500.13 MHz): δ 1.13 (t, 3H, CH2CH3, J = 7.0 Hz), 1.67–1.73 (m, 6H, Adamantane-H), 1.96 (s, 6H, Adamantane-H), 2.03 (s, 3H, Adamantane-H), 2.88 (s, 4H, Piperazine-H), 3.09 (s, 4H, Piperazine-H), 4.17 (q, 2H, CH2CH3, J = 7.0 Hz), 5.08 (s, 2H, CH2), 6.46–6.83 (m, 3H, Ar—H), 7.15–7.17 (m, 2H, Ar—H). 13C NMR (CDCl3, 125.76 MHz): δ 13.81 (CH2CH3), 27.95, 35.24, 36.31, 39.90 (Adamantane-C), 43.43 (CH2CH3), 49.40, 50.37 (Piperazine-C), 68.80 (CH2), 116.32, 119.99, 129.12, 151.27 (Ar—C), 156.10 (Triazole C-5), 168.75 (C=S).
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.95 to 0.98 Å) and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2 or 1.5 (for methyl groups) Ueq(C).
Figures
Fig. 1.
ORTEP-style plot of title compound with labeling. Ellipsoids are given at the 50% probability level.
Crystal data
| C25H35N5S | F(000) = 944 |
| Mr = 437.65 | Dx = 1.220 Mg m−3 |
| Orthorhombic, Pna21 | Cu Kα radiation, λ = 1.54184 Å |
| Hall symbol: P 2c -2n | Cell parameters from 728 reflections |
| a = 27.382 (4) Å | θ = 3.7–70.5° |
| b = 6.5083 (7) Å | µ = 1.36 mm−1 |
| c = 13.369 (2) Å | T = 293 K |
| V = 2382.4 (5) Å3 | Prism, colourless |
| Z = 4 | 0.16 × 0.06 × 0.02 mm |
Data collection
| Oxford Diffraction Xcalibur Gemini R diffractometer | 3016 independent reflections |
| Radiation source: Enhance (Cu) X-ray Source | 1828 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.086 |
| Detector resolution: 10.2673 pixels mm-1 | θmax = 70.7°, θmin = 4.6° |
| ω scans | h = −27→32 |
| Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2010) | k = −7→7 |
| Tmin = 0.919, Tmax = 1.000 | l = −9→16 |
| 5844 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.057 | H-atom parameters constrained |
| wR(F2) = 0.128 | w = 1/[σ2(Fo2) + (0.0292P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.00 | (Δ/σ)max = 0.001 |
| 3016 reflections | Δρmax = 0.17 e Å−3 |
| 282 parameters | Δρmin = −0.18 e Å−3 |
| 1 restraint | Absolute structure: Flack (1983), 632 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.00 (4) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.47530 (6) | 0.0990 (2) | 0.88657 (13) | 0.0661 (4) | |
| N1 | 0.40642 (17) | 0.4993 (7) | 1.0508 (3) | 0.0559 (11) | |
| N2 | 0.42569 (16) | 0.4251 (7) | 0.9622 (3) | 0.0533 (11) | |
| N3 | 0.44288 (16) | 0.2016 (7) | 1.0746 (3) | 0.0495 (10) | |
| N4 | 0.37594 (16) | 0.5467 (7) | 0.8271 (4) | 0.0536 (11) | |
| N5 | 0.30519 (17) | 0.5670 (7) | 0.6693 (3) | 0.0540 (11) | |
| C1 | 0.41610 (19) | 0.3589 (8) | 1.1182 (4) | 0.0486 (12) | |
| C2 | 0.39947 (19) | 0.3789 (8) | 1.2243 (4) | 0.0498 (12) | |
| C3 | 0.3646 (2) | 0.1977 (9) | 1.2515 (5) | 0.0634 (15) | |
| H3A | 0.3370 | 0.1958 | 1.2060 | 0.076* | |
| H3B | 0.3819 | 0.0681 | 1.2452 | 0.076* | |
| C4 | 0.3465 (3) | 0.2256 (11) | 1.3600 (5) | 0.0765 (19) | |
| H4 | 0.3253 | 0.1100 | 1.3776 | 0.092* | |
| C5 | 0.3177 (2) | 0.4225 (11) | 1.3669 (6) | 0.0805 (19) | |
| H5A | 0.3045 | 0.4378 | 1.4339 | 0.097* | |
| H5B | 0.2906 | 0.4186 | 1.3202 | 0.097* | |
| C6 | 0.3508 (3) | 0.6039 (11) | 1.3427 (5) | 0.0764 (19) | |
| H6 | 0.3323 | 0.7322 | 1.3478 | 0.092* | |
| C7 | 0.3692 (2) | 0.5759 (9) | 1.2349 (5) | 0.0671 (16) | |
| H7A | 0.3416 | 0.5701 | 1.1897 | 0.081* | |
| H7B | 0.3891 | 0.6932 | 1.2162 | 0.081* | |
| C8 | 0.4414 (2) | 0.3859 (11) | 1.2987 (4) | 0.0672 (16) | |
| H8A | 0.4627 | 0.4999 | 1.2824 | 0.081* | |
| H8B | 0.4602 | 0.2601 | 1.2937 | 0.081* | |
| C9 | 0.4223 (3) | 0.4110 (12) | 1.4070 (5) | 0.082 (2) | |
| H9 | 0.4499 | 0.4153 | 1.4537 | 0.099* | |
| C10 | 0.3897 (3) | 0.2300 (12) | 1.4314 (5) | 0.092 (2) | |
| H10A | 0.3781 | 0.2413 | 1.4997 | 0.110* | |
| H10B | 0.4081 | 0.1033 | 1.4254 | 0.110* | |
| C11 | 0.3934 (3) | 0.6097 (11) | 1.4138 (5) | 0.089 (2) | |
| H11A | 0.3815 | 0.6282 | 1.4816 | 0.107* | |
| H11B | 0.4143 | 0.7250 | 1.3975 | 0.107* | |
| C12 | 0.4659 (2) | 0.0198 (9) | 1.1198 (5) | 0.0650 (17) | |
| H12A | 0.4599 | −0.0992 | 1.0779 | 0.078* | |
| H12B | 0.4514 | −0.0060 | 1.1848 | 0.078* | |
| C13 | 0.5207 (2) | 0.0501 (14) | 1.1319 (6) | 0.096 (3) | |
| H13A | 0.5352 | −0.0745 | 1.1563 | 0.143* | |
| H13B | 0.5267 | 0.1593 | 1.1786 | 0.143* | |
| H13C | 0.5348 | 0.0847 | 1.0683 | 0.143* | |
| C14 | 0.4485 (2) | 0.2439 (8) | 0.9739 (4) | 0.0487 (12) | |
| C15 | 0.42363 (18) | 0.5519 (8) | 0.8724 (4) | 0.0561 (14) | |
| H15A | 0.4318 | 0.6926 | 0.8895 | 0.067* | |
| H15B | 0.4477 | 0.5031 | 0.8246 | 0.067* | |
| C16 | 0.3704 (2) | 0.3810 (9) | 0.7556 (5) | 0.0640 (15) | |
| H16A | 0.3784 | 0.2514 | 0.7875 | 0.077* | |
| H16B | 0.3929 | 0.4013 | 0.7004 | 0.077* | |
| C17 | 0.3181 (2) | 0.3728 (9) | 0.7154 (5) | 0.0670 (17) | |
| H17A | 0.3153 | 0.2632 | 0.6666 | 0.080* | |
| H17B | 0.2957 | 0.3436 | 0.7699 | 0.080* | |
| C18 | 0.3126 (2) | 0.7352 (11) | 0.7401 (5) | 0.077 (2) | |
| H18A | 0.2907 | 0.7184 | 0.7964 | 0.092* | |
| H18B | 0.3049 | 0.8646 | 0.7078 | 0.092* | |
| C19 | 0.3642 (2) | 0.7407 (10) | 0.7770 (5) | 0.0707 (17) | |
| H19A | 0.3863 | 0.7619 | 0.7211 | 0.085* | |
| H19B | 0.3683 | 0.8540 | 0.8234 | 0.085* | |
| C20 | 0.2619 (2) | 0.5739 (9) | 0.6120 (4) | 0.0587 (14) | |
| C21 | 0.2322 (2) | 0.4026 (11) | 0.5996 (4) | 0.0650 (16) | |
| H21 | 0.2393 | 0.2810 | 0.6331 | 0.078* | |
| C22 | 0.1913 (2) | 0.4140 (12) | 0.5361 (5) | 0.0732 (18) | |
| H22 | 0.1719 | 0.2983 | 0.5260 | 0.088* | |
| C23 | 0.1798 (2) | 0.5956 (12) | 0.4890 (5) | 0.0775 (19) | |
| H23 | 0.1523 | 0.6035 | 0.4482 | 0.093* | |
| C24 | 0.2089 (3) | 0.7651 (12) | 0.5023 (5) | 0.0766 (19) | |
| H24 | 0.2006 | 0.8880 | 0.4711 | 0.092* | |
| C25 | 0.2496 (2) | 0.7563 (10) | 0.5605 (4) | 0.0688 (16) | |
| H25 | 0.2695 | 0.8714 | 0.5665 | 0.083* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0731 (8) | 0.0694 (8) | 0.0558 (8) | 0.0032 (8) | 0.0078 (9) | −0.0078 (9) |
| N1 | 0.061 (3) | 0.059 (3) | 0.048 (3) | 0.003 (2) | 0.000 (2) | −0.004 (2) |
| N2 | 0.059 (2) | 0.056 (3) | 0.045 (2) | 0.000 (2) | 0.003 (2) | 0.003 (2) |
| N3 | 0.053 (2) | 0.050 (2) | 0.046 (2) | 0.002 (2) | −0.003 (2) | −0.005 (2) |
| N4 | 0.056 (2) | 0.053 (3) | 0.052 (2) | 0.001 (2) | −0.006 (2) | 0.001 (2) |
| N5 | 0.058 (3) | 0.048 (2) | 0.056 (3) | −0.001 (2) | −0.010 (2) | 0.004 (2) |
| C1 | 0.048 (3) | 0.046 (3) | 0.051 (3) | −0.001 (2) | −0.005 (3) | 0.005 (2) |
| C2 | 0.051 (3) | 0.047 (3) | 0.052 (3) | −0.001 (3) | −0.002 (3) | −0.003 (2) |
| C3 | 0.073 (4) | 0.059 (3) | 0.058 (4) | −0.006 (3) | 0.010 (3) | −0.007 (3) |
| C4 | 0.102 (5) | 0.066 (4) | 0.062 (4) | −0.014 (4) | 0.024 (4) | −0.007 (3) |
| C5 | 0.078 (4) | 0.087 (5) | 0.077 (5) | −0.008 (4) | 0.023 (4) | −0.015 (4) |
| C6 | 0.089 (4) | 0.070 (4) | 0.070 (4) | 0.012 (4) | 0.016 (4) | −0.011 (3) |
| C7 | 0.085 (4) | 0.054 (3) | 0.063 (4) | 0.011 (3) | 0.008 (3) | −0.004 (3) |
| C8 | 0.066 (3) | 0.082 (4) | 0.055 (4) | −0.000 (3) | −0.005 (3) | −0.011 (3) |
| C9 | 0.090 (4) | 0.106 (5) | 0.051 (4) | 0.007 (5) | −0.012 (4) | −0.012 (4) |
| C10 | 0.133 (7) | 0.087 (5) | 0.056 (4) | 0.025 (5) | 0.017 (5) | 0.003 (4) |
| C11 | 0.117 (6) | 0.078 (4) | 0.072 (5) | −0.007 (5) | 0.012 (4) | −0.030 (3) |
| C12 | 0.082 (4) | 0.057 (3) | 0.056 (3) | 0.018 (3) | 0.007 (4) | 0.010 (3) |
| C13 | 0.072 (4) | 0.143 (7) | 0.072 (4) | 0.038 (5) | 0.004 (4) | 0.019 (5) |
| C14 | 0.048 (3) | 0.055 (3) | 0.043 (3) | −0.006 (3) | 0.001 (3) | 0.000 (2) |
| C15 | 0.058 (3) | 0.063 (3) | 0.048 (3) | −0.002 (3) | −0.000 (3) | 0.010 (3) |
| C16 | 0.071 (3) | 0.049 (3) | 0.072 (4) | 0.005 (3) | −0.010 (3) | −0.004 (3) |
| C17 | 0.077 (4) | 0.051 (3) | 0.073 (4) | −0.002 (3) | −0.015 (3) | 0.006 (3) |
| C18 | 0.083 (4) | 0.063 (4) | 0.084 (5) | 0.015 (4) | −0.019 (4) | −0.009 (4) |
| C19 | 0.084 (4) | 0.058 (4) | 0.070 (4) | 0.000 (4) | −0.012 (4) | 0.005 (3) |
| C20 | 0.059 (3) | 0.062 (3) | 0.055 (3) | 0.006 (3) | 0.001 (3) | −0.001 (3) |
| C21 | 0.061 (3) | 0.071 (4) | 0.062 (4) | −0.006 (3) | −0.002 (3) | 0.003 (3) |
| C22 | 0.061 (3) | 0.089 (5) | 0.070 (4) | 0.001 (4) | −0.006 (3) | −0.005 (4) |
| C23 | 0.069 (4) | 0.099 (6) | 0.064 (4) | 0.020 (4) | −0.011 (3) | −0.011 (4) |
| C24 | 0.086 (5) | 0.082 (5) | 0.062 (4) | 0.023 (4) | −0.018 (4) | −0.001 (4) |
| C25 | 0.077 (4) | 0.064 (4) | 0.065 (4) | 0.007 (3) | −0.012 (4) | 0.006 (3) |
Geometric parameters (Å, º)
| S1—C14 | 1.670 (6) | C9—C11 | 1.520 (10) |
| N1—C1 | 1.311 (7) | C9—H9 | 0.9800 |
| N1—N2 | 1.384 (6) | C10—H10A | 0.9700 |
| N2—C14 | 1.344 (7) | C10—H10B | 0.9700 |
| N2—C15 | 1.459 (7) | C11—H11A | 0.9700 |
| N3—C14 | 1.383 (7) | C11—H11B | 0.9700 |
| N3—C1 | 1.388 (7) | C12—C13 | 1.522 (9) |
| N3—C12 | 1.471 (7) | C12—H12A | 0.9700 |
| N4—C15 | 1.440 (7) | C12—H12B | 0.9700 |
| N4—C16 | 1.449 (7) | C13—H13A | 0.9600 |
| N4—C19 | 1.465 (7) | C13—H13B | 0.9600 |
| N5—C20 | 1.412 (7) | C13—H13C | 0.9600 |
| N5—C17 | 1.450 (8) | C15—H15A | 0.9700 |
| N5—C18 | 1.461 (8) | C15—H15B | 0.9700 |
| C1—C2 | 1.495 (8) | C16—C17 | 1.530 (8) |
| C2—C8 | 1.518 (8) | C16—H16A | 0.9700 |
| C2—C7 | 1.533 (8) | C16—H16B | 0.9700 |
| C2—C3 | 1.560 (8) | C17—H17A | 0.9700 |
| C3—C4 | 1.544 (8) | C17—H17B | 0.9700 |
| C3—H3A | 0.9700 | C18—C19 | 1.496 (9) |
| C3—H3B | 0.9700 | C18—H18A | 0.9700 |
| C4—C5 | 1.509 (9) | C18—H18B | 0.9700 |
| C4—C10 | 1.520 (10) | C19—H19A | 0.9700 |
| C4—H4 | 0.9800 | C19—H19B | 0.9700 |
| C5—C6 | 1.525 (9) | C20—C21 | 1.390 (8) |
| C5—H5A | 0.9700 | C20—C25 | 1.413 (8) |
| C5—H5B | 0.9700 | C21—C22 | 1.408 (9) |
| C6—C11 | 1.504 (10) | C21—H21 | 0.9300 |
| C6—C7 | 1.538 (9) | C22—C23 | 1.376 (10) |
| C6—H6 | 0.9800 | C22—H22 | 0.9300 |
| C7—H7A | 0.9700 | C23—C24 | 1.373 (10) |
| C7—H7B | 0.9700 | C23—H23 | 0.9300 |
| C8—C9 | 1.548 (9) | C24—C25 | 1.361 (9) |
| C8—H8A | 0.9700 | C24—H24 | 0.9300 |
| C8—H8B | 0.9700 | C25—H25 | 0.9300 |
| C9—C10 | 1.514 (11) | ||
| C1—N1—N2 | 105.6 (4) | C6—C11—C9 | 110.2 (6) |
| C14—N2—N1 | 112.6 (4) | C6—C11—H11A | 109.6 |
| C14—N2—C15 | 127.6 (5) | C9—C11—H11A | 109.6 |
| N1—N2—C15 | 119.5 (4) | C6—C11—H11B | 109.6 |
| C14—N3—C1 | 108.7 (4) | C9—C11—H11B | 109.6 |
| C14—N3—C12 | 120.9 (5) | H11A—C11—H11B | 108.1 |
| C1—N3—C12 | 130.3 (4) | N3—C12—C13 | 111.2 (5) |
| C15—N4—C16 | 112.9 (5) | N3—C12—H12A | 109.4 |
| C15—N4—C19 | 111.8 (5) | C13—C12—H12A | 109.4 |
| C16—N4—C19 | 108.5 (5) | N3—C12—H12B | 109.4 |
| C20—N5—C17 | 117.6 (5) | C13—C12—H12B | 109.4 |
| C20—N5—C18 | 116.4 (5) | H12A—C12—H12B | 108.0 |
| C17—N5—C18 | 110.1 (5) | C12—C13—H13A | 109.5 |
| N1—C1—N3 | 109.4 (5) | C12—C13—H13B | 109.5 |
| N1—C1—C2 | 122.0 (5) | H13A—C13—H13B | 109.5 |
| N3—C1—C2 | 128.6 (5) | C12—C13—H13C | 109.5 |
| C1—C2—C8 | 113.2 (4) | H13A—C13—H13C | 109.5 |
| C1—C2—C7 | 108.9 (5) | H13B—C13—H13C | 109.5 |
| C8—C2—C7 | 108.8 (5) | N2—C14—N3 | 103.6 (5) |
| C1—C2—C3 | 110.0 (4) | N2—C14—S1 | 128.2 (4) |
| C8—C2—C3 | 109.4 (5) | N3—C14—S1 | 128.1 (4) |
| C7—C2—C3 | 106.3 (5) | N4—C15—N2 | 111.6 (4) |
| C4—C3—C2 | 109.0 (5) | N4—C15—H15A | 109.3 |
| C4—C3—H3A | 109.9 | N2—C15—H15A | 109.3 |
| C2—C3—H3A | 109.9 | N4—C15—H15B | 109.3 |
| C4—C3—H3B | 109.9 | N2—C15—H15B | 109.3 |
| C2—C3—H3B | 109.9 | H15A—C15—H15B | 108.0 |
| H3A—C3—H3B | 108.3 | N4—C16—C17 | 110.8 (5) |
| C5—C4—C10 | 110.7 (6) | N4—C16—H16A | 109.5 |
| C5—C4—C3 | 109.0 (6) | C17—C16—H16A | 109.5 |
| C10—C4—C3 | 110.0 (6) | N4—C16—H16B | 109.5 |
| C5—C4—H4 | 109.0 | C17—C16—H16B | 109.5 |
| C10—C4—H4 | 109.0 | H16A—C16—H16B | 108.1 |
| C3—C4—H4 | 109.0 | N5—C17—C16 | 110.3 (5) |
| C4—C5—C6 | 109.4 (5) | N5—C17—H17A | 109.6 |
| C4—C5—H5A | 109.8 | C16—C17—H17A | 109.6 |
| C6—C5—H5A | 109.8 | N5—C17—H17B | 109.6 |
| C4—C5—H5B | 109.8 | C16—C17—H17B | 109.6 |
| C6—C5—H5B | 109.8 | H17A—C17—H17B | 108.1 |
| H5A—C5—H5B | 108.2 | N5—C18—C19 | 111.3 (5) |
| C11—C6—C5 | 110.3 (6) | N5—C18—H18A | 109.4 |
| C11—C6—C7 | 110.0 (5) | C19—C18—H18A | 109.4 |
| C5—C6—C7 | 107.6 (6) | N5—C18—H18B | 109.4 |
| C11—C6—H6 | 109.6 | C19—C18—H18B | 109.4 |
| C5—C6—H6 | 109.6 | H18A—C18—H18B | 108.0 |
| C7—C6—H6 | 109.6 | N4—C19—C18 | 109.7 (6) |
| C2—C7—C6 | 111.2 (5) | N4—C19—H19A | 109.7 |
| C2—C7—H7A | 109.4 | C18—C19—H19A | 109.7 |
| C6—C7—H7A | 109.4 | N4—C19—H19B | 109.7 |
| C2—C7—H7B | 109.4 | C18—C19—H19B | 109.7 |
| C6—C7—H7B | 109.4 | H19A—C19—H19B | 108.2 |
| H7A—C7—H7B | 108.0 | C21—C20—N5 | 122.0 (5) |
| C2—C8—C9 | 111.2 (5) | C21—C20—C25 | 118.4 (5) |
| C2—C8—H8A | 109.4 | N5—C20—C25 | 119.4 (6) |
| C9—C8—H8A | 109.4 | C20—C21—C22 | 119.7 (6) |
| C2—C8—H8B | 109.4 | C20—C21—H21 | 120.1 |
| C9—C8—H8B | 109.4 | C22—C21—H21 | 120.1 |
| H8A—C8—H8B | 108.0 | C23—C22—C21 | 120.2 (7) |
| C10—C9—C11 | 110.0 (7) | C23—C22—H22 | 119.9 |
| C10—C9—C8 | 108.5 (6) | C21—C22—H22 | 119.9 |
| C11—C9—C8 | 108.8 (6) | C24—C23—C22 | 119.9 (6) |
| C10—C9—H9 | 109.9 | C24—C23—H23 | 120.0 |
| C11—C9—H9 | 109.9 | C22—C23—H23 | 120.0 |
| C8—C9—H9 | 109.9 | C25—C24—C23 | 121.1 (7) |
| C9—C10—C4 | 109.8 (6) | C25—C24—H24 | 119.5 |
| C9—C10—H10A | 109.7 | C23—C24—H24 | 119.5 |
| C4—C10—H10A | 109.7 | C24—C25—C20 | 120.6 (7) |
| C9—C10—H10B | 109.7 | C24—C25—H25 | 119.7 |
| C4—C10—H10B | 109.7 | C20—C25—H25 | 119.7 |
| H10A—C10—H10B | 108.2 | ||
| C1—N1—N2—C14 | −1.9 (6) | C10—C9—C11—C6 | −58.7 (7) |
| C1—N1—N2—C15 | −176.4 (5) | C8—C9—C11—C6 | 60.0 (8) |
| N2—N1—C1—N3 | 2.4 (6) | C14—N3—C12—C13 | 74.2 (7) |
| N2—N1—C1—C2 | −177.8 (5) | C1—N3—C12—C13 | −101.9 (7) |
| C14—N3—C1—N1 | −2.2 (6) | N1—N2—C14—N3 | 0.5 (6) |
| C12—N3—C1—N1 | 174.2 (5) | C15—N2—C14—N3 | 174.4 (5) |
| C14—N3—C1—C2 | 178.0 (5) | N1—N2—C14—S1 | 178.2 (4) |
| C12—N3—C1—C2 | −5.6 (9) | C15—N2—C14—S1 | −7.9 (9) |
| N1—C1—C2—C8 | −118.6 (6) | C1—N3—C14—N2 | 1.0 (6) |
| N3—C1—C2—C8 | 61.1 (8) | C12—N3—C14—N2 | −175.9 (5) |
| N1—C1—C2—C7 | 2.5 (7) | C1—N3—C14—S1 | −176.7 (4) |
| N3—C1—C2—C7 | −177.7 (5) | C12—N3—C14—S1 | 6.4 (8) |
| N1—C1—C2—C3 | 118.7 (6) | C16—N4—C15—N2 | −88.9 (6) |
| N3—C1—C2—C3 | −61.5 (7) | C19—N4—C15—N2 | 148.5 (5) |
| C1—C2—C3—C4 | −178.0 (5) | C14—N2—C15—N4 | 107.8 (6) |
| C8—C2—C3—C4 | 57.1 (7) | N1—N2—C15—N4 | −78.7 (6) |
| C7—C2—C3—C4 | −60.2 (7) | C15—N4—C16—C17 | 175.9 (5) |
| C2—C3—C4—C5 | 62.4 (7) | C19—N4—C16—C17 | −59.7 (6) |
| C2—C3—C4—C10 | −59.2 (7) | C20—N5—C17—C16 | 168.3 (5) |
| C10—C4—C5—C6 | 58.4 (7) | C18—N5—C17—C16 | −55.2 (7) |
| C3—C4—C5—C6 | −62.7 (8) | N4—C16—C17—N5 | 57.9 (7) |
| C4—C5—C6—C11 | −58.6 (7) | C20—N5—C18—C19 | −165.7 (5) |
| C4—C5—C6—C7 | 61.4 (8) | C17—N5—C18—C19 | 57.2 (7) |
| C1—C2—C7—C6 | 179.7 (5) | C15—N4—C19—C18 | −174.4 (5) |
| C8—C2—C7—C6 | −56.5 (7) | C16—N4—C19—C18 | 60.4 (6) |
| C3—C2—C7—C6 | 61.2 (6) | N5—C18—C19—N4 | −59.8 (7) |
| C11—C6—C7—C2 | 58.1 (8) | C17—N5—C20—C21 | 0.2 (8) |
| C5—C6—C7—C2 | −62.1 (7) | C18—N5—C20—C21 | −133.6 (6) |
| C1—C2—C8—C9 | 178.9 (5) | C17—N5—C20—C25 | −176.0 (6) |
| C7—C2—C8—C9 | 57.6 (7) | C18—N5—C20—C25 | 50.2 (7) |
| C3—C2—C8—C9 | −58.2 (7) | N5—C20—C21—C22 | −175.6 (5) |
| C2—C8—C9—C10 | 59.9 (8) | C25—C20—C21—C22 | 0.6 (8) |
| C2—C8—C9—C11 | −59.7 (8) | C20—C21—C22—C23 | −2.1 (9) |
| C11—C9—C10—C4 | 58.0 (7) | C21—C22—C23—C24 | 1.3 (10) |
| C8—C9—C10—C4 | −60.8 (8) | C22—C23—C24—C25 | 1.0 (10) |
| C5—C4—C10—C9 | −58.6 (7) | C23—C24—C25—C20 | −2.5 (10) |
| C3—C4—C10—C9 | 61.9 (8) | C21—C20—C25—C24 | 1.7 (9) |
| C5—C6—C11—C9 | 58.9 (7) | N5—C20—C25—C24 | 178.0 (6) |
| C7—C6—C11—C9 | −59.6 (8) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C15—H15A···S1i | 0.97 | 2.90 | 3.836 (5) | 162 |
| C5—H5A···C20ii | 0.97 | 2.80 | 3.750 (6) | 167 |
Symmetry codes: (i) x, y+1, z; (ii) x, y, z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FY2061).
References
- Al-Abdullah, E. S., Asiri, H. H., El-Emam, A. A. & Ng, S. W. (2012). Acta Cryst. E68, o531. [DOI] [PMC free article] [PubMed]
- Al-Deeb, O. A., Al-Omar, M. A., El-Brollosy, N. R., Habib, E. E., Ibrahim, T. M. & El-Emam, A. A. (2006). Arzneim. Forsch. Drug. Res. 56, 40–47. [DOI] [PubMed]
- Al-Omar, M. A., Al-Abdullah, E. S., Shehata, I. A., Habib, E. E., Ibrahim, T. M. & El-Emam, A. A. (2010). Molecules, 15, 2526–2550. [DOI] [PMC free article] [PubMed]
- Al-Tamimi, A.-M. S., Bari, A., Al-Omar, M. A., Alrashood, K. A. & El-Emam, A. A. (2010). Acta Cryst. E66, o1756. [DOI] [PMC free article] [PubMed]
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst. 27, 435.
- El-Emam, A. A., Al-Deeb, O. A., Al-Omar, M. A. & Lehmann, J. (2004). Bioorg. Med. Chem. 12, 5107–5113. [DOI] [PubMed]
- El-Emam, A. A., Alrashood, K. A., Al-Tamimi, A.-M. S., Ng, S. W. & Tiekink, E. R. T. (2012). Acta Cryst. E68, o657–o658. [DOI] [PMC free article] [PubMed]
- El-Emam, A. A. & Ibrahim, T. M. (1991). Arzneim. Forsch. Drug. Res. 41, 1260–1264. [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Kadi, A. A., Al-Abdullah, E. S., Shehata, I. A., Habib, E. E., Ibrahim, T. M. & El-Emam, A. A. (2010). Eur. J. Med. Chem. 45, 5006–5011. [DOI] [PubMed]
- Kadi, A. A., El-Brollosy, N. R., Al-Deeb, O. A., Habib, E. E., Ibrahim, T. M. & El-Emam, A. A. (2007). Eur. J. Med. Chem. 42, 235–242. [DOI] [PubMed]
- Lahsasni, S., El-Emam, A. A., El-Brollosy, N. R., Quah, C. K. & Fun, H.-K. (2012). Acta Cryst. E68, o1439–o1440. [DOI] [PMC free article] [PubMed]
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Oxford Diffraction (2010). CrysAlis PRO, CrysAlis CCD and CrysAlis RED Oxford Diffraction Ltd, Yarnton, Oxfordshire, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Vernier, V. G., Harmon, J. B., Stump, J. M., Lynes, T. L., Marvel, M. P. & Smith, D. H. (1969). Toxicol. Appl. Pharmacol. 15, 642–665. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S160053681202990X/fy2061sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681202990X/fy2061Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681202990X/fy2061Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report