Abstract
The asymmetric unit of the title salt C8H22N4 2+·2C7H5O2 −, comprises two independent pairs of half a 2,2′-(piperazine-1,4-diyl)diethanaminium dication plus a benzoate anion. The dications are symmetrical and lie across crystallographic centres of inversion. The crystal structure was refined as a two-component pseudo-merohedral twin using the twin law 001 0-10 100 [he domain fractions are 0.8645 (8) and 0.1355 (8)]. The anions and cations are linked by N—H⋯O hydrogen bonds and weak N—H⋯O intermolecular interactions to form infinite two-dimensional networks parallel to [101]. The conformation adopted by the cation in the crystal structure is very similar to that adopted by the same cation in the structures of the 2-hydroxybenzoate [Cukrowski et al. (2012 ▶). Acta Cryst, E68, o2387], the nitrate and the tetrahydrogen pentaborate salts.
Related literature
For the structures of the 2-hydroxybenzoate, the nitrate and the tetrahydrogen pentaborate salts of the 1,4-di(2-ammonioethyl)piperazine cation, see: Cukrowski et al. (2012 ▶); Junk & Smith (2005 ▶); Jiang et al. (2009 ▶), respectively.
Experimental
Crystal data
C8H22N4 2+·2C7H5O2 −
M r = 416.52
Monoclinic,

a = 19.5300 (4) Å
b = 6.6694 (2) Å
c = 19.6178 (4) Å
β = 115.989 (1)°
V = 2296.89 (10) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 180 K
0.28 × 0.23 × 0.12 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (SORTAV; Blessing, 1995 ▶) T min = 0.910, T max = 0.991
20137 measured reflections
5194 independent reflections
3970 reflections with I > 2σ(I)
R int = 0.031
Refinement
R[F 2 > 2σ(F 2)] = 0.040
wR(F 2) = 0.110
S = 1.02
5194 reflections
290 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.16 e Å−3
Δρmin = −0.18 e Å−3
Data collection: COLLECT (Nonius, 1998 ▶); cell refinement: SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO (Otwinowski & Minor, 1997 ▶), SCALEPACK and SORTAV (Blessing, 1995 ▶); program(s) used to solve structure: SIR92 (Altomare et al., 1994 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶), POV-RAY (Cason, 2004 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812030115/jj2134sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812030115/jj2134Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812030115/jj2134Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1A⋯O15i | 0.945 (18) | 1.833 (19) | 2.7739 (17) | 173.0 (15) |
| N1—H1B⋯O15 | 0.902 (16) | 1.895 (17) | 2.7836 (15) | 167.8 (14) |
| N1—H1B⋯O14 | 0.902 (16) | 2.632 (16) | 3.2580 (16) | 127.2 (13) |
| N1—H1C⋯O14ii | 0.925 (18) | 1.887 (18) | 2.7660 (16) | 157.9 (14) |
| N1′—H1′A⋯O14′ | 0.882 (19) | 1.857 (19) | 2.7355 (17) | 174.2 (15) |
| N1′—H1′B⋯O14′iii | 0.897 (17) | 1.908 (17) | 2.7836 (16) | 164.8 (15) |
| N1′—H1′B⋯O15′iii | 0.897 (17) | 2.533 (16) | 3.1858 (16) | 130.1 (13) |
| N1′—H1′C⋯O15′iv | 0.916 (18) | 1.934 (18) | 2.7585 (16) | 148.8 (14) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii) 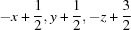 ; (iv)
; (iv)  .
.
Acknowledgments
The authors thank Dr John E. Davies of the University of Cambridge (England) for the data collection.
supplementary crystallographic information
Comment
The title compound [C8H22N42+ 2(C7H5O2-)] (1) was obtained as an unintended product during an attempt to prepare a benzoate salt of a singly protonated N,N'-di(2-aminoethyl)-2-aminoethane-1-ammonium ion (C6H19N4+ C7H5O2-). This occurred because the starting material, instead of being pure N,N'-di(2-aminoethyl)-ethane-1,2-diamine, (C6H18N4), was a mixture of that compound and 1,4-di(2-aminoethyl)piperazine (C8H22N4) see Cukrowski, et al. (2012).
The asymmetric unit of the title compound, C8H22N42+, 2(C7H5O2-), 1, is a salt with two independent pairs of half a C8H22N42+ cation plus a C7H5O2- anion. The C8H22N42+ cations are symmetrical and lie across crystallographic centres of inversion (Fig. 1). The crystal structure was refined as a two-component pseudo- merohedral twin using the twin law 0 0 1 0 - 1 0 1 0 0. The fractional contribution refined to 0.1355 (8).
All three H atoms of each ammonium group in the cations of 1 are hydrogen bonded to the O atoms of the carboxylate groups of the anions. For each ammonium group, one H atom forms a bifurcated hydrogen bond to both of the O atoms of the carboxylate group of one anion, whereas the other two H atoms each form single hydrogen bonds to one O atom of the carboxylate group of each of two additional anions (Fig. 2). Thus both the O atoms of each carboxylate group are each acceptors for two hydrogen bonds. N—H···O hydrogen bonds and weak N—H···O intermolecular interactions link the cations and anions to form a two-dimensional network with layers parallel to the [101] plane (Fig. 2). Each of the two independent cation-anion pairs form the content of alternate network layers.
The conformation adopted by the C8H22N42+ cation in the crystal structure of 1 is very similar to the conformations adopted by the same cation in the crystal structures of the 2-hydroxybenzoate (Cukrowski, et al., 2012), the nitrate (Junk & Smith, 2005) and the tetrahydrogenpentaborate (Jiang, et al., 2009) salts despite the differences in the size and shape of the anions in the various structures.
Experimental
2 ml of a.3.32 M aqueous solution of what was claimed by the supplier (QinHuangDao JinLei Chemical Co.Ltd) to be N,N'-di(2-aminoethyl)-ethane-1,2-diamine, but which turned out to be a mixture of that compound (C6H18N4, 6.64n mmol) and 1,4-di(2-aminoethyl)piperazine (C8H20N4, 5.57(1-n) mmol) was added to 0.78 g of benzoic acid (6.96 mmol), resulting in a clear colourless solution. 0.2 ml of ethanol was added to the solution and the mixture was heated for 3 h at 70 °C. The solution was cooled to room temperature and then left covered for six days and then allowed to slowly evaporate by covering the container with perforated aluminium foil. Yellow crystals were obtained after four days of slow evaporation.
Refinement
The crystal structure was refined as a two component pseudo-merohedral twin using the twin law 0 0 1 0 - 1 0 1 0 0. The fractional contribution refined to 0.1355 (8). H1A, H1B and H1C were located by a difference map and their coordinates were refined. All of the remaining H atoms were placed in their calculated positions and then refined using the riding model with Atom—H lengths of 0.95Å, (CH) or 0.99Å (CH2). Isotropic displacement parameters for all hydrogen atoms were set to 1.20 times Ueq of the parent atom.
Figures
Fig. 1.
Molecular structure of the title compound showing the atom labeling scheme and 50° probability displacement ellipsoids.
Fig. 2.
Packing diagram of the title compound viewed offset from along the b axis. Dashed lines indicate N—H···O and O—H···O hydrogen bonds. The intermolecular N—H···O hydrogen bonds form a two-dimensional network.
Crystal data
| C8H22N42+·2C7H5O2− | F(000) = 896 |
| Mr = 416.52 | Dx = 1.204 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71070 Å |
| Hall symbol: -P 2yn | Cell parameters from 12295 reflections |
| a = 19.5300 (4) Å | θ = 1.0–27.5° |
| b = 6.6694 (2) Å | µ = 0.08 mm−1 |
| c = 19.6178 (4) Å | T = 180 K |
| β = 115.989 (1)° | Block, yellow |
| V = 2296.89 (10) Å3 | 0.28 × 0.23 × 0.12 mm |
| Z = 4 |
Data collection
| Nonius KappaCCD diffractometer | 5194 independent reflections |
| Radiation source: fine-focus sealed tube | 3970 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.031 |
| Thin slice ω and φ scans | θmax = 27.5°, θmin = 3.6° |
| Absorption correction: multi-scan (SORTAV; Blessing, 1995) | h = −25→22 |
| Tmin = 0.910, Tmax = 0.991 | k = −7→8 |
| 20137 measured reflections | l = −25→25 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.040 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.110 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0702P)2] where P = (Fo2 + 2Fc2)/3 |
| 5194 reflections | (Δ/σ)max = 0.001 |
| 290 parameters | Δρmax = 0.16 e Å−3 |
| 0 restraints | Δρmin = −0.18 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.19472 (7) | 0.2447 (2) | 0.19552 (7) | 0.0375 (3) | |
| H1A | 0.1776 (9) | 0.374 (3) | 0.2008 (8) | 0.045* | |
| H1B | 0.2431 (9) | 0.223 (2) | 0.2302 (9) | 0.045* | |
| H1C | 0.1652 (9) | 0.142 (3) | 0.2002 (8) | 0.045* | |
| C2 | 0.19529 (8) | 0.2437 (3) | 0.12037 (8) | 0.0491 (4) | |
| H2A | 0.2127 | 0.1110 | 0.1116 | 0.059* | |
| H2B | 0.2319 | 0.3457 | 0.1198 | 0.059* | |
| C3 | 0.11811 (9) | 0.2872 (3) | 0.05773 (8) | 0.0512 (4) | |
| H3A | 0.0994 | 0.4156 | 0.0686 | 0.061* | |
| H3B | 0.1226 | 0.3034 | 0.0097 | 0.061* | |
| N4 | 0.06208 (7) | 0.12951 (18) | 0.04783 (6) | 0.0418 (3) | |
| C5 | −0.01461 (8) | 0.2057 (2) | 0.00103 (8) | 0.0458 (4) | |
| H5A | −0.0182 | 0.2489 | −0.0487 | 0.055* | |
| H5B | −0.0242 | 0.3241 | 0.0260 | 0.055* | |
| C6 | 0.07429 (8) | −0.0487 (2) | 0.01107 (8) | 0.0483 (4) | |
| H6A | 0.1254 | −0.1048 | 0.0430 | 0.058* | |
| H6B | 0.0726 | −0.0106 | −0.0384 | 0.058* | |
| C7 | 0.46941 (7) | 0.2132 (2) | 0.30262 (7) | 0.0347 (3) | |
| C8 | 0.51949 (8) | 0.3595 (3) | 0.30237 (8) | 0.0470 (4) | |
| H8 | 0.5013 | 0.4909 | 0.2852 | 0.056* | |
| C9 | 0.59622 (9) | 0.3155 (3) | 0.32714 (9) | 0.0583 (5) | |
| H9 | 0.6304 | 0.4175 | 0.3276 | 0.070* | |
| C10 | 0.62302 (9) | 0.1248 (3) | 0.35110 (9) | 0.0566 (5) | |
| H10 | 0.6755 | 0.0952 | 0.3680 | 0.068* | |
| C11 | 0.57335 (9) | −0.0231 (3) | 0.35052 (8) | 0.0512 (4) | |
| H11 | 0.5915 | −0.1552 | 0.3664 | 0.061* | |
| C12 | 0.49691 (8) | 0.0210 (2) | 0.32676 (8) | 0.0415 (3) | |
| H12 | 0.4631 | −0.0809 | 0.3270 | 0.050* | |
| C13 | 0.38687 (7) | 0.2669 (2) | 0.27915 (7) | 0.0340 (3) | |
| O14 | 0.36433 (5) | 0.43634 (15) | 0.25215 (5) | 0.0429 (2) | |
| O15 | 0.34467 (5) | 0.13526 (15) | 0.28820 (6) | 0.0435 (3) | |
| N1' | 0.18677 (7) | 0.8130 (2) | 0.69616 (7) | 0.0387 (3) | |
| H1'A | 0.2019 (9) | 0.704 (3) | 0.6812 (9) | 0.046* | |
| H1'B | 0.2193 (9) | 0.845 (2) | 0.7439 (10) | 0.046* | |
| H1'C | 0.1828 (9) | 0.917 (3) | 0.6640 (9) | 0.046* | |
| C2' | 0.11280 (9) | 0.7645 (3) | 0.69548 (8) | 0.0490 (4) | |
| H2'A | 0.0946 | 0.8814 | 0.7141 | 0.059* | |
| H2'B | 0.1193 | 0.6506 | 0.7301 | 0.059* | |
| C3' | 0.05450 (8) | 0.7108 (3) | 0.61682 (8) | 0.0484 (4) | |
| H3'A | 0.0703 | 0.5854 | 0.6006 | 0.058* | |
| H3'B | 0.0049 | 0.6857 | 0.6177 | 0.058* | |
| N4' | 0.04519 (6) | 0.86872 (18) | 0.56193 (6) | 0.0409 (3) | |
| C5' | 0.00761 (8) | 1.0480 (2) | 0.57298 (8) | 0.0458 (4) | |
| H5'A | 0.0379 | 1.1020 | 0.6246 | 0.055* | |
| H5'B | −0.0434 | 1.0114 | 0.5683 | 0.055* | |
| C6' | 0.00043 (8) | 0.7944 (2) | 0.48472 (8) | 0.0441 (4) | |
| H6'A | −0.0507 | 0.7539 | 0.4785 | 0.053* | |
| H6'B | 0.0256 | 0.6747 | 0.4762 | 0.053* | |
| C7' | 0.19900 (7) | 0.3513 (2) | 0.53125 (7) | 0.0351 (3) | |
| C8' | 0.17823 (8) | 0.5420 (2) | 0.50088 (8) | 0.0425 (3) | |
| H8' | 0.1815 | 0.6515 | 0.5332 | 0.051* | |
| C9' | 0.15272 (9) | 0.5741 (3) | 0.42383 (9) | 0.0515 (4) | |
| H9' | 0.1377 | 0.7044 | 0.4032 | 0.062* | |
| C10' | 0.14927 (9) | 0.4151 (3) | 0.37717 (9) | 0.0533 (4) | |
| H10' | 0.1315 | 0.4365 | 0.3243 | 0.064* | |
| C11' | 0.17134 (9) | 0.2262 (3) | 0.40677 (8) | 0.0518 (4) | |
| H11' | 0.1699 | 0.1181 | 0.3746 | 0.062* | |
| C12' | 0.19571 (8) | 0.1943 (2) | 0.48389 (8) | 0.0440 (3) | |
| H12' | 0.2102 | 0.0634 | 0.5043 | 0.053* | |
| C13' | 0.22236 (7) | 0.3122 (2) | 0.61444 (7) | 0.0355 (3) | |
| O14' | 0.23245 (6) | 0.46100 (17) | 0.65735 (5) | 0.0521 (3) | |
| O15' | 0.23097 (6) | 0.13489 (16) | 0.63648 (6) | 0.0478 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0311 (6) | 0.0325 (7) | 0.0436 (6) | −0.0018 (5) | 0.0115 (5) | −0.0006 (5) |
| C2 | 0.0417 (8) | 0.0570 (10) | 0.0495 (8) | −0.0097 (7) | 0.0207 (6) | −0.0086 (7) |
| C3 | 0.0576 (9) | 0.0497 (10) | 0.0432 (8) | −0.0072 (8) | 0.0193 (7) | 0.0004 (7) |
| N4 | 0.0427 (7) | 0.0410 (7) | 0.0360 (6) | −0.0020 (5) | 0.0119 (5) | −0.0013 (5) |
| C5 | 0.0519 (9) | 0.0402 (8) | 0.0360 (7) | 0.0059 (7) | 0.0107 (6) | −0.0001 (6) |
| C6 | 0.0448 (8) | 0.0519 (10) | 0.0428 (7) | 0.0073 (7) | 0.0144 (6) | −0.0012 (7) |
| C7 | 0.0362 (7) | 0.0386 (8) | 0.0291 (6) | 0.0007 (6) | 0.0141 (5) | −0.0019 (5) |
| C8 | 0.0428 (8) | 0.0523 (10) | 0.0458 (8) | −0.0024 (7) | 0.0195 (6) | 0.0051 (7) |
| C9 | 0.0424 (9) | 0.0790 (14) | 0.0564 (9) | −0.0083 (9) | 0.0242 (7) | 0.0073 (8) |
| C10 | 0.0378 (8) | 0.0872 (14) | 0.0471 (8) | 0.0120 (9) | 0.0209 (6) | 0.0019 (8) |
| C11 | 0.0468 (8) | 0.0586 (11) | 0.0473 (8) | 0.0164 (8) | 0.0198 (6) | 0.0009 (7) |
| C12 | 0.0405 (8) | 0.0430 (9) | 0.0401 (7) | 0.0045 (6) | 0.0169 (6) | −0.0012 (6) |
| C13 | 0.0375 (7) | 0.0331 (8) | 0.0299 (6) | 0.0004 (6) | 0.0133 (5) | −0.0028 (5) |
| O14 | 0.0447 (5) | 0.0350 (6) | 0.0472 (5) | 0.0067 (4) | 0.0184 (4) | 0.0049 (4) |
| O15 | 0.0346 (5) | 0.0389 (6) | 0.0540 (6) | −0.0003 (4) | 0.0167 (4) | 0.0055 (4) |
| N1' | 0.0444 (7) | 0.0342 (7) | 0.0334 (6) | −0.0010 (5) | 0.0132 (5) | 0.0019 (5) |
| C2' | 0.0499 (9) | 0.0546 (10) | 0.0447 (8) | −0.0003 (7) | 0.0227 (7) | 0.0058 (7) |
| C3' | 0.0402 (8) | 0.0472 (10) | 0.0529 (9) | −0.0060 (7) | 0.0159 (6) | 0.0023 (7) |
| N4' | 0.0353 (6) | 0.0389 (7) | 0.0423 (6) | −0.0011 (5) | 0.0113 (5) | −0.0021 (5) |
| C5' | 0.0419 (8) | 0.0485 (9) | 0.0432 (8) | −0.0010 (7) | 0.0152 (6) | −0.0082 (7) |
| C6' | 0.0376 (7) | 0.0406 (9) | 0.0476 (8) | −0.0017 (6) | 0.0127 (6) | −0.0088 (6) |
| C7' | 0.0308 (6) | 0.0379 (8) | 0.0375 (7) | −0.0013 (6) | 0.0159 (5) | 0.0026 (6) |
| C8' | 0.0444 (8) | 0.0390 (9) | 0.0466 (8) | 0.0006 (6) | 0.0223 (6) | 0.0053 (6) |
| C9' | 0.0560 (9) | 0.0530 (10) | 0.0492 (8) | 0.0069 (8) | 0.0265 (7) | 0.0185 (8) |
| C10' | 0.0478 (8) | 0.0758 (13) | 0.0375 (7) | 0.0002 (8) | 0.0198 (6) | 0.0123 (8) |
| C11' | 0.0554 (9) | 0.0616 (11) | 0.0402 (8) | −0.0002 (8) | 0.0226 (7) | −0.0054 (7) |
| C12' | 0.0472 (8) | 0.0404 (8) | 0.0425 (8) | 0.0024 (7) | 0.0179 (6) | 0.0008 (6) |
| C13' | 0.0312 (7) | 0.0365 (8) | 0.0388 (7) | 0.0026 (6) | 0.0154 (5) | 0.0036 (6) |
| O14' | 0.0690 (7) | 0.0428 (7) | 0.0385 (5) | 0.0114 (5) | 0.0181 (5) | −0.0018 (5) |
| O15' | 0.0582 (7) | 0.0390 (6) | 0.0432 (5) | −0.0003 (5) | 0.0195 (5) | 0.0086 (4) |
Geometric parameters (Å, º)
| N1—C2 | 1.4792 (19) | N1'—C2' | 1.475 (2) |
| N1—H1A | 0.945 (18) | N1'—H1'A | 0.882 (19) |
| N1—H1B | 0.902 (16) | N1'—H1'B | 0.897 (17) |
| N1—H1C | 0.925 (18) | N1'—H1'C | 0.916 (18) |
| C2—C3 | 1.498 (2) | C2'—C3' | 1.505 (2) |
| C2—H2A | 0.9900 | C2'—H2'A | 0.9900 |
| C2—H2B | 0.9900 | C2'—H2'B | 0.9900 |
| C3—N4 | 1.468 (2) | C3'—N4' | 1.460 (2) |
| C3—H3A | 0.9900 | C3'—H3'A | 0.9900 |
| C3—H3B | 0.9900 | C3'—H3'B | 0.9900 |
| N4—C5 | 1.4625 (19) | N4'—C6' | 1.4640 (18) |
| N4—C6 | 1.463 (2) | N4'—C5' | 1.468 (2) |
| C5—C6i | 1.506 (2) | C5'—C6'ii | 1.502 (2) |
| C5—H5A | 0.9900 | C5'—H5'A | 0.9900 |
| C5—H5B | 0.9900 | C5'—H5'B | 0.9900 |
| C6—C5i | 1.506 (2) | C6'—C5'ii | 1.502 (2) |
| C6—H6A | 0.9900 | C6'—H6'A | 0.9900 |
| C6—H6B | 0.9900 | C6'—H6'B | 0.9900 |
| C7—C8 | 1.383 (2) | C7'—C12' | 1.383 (2) |
| C7—C12 | 1.390 (2) | C7'—C8' | 1.388 (2) |
| C7—C13 | 1.5127 (18) | C7'—C13' | 1.5125 (18) |
| C8—C9 | 1.389 (2) | C8'—C9' | 1.385 (2) |
| C8—H8 | 0.9500 | C8'—H8' | 0.9500 |
| C9—C10 | 1.378 (3) | C9'—C10' | 1.383 (3) |
| C9—H9 | 0.9500 | C9'—H9' | 0.9500 |
| C10—C11 | 1.380 (3) | C10'—C11' | 1.375 (3) |
| C10—H10 | 0.9500 | C10'—H10' | 0.9500 |
| C11—C12 | 1.387 (2) | C11'—C12' | 1.389 (2) |
| C11—H11 | 0.9500 | C11'—H11' | 0.9500 |
| C12—H12 | 0.9500 | C12'—H12' | 0.9500 |
| C13—O14 | 1.2436 (17) | C13'—O15' | 1.2447 (17) |
| C13—O15 | 1.2690 (17) | C13'—O14' | 1.2599 (17) |
| C2—N1—H1A | 105.8 (9) | C2'—N1'—H1'A | 106.7 (11) |
| C2—N1—H1B | 106.8 (9) | C2'—N1'—H1'B | 107.8 (10) |
| H1A—N1—H1B | 111.6 (14) | H1'A—N1'—H1'B | 110.8 (14) |
| C2—N1—H1C | 111.9 (10) | C2'—N1'—H1'C | 111.8 (10) |
| H1A—N1—H1C | 113.4 (14) | H1'A—N1'—H1'C | 109.5 (14) |
| H1B—N1—H1C | 107.2 (14) | H1'B—N1'—H1'C | 110.2 (15) |
| N1—C2—C3 | 111.87 (13) | N1'—C2'—C3' | 111.07 (12) |
| N1—C2—H2A | 109.2 | N1'—C2'—H2'A | 109.4 |
| C3—C2—H2A | 109.2 | C3'—C2'—H2'A | 109.4 |
| N1—C2—H2B | 109.2 | N1'—C2'—H2'B | 109.4 |
| C3—C2—H2B | 109.2 | C3'—C2'—H2'B | 109.4 |
| H2A—C2—H2B | 107.9 | H2'A—C2'—H2'B | 108.0 |
| N4—C3—C2 | 113.21 (13) | N4'—C3'—C2' | 112.22 (13) |
| N4—C3—H3A | 108.9 | N4'—C3'—H3'A | 109.2 |
| C2—C3—H3A | 108.9 | C2'—C3'—H3'A | 109.2 |
| N4—C3—H3B | 108.9 | N4'—C3'—H3'B | 109.2 |
| C2—C3—H3B | 108.9 | C2'—C3'—H3'B | 109.2 |
| H3A—C3—H3B | 107.8 | H3'A—C3'—H3'B | 107.9 |
| C5—N4—C6 | 108.42 (11) | C3'—N4'—C6' | 110.11 (12) |
| C5—N4—C3 | 109.47 (12) | C3'—N4'—C5' | 112.79 (12) |
| C6—N4—C3 | 111.96 (12) | C6'—N4'—C5' | 108.54 (11) |
| N4—C5—C6i | 111.48 (13) | N4'—C5'—C6'ii | 110.65 (12) |
| N4—C5—H5A | 109.3 | N4'—C5'—H5'A | 109.5 |
| C6i—C5—H5A | 109.3 | C6'ii—C5'—H5'A | 109.5 |
| N4—C5—H5B | 109.3 | N4'—C5'—H5'B | 109.5 |
| C6i—C5—H5B | 109.3 | C6'ii—C5'—H5'B | 109.5 |
| H5A—C5—H5B | 108.0 | H5'A—C5'—H5'B | 108.1 |
| N4—C6—C5i | 111.13 (12) | N4'—C6'—C5'ii | 111.14 (12) |
| N4—C6—H6A | 109.4 | N4'—C6'—H6'A | 109.4 |
| C5i—C6—H6A | 109.4 | C5'ii—C6'—H6'A | 109.4 |
| N4—C6—H6B | 109.4 | N4'—C6'—H6'B | 109.4 |
| C5i—C6—H6B | 109.4 | C5'ii—C6'—H6'B | 109.4 |
| H6A—C6—H6B | 108.0 | H6'A—C6'—H6'B | 108.0 |
| C8—C7—C12 | 118.90 (13) | C12'—C7'—C8' | 119.15 (12) |
| C8—C7—C13 | 119.50 (13) | C12'—C7'—C13' | 119.82 (13) |
| C12—C7—C13 | 121.57 (12) | C8'—C7'—C13' | 120.99 (13) |
| C7—C8—C9 | 120.39 (16) | C9'—C8'—C7' | 120.50 (14) |
| C7—C8—H8 | 119.8 | C9'—C8'—H8' | 119.7 |
| C9—C8—H8 | 119.8 | C7'—C8'—H8' | 119.7 |
| C10—C9—C8 | 120.31 (16) | C10'—C9'—C8' | 119.60 (15) |
| C10—C9—H9 | 119.8 | C10'—C9'—H9' | 120.2 |
| C8—C9—H9 | 119.8 | C8'—C9'—H9' | 120.2 |
| C9—C10—C11 | 119.78 (15) | C11'—C10'—C9' | 120.50 (14) |
| C9—C10—H10 | 120.1 | C11'—C10'—H10' | 119.7 |
| C11—C10—H10 | 120.1 | C9'—C10'—H10' | 119.7 |
| C10—C11—C12 | 120.04 (16) | C10'—C11'—C12' | 119.65 (16) |
| C10—C11—H11 | 120.0 | C10'—C11'—H11' | 120.2 |
| C12—C11—H11 | 120.0 | C12'—C11'—H11' | 120.2 |
| C11—C12—C7 | 120.57 (14) | C7'—C12'—C11' | 120.56 (15) |
| C11—C12—H12 | 119.7 | C7'—C12'—H12' | 119.7 |
| C7—C12—H12 | 119.7 | C11'—C12'—H12' | 119.7 |
| O14—C13—O15 | 123.91 (12) | O15'—C13'—O14' | 123.96 (12) |
| O14—C13—C7 | 118.44 (12) | O15'—C13'—C7' | 118.02 (12) |
| O15—C13—C7 | 117.65 (12) | O14'—C13'—C7' | 118.01 (12) |
| N1—C2—C3—N4 | −66.32 (18) | N1'—C2'—C3'—N4' | −56.03 (18) |
| C2—C3—N4—C5 | 164.89 (13) | C2'—C3'—N4'—C6' | 168.40 (13) |
| C2—C3—N4—C6 | −74.84 (16) | C2'—C3'—N4'—C5' | −70.19 (16) |
| C6—N4—C5—C6i | 57.36 (17) | C3'—N4'—C5'—C6'ii | 179.90 (11) |
| C3—N4—C5—C6i | 179.76 (12) | C6'—N4'—C5'—C6'ii | −57.80 (16) |
| C5—N4—C6—C5i | −57.14 (17) | C3'—N4'—C6'—C5'ii | −177.99 (12) |
| C3—N4—C6—C5i | −178.01 (11) | C5'—N4'—C6'—C5'ii | 58.10 (16) |
| C12—C7—C8—C9 | −1.1 (2) | C12'—C7'—C8'—C9' | 1.6 (2) |
| C13—C7—C8—C9 | 176.94 (13) | C13'—C7'—C8'—C9' | −176.24 (13) |
| C7—C8—C9—C10 | 1.0 (2) | C7'—C8'—C9'—C10' | −1.2 (2) |
| C8—C9—C10—C11 | −0.1 (2) | C8'—C9'—C10'—C11' | −0.3 (2) |
| C9—C10—C11—C12 | −0.8 (2) | C9'—C10'—C11'—C12' | 1.4 (2) |
| C10—C11—C12—C7 | 0.7 (2) | C8'—C7'—C12'—C11' | −0.5 (2) |
| C8—C7—C12—C11 | 0.28 (19) | C13'—C7'—C12'—C11' | 177.32 (13) |
| C13—C7—C12—C11 | −177.74 (12) | C10'—C11'—C12'—C7' | −0.9 (2) |
| C8—C7—C13—O14 | 7.89 (17) | C12'—C7'—C13'—O15' | −6.79 (19) |
| C12—C7—C13—O14 | −174.10 (12) | C8'—C7'—C13'—O15' | 171.02 (13) |
| C8—C7—C13—O15 | −172.22 (12) | C12'—C7'—C13'—O14' | 172.71 (13) |
| C12—C7—C13—O15 | 5.79 (17) | C8'—C7'—C13'—O14' | −9.48 (19) |
Symmetry codes: (i) −x, −y, −z; (ii) −x, −y+2, −z+1.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···O15iii | 0.945 (18) | 1.833 (19) | 2.7739 (17) | 173.0 (15) |
| N1—H1B···O15 | 0.902 (16) | 1.895 (17) | 2.7836 (15) | 167.8 (14) |
| N1—H1B···O14 | 0.902 (16) | 2.632 (16) | 3.2580 (16) | 127.2 (13) |
| N1—H1C···O14iv | 0.925 (18) | 1.887 (18) | 2.7660 (16) | 157.9 (14) |
| N1′—H1′A···O14′ | 0.882 (19) | 1.857 (19) | 2.7355 (17) | 174.2 (15) |
| N1′—H1′B···O14′v | 0.897 (17) | 1.908 (17) | 2.7836 (16) | 164.8 (15) |
| N1′—H1′B···O15′v | 0.897 (17) | 2.533 (16) | 3.1858 (16) | 130.1 (13) |
| N1′—H1′C···O15′vi | 0.916 (18) | 1.934 (18) | 2.7585 (16) | 148.8 (14) |
Symmetry codes: (iii) −x+1/2, y+1/2, −z+1/2; (iv) −x+1/2, y−1/2, −z+1/2; (v) −x+1/2, y+1/2, −z+3/2; (vi) x, y+1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: JJ2134).
References
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst. 27, 435.
- Blessing, R. H. (1995). Acta Cryst. A51, 33–38. [DOI] [PubMed]
- Cason, C. J. (2004). POV-RAY for Windows Persistence of Vision Raytracer Pty Ltd, Victoria, Australia. URL: http://www.povray.org.
- Cukrowski, I., Adeyinka, A. S. & Liles, D. C. (2012). Acta Cryst E68, o2387. [DOI] [PMC free article] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Jiang, X., Liu, H.-X., Wu, S.-L. & Liang, Y.-X. (2009). Jiegou Huaxue (Chin. J. Struct. Chem), 28, 723–729.
- Junk, P. C. & Smith, M. K. (2005). C. R. Chim. 8, 189–198.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Nonius (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812030115/jj2134sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812030115/jj2134Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812030115/jj2134Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




