Summary
Human neonates are at significantly greater risk of serious infection than immunocompetent adults. In particular, very low birth weight infants in the neonatal intensive care nursery are at high risk of developing life-threatening bacterial and fungal infections. Recent studies have identified Th17 cells as critical mediators of immunity to bacterial and fungal infections at epithelial barriers. Little is known, however, about the ontogeny of Th17 responses in humans. The frequency of serious bacterial infections in preterm infants and the importance of Th17 cells in providing protection against such infections in animal studies prompted us to study Th17 development in human neonates. NaÔve CD4 T cells from extremely preterm infants, term infants, and adults were assayed for their capacity to develop into Th17 effector cells. Surprisingly, Th17 capacity was inversely related to developmental age. Neonates expressed higher levels of IL-23R, RORγt, and STAT3 prior to activation and showed a significant Th17 bias after activation. In contrast, adult cells expressed more TBX21 with a corresponding Th1 bias. CD161 expression on Th17 precursors was also developmentally regulated. Our results suggest there is significant developmental regulation of CD4 effector lineages with a strong bias toward Th17 development early in life.
Keywords: Th17 cell, premature infant, CD161, neonatal immunity, T-helper subsets
Introduction
Developmental limitations of immune function place human neonates at significantly greater risk of serious infection than immunologically competent adults.[1] Intracellular pathogens such as cytomegalovirus and herpes simplex virus, which typically cause only limited disease in adults, often result in severe systemic infections in infants due to poor cell-mediated immunity.[2] Very low birth weight (<1500 g) (VLBW) preterm infants additionally suffer frequent extracellular bacterial and fungal infections at rates that vary inversely with birth weight, suggesting a continuum of immunologic development. [3]
Immune responses are strongly influenced by the type of CD4 T cells participating in the response.[4] Th1 cells, which promote cell-mediated immunity to intracellular pathogens, develop from naÔve CD4 T cells after activation via T cell receptor engagement in the presence of IL-12. Th1 cells express the Th1 lineage-associated transcription factor Tbet that drives secretion of IFN-γ the signature cytokine of Th1 cells. Th2 cells develop after activation in the presence of IL-4 and express the lineage-associated transcription factor GATA3. Th2 cells secrete IL-4, IL-5, IL-10, and IL-13, and are important in responses against helminthes and environmental allergens. A more recently discovered subset, Th17 cells, express the lineage-associated transcription factor RORγt and secrete IL-17A and IL-17F as well as IL-21 and IL-22. Th17 cells are critical in protecting against bacterial and fungal infections and in linking the innate and adaptive immune responses, particularly at epithelial surfaces.[5–11] Patients deficient in Th17 function due to mutations in IL-17 or IL-6 signaling pathways suffer intractable fungal infections (chronic mucocutaneous candidiasis) and bacterial infections (Job’s syndrome) at epithelial barriers.[12, 13] In mice, activation of naÔve T cells in the presence of TGF-β and IL-6 is sufficient to generate Th17 cells, with IL-1, IL-21, and IL-23 all contributing to full phenotype development.[14–18] In humans, the ideal conditions for Th17 development are less clear, but IL-1, IL-6, IL-23, TGF-β, and PGE2 have all been reported to play a role.[19–24]
Currently, very little is known about the ontogeny of Th17 cells in humans. Term infant cord blood has been reported to contain a population of CD161+ CD4+ cells that preferentially develop into Th17 cells when compared to CD161− CD4+ cells, but the timing of the development of this population is unknown.[25] Given the clinical importance of bacterial infections in VLBW preterm infants and the growing recognition of the importance of Th17 cells in protection from bacterial infections at epithelial surfaces, we investigated the capacity of preterm and term infants to develop Th17 cells. We hypothesized that VLBW infants would demonstrate developmental limitations in Th17 capacity similar to the well-documented developmental limitations in Th1 capacity previously observed in term infants.[2] Surprisingly, we found that Th17 lineage capacity inversely correlated with age such that T cells from extremely preterm infants had the greatest tendency to develop into Th17 cells, followed by term infants, and then by adult naÔve T cells which demonstrated little or no ability to become Th17 cells. This was in contrast with Th1 responses in which adults showed a significant Th1 bias compared to infants. Taken together, our results suggest that human Th17 responses are highly developmentally regulated.
Results
Developmental Regulation of Th17, Th1 and Th2 Capacity
We began by asking whether CD4 T cells from preterm and term infants have the capacity to develop into Th17 cells or if they are restricted in their lineage development. To determine the Th17 capacity of neonatal CD4 T cells, we purified CD45RA+ CD45RO− CD4+ naÔve T cells from preterm and term infant cord blood and adult peripheral blood and stimulated them in vitro with anti-CD3/CD28 microbeads under Th0 conditions (only low-dose IL-2 was added) or Th17 polarizing conditions (with IL-1, IL-6, IL-23, low-dose TGF-β and low-dose IL-2 added). As a control, memory CD45RA− CD45RO+ CD4+ T cells from adults were also stimulated. After 3 days, supernatants were collected and assayed by multiplex bead array and ELISA for Th17 cytokines (IL-17 and IL-21), Th1 cytokines (IFN-γ), and Th2 cytokines (IL-4, IL-10, and IL-13). Under Th0 conditions, little IL-17 or IL-21 was detected in any of the samples except the adult memory cells (Figures 1 and S1). Consistent with previous reports[26] preterm cells produced more IL-4 (p<0.05 vs. term and adult RA) and IL-10 (p<0.01 vs. adult RA) with a trend toward more IL-13 as well, suggesting a significant Th2 bias (Figures 1 and S2). Under Th17 conditions, both preterm and term infants produced more IL-17 compared to adult naÔve cells (p<0.01) (Figure 1). Preterm infants also produced significantly more IL-21 (Figure S1). Even under the influence of Th17 polarizing conditions, neonatal cells maintained a significant Th2 bias compared to adult naÔve cells with higher levels of IL-4 (p<0.01 preterm vs. adult, p<0.05 term vs. adult), IL-10 (p<0.01 for term or preterm vs. adult), and IL-13 (p<0.05 term vs. adult). IL-22 was not detected at significant levels in any of the samples. Similar results were seen in separate experiments with different patient samples after 6 days of culture, suggesting that these patterns of cytokine secretion are temporally robust.
Figure 1.
Preterm and term infant naÔve CD4 T cells secrete significantly more Th17 and Th2 cytokines. Purified naÔve CD4 T cells from preterm infants (○), term infants (□), or adults (∆), or memory CD4 T cells from adults (∇) were stimulated in vitro with CD3/CD28 beads under Th0 conditions with only IL-2 added (open symbols) or under Th17-polarizing conditions with IL-1, IL-2, IL-6, IL-23 and low-dose TGF-β added (closed symbols). On day 3, supernatants were collected and assayed by multiplex cytokine bead array analysis. Each symbol represents a separate patient. Bars indicate mean values and significant differences between groups (Mann-Whitney test) are indicated.
To determine if the higher levels of Th17 cytokines observed in infant samples correlated with greater numbers of Th17 cells, we analyzed cultured preterm, term, and adult samples at the single cell level by intracellular cytokine staining and flow cytometry. Samples were cultured under Th0 or Th17 conditions for 6 days, then re-stimulated with PMA, ionomycin, and brefeldin A for 5 hours prior to intracellular cytokine staining. In agreement with the multiplex and ELISA data, both preterm and term infants were capable of developing distinct Th17 populations (Figure 2). In contrast, naÔve RA+ RO− adult cells rarely developed significant numbers of Th17 cells, instead showing higher numbers of Th1 cells. In adult samples, Th17 cells were found only in cultured RO+RA− memory cells.
Figure 2.
Th17 cells develop from neonatal but not from naÔve adult T cells. Purified (A) VLBW preterm, (B) term, (C) adult naÔve, and (D) adult memory CD4+ T cells were stimulated with CD3/CD28 beads under Th17 polarizing conditions. On day 6 of culture, the cells were re-stimulated with PMA, ionomycin, and brefeldin A for 5 hours, then fixed, permeabilized, and stained for IL-17 and IFN-γ. Shown are representative plots of 10 or more similar experiments.
Gene expression of Receptors and Transcription Factors
A number of signaling pathways including IL-23/IL-6/Stat3 and TGF-β and are known to be important for RORγt induction and Th17 function. To determine if differential gene expression in the upstream signaling pathways for Th17 induction in neonatal CD4 T cells was responsible for increased Th17 development compared to adult cells, we conducted a limited gene expression profile of key upstream receptors and transcription factors. RNA was collected from purified preterm, term, and adult naÔve cells and real time PCR analysis was performed to assess gene expression of the receptors of the cytokines added under Th17 conditions including IL-1R, IL-6R, IL-23R, TGF-βRI and TGF-βRII as well as for the T-cell fate-determining transcription factors RORC(RORγt) and TBX21(Tbet). As shown in Figure 3, unmanipulated naÔve neonatal cells already showed higher expression levels of many important Th17 genes including IL-23R, STAT3, and RORC, which were 36-, 3.3-, and 3.2-fold higher respectively in preterm infants than adult cells and 98-, 2.2-, and 4.2-fold higher in term infants than adult cells. In contrast, TBX21 expression was significantly lower with 0.3-fold expression in preterm and 0.2-fold expression in term infants compared to adults. These results suggest that even before activation, neonatal cells are predisposed toward Th17 responses whereas adult cells are predisposed toward Th1 responses.
Figure 3.
Th17 gene expression in resting naÔve neonatal CD4+ cells relative to adult cells. Real time PCR for the indicated genes was performed on samples from purified naÔve preterm, term, or adult T cells prior to activation. Fold differences in gene expression were calculated using the ΔΔCt method. Bars indicate averages of 4 independent pairings of neonatal and adult samples.
Influence of IFN-γ on Adult Th17 Development
In mice, IFN-γ is known to inhibit Th17 development.[27] Given that adult naÔve CD4 T cells produce more IFN-γ than neonatal cells, we sought to determine whether autocrine IFN-γ signaling was responsible for the apparent limitation on adult Th17 development. Sorted naÔve RA+RO− CD4+ cells were stimulated under Th17 conditions with and without the addition of 10 µg/ml anti-IFN-γ antibody. Although addition of the antibody successfully reduced IFN-γ in the supernatants to undetectable levels (not shown), it had minimal effect on adult Th17 development, suggesting that other mechanisms contribute to the limitation of adult Th17 differentiation (Figure 4).
Figure 4.
Inhibition of IFN-γ does not increase IL-17 production from adult naÔve cells. Purified adult CD4+RA+RO− cells were cultured under Th17 conditions with or without 10 µg/ml of anti-IFN-γ added. On day 3, IL-17 in the supernatant was measured by multiplex cytokine bead array.
Role of CD161+ Th17 Precursors
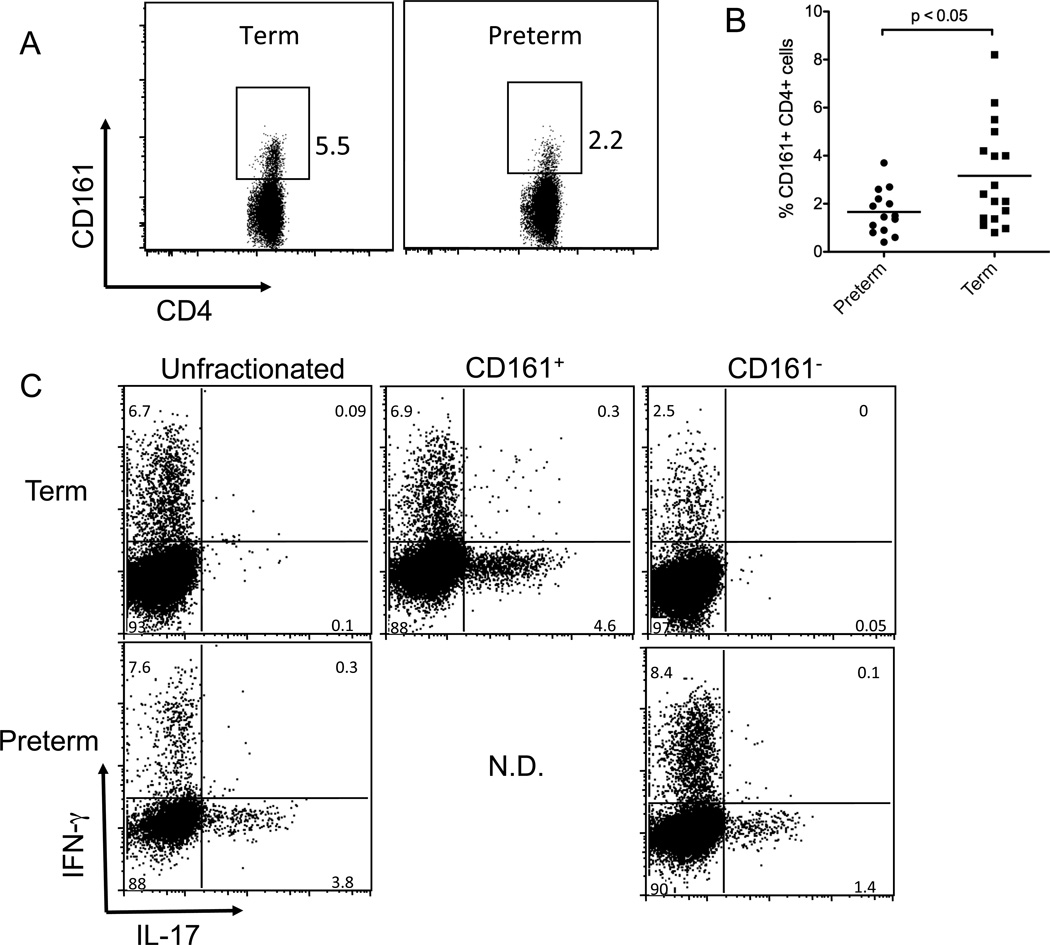
Cosmi et al have reported that cord blood Th17 cells develop primarily from a CD161+ CD4+ precursor population and that this population is largely absent in adult naÔve cells. [25] Given that preterm infants T cells generate equivalent or higher levels of IL-17 and IL-21 and show more Th17 cells by flow cytometry after culture under Th17 conditions, we considered that preterm infants might have higher numbers of the CD161+ Th17 precursor population. Preterm and term cells were analyzed by flow cytometry to determine the CD161+ fraction within the live CD3+CD4+ T cell population after gating out contaminating CD56+ NK cells, CD8+, CD16+, and CD25+ cells. In spite of higher capacity for Th17 differentiation, preterm infants had significantly fewer CD161+ Th17 precursor cells prior to stimulation (p<0.05) (Figure 5) suggesting that Th17 cells can develop from CD161− precursors in preterm infants.
Figure 5.
Preterm infants generate significant Th17 responses but have fewer CD161+ CD4+ Th17 precursor cells than term infants. CD4 cells were purified from preterm and term cord blood samples as described then preserved in liquid nitrogen. On the day of analysis, cells were thawed, washed, and stained with antibodies for CD3, CD4, CD161, CD56, and CD25 and with vital dye then analyzed by flow cytometry (A). Shown are representative plots of term and preterm samples with the percentages of CD161+CD4+CD3+ cells indicated after gating off of dead cells and CD56+ or CD25+ cells. The data for all the patient samples are summarized in (B), with bars representing the mean. To determine if Th17 cells could develop from CD161− precursors, samples were depleted of CD161+ cells using magnetic beads. Unfractionated cells and the CD161+ and CD161− fractions were stimulated separately for 6 days under Th17 conditions then re-stimulated and analyzed for IFN-γ and IL-17 production by flow cytometry. Recovery and growth of preterm CD161+ cells was consistently poor and is omitted from the figure. Shown are representative data from one of four experiments.
To determine the relative contribution of CD161+ precursors to the Th17 population in preterm and term infants, we assessed Th17 development from the CD161+ and CD161− fractions in preterm and term infants. CD161+ and CD161-depleted fractions were purified from preterm and term cord CD4+ cells by magnetic bead depletion. The CD161+ and CD161− fractions were then stimulated under Th17 conditions (Figure 5C). Consistent with our flow cytometry data (Figures 5A and 5B), fewer CD161+ cells were obtained from preterm blood and these cells were difficult to culture. (In four separate attempts we were unable to culture enough CD161+ cells from preterm samples to analyze.) However, the remaining CD161− cells still developed a robust Th17 response. In contrast, significant numbers of CD161+ cells were obtained from most term samples. These consistently showed robust Th17 development while the CD161− fraction from term infants demonstrated weak Th17 development (Figure 5C). Together these results suggest that CD161 expression on Th17 precursors is also developmentally regulated.
Discussion
Infections remain a leading cause of infant morbidity and mortality worldwide.[28] Preterm infants in particular suffer numerous bacterial and fungal infections with nearly 40% of extremely low birth weight infants having a positive blood culture prior to discharge.[3] These infections have serious consequences: in spite of antibiotics and advanced medical care, infected VLBW preterm infants are significantly more likely to die,[3] to develop chronic lung disease,[29, 30] and to suffer poor neurologic outcomes.[31, 32] Some of these infections are undoubtedly related to catheters and other invasive aspects of intensive medical care, but many occur spontaneously across epithelial barriers due to developmental limitations of the immune system.
Th17 cells and their cytokines are now recognized to be critical players in antibacterial and antifungal immunity at epithelial surfaces. In animal models, IL-17 and IL-22 have been shown to be important for immunity to infections from Klebsiella, Citrobacter, Salmonella, and Candida species, all of which are important pathogens in the neonatal period. [3, 7–9, 14, 33, 34] In humans, patients with defects in Th17 function secondary to defects in the IL-6 or IL-17 signal transduction pathways suffer numerous infections from Staphylococcal, streptococcal, and fungal species- organisms that are also common culprits in neonatal sepsis and pneumonia.[12, 13]
We hypothesized that developmental limitations on Th17 function might contribute to neonatal susceptibility to infections, particularly in VLBW infants in whom extracellular bacterial and fungal infections are so common. Using purified populations of naÔve CD4 T cells from preterm, term, and adult patients, we tested the potential of these cells to develop into Th17 cells by stimulating them in vitro under neutral and Th17 polarizing conditions. In contrast to our hypothesis, we found that cells from term and preterm infants were quite capable of developing into Th17 cells. In fact, preterm infant CD4 T cells showed similar or greater Th17 differentiation compared to term infant samples, which in turn produced significantly more Th17 cytokines than naÔve adult samples. (Figures 1, 2, and 4) Consistent with previous reports,[26] we also saw a Th2 bias in the preterm samples with more IL-4, IL-13, and IL-10 production, and an overall impairment of IFN-γ secretion in both term and preterm cells.[35–37] Taken together, our results show there is significant developmental regulation of CD4 helper lineage development with Th17 and Th2 being favored over Th1 development early in life.
The mechanism for increased Th17 potential in infants relative to adults appears to involve increased expression of key upstream Th17 signaling components and transcription factors at baseline in naÔve neonatal CD4 T cells. We induced Th17 development using TCR activation in the presence of a cocktail of cytokines including IL-23, IL-6, IL-1, and TGF-β. Interestingly expression of IL-23R, STAT3, RORC, IL6ST(gp130), and TGFβR1 all were higher in resting naÔve neonatal cells than in adult cells suggesting that neonatal cells were preprogrammed to have a Th17 bias. In contrast, adult cells had lower levels of IL23R, STAT3, and RORC (but higher levels of TBX21), and very few adult cells successfully differentiated into Th17 cells. Aside from differences in cytokine receptor and transcription factor expression, epigenetic mechanisms could also be playing a role in the developmental regulation of Th17 function as reported for other cytokines.[38] IFN-γ-mediated suppression of Th17 development in the adult samples does not appear to dictate poor adult Th17 differentiation because neutralization of IFN-γ had no effect (Figure 3).
Our results indicate that expression of CD161, which has been reported by Cosmi et al [25] to mark Th17 precursor cells in cord blood, is developmentally regulated as well. Preterm infants have fewer CD161+ cells than term infants yet are capable of generating significant Th17 responses in vitro (Figures 1 and 5). In contrast to term infant CD4 T cells, in which most of the Th17 potential is contained in the CD161+ fraction, CD161− cells from preterm infants can give rise to Th17 cells in vitro (Figure 5), suggesting that up-regulation of CD161 on Th17 precursors occurs late in gestation. Preterm CD161+ cells may also give rise to Th17 cells, but we were unable to isolate and culture sufficient numbers of these to assess this. CD161+ cells do not pass through a CD161+ state in vitro prior to differentiating into Th17 cells, and in fact CD161 was down-regulated on all our samples under our activation conditions (data not shown). To date the function of CD161 on Th17 cells is unclear. It is possible that CD161 is important for full function of Th17 cells at mucosal surfaces in vivo, and its low expression on Th17 precursors in preterm infants could contribute to their susceptibility to bacterial infections. In adults, these cells may have already migrated to the gut and lung and other epithelial barriers, and are found only rarely in peripheral blood, which limits the ability to culture Th17 cells from adult naÔve cells in vitro.
Our study is one of only a few reports of T-helper subset development in VLBW infants, and to our knowledge is the only report on the ontogeny of human Th17 capacity from premature infants to adults. We went to significant effort to use only very pure naÔve CD4+ populations and used anti-CD3 and anti-CD28 stimulation to ensure cytokine production was exclusively T-cell-derived and not due to contaminating memory cells in the adult samples. The study is limited in that, although reflective of typical clinical situations, the preterm samples might not reflect normal in utero development since by definition preterm delivery is not a normal event, and all preterm samples were exposed to maternal prenatal steroids. To minimize the possibility of inflammatory cytokines influencing the results, patients were excluded if maternal chorioamnionitis was suspected. The limited IL-17 produced by adult cells in our system is in agreement with Cosmi, et al [25] and Evans, et al [39] who were unable to see significant Th17 development from naÔve adult cells. In contrast, contrast Wilson, et al [40] and Acosta-Rodriguez, et al [41] saw modest but easily detectable IL-17 production. These differences may be due to the prolonged culture systems used in the second two references which could eventually overcome the inherent limitations of adult cells in differentiating into Th17 cells or could allow minor populations of contaminating memory Th17 cells to expand. Regardless, in our system comparing parallel cultures of purified neonatal and adult naÔve CD4 cells, there was clear bias toward Th17 development in the neonatal samples.
Why then are preterm infants so susceptible to infections at mucosal interfaces? Although we have shown that they have the capacity to become Th17 cells in vitro, preterm CD4 T cells may fail to do so in vivo as a result of inadequate antigen presentation and cytokine signals since antigen presenting cell responses to TLR ligands are known to be developmentally regulated.[42] Preterm CD4 T cells also may be unprepared to localize to mucosal barriers due to inadequate expression of CD161, decreased CCR6, or other characteristic Th17 genes. Homing signals such as CCL20 in the gut are induced in response to TLR-mediated epithelial recognition of intestinal flora. Preterm infants lacking normal flora may lack the ability to attract Th17 cells mucosal interfaces and may lack the cytokines necessary for Th17 differentiation. Also, although the present study focuses on Th17 cells, many other cell types support mucosal barrier function by producing IL-17 and IL-22 including NK T cells, γδ T cells, and lymphoid tissue inducer cells. These cells may be particularly important in bridging early innate responses to infection with adaptive immunity, but little is known about their functional capacity in preterm infants. Poor mucosal immunity in preterm infants may reflect developmental limitations of one or more of these other cell types. Interestingly, the capacity to produce IL-17 in preterm infants is associated with a lower risk of blood stream infections and chronic lung disease suggesting that IL-17 pathways are clinically important.[43, 44] Future studies investigating the cellular sources of IL-17 and IL-22 across development as well as ways to augment these defenses in Th17 and other cell types will be of great interest.
In summary, we have shown that, compared to adult cells, neonatal CD4 T cells exhibit a significant Th17 bias in addition to the previously reported Th2 bias but are restricted in Th1 responses. In neonatal cells, multiple genes important for Th17 function including IL23R, RORC, and STAT3, are expressed at higher levels than in adults. In preterm infants, Th17 can develop from CD161− cells whereas in term infants Th17 cells develop primarily from a CD161+ precursor population suggesting a developmental progression of the Th17 lineage. After birth, newborn infants must transition from the sterile intrauterine environment to the outside world where they are rapidly and heavily colonized with a multitude of commensal bacterial species. A bias toward Th17 responses may facilitate this transition and help keep colonizing microbes in check.
Methods
Isolation of naÔve CD4+ T cells
Umbilical venous cord blood was drawn in a sterile manner from the placentas of 18 healthy term infants (>37 weeks gestational age (GA)) and 16 very low birth weight preterm infants (24 weeks to 31 weeks GA). Infants were excluded if chorioamnionitis was suspected. Mothers of all preterm infants received antenatal steroids prior to delivery. Adult peripheral blood samples were obtained by sterile venipuncture from 12 healthy volunteers or adult buffy coats were commercially obtained (Research Blood Components, LLC). All studies were approved by the UAB Internal Review Board, and informed consent was obtained from all participants.
CD4+ T cells were isolated using RosetteSep Human CD4+ T cell enrichment cocktail (Stem Cell Technologies) according to the manufacturer’s instructions. The cells were then incubated with anti-CD45RO antibody (eBioscience) followed by a depletion step with magnetic goat-anti-mouse beads to remove any residual non-CD4 cells as well as any CD45RO+ cells. The resulting cord cells were consistently >97% pure CD4+ CD45RA+CD45RO− T cells while adult samples had residual CD45RO+ memory cells. All adult samples were further purified to >97% by staining with antibodies against CD3, CD4, CD45RA, CD45RO (eBioscience) and flow cytometry sorting for the CD3+CD4+CD45RA+CD45RO− naÔve fraction. For some samples, sorted CD45RO+ cells were saved and used as positive controls for cytokine production.
Stimulation of CD4 T cells
Purified cord and adult CD4 T cells were cultured for 3 days in a 24-well plate (Costar) at a density of 106 cells per well with Human T-Activator CD3/CD28 Dynabeads (Invitrogen) at a 1:1 bead to cell ratio in T cell media (Iscove’s Modified Dulbecco’s Media supplemented with 10% fetal calf serum (HyClone), 2 mM L-Glutamine (Cellgro), 100 ug/ml Penicillin- Streptomycin (Cellgro), 1 µg/ml Fungizone (Gibco), 110 µg/ml NaPyruvate (Sigma), 50 µM 2- mercaptoethanol (Sigma- Aldrich)). For neutral conditions, only IL-2 (Peprotech) at 25 U/ml was added. For Th17 polarizing conditions, IL-1 at 10 ng/ml (Peprotech), IL-6 at 10 ng/ml (Peprotech), IL-23 at 10 ng/ml (R&D Systems), and TGF-β at 3 ng/ml (R&D Systems) were added. After incubation for 72 hours at 37°C in 7% CO2 humidified air, supernatants were harvested and stored at −20°C for further cytokine determination. Remaining cells underwent surface and intracellular staining and flow cytometry. For IFN-γ neutralization experiments, 10 µg/ml anti-IFN-γ (R&D Systems) was added the cultures.
Flow Cytometry
For intracellular cytokine staining, cells were cultured for 5 hours with 10 ng/ml PMA, 500 ng/ml ionomycin and 5 µg/ml brefeldin A (all from Sigma). 106 cells were stained for viability with Live/Dead dye (Invitrogen), then fixed, permeabilized (Cytofix/Cytoperm; BD Biosciences) and stained with anti-human IL-17A and anti-human IFN-γ (eBioscience). For surface CD161 staining, cells were stained for viability, then stained with anti-CD3, anti-CD4, and anti-CD161. To eliminate non-CD4 T cells from the analyses, anti-CD8, anti-CD25, anti-CD56, and anti-CD16 were added and excluded as a “dump” channel. Data for all samples were collected on an LSRII flow cytometer (BD), and analyzed using FlowJo software (Treestar).
Cytokine Analysis
Culture supernatants were analyzed for IL-4, IL-10, IL-13, IL-17, and IFN-γ using Milliplex MAP Human Cytokine Immunoassay technology (Millipore) per the manufacturer’s instructions. IL-21 in the supernatants was quantified by commercial ELISA (eBioscience).
Real Time PCR
RNA was isolated from purified CD4 T cells using Trizol reagent (Invitrogen), and first strand synthesis was accomplished with a Superscript III kit (Invitrogen) all per the manufacturer’s instructions. Real time PCR was performed with a BioRad iCycler system. Primer pairs used are listed in Table S1 in Supplementary data.
Statistics
Statistical analysis was performed using Prism software (GraphPad Software, Inc.) Differences between cytokine measurements and surface CD161 expression were deemed significant if the calculated two-tailed P-value of the non-parametric Mann-Whitney test was less than 0.05.
Supplementary Material
Acknowledgements
This work was supported by the University of Alabama at Birmingham Department of Pediatrics. DAR also received salary support from NIH R00HD056222.
Abbreviations
- VLBW
Very low birth weight
Footnotes
Conflict -of-interest: The authors declare no competing financial or commercial interests.
References
- 1.Lewis DB, Wilson CB. Developmental Immunology and Role of Host Defenses in Fetal and Neonatal Susceptibility to Infection. In: Remington JS, Klein JO, editors. Infectious Diseases of the Fetus and Newborn Infant. Seventh Edn. Philadelphia: Elsevier Saunders; 2011. pp. 80–191. [Google Scholar]
- 2.Randolph DA, Lewis DB. Transient deficiencies of T-cell-mediated immunity in the neonate. Adv Exp Med Biol. 2006;582:55–69. doi: 10.1007/0-387-33026-7_6. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 6.Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol. 2009;159:109–119. doi: 10.1111/j.1365-2249.2009.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 10.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int Immunol. 2008;20:1361–1368. doi: 10.1093/intimm/dxn106. [DOI] [PubMed] [Google Scholar]
- 12.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 15.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 18.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong E, Suddason T, Lord GM. Translational mini-review series on Th17 cells: development of mouse and human T helper 17 cells. Clin Exp Immunol. 2009;159:148–158. doi: 10.1111/j.1365-2249.2009.04041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 24.Chizzolini C, Chicheportiche R, Alvarez M, de Rham C, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasparoni A, Ciardelli L, Avanzini A, Castellazzi AM, Carini R, Rondini G, Chirico G. Age-related changes in intracellular TH1/TH2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol Neonate. 2003;84:297–303. doi: 10.1159/000073638. [DOI] [PubMed] [Google Scholar]
- 27.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 28.Vergnano S, Sharland M, Kazembe P, Mwansambo C, Heath PT. Neonatal sepsis: an international perspective. Arch Dis Child Fetal Neonatal Ed. 2005;90:F220–F224. doi: 10.1136/adc.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, Martin C. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140:171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Ronquillo L, Tellez-Zenteno JF, Weder-Cisneros N, Salinas-Ramirez V, Zapata-Pallagi JA, da Silva O. Risk factors for the development of bronchopulmonary dysplasia: a case-control study. Arch Med Res. 2004;35:549–553. doi: 10.1016/j.arcmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. doi: 10.1016/j.jpeds.2008.02.033. 175 e171. [DOI] [PubMed] [Google Scholar]
- 32.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 33.Schulz SM, Kohler G, Holscher C, Iwakura Y, Alber G. IL-17A is produced by Th17, gammadelta T cells and other CD4- lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int Immunol. 2008;20:1129–1138. doi: 10.1093/intimm/dxn069. [DOI] [PubMed] [Google Scholar]
- 34.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, Edgerton M, Gaffen SL, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) Regulatory T Cells Promote Th17 Cells In Vitro and Enhance Host Resistance in Mouse Candida albicans Th17 Cell Infection Model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L, Cohen AC, Lewis DB. Impaired allogeneic activation and T-helper 1 differentiation of human cord blood naive CD4 T cells. Biol Blood Marrow Transplant. 2006;12:160–171. doi: 10.1016/j.bbmt.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Burchett SK, Corey L, Mohan KM, Westall J, Ashley R, Wilson CB. Diminished interferon-gamma and lymphocyte proliferation in neonatal and postpartum primary herpes simplex virus infection. J Infect Dis. 1992;165:813–818. doi: 10.1093/infdis/165.5.813. [DOI] [PubMed] [Google Scholar]
- 37.Lewis DB, Yu CC, Meyer J, English BK, Kahn SJ, Wilson CB. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J Clin Invest. 1991;87:194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol. 2002;168:2820–2827. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 39.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of toll-like receptor activated monocytes. PNAS. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 41.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1b and 6 but not transforming growth factor-b are essential for the differentiation of interleukin 17-producing human helper T cells. Nat Immun. 2007;8:942–948. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 42.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ambalavanan N, Carlo WA, D'Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schelonka RL, Maheshwari A, Carlo WA, Taylor S, Hansen NI, Schendel DE, Thorsen P, Skogstrand K, Hougaard DM, Higgins RD. T cell cytokines and the risk of blood stream infection in extremely low birth weight infants. Cytokine. 2010;53:249–255. doi: 10.1016/j.cyto.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.