Abstract
Our recent paper examined how pelvic fins and their musculature form developmentally and how these mechanisms have evolved within the vertebrate lineage, a process fundamental to the tetrapod transition. The transition from the water onto the land is among one of the most well studied steps in the evolutionary history of vertebrates, yet the genetic basis of this evolutionary transition is little studied and ill-defined. The advent of these terrestrial species resulted in a shift in locomotor strategies from the rhythmic undulating muscles of the fish body to a reliance upon powerful weight bearing muscles of the limbs to generate movement. We demonstrated that the pelvic fin muscles of bony fish are generated by a mechanism that has features of both of limb/fin muscle formation in tetrapods and primitive cartilaginous fish. We hypothesize that the adoption of the fully derived mode of hindlimb muscle formation, was a further modification of the mode of development deployed to generate pelvic fin muscles, a shift in overall muscle bioarchitecture we believe was critical to the success of the tetrapod transition.
Keywords: muscle, evolution, fin, limb, zebrafish, tetrapod
Four-Footed Vertebrates’ Evolved from the Lobe-Finned Fishes
The earliest tetrapods “four-footed vertebrates” evolved from the lobe-finned fishes over the 60 million years of the Devonian period, a period when extinct and modern major fish groups diversified.1-3 The advent of these terrestrial species resulted in a shift in locomotor strategies from the rhythmic undulating muscles of the fish body to a reliance upon powerful weight bearing muscles of the limbs to generate movement.4,5 The fossil record has provided significant insight into how the skeleton has evolved from a fin into a limb. The evolution of the skeleton provided the required scaffold for the evolving limb muscles to attach. However, as muscle, unlike bone, is rarely ever preserved within individual fossils, how different muscles arose is not clearly represented within the fossil record. Therefore, almost nothing is known about how this evolutionary shift in muscle development occurred and even less is known concerning the changes in the developmental mechanisms involved in generating these distinct changes in overall bioarchitecture.
Developmental Mechanism that Generate the Muscles of Terrestrial Tetrapods
Fortunately, cellular and molecular biology in the post-genomic era has provided significant insights into the developmental mechanism that generate the muscles of terrestrial tetrapods limbs. Limb muscle development has been well characterized in amniotes, a group which represents most land dwelling vertebrates, including man and modern research models—chicks and mice. In these species, cells that will form the limb muscle (known as myoblasts or muscle precursors) migrate from the somites into the limb bud, where they proliferate and then differentiate to form the future muscle masses6,7 (Fig. 1). This developmental process requires these cells to undergo an epithelial to mesenchymal transition, delamination, migration, proliferation and differentiation. Many of the genes that coordinate limb muscle formation have been identified (for a review see ref. 8). Among these genes is the homeobox containing gene lbx. Lbx is a marker of limb muscle precursors and its expression is maintained within both the mesenchymal myoblasts as they migrate to the limb and within post-migratory myoblasts within the forming muscle masses.9-12 This mechanism is considered as the most relatively “recent” to evolve, and has been termed the “derived mode” where derived is defined as a trait that is present (or absent) in an organism, but was absent (or present, respectively) in their last common ancestor. Both the forelimbs and hindlimbs in amniote species utilize this relatively more recent “derived” mechanism characterized by the migration of lbx positive mesenchymal precursor cells from the ventro-lateral or hypaxial region of limb level somites to generate limb musculature.
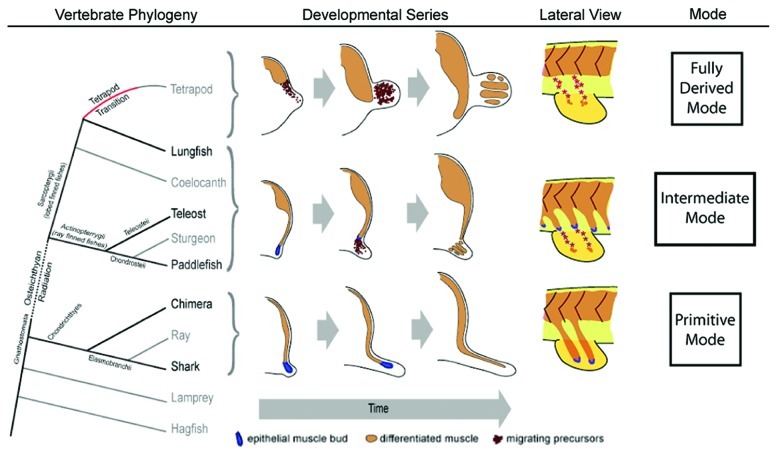
Figure 1. Pelvic fin and hindlimb developmental mechanisms mapped onto the vertebrate phylogeny. Vertebrate phylogeny (left). Schematic of the vertebrate phylogeny. Tetrapod transition, red line; ostechthyan radiation, dotted line; species used in our study, bold text. Developmental progression in cross-sections (center). Cartoon of cross sections depicting the developmental progression of fin muscle forming mechanisms described from a pre-fin/limb bud stage to a fin/limb stage containing differentiated muscle. Lateral view cartoon of each developmental mode (right). Modified from Cole NJ, et al. Development and evolution of the muscles of the pelvic fin. PLoS Biol 2011; 9:e1001168; 10.1371/journal.pbio.1001168.
Ancestral Fin Muscle Development in the Paired Fins of Sharks
In contrast a distinct, primitive or “ancestral” developmental mode of fin muscle development was shown to occur within the paired fins of cartilaginous fish, sharks and chimeras.13 (Fig. 1). Cartilaginous fish occupy a basal position in the vertebrate phylogeny (Fig. 1) and are thought to retain many ancestral traits, in particular the ancestral form of the paired fins.13 In cartilaginous fish fins, the muscles originate as direct extensions of epithelial muscle buds which extend from the ventral ends of fin level myotomes and invade the developing fin mesenchyme where they differentiate into muscle while still in contact with the myotome13 (Fig. 1). Neyt et al.13 confirmed the presence of this developmental mechanism in sharks using immunocytochemistry and considered this direct epithelial somitic extension represents the primitive or ancestral mode of muscle bioarchitecture in ancestral embryonic paired fin muscles.
It is well established that our limbs evolved from the paired fins of ancestral fish species. The pectoral and pelvic fins of fish are homologous to the tetrapod fore and hindlimb, respectively. The remnants of this common ancestry can still be seen today with the expression of similar genes and proteins during the development of fish fins and vertebrate limbs.14 Neyt et al.13 also showed that the same “recent” or derived developmental mechanism of muscle formation was shown in the pectoral fins of teleosts (evolutionary forerunner to the forelimb).
However, despite the relevance of pelvic fin initiation to amniote hindlimb development, examination of the development of pelvic fins and corresponding musculature is a neglected area of research. This is a matter of some importance since in the course of the evolution of terrestrial species from water dwelling ancestors, a shift to a “rear wheel drive” mode of locomotion occurred15 such that the hindlimbs and large powerful hindlimb muscles became the dominant force in locomotion. This necessarily involved the evolution of changes to the mechanisms that generate of the pelvic fin muscles during development. Despite the importance of this process to the evolution of vertebrate phylogeny almost nothing is known the developmental mechanisms that were altered to facilitate this striking alteration in morphology. Prior to our study only two, very distinct, developmental mechanisms had been described, which are deployed to generate the muscles of pectoral fins and vertebrate limbs, but the process that generated the pelvic fin muscles of bony fish remained unknown.
Examining Pelvic Fin Muscle Formation
To investigate this question we examined pelvic muscle development in a range of species with the aim of answering two outstanding questions: (1) What was the developmental mechanism(s) at work in pelvic fins of fishes? (2) What changes resulted in the steps necessary for the evolution of these developmental mechanisms, which consequently allowed the generation of the different muscle morphologies necessary for the tetrapod transition. We postulated that three observations were possible. Either, (1) pelvic fin muscles were generated from a completely new mechanism or that (2) one of the existing mechanisms that had previously been characterized was altered/modified/re-utilized, or (3) the primitive mode found in cartilaginous fish orchestrated the formation of pelvic fin muscles. The zebrafish provided an opportunity to study these mechanisms in a genetically tractable model combining embryological manipulability with optical clarity of the early embryo and larvae, which would allow simple visualization of cell biological events directly in vivo.
In order to determine where pelvic fin muscle came from we performed orthotopic somite transplantation, to pinpoint the origin, intermediate and final position of the derivatives of the somite, between strains of transgenic zebrafish. This would allow us to determine if pelvic fin muscles have a somitic origin. The pelvic fins of zebrafish form at 3 weeks after fertilisation and significantly after the formation of pectoral fins which occurs by 48 hpf. (Fig. 2A and B). We previously found that techniques such as lipophilic DiI labeling and uncaged fluorescein fate mapping13 were not robust enough and too short-lived for determining the origins of the pelvic fin muscle (5 weeks from somite label to fin muscle differentiation). Thus, we first needed to develop a long-term fate mapping strategy, which would allow us to examine the developmental origin of pelvic fin muscle precursors.
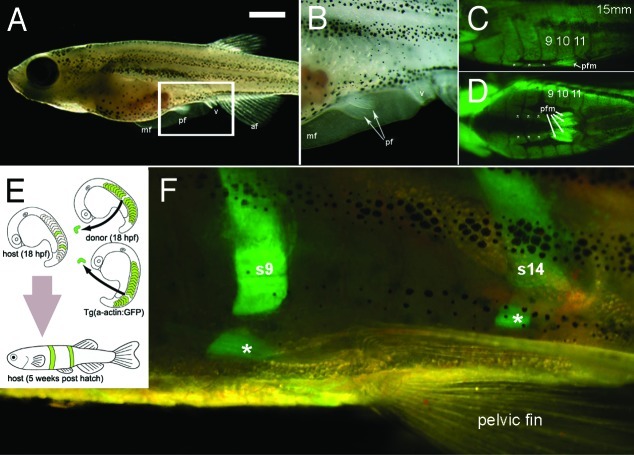
Figure 2. Pelvic fins, musculature and transplantation in zebrafish. (A) Lateral view of a 5 week old zebrafish. Scale bar= 1 mm. (B) Higher magnification view of area boxed in (A). (C and D) Lateral view (C) and ventral view (D), of green fluorescent protein (GFP) expression in muscle of the Tg(acta1:GFP)zf13 zebrafish. The pelvic fin muscles (pfm) and line of muscles ventral to and separate from each myotome can be seen (white asterisks). (E) Cartoon detailing method of double transplant of two GFP positive somites into wild type host. (F) Detail of pelvic fin region of GFP positive donor somite 9 and 14 in to wild type host zebrafish. The donor GFP somite does not form pelvic fin muscles in the host but does contribute to the muscles (white asterisks) ventral to and separate from the corresponding somite. Abbreviations mf, median fin; pf; pelvic fin, v; vent, af, anal fin, pfm pelvic fin muscle, s9, s14 somites 9 and 14.
When initially developing the technique we first transplanted donor somites expressing GFP from the α-actin GFP fish16 which expresses GFP in all skeletal muscle (Fig. 2C and D) into wild type hosts (Fig. 2E and F).
Transplant surgery was performed on donor and host embryos at 15 somite stage (18 hpf). A single somite was carefully removed from the host while at the same time a single somite was removed from the donor and transferred into the host, and placed into the space where the host somite had been removed. The incision closed up over the next few hours and approximately 20% of the embryos survived through to adulthood. The transplanted tissue gave rise to normal developed structures and so we were able to observe the contribution of the donor somite to muscle growth of the host throughout development in real time in the living fish. We noticed that immediately ventral to, and separate from, each myotome was a muscle (Fig. 2F asterisks). Somites transplanted at any level would contribute to one of these blocks ventral to the corresponding myotome. The purpose of this muscle block is unclear but it may provide a rigid rod of muscle for the pelvic fins to contract against, rather like a rod running along the ventral surface of the fish. In the absence of any connection of the pelvic fin to the spine this may be required to prevent the fish “buckling” during contraction.
Transplantation could also provide the ability to visualize both the host and donor muscle in vivo. To do this we generated a zebrafish with RFP skeletal muscle (α actin-mCherrypc14 line). We next transplanted RFP expressing donor somites into GFP expressing hosts (Fig. 3). Specifically, we transplanted individual somites orthotypically from embryos carrying the muscle specific RFP transgene, into equivalent somite region of a stage matched host transgenic line of zebrafish that drives GFP expression from the same skeletal muscle-specific promoter16 (Fig. 3). Thus all the tissue generated in the host by the donor somite would be labeled red in a fish with green muscle allowing the clear visualization of the donor somite and its derivatives. Successfully operated embryos developed to adulthood and during this time were regularly documented to determine which musculature contains differentiated RFP positive cells.
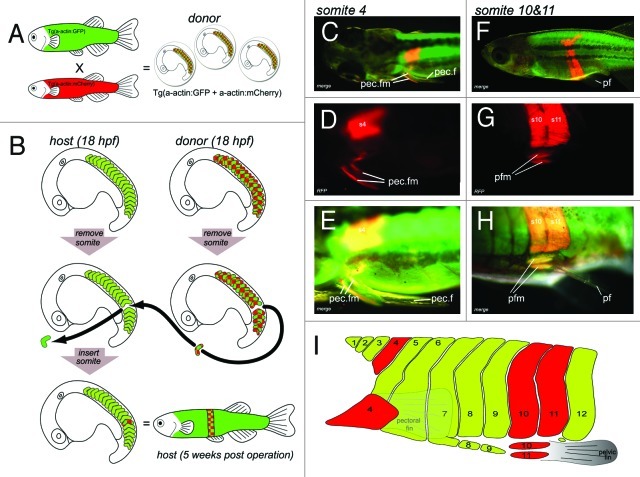
Figure 3. Transgenic somite transplantation in D. rerio confirms pectoral fin muscle derive from somite 4 and pelvic fin muscles derive from myotomal extension of somite 10 and 11. (A and B) Cartoon detailing the method used for double transgenic transplant of somites. (A) Generation of donor embryos. Tg(acta1:mCherry)pc4 fish were mated with Tg(acta1:GFP)zf13. GFP positive donors were used as GFP expression was brighter at early stage (15 somite stage) which aided the the transplantation process. The chequered pattern of the donor somites in the figure represents this double expression of both mChery and GFP in the double transgenic donor embryos. (B) At 18 h post fertilization a somite was removed from stage matched host embryo. The equivalent somite was removed from the donor and transplanted into the host. The operated embryos were grown for 5 weeks. (C–H) Results of transplanting somites 4, 10 and 11. (C–E) Somite 4 contributes to pectoral fin muscles. (C) Dorsal view of a 5-d post fertilization larvae. Higher magnification in (D and E). (F–H) Myotomal extensions derived from somites 10 and 11 generate the pelvic fin muscles. (F) Lateral view of 5-week-old juvenile. Higher magnification in G and H. Somites 10 and 11 contributing to the pelvic fin muscles. (I) Cartoon of lateral view of zebrafish flank detailing our observations of donor somite (red) that contributes the pectoral fin and pelvic fin muscles in our transplant experiments. Modified from Cole NJ, et al. Development and evolution of the muscles of the pelvic fin. PLoS Biol 2011; 9:e1001168; 10.1371/journal.pbio.1001168.
In our final transplantation strategy we aimed to demonstrate that the donor somite contained only somitic tissue and that this only ever generated donor-derived muscle in the host. In order to do this performed a triple transgenic fluorescent transplant strategy. Two additional fish lines were generated, an α-actin BFP fish (blue skeletal muscle) and a ubiquitously expressed β-actin mCherry fish, which has mCherry in every cell. We transplanted donor GFP muscle/β-actin RFP into BFP muscle hosts. If the donor somite contributes to non-muscle tissue this tissue will be red in the host (see supplementary figure in Cole et al.17).
To test our long-term fate mapping strategy we transplanted somite 4 from mCherry donor to GFP host. The 4th somite is known to give rise to the pectoral fin muscles which is formed by 48 hpf in zebrafish, as demonstrated by Neyt et al.13 using lipophilic DiI labeling. Similarly, when we transplanted somite 4, the pectoral fin musculature of the host consisted of functioning donor tissue. The donor tissue grew in the host for the entire life of the fish (Fig. 3C–E) and appeared to function normally such that no observable differences to the pectoral fin on the un-operated side were seen. This data confirmed that pectoral fin muscles do indeed form by the migration of precursors from somite 4 and that we had a workable long-term fate mapping strategy.
The origin of pelvic fin muscles was investigated by transplanting every somite from 8 to 14. The results clearly demonstrated that it is somites 10 and 11 that contribute to the pelvic fin muscles in zebrafish (Fig. 3F–H). Somites anterior to 10 and posterior to 11 do not contribute precursors to pelvic fin muscles.17 We also found that if the host transplant was not initially placed ventrally enough in the host during the operation then the donor precursors would fail to contribute to the pelvic fin muscles even though the a large portion of the body wall muscle would be made from the donor tissue.17 It was clear the ventral tip of the extension was the source of the pelvic fin muscle precursors.
We then applied the more traditional methods of histology combined with immuno-histochemistry and in situ-hybridization to generate a complete developmental time series of pelvic fin muscle formation in the zebrafish. We analyzed expression of known players in muscle developmental mechanisms, including lbx and pax3, which mark the migrating muscle precursors. We found initially that a myotomal extension grew down toward the site of the future fin, and once in position, delamination and migration of lbx positive precursors occurred to deliver myoblasts to the fin. The data we obtained from examining pelvic fin muscle formation in zebrafish revealed that the muscles form in a process that appeared to be a combination of the primitive and derived mechanisms previously described.
Pelvic Fin Muscle Formation in Species at Key Points in the Vertebrate Phylogeny
Next we examined the developmental mechanism at work during pelvic fin muscle formation in a range of phylogenetically important living fish species. These species were chosen because they are positioned at key points of the vertebrate phylogeny and we were able to obtain living embryos from them. These species represent a snapshot of steps in evolutionary time in the evolution of fish. We thought that these species would reflect the mechanisms of development from their ancestry and would therefore provide an insight into the evolution of pelvic fin/hindlimb muscle developmental mechanisms. We examined pelvic fin muscle development in embryos from a basal bony fish, the North American paddlefish (Polyodon spathula), positioned close to the sarcopterygian radiation. We also examined fin muscle formation in a sarcopterygian fish, the Australian lungfish (Neoceratodus forsteri). Australian lungfish represent the most closely related extant fish species to tetrapods. Finally we also examined pelvic fin muscle development in the cartilaginous fish [(the bamboo shark, Chiloscyllium punctatum), and the chimera (Callorhinchus milii)], which represent basal fish with paired fins. We confirmed that the pelvic fin musculature of the cartilaginous fish (shark and chimera) developed by the “primitive mode” of myotomal extension without lbx expression. The fin muscles are an extension of the body wall and are delivered directly into the developing fin without any lbx positive precursor migration.
In contrast, in the lungfish and paddlefish, like the zebrafish, the pelvic muscle development also initially involves the primitive mode of epithelial extension and as the extension reaches the developing pelvic fin bud, the tip of the extension undergoes an epithelial to mesenchymal transition, and lbx positive migratory precursor cells undergo migration into the fin. These precursors then spread into the fin to lay down the fin muscles. This migratory phase of this mechanism mirrors that seen in amniotes yet the extension phase reflects the primitive mode of the cartilaginous fishes. Therefore we conclude that the mechanism of pelvic fin development in the bony fish represents and intermediate mechanisms between that of primitive mode seen in the basal cartilaginous fishes and the derived mode of tetrapods.
Therefore we described and characterized an intermediate mode of muscle formation, which may reflect a transitional process, offering a window into the steps necessary for the evolution of powerful hindlimb muscles required for the tetrapod transition onto land. Importantly it shows that the processes or tools required for the expansion and long range migration of muscle precursors to build powerful hindlimbs supporting the weight of the animal required were already present in bony fish to facilitate this transition to occur.
Conclusions
These results demonstrate that the pelvic fin muscles of bony fish are generated by a mechanism that has features of both of limb/fin muscle formation of tetrapods and primitive cartilaginous fish. They further show that in contrast to the derived mode of fin muscle formation in the zebrafish pectoral fin, a third intermediate developmental mode occurs during pelvic fin muscle formation (Fig. 1). This “third way” generates pelvic fin muscle in the pelvic fins of all fish species that evolved after the osteichtyan radiation (Fig. 1). Collectively the results of our studies have formed the basis of a simple hypothesis- a primitive morphogenesis involving the continual ventral extension of epithelial somitic buds was originally used to generate all lateral muscle, including fin musculature. However this mechanism was genetically altered prior to the tetrapod transition to create migratory myoblasts within fin level somites. We hypothesize that the adoption of the fully derived mode of hindlimb muscle formation, was a further modification of the “third way” observed in pelvic fins, and was an evolutionary innovation critical to the success of the tetrapod transition.
The knowledge gleaned from this research has advanced our understanding of how different locomotor strategies have developed in the vertebrate clade, and has particular resonance for our understanding of the tetrapod transition on to land, which involved the deployment of a limb muscle dominant locomotor strategy over a myotomal-based system.
Glossary
Abbreviations:
- pec.f
pectoral fin
- pec.fm
pectoral fin muscle
- Pf
pelvic fin
- rfp
red fluorescent protein
- pfm
pelvic fin muscle
- me
myotomal extension
- s4,s10,s11,s12
somite numbered from anterior to posterior
- hpf
hours post-fertilization
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/20969
References
- 1.Clack JA. Gaining ground: the origin and evolution of tetrapods. 2011 Bloomington, Ind.: Indiana University Press. [Google Scholar]
- 2.Wells HG. A short history of the world. 1922 London: Cassell. [Google Scholar]
- 3.Zimmer C. At the water's edge: macroevolution and the transformation of life. 1998 New York; London: Free Press. [Google Scholar]
- 4.Kardong KV. Vertebrates: comparative anatomy, function, evolution. Dubuque, Iowa 1995; Oxford: Wm. C. Brown. [Google Scholar]
- 5.Goodrich ES. Studies on the structure and development of vertebrates. New York 1958; Dover; London: Constable:906. [Google Scholar]
- 6.Christ B, Jacob HJ, Jacob M. Experimental analysis of the origin of the wing musculature in avian embryos. Anat Embryol (Berl) 1977;150:171–86. doi: 10.1007/BF00316649. [DOI] [PubMed] [Google Scholar]
- 7.Williams BA, Ordahl CP. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development. 1994;120:785–96. doi: 10.1242/dev.120.4.785. [DOI] [PubMed] [Google Scholar]
- 8.Vasyutina E, Birchmeier C. The development of migrating muscle precursor cells. Anat Embryol (Berl) 2006;211(Suppl 1):37–41. doi: 10.1007/s00429-006-0118-9. [DOI] [PubMed] [Google Scholar]
- 9.Mennerich D, Schäfer K, Braun T. Pax-3 is necessary but not sufficient for lbx1 expression in myogenic precursor cells of the limb. Mech Dev. 1998;73:147–58. doi: 10.1016/S0925-4773(98)00046-X. [DOI] [PubMed] [Google Scholar]
- 10.Schäfer K, Braun T. Early specification of limb muscle precursor cells by the homeobox gene Lbx1h. Nat Genet. 1999;23:213–6. doi: 10.1038/13843. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich S, Schubert FR, Healy C, Sharpe PT, Lumsden A. Specification of the hypaxial musculature. Development. 1998;125:2235–49. doi: 10.1242/dev.125.12.2235. [DOI] [PubMed] [Google Scholar]
- 12.Mankoo BS, Collins NS, Ashby P, Grigorieva E, Pevny LH, Candia A, et al. Mox2 is a component of the genetic hierarchy controlling limb muscle development. Nature. 1999;400:69–73. doi: 10.1038/21892. [DOI] [PubMed] [Google Scholar]
- 13.Neyt C, Jagla K, Thisse C, Thisse B, Haines L, Currie PD. Evolutionary origins of vertebrate appendicular muscle. Nature. 2000;408:82–6. doi: 10.1038/35040549. [DOI] [PubMed] [Google Scholar]
- 14.Mercader N. Early steps of paired fin development in zebrafish compared with tetrapod limb development. Dev Growth Differ. 2007;49:421–37. doi: 10.1111/j.1440-169X.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- 15.Boisvert CA. The pelvic fin and girdle of Panderichthys and the origin of tetrapod locomotion. Nature. 2005;438:1145–7. doi: 10.1038/nature04119. [DOI] [PubMed] [Google Scholar]
- 16.Higashijima S, Okamoto H, Ueno N, Hotta Y, Eguchi G. High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev Biol. 1997;192:289–99. doi: 10.1006/dbio.1997.8779. [DOI] [PubMed] [Google Scholar]
- 17.Cole NJ, Hall TE, Don EK, Berger S, Boisvert CA, Neyt C, et al. Development and evolution of the muscles of the pelvic fin. PLoS Biol. 2011;9:e1001168. doi: 10.1371/journal.pbio.1001168. [DOI] [PMC free article] [PubMed] [Google Scholar]