Abstract
The aim of mitosis is to produce two daughter nuclei, each containing a chromosome complement identical to that of the mother nucleus. This can be accomplished through a variety of strategies, with “open” and “closed” modes of mitosis positioned at the opposite ends of the spectrum and a range of intermediate patterns in between. In the “closed” mitosis, the nuclear envelope remains intact throughout the nuclear division. In the “open” division type, the envelope of the original nucleus breaks down early in mitosis and reassembles around the segregated daughter genomes. In any case, the nuclear membrane has to remodel to accommodate the mitotic spindle assembly, chromosome segregation and formation of the daughter nuclei. We have recently shown that within the fission yeast clade, the mitotic control of the nuclear surface area may determine the choice between the nuclear envelope breakdown and a fully “closed” division. Here we discuss our data and argue that comparative cell biology studies using two fission yeast species, Schizosaccharomyces pombe and Schizosaccharomyces japonicus, could provide unprecedented insights into physiology and evolution of mitosis.
Keywords: mitosis, nuclear envelope, nuclear surface, spindle, nuclear pore
The fission yeast genus, Schizosaccharomyces, forms an ancient branch within Ascomycete fungi and consists of four known species, each propagating through medial fission.1 Schizosaccharomyces pombe (S. pombe) is a commonly used model organism that undergoes a typical “closed” mitosis where the mitotic spindle is assembled inside the nucleus and the nuclear membrane remains intact throughout chromosome segregation.2,3 This mode of nuclear division is commonly used in lower eukaryotes, at least in those that emerged as popular genetically tractable experimental systems.3 We and Niki and colleagues have recently shown that an early diverging species in the fission yeast clade, Schizosaccharomyces japonicus (S. japonicus) undergoes an unusual form of mitosis, where the spindle forms in the nucleus but the nuclear envelope (NE) abruptly ruptures during late anaphase.4,5 The loss of the nucleocytoplasmic integrity is transient and the nuclear envelope seals soon after the completion of nuclear division. The dramatic variation between the mitotic scenarios in S. pombe and S. japonicus allows for direct probing of the nuclear membrane behavior during mitosis using a comparative approach, and it may advance our understanding of evolution of mitosis in eukaryotes.
Cytology of Mitosis in Schizosaccharomyces japonicus
Initial sequence of events during mitosis in S. japonicus is fairly similar to that in S. pombe. The mitotic microtubule-based spindle assembles inside an intact nucleus at the duplicated spindle pole bodies (SPBs) that reside at the cytoplasmic face of the NE throughout interphase but become embedded into the NE upon mitotic commitment.6,7 The spindle rapidly extends to “metaphase” length spanning the nuclear diameter.4 Both yeasts have three similarly sized chromosomes1 and the chromosomal dynamics can be monitored using both kinetochore4 and chromatin marker protein.5 Kinetochore microtubules rapidly capture and align chromosomes equidistantly from the spindle poles forming a metaphase plate. Upon anaphase A onset, the kinetochore pairs split and the sister chromosomes travel to the poles of the mitotic spindle that at this stage remains short. Immediately after that, cells enter anaphase B that is characterized by rapid spindle elongation and changes in the nuclear shape.
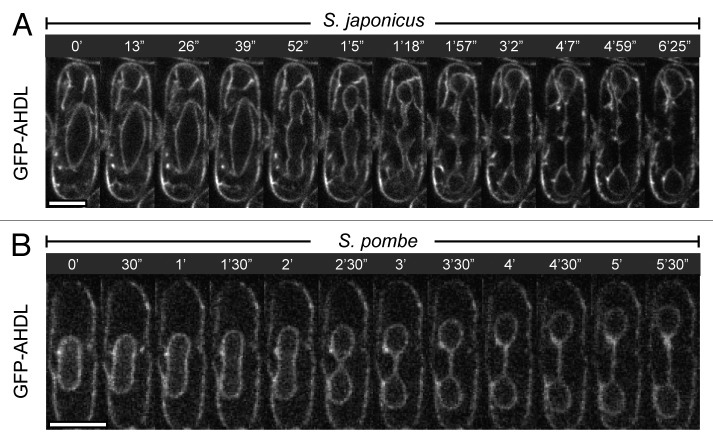
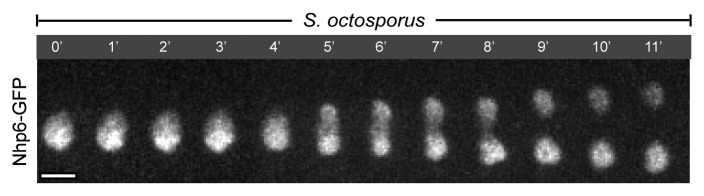
At this stage, the mitotic programs in two yeasts begin to differ. In S. pombe, the spindle remains straight throughout anaphase and the nucleus divides into two through a dumbbell shaped intermediate. On the other hand, the elongating spindles buckle severely inside S. japonicus nuclei, which in turn, assume a diamond and often, a bow shape.4 Since the outer NE is continuous with the endoplasmic reticulum (ER), the behavior of the nuclear membrane at this stage is best visualized by imaging the bright artificial marker GFP-AHDL that diffuses throughout the lumen of the entire ER (Fig. 1), or endogenous membrane-spanning markers of the cisternal ER, such as Sec63-GFP or Ost1-GFP.4 The dynamic process of nuclear elongation into a diamond shape with its frequent bending into a bow culminates in a dramatic equatorial rupture of the NE followed by virtually instantaneous intermixing between the nuclear and the cytosolic components.4 The physical breakage of the NE was confirmed at an ultra-structural level by Niki and colleagues.5 A single expanding tear appears to relieve the strain and the NE relaxes into a more rounded conformation.4 Simultaneously, the mitotic spindle straightens abruptly, indicating that it had been experiencing the compressive stress when confined in the nucleus.4 The NE resolves into three parts—the two daughter nuclei and a short-lived medial compartment. The mitosis is completed when the nuclear integrity is restored, the nucleoplasmic markers are reimported into the newly formed daughter nuclei and the spindle disassembles. Thus, S. japonicus undergoes mitosis of what could be classified as a “semi-open” type, where the spindle is assembled in a “closed” manner but the NE still fragments late in anaphase. This mode of mitosis is unusual for the fission yeast clade since not only S. pombe but also Schizosaccharomyces octosporus (S. octosporus) maintain the nuclear integrity throughout division (Fig. 2).
Figure 1. S. japonicus and S. pombe exhibit distinct nuclear envelope dynamics during mitosis. Time-lapse single plane images of mitotic S. japonicus (A) and S. pombe (B) cells expressing the artificial ER marker GFP-AHDL. Note the abrupt rupture of the nuclear envelope (at time point 52”) in S. japonicus. The anaphase nucleus in S. pombe divides through a dumbbell shaped intermediate. Time is in minutes and seconds. Scale bars represent 5 µm.
Figure 2. S. octosporus undergoes “closed” mitosis. Time-lapse maximum projection images of a mitotic S. octosporus cell expressing the nuclear marker Nhp6-GFP. Note that Nhp6-GFP is restricted to the nucleus throughout mitosis. Similar to S. pombe, the mitotic S. octosporus nucleus divides through a dumbbell shaped intermediate. Time is in minutes. Scale bar represents 2.5 µm.
Another curious aspect of the mitotic division in S. japonicus is unusual dynamics of the nuclear pore complexes (NPCs). Shortly after the onset of anaphase B spindle elongation, the NPC markers (Cut11, Nup85, Nup132 and Nup189), exhibit a net motion toward the mitotic poles, effectively clearing the nuclear equator.4 Tts1, a transmembrane ER protein that localizes preferentially to the curved membrane regions including the nuclear pores, exhibits a similar behavior.4 Three-dimensional reconstructions of the anaphase nuclei clearly show an increase in the nuclear pore density at the poles of the dividing nucleus as compared with the medial portion.5 We did not observe mitosis-specific dissociation of the core nucleoporins from the nuclear envelope.4 Interestingly, throughout the cell cycle in S. japonicus, the nuclear basket proteins, Mlp homologs Nup211 and Alm1 exhibit a diffuse nucleoplasmic localization in addition to their association with the nuclear pores (our unpublished data). This is in contrast to the situation in S. pombe and budding yeast where the Mlp proteins localize exclusively to the nuclear rim.8,9 The nucleoplasmic pool is released into the cytoplasm upon the NE breakage and it is reimported into the nucleus when mitosis is complete (our unpublished data). So far, this is the only observed structural dissimilarity between NPCs in S. pombe and S. japonicus. However, since the degree of Mlp association with the nuclear pores does not change during mitosis, we do not believe that their differential localization could be directly responsible for the variance in mitotic scenarios.
The NPC “sliding” occurs concurrently with the bulk movement of the mitotic chromosomes toward the spindle poles, which suggests a possible association between the chromatin and the nuclear pores. During anaphase, chromosomes move toward the SPBs and the general shape of the chromatin correlates with the distribution of the core nucleoporins.5 It is worth mentioning that the anaphase chromatin in S. japonicus appears less condensed than in S. pombe, based on imaging of GFP-tagged histone H3.5 This provides an unprecedented opportunity to visualize chromosome segregation in the yeast system using light microscopy. The nucleolus does not persist through mitosis in S. japonicus,4 as it does in S. pombe and other organisms undergoing “closed” nuclear division.10 Imaging of the nucleolar markers4 (the ribosome biogenesis proteins Erb111 and Fib112) and transmission electron microscopy5 reveal that this organelle stretches in anaphase B following mitotic spindle elongation in S. japonicus. Upon NE rupture the stretched mother nucleolus breaks into three compartments. Each of the daughter nuclei inherits one of the smaller polar nucleolar fragments likely associated with the nucleolar-organizing regions on the chromosomes (rDNA arrays). The largest medial compartment partially surrounded by the NPC-free NE is discarded in the cytoplasm in between the segregated daughter genomes.4 It rapidly disperses, concomitantly with a visible increase in the size of the daughter nucleoli, suggesting that the protein components of the mother nucleolus are recycled to build the daughters. Thus, similar to the higher eukaryotes, the nucleolus in S. japonicus exhibits a dispersive behavior during mitosis, although the nucleolar disassembly occurs in late anaphase rather than in prophase. Similar nucleolar dynamics have been observed in the filamentous Ascomycete Aspergellus nidulans.13
To conclude, the fission yeast S. japonicus exhibits the following cytological features that set it apart from the related, commonly studied species S. pombe and could be exploited to gain important insights into mechanisms underlying mitotic division and structuring the mitotic nucleus in eukaryotes. First, and perhaps most important, the NE breaks in anaphase to be reassembled after mitosis. Second, the bulk of the nucleolar material disperses upon the nuclear envelope breakage and the daughter nucleoli must be built de novo. Third, the sheer size of an S. japonicus cell (the linear dimensions of which are approximately twice as large as in S. pombe) and the less compacted chromatin allow for detailed observations of the chromosome and the nuclear envelope dynamics.
Geometry and Mechanics of the Nuclear Division
A “closed” division mode implies constancy of the nuclear volume during division of a spherical nucleus into two daughters. Since both volume and surface area depend on the radius of a sphere with volume scaling to the power of 3 and the surface area scaling to the power of 2, the surface area of the dividing “closed” nucleus must increase in order to accommodate its division. Indeed, the dividing S. pombe nucleus increases its surface area by approximately 30% within a fairly short time it takes to complete anaphase.14 We have found that there is no addition of new membranes to the dividing nucleus in S. japonicus.4 Therefore, the only remaining alternative is to reduce the nuclear volume by NE breakage to form smaller daughter nuclei. This is exactly what we observed—the experimentally determined values of combined volume of the two daughter nuclei in S. japonicus agree with predictions and are approximately 70% that of the mother nuclei.4
The ER is continuous with the NE and therefore, could potentially serve as a considerable membrane reservoir in any dividing cell. So why does the dividing S. japonicus nucleus fail to draw on this resource? One possible explanation could be the presence of a physical barrier between the NE and the peripheral ER that is abrogated during mitosis in S. pombe but not S. japonicus. Whether there indeed exists a junction-like barrier between the outer NE and the ER or it is simply the matter of structuring the inner NE, for instance by modifying its association with chromatin and therefore limiting the ability to expand the nucleus,15,16 such mechanisms could control membrane partitioning between the NE and the ER. An additional aspect of controlling the nuclear surface area could involve an upregulation of the membrane biosynthesis just before mitosis and assembly of a specific ER compartment that could serve as a source of membranes for the dividing nucleus in S. pombe and other organisms with “closed” mitosis.16-18 Supporting this hypothesis, inhibition of membrane biosynthesis by treating S. pombe cells at G2/M boundary with cerulenin, an inhibitor of fatty acid synthase, results in failure of nuclear division.4
An important aspect of the “closed” mitosis is intranuclear assembly and elongation of the mitotic spindle. Elongating spindles produce pushing force that acts on the NE and are capable of deforming it when the microtubule minus ends directly encounter the membrane instead of being properly anchored at the SPBs.19 Since the SPBs reinforce the NE, the elongating spindle will also experience compressive stress along the longitudinal direction when pushing against the NE. When the NE surface area increases in parallel with spindle elongation, like in S. pombe, the compressive stress is negligible. However, when the nuclear surface area does not increase, as in S. japonicus or in cerulenin-treated S. pombe cells, the compressive stress leads to spindle buckling that could potentially trigger spindle collapse.4 In S. japonicus, this problem is ultimately resolved by the NE breakdown that allows the severely bent spindle to straighten and complete chromosome segregation.4 However, since S. pombe lacks the mechanism to break down the NE, anaphase spindles indeed collapse and chromosome segregation fails when the membrane reservoir is not available.4 This underscores the relative fragility of the mitotic spindle in relation to the NE. It would be of interest, for instance, to compare its mechanical properties including microtubule cross-linking requirements for spindle mid-zone construction in two yeasts.
In summary, we propose that scaling considerations dictate either nuclear surface expansion or the NE breakdown during mitosis. It is tempting to speculate that they could have played a central role in emergence of distinct mitotic strategies across eukaryotes.
Nuclear Envelope Breakdown in Schisosaccharomyces japonicus
As discussed above, in the absence of the membrane reservoir S. japonicus appears to have evolved a mechanism to break down the NE in late anaphase. Importantly, it is an active mechanism rather than the passive tearing of the membrane by the spindle forces: when cells undergo mitosis in the absence of the mitotic spindle, the NE still breaks down.4 This occurs however, with a slightly slower kinetics—instead of a virtually instantaneous rupture of the NE, the nuclear markers now take longer time to redistribute throughout the cellular volume.4 It is likely that in the presence of the elongating spindle, the first tear in the NE becomes the only, rapidly expanding one, while in the absence of the spindle force, the NE still gets permeabilized but in a fairly delocalized manner. This requirement for microtubule forces to trigger the timely and efficient NE breakdown is reminiscent of the situation in other experimental systems. In a mammalian model of the NE breakdown (NEBD), the partial tearing of the membrane by centrosomal microtubules is followed by a rapid collapse of the entire NE, including NPC disassembly and depolymerization of the nuclear lamina.20 Although the NEBD is still able to occur when the microtubule network is compromised, it is delayed significantly.21 In the Basidiomycete fungus Ustilago maydis the pulling forces generated by microtubules also appear to mediate the NE rupture.22 Interestingly, while the NE morphology appears normal in the absence of microtubules, the NE permeabilization, as judged by the loss of compartmentalization of nuclear resident proteins, still occurs.22 Thus, microtubule-based forces appear to facilitate the NE breakdown but are not essential for this process.
In higher eukaryotes, the changes in NPC permeability mark the first step in NEBD and partial disassembly of the NPCs precedes disassembly of lamins and dissociation of the inner nuclear membrane (INM) proteins.23,24 The nuclear pore is a massive and highly organized protein complex that contains over a hundred nucleoporins. FxFG, GLGF and WD-40 repeat-containing and non-repeat nucleoporin types play distinct functional roles, either in mediating recognition and interaction with the transported cargo, or in maintaining NPC structure (for review see ref. 25). Accumulated biochemical evidence suggests that at least in cultured mammalian cells, phosphorylation of GLFG repeat-containing nucleoporins on multiple sites by the concerted action of mitotic kinases such as Cdk1 and (NIMA) never in mitosis A-related kinase (Nek) may trigger their disassociation from the NPCs and initiate the disassembly process of the remaining core nucleoporins during prophase.26 Similarly, phosphorylation of nucleoporins by Cdk1 and NIMA kinases seems to occur during semi-open mitosis in the filamentous fungus Aspergillus nidulans, with the structural components of NPC remaining at the NE throughout mitosis but with the FG-repeat nucleoporins dispersing in the cytoplasm already in prophase.27,28 While we did not observe any mitosis-specific dissociation of core nucleoporins from the NPCs in S. japonicus, it would be interesting to test if the peripheral FG-repeat nucleoporin Nup98 and its interacting protein, the WD-repeat homolog Rae1/Gle2 shown to reside at the vertex of NPC disassembly in higher eukaryotes, remain associated with the nuclear pores throughout nuclear division.
In higher eukaryotes, the Ran-GTP gradient that drives the nucleocytoplasmic transport, is maintained across the NE during interphase by localizing RanGAP to the cytoplasm and activating RanGEF in the nucleus (for review see ref. 29). While the nucleocytoplasmic trafficking is modified during “closed” nuclear division to allow mitosis-specific trafficking events to take place, the overall Ran system remains functional.18 Interestingly, during meiosis II in S. pombe, RanGAP enters the nucleus that coincides with the nuclear marker dispersal, despite the seemingly intact NE and the NPC structure (“virtual” NEBD).30,31 However, in S. japonicus mitosis, the specific nuclear and cytoplasmic markers remain compartmentalized4 and RanGAP is excluded from the nucleus right up to the point of the NE breakage (our unpublished data), suggesting that the Ran system is likely downstream of the NE breakdown machinery.
Possible mechanisms of the NE breakage could involve mitosis-specific modifications of the INM proteins modulating the NE strength and rigidity. Supporting this hypothesis, S. japonicus cells lacking the INM protein Lem2, exhibit precocious loss of the nucleocytoplasmic compartmentalization immediately after onset of anaphase B spindle elongation.4 The vertebrate homolog of Lem2 was also implicated in maintaining NE structural integrity.32 Most likely, Lem2 also participates in restoring the NE function after mitosis—the post-mitotic recruitment of nuclear markers is delayed in lem2Δ cells and Lem2 itself is enriched at the intersection between the spindle and the NE in the resealing daughter nuclei.4 It is possible that in fungi, a lineage that lacks lamins, a network of the INM proteins could fulfill their function in supporting the nuclear membrane.33,34
Vesicular trafficking events or ER membrane remodeling could also provide a potential mechanism for regulating the NE breakdown during mitosis in S. japonicus. For instance, reversing the direction of ER-Golgi vesicle transport delays the nuclear marker dispersion during meiosis in S. pombe suggesting that limitation of membrane components during forespore membrane formation may account for the NE permeability changes.30 Functional links between the vesicular trafficking and the NE morphology were also reported in budding yeast.35 Finally, NE permeabilization could be facilitated by mitosis-specific restructuring of the outer NE mediated by ER remodeling proteins including the homotypic membrane fusion machinery (for review see ref. 36).
Any of the possible mechanisms of NE breakdown must be entrained into the cell cycle machinery to ensure that the NE breakage occurs only at a specific stage of mitosis. In S. japonicus, the NE rupture takes place in late anaphase B4 and it is of immediate interest to identify the exact signaling pathway that triggers the NE permeabilization. As discussed above, in mammalian cells and Aspergillus nidulans, Cdk1 and NIMA kinases control the NPC disassembly in prophase.26,28 In the “virtual” NEBD occurring in S. pombe meiosis II, it is the septation initiation kinase network (SIN) that appears to control the permeabilization of the nuclear membrane at metaphase to anaphase transition.30,31 Disruption of the SIN-related mitotic exit network (MEN) in Ustilago maydis leads to incomplete NE removal.22 Thus, it appears that signaling inputs from various cell cycle regulators might co-evolve with effectors at the nuclear periphery to achieve timely nuclear envelope breakdown in a variety of physiological contexts.
In conclusion, the distinct features of mitotic division in S. japonicus, a genetically tractable unicellular model organism, offer an exciting opportunity to address fundamental questions pertaining to the mitotic nuclear envelope breakdown and reassembly in eukaryotes. Using this system it will be possible to understand how chromosome segregation, NPC dynamics and nuclear membrane remodeling are coordinated during the NE breakdown and how the nucleocytoplasmic integrity is reestablished following mitosis. On the other hand, comparative and synthetic studies using the related fission yeast species, S japonicus and S. pombe, will hopefully shed light on the evolution of physiologically diverse modes of nuclear division.
Acknowledgments
We are grateful to E. Makeyev for comments on the manuscript and Singapore Millenium Foundation for funding.
Glossary
Abbreviations:
- ER
endoplasmic reticulum
- NE
nuclear envelope
- INM
inner nuclear membrane
- NEBD
nuclear envelope breakdown
- NPC
nuclear pore complex
- SPBs
spindle pole bodies
- SIN
septation initiation network
- MEN
mitotic exit network
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/19514
References
- 1.Rhind N, Chen Z, Yassour M, Thompson DA, Haas BJ, Habib N, et al. Comparative functional genomics of the fission yeasts. Science. 2011;332:930–6. doi: 10.1126/science.1203357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka K, Kanbe T. Mitosis in the fission yeast Schizosaccharomyces pombe as revealed by freeze-substitution electron microscopy. J Cell Sci. 1986;80:253–68. doi: 10.1242/jcs.80.1.253. [DOI] [PubMed] [Google Scholar]
- 3.Heath IB. Variant mitoses in lower eukaryotes: indicators of the evolution of mitosis. Int Rev Cytol. 1980;64:1–80. doi: 10.1016/S0074-7696(08)60235-1. [DOI] [PubMed] [Google Scholar]
- 4.Yam C, He Y, Zhang D, Chiam KH, Oliferenko S. Divergent strategies for controlling the nuclear membrane satisfy geometric constraints during nuclear division. Curr Biol. 2011;21:1314–9. doi: 10.1016/j.cub.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 5.Aoki K, Hayashi H, Furuya K, Sato M, Takagi T, Osumi M, et al. Breakage of the nuclear envelope by an extending mitotic nucleus occurs during anaphase in Schizosaccharomyces japonicus. Genes Cells. 2011;16:911–26. doi: 10.1111/j.1365-2443.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- 6.Ding R, West RR, Morphew DM, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–79. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horio T, Kimura N, Basaki A, Tanaka Y, Noguchi T, Akashi T, et al. Molecular and structural characterization of the spindle pole bodies in the fission yeast Schizosaccharomyces japonicus var japonicus. Yeast. 2002;19:1335–50. doi: 10.1002/yea.921. [DOI] [PubMed] [Google Scholar]
- 8.Chen XQ, Du X, Liu J, Balasubramanian MK, Balasundaram D. Identification of genes encoding putative nucleoporins and transport factors in the fission yeast Schizosaccharomyces pombe: a deletion analysis. Yeast. 2004;21:495–509. doi: 10.1002/yea.1115. [DOI] [PubMed] [Google Scholar]
- 9.Strambio-de-Castillia C, Blobel G, Rout MP. Proteins connecting the nuclear pore complex with the nuclear interior. J Cell Biol. 1999;144:839–55. doi: 10.1083/jcb.144.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granot D, Snyder M. Segregation of the nucleolus during mitosis in budding and fission yeast. Cell Motil Cytoskeleton. 1991;20:47–54. doi: 10.1002/cm.970200106. [DOI] [PubMed] [Google Scholar]
- 11.Pestov DG, Stockelman MG, Strezoska Z, Lau LF. ERB1, the yeast homolog of mammalian Bop1, is an essential gene required for maturation of the 25S and 5.8S ribosomal RNAs. Nucleic Acids Res. 2001;29:3621–30. doi: 10.1093/nar/29.17.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tollervey D, Lehtonen H, Carmo-Fonseca M, Hurt EC. The small nucleolar RNP protein NOP1 (fibrillarin) is required for pre-rRNA processing in yeast. EMBO J. 1991;10:573–83. doi: 10.1002/j.1460-2075.1991.tb07984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ukil L, De Souza CP, Liu HL, Osmani SA. Nucleolar separation from chromosomes during Aspergillus nidulans mitosis can occur without spindle forces. Mol Biol Cell. 2009;20:2132–45. doi: 10.1091/mbc.E08-10-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim HWG, Huber G, Torii Y, Hirata A, Miller J, Sazer S. Vesicle-like biomechanics governs important aspects of nuclear geometry in fission yeast. PLoS ONE. 2007;2:e948. doi: 10.1371/journal.pone.0000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell JL, Lorenz A, Witkin KL, Hays T, Loidl J, Cohen-Fix O. Yeast nuclear envelope subdomains with distinct abilities to resist membrane expansion. Mol Biol Cell. 2006;17:1768–78. doi: 10.1091/mbc.E05-09-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster M, Witkin KL, Cohen-Fix O. Sizing up the nucleus: nuclear shape, size and nuclear-envelope assembly. J Cell Sci. 2009;122:1477–86. doi: 10.1242/jcs.037333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitoh S, Takahashi K, Nabeshima K, Yamashita Y, Nakaseko Y, Hirata A, et al. Aberrant mitosis in fission yeast mutants defective in fatty acid synthetase and acetyl CoA carboxylase. J Cell Biol. 1996;134:949–61. doi: 10.1083/jcb.134.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez Y, Meerbrey K, Chong J, Torii Y, Padte NN, Sazer S. Nuclear shape, growth and integrity in the closed mitosis of fission yeast depend on the Ran-GTPase system, the spindle pole body and the endoplasmic reticulum. J Cell Sci. 2009;122:2464–72. doi: 10.1242/jcs.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng L, Schwartz C, Magidson V, Khodjakov A, Oliferenko S. The spindle pole bodies facilitate nuclear envelope division during closed mitosis in fission yeast. PLoS Biol. 2007;5:e170. doi: 10.1371/journal.pbio.0050170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. doi: 10.1016/S0092-8674(01)00627-4. [DOI] [PubMed] [Google Scholar]
- 21.Salina D, Bodoor K, Eckley DM, Schroer TA, Rattner JB, Burke B. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 2002;108:97–107. doi: 10.1016/S0092-8674(01)00628-6. [DOI] [PubMed] [Google Scholar]
- 22.Straube A, Weber I, Steinberg G. A novel mechanism of nuclear envelope break-down in a fungus: nuclear migration strips off the envelope. EMBO J. 2005;24:1674–85. doi: 10.1038/sj.emboj.7600644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenart P, Rabut G, Daigle N, Hand AR, Terasaki M, Ellenberg J. Nuclear envelope breakdown in starfish oocytes proceeds by partial NPC disassembly followed by a rapidly spreading fenestration of nuclear membranes. J Cell Biol. 2003;160:1055–68. doi: 10.1083/jcb.200211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KK, Gruenbaum Y, Spann P, Liu J, Wilson KL. C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Mol Biol Cell. 2000;11:3089–99. doi: 10.1091/mbc.11.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hetzer MW, Walther TC, Mattaj IW. Pushing the envelope: structure, function, and dynamics of the nuclear periphery. Annu Rev Cell Dev Biol. 2005;21:347–80. doi: 10.1146/annurev.cellbio.21.090704.151152. [DOI] [PubMed] [Google Scholar]
- 26.Laurell E, Beck K, Krupina K, Theerthagiri G, Bodenmiller B, Horvath P, et al. Phosphorylation of Nup98 by multiple kinases is crucial for NPC disassembly during mitotic entry. Cell. 2011;144:539–50. doi: 10.1016/j.cell.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 27.De Souza CP, Horn KP, Masker K, Osmani SA. The SONB(NUP98) nucleoporin interacts with the NIMA kinase in Aspergillus nidulans. Genetics. 2003;165:1071–81. doi: 10.1093/genetics/165.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Souza CP, Osmani AH, Hashmi SB, Osmani SA. Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Curr Biol. 2004;14:1973–84. doi: 10.1016/j.cub.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 29.Clarke PR, Zhang C. Spatial and temporal coordination of mitosis by Ran GTPase. Nat Rev Mol Cell Biol. 2008;9:464–77. doi: 10.1038/nrm2410. [DOI] [PubMed] [Google Scholar]
- 30.Arai K, Sato M, Tanaka K, Yamamoto M. Nuclear compartmentalization is abolished during fission yeast meiosis. Curr Biol. 2010;20:1913–8. doi: 10.1016/j.cub.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Asakawa H, Kojidani T, Mori C, Osakada H, Sato M, Ding DQ, et al. Virtual breakdown of the nuclear envelope in fission yeast meiosis. Curr Biol. 2010;20:1919–25. doi: 10.1016/j.cub.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 32.Ulbert S, Antonin W, Platani M, Mattaj IW. The inner nuclear membrane protein Lem2 is critical for normal nuclear envelope morphology. FEBS Lett. 2006;580:6435–41. doi: 10.1016/j.febslet.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 33.Hiraoka Y, Maekawa H, Asakawa H, Chikashige Y, Kojidani T, Osakada H, et al. Inner nuclear membrane protein Ima1 is dispensable for intranuclear positioning of centromeres. Genes Cells. 2011;16:1000–11. doi: 10.1111/j.1365-2443.2011.01544.x. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez Y, Saito A, Sazer S. Fission yeast Lem2 and Man1 perform fundamental functions of the animal cell nuclear lamina. Nucleus. 2012;3 doi: 10.4161/nucl.18824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webster MT, McCaffery JM, Cohen-Fix O. Vesicle trafficking maintains nuclear shape in Saccharomyces cerevisiae during membrane proliferation. J Cell Biol. 2010;191:1079–88. doi: 10.1083/jcb.201006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu J, Prinz WA, Rapoport TA. Weaving the Web of ER Tubules. Cell. 2011;147:1226–31. doi: 10.1016/j.cell.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]