Abstract
Somatic mutation of Isocitrate dehydrogenase 1 (IDH1) at the locus of R132 (IDH1R132H) occurs in > 70% of WHO grade II-III gliomas and secondary glioblastomas. To date it remains unknown whether the mutation is restricted to glial tumor cells. Microglial cells are the resident macrophages in the central nervous system. Tumor-infiltrating microglial cells/macrophages are major stromal cellular components of malignant gliomas and substantially contribute to the tumor mass. Differential identification of the IDH1R132H mutant cellular components is of particular importance for understanding of the mutation-associated tumor biology. Here we discovered that a significant portion of CD68+, Iba1+, CX3CR1+ microglial cells/macrophages also harbor the IDH1R132H mutation. The findings provide novel insights for understanding the mutation-associated tumor biology relevant to clinical applications as a predictive and/or prognostic marker or therapeutic target.
Keywords: IDH1 mutation, cell motility, glioma, macrophages, microglia
Introduction
Isocitrate dehydrogenase (IDH) is a member of the β-decarboxylating dehydrogenase family of enzymes and catalyzes the oxidative decarboxylation of 2R, 3S-isocitrate to yield 2-oxoglutarate (α -KG).1 There are three isoforms of IDH namely IDH1, IDH2 and IDH3. Somatic mutation of IDH1 at the locus of R132 (IDH1R132H) occurs in > 70% of WHO grade II-III gliomas and secondary glioblastomas.2 Acute myeloid leukemia (AML) is the only non-central nervous system (CNS) tumor in which a substantial percentage of the mutations are present as well.3 In other cancer types mutation of IDH1 is infrequent. A mutation-specific antibody was raised against the mutated enzyme (anti-mIDH1R132H) and immunostaining is becoming an alternative screening tool for the presence of the mutation (Dianova GmbH, Germany)4 to be applied prior to DNA sequencing. In immunostained glioma sections we discovered cells with microglial features which appeared to be positive for the mutated IDH1R132H. Following up on this observation we initiated the present investigation aiming at proving the microglial lineage of these immunopositive cells. Elucidation of such IDH1R132H mutant cellular components directs the unravelling glioma tumorigenesis. Microglial cells are the resident macrophages participating in the active innate immune defense in the CNS. Phagocytosis, as a mechanism of innate immune defense, is typically inhibited in gliomas.5,6 Because microglial cells largely arise from bone marrow they share functional and physical properties with cells of myeloid origin and the common origin may well account for the shared genetic changes in glioma and AML. More importantly, human glioma-infiltrating microglia/macrophages (GIMs) are known to substantially contribute to the tumor mass7 (Fig. 1A). In the present study we specifically explored whether microglial cells/macrophages residing in glioma samples share the IDH1R132H mutation with glial tumor cells.
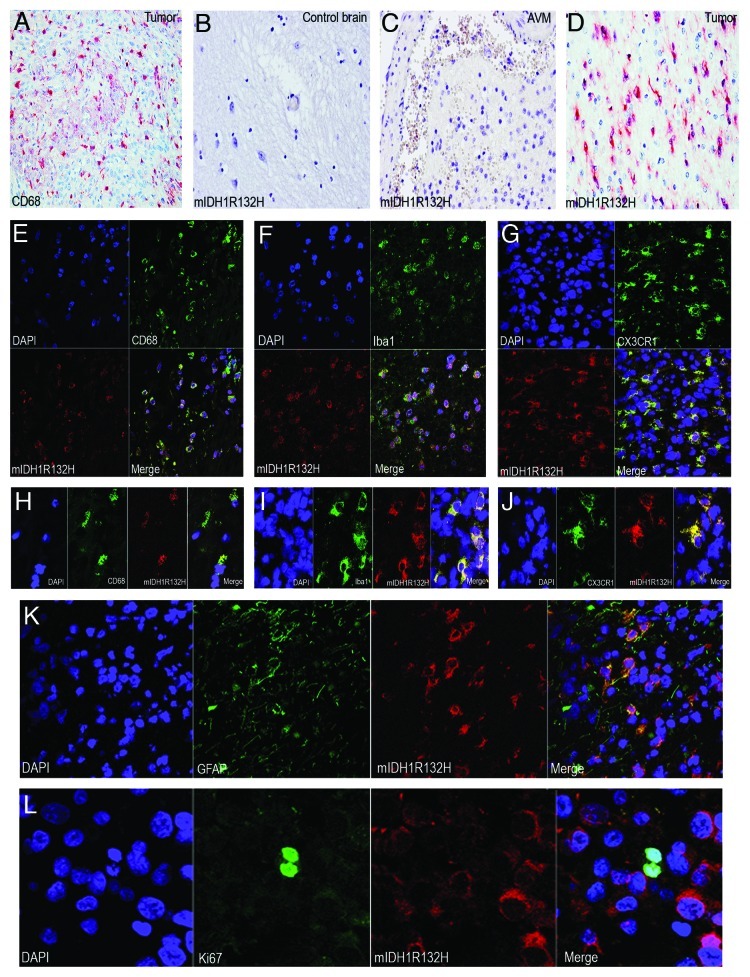
Figure 1. Mutant IDH1R132H detected in glioma-infiltrating microglia/macrophages (GIMs). (A) GIMs as labeled by CD68 significantly contribute to the cellular components of a glioma. (B) Mutant IDH1R132H is not detected in a control brain. (C) Mutant IDH1R132H is not detected in glial cells around an AVM. (D) Mutant IDH1R132H is detected in a glioma. (E) GIMs double positive for CD68 and mutant IDH1R132H are shown in overview. (F) GIMs double positive for Iba1 and mutant IDH1R132H are shown in overview. (G) GIMs double positive for CX3CR1 and mutant IDH1R132H are shown in overview. (H) Detail of GIMs double positive for CD68 and mutant IDH1R132H. (I) Detail of GIMs double positive for Iba1 and mutant IDH1R132H. (J) Detail of GIMs double positive for CX3CR1 and mutant IDH1R132H. (K) Glial tumor cells co-express GFAP and mutant IDH1R132H. (I) Mutant IDH1R132H positive cells do not co-express Ki67.
Results and Discussion
The mutation-specific antibody enables robust detection of the mutation in routine biopsy samples. The specificity of the antibody is confirmed by specifically staining the IDH1R132H mutant cells only while not cells in normal or non-neoplastic brains4 (Fig. 1B–D). The cellular composition of diffusely infiltrating gliomas is notoriously heterogeneous. In addition to neoplastic glial cells which harbour the IDH1 mutation (Fig. 1K) as identified by double positive cells of mIDH1R132H and glial fibrillary acidic protein (GFAP, the classical marker for astrocytes), tumor samples generally contain reactive glial cells, vascular cells and phagocytic elements such as macrophages and microglia. We extended our investigations with a cohort of 60 patients with gliomas in which IDH1R132H mutation had previously been determined by DNA sequence analysis. The mutation was also confirmed at the protein level by using the anti-mIDH1R132H antibody for each case (Fig. 1D). A cohort of control brains including ten non-neoplastic autopsy brains and resection specimens of six arteriovenous malformation (AVM) were included in this study, and none of these controls appeared to be positive for the anti-mIDH1R132H (Fig. 1B and C). In order to locate microglial cells/macrophages, we used CD68, Iba1 and CX3CR1 as markers for microglial cells in double immunofluorescence labeling experiments with the anti-mIDH1R132H. Initially, we randomly counted 200 CD68+ microglial cells in non-overlapping regions in each sample and found 26 ~86% CD68+ microglial cells to be immunopositive for the mutant IDH1R132H (Fig. 1E and H). To further confirm the lineage of these cells we used additional microglial markers (Iba1 and CX3CR1) in 25 samples, and found 16 ~68% Iba1+ (Fig. 1F and I) and 20 ~56% CX3CR1+ (Fig. 1G and J) microglial cells to be immunopositive for the mutant IDH1R132H. Morphological signs of phagocytosis are largely not observed in CD68+, Iba1+ and CX3CR1+ microglia/macrophages. The findings demonstrate that part of the IDH1R132H mutant cells are either true microglial cells or fusions of glial tumor cells and microgalia/macrophages. It is well-known that macrophages are capable of fusing with tumor cells.8 This corroborates the hypothesis that microglial cells are actively taking part in glial tumorigenesis.9
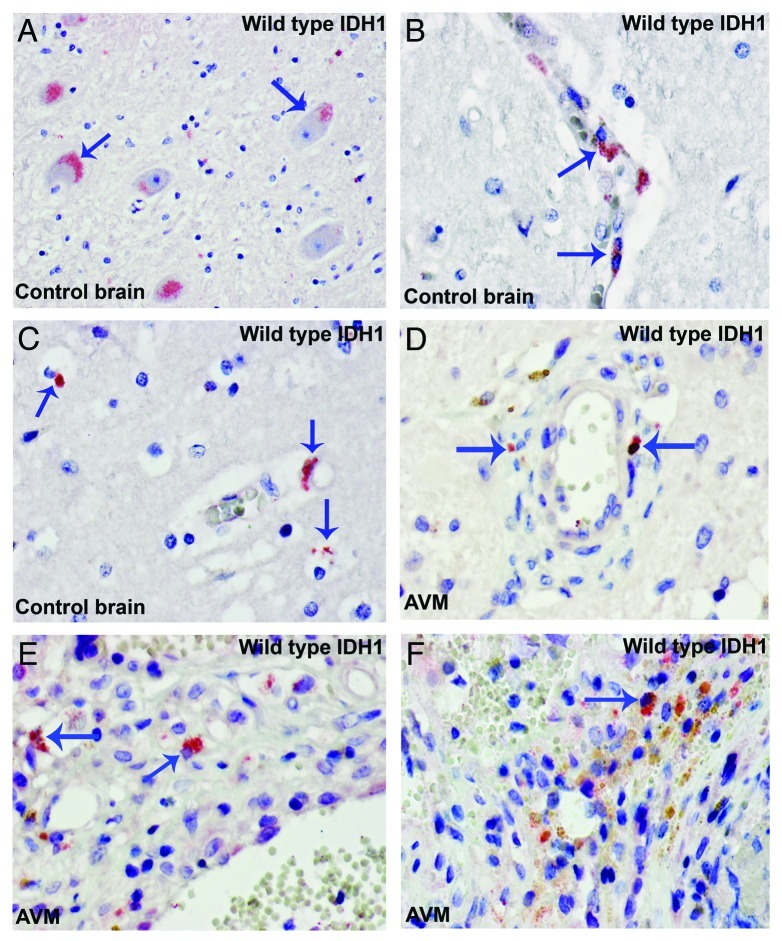
Tumor progression of gliomas depends on both cellular proliferation and migration which are mutually exclusive phenomena at the level of individual cells.10-12 Microglial cells are highly motile and show a distinct migratory behavior. Microglial cells fit well into the group of highly migratory cells (invasive tumor component) which display little mitotic activity.9,13 Indeed, the large majority of mutant IDH1R132H cells did not co-express the cell proliferation marker Ki67 (Fig. 1I). These non-glial cells harbour genetic aberrations of the tumor cells, reminiscent of tumor–macrophage hybrids which have been supposed to be the origin of human metastatic cancers by fusogenicity.8 Such fused cells would combine the migratory potential of macrophages and the proliferative potential of the tumor cells.9 We also examined the expression pattern of wild type IDH1 (WT-IDH1) in control brain samples and WT-IDH1 is largely detected in neurons and microglia/macrophages (Fig. 2). It seems that the expression of WT-IDH1 in normal or non-neoplastic brain is restricted to cells with high energetic demands, and the expression seems to be induced in other cells under pathological situations where cellular energy requirements have increased.
Figure 2. Wild type IDH1 is expressed in neuron and microglia/macrophages. (A) Control brain. WT IDH1 is expressed in neuron (arrows). (B) Control brain. WTIDH1 is expressed in perivascular microglia (arrows). (C) Control brain. WTIDH1 is expressed in parenchymal microglia (arrows). (D to F) WTIDH1 is expressed in microglia/macrophages present in samples of arteriovenous malformations (AVM) (arrows).
In summary, our findings demonstrate that the cell group of microglial cells/macrophages is an important target for IDH1R132H mutation in glial tumors. The findings suggest that blood borne cells like microglia/macrophages serve as an additional tumor component contributing in particular to the tumor infiltrative behavior. The data provide novel insights/clues for therapeutic strategies against malignant gliomas.
Materials and Methods
Patients and tissue samples
The study was conducted with the approval of the institutional ethical review board. A cohort of 60 patients with gliomas was used for this study. All samples were known with IDH1 mutation at locus R132H determined by DNA sequence analysis. Control brain samples were from autopsy brains without CNS diseases (n = 10) and arteriovenous malformations (AVM, n = 6).
Antibodies
Antibodies specifically targeting the IDH1 R132H mutation (1: 250; Dianova GmbH, Germany); CD68 (1: 150; Abbiotec); Iba1 (1: 100; Bioss Inc.); CX3CR1 (1:100; Sigma-Aldrich); GFAP (1: 50; Dako), Ki67 (1:100; Dako) and wild type IDH1 (WT IDH1) (1: 100; Epitomics) were used for this study. Corresponding secondary antibodies conjugated with AP, FITC and Cy3 were used.
Immunostaining and confocal laser scanning microscope (CLSM)
The procedures for immunohistochemistry, double immunofluorescent staining and CLSM were performed as previously published.14-16
Acknowledgments
We thanks Drs W.N. Dinjens and H.J. Dubbink for providing the DNA sequencing date.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/20836
References
- 1.Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med. 2010;16:387–97. doi: 10.1016/j.molmed.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339–44. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118:599–601. doi: 10.1007/s00401-009-0595-z. [DOI] [PubMed] [Google Scholar]
- 5.Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–25. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011;59:472–85. doi: 10.1002/glia.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50:305–11. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 8.Pawelek JM, Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis. Nat Rev Cancer. 2008;8:377–86. doi: 10.1038/nrc2371. [DOI] [PubMed] [Google Scholar]
- 9.Huysentruyt LC, Akgoc Z, Seyfried TN. Hypothesis: are neoplastic macrophages/microglia present in glioblastoma multiforme? ASN Neuro. 2011;3:183–93. doi: 10.1042/AN20110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng PP, Severijnen LA, van der Weiden M, Willemsen R, Kros JM. Cell proliferation and migration are mutually exclusive cellular phenomena in vivo: implications for cancer therapeutic strategies. Cell Cycle. 2009;8:950–1. doi: 10.4161/cc.8.6.7851. [DOI] [PubMed] [Google Scholar]
- 11.Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53:799–808. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- 12.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–36. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 13.Kofler J, Wiley CA. Microglia: key innate immune cells of the brain. Toxicol Pathol. 2011;39:103–14. doi: 10.1177/0192623310387619. [DOI] [PubMed] [Google Scholar]
- 14.Zheng PP, Hop WC, Luider TM, Sillevis Smitt PA, Kros JM. Increased levels of circulating endothelial progenitor cells and circulating endothelial nitric oxide synthase in patients with gliomas. Ann Neurol. 2007;62:40–8. doi: 10.1002/ana.21151. [DOI] [PubMed] [Google Scholar]
- 15.Zheng PP, van der Weiden M, Kros JM. Hela l-CaD is implicated in the migration of endothelial cells/endothelial progenitor cells in human neoplasms. Cell Adh Migr. 2007;1:84–91. doi: 10.4161/cam.1.2.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng PP, Weiden M, Sillevis Smitt PA, Luider TM, Kros JM. Hela /-CaD undergoes a DNA replication-associated switch in localization from the cytoplasm to the nuclei of endothelial cells/endothelial progenitor cells in human tumor vasculature. Cancer Biol Ther. 2007;6:886–90. doi: 10.4161/cbt.6.6.4091. [DOI] [PubMed] [Google Scholar]