Abstract
Overcoming resistance to chemotherapy is the main therapeutic challenge in the treatment of acute lymphocytic leukemia (ALL). Interactions between leukemia cells and the microenvironment promote leukemia cell survival and confer resistance to chemotherapy. Hypoxia is an integral component of bone marrow (BM) microenvironment. Hypoxia-inducible factor-1α (HIF-1), a key regulator of the cellular response to hypoxia, regulates cell growth and metabolic adaptation to hypoxia. HIF-1α expression, analyzed by Reverse Phase Protein Arrays in 92 specimens from newly diagnosed patients with pre-B-ALL, had a negative prognostic impact on survival (p = 0.0025). Inhibition of HIF-1α expression by locked mRNA antagonist (LNA) promoted chemosensitivity under hypoxic conditions, while pharmacological or genetic stabilization of HIF-1α under normoxia inhibited cell growth and reduced apoptosis induction by chemotherapeutic agents. Co-culture of pre-B ALL or REH cells with BM-derived mesenchymal stem cells (MSC) under hypoxia resulted in further induction of HIF-1α protein and acquisition of the glycolytic phenotype, in part via stroma-induced AKT/mTOR signaling. mTOR blockade with everolimus reduced HIF-1α expression, diminished glucose uptake and glycolytic rate and partially restored the chemosensitivity of ALL cells under hypoxia/stroma co-cultures. Hence, mTOR inhibition or blockade of HIF-1α-mediated signaling may play an important role in chemosensitization of ALL cells under hypoxic conditions of the BM microenvironment.
Keywords: HIF-1α, chemoresistance, ALL, hypoxia, microenvironment
Introduction
Adult acute lymphocytic leukemia (ALL) is an aggressive lymphoproliferative disorder with high complete remission (CR) rates (91%) to frontline chemotherapy, but relapse remains common with an estimated median survival time of 35 months.1,2 Persistence of minimal residual disease (MRD) after the 1st cycle of induction chemotherapy is highly predictive for subsequent relapse and shorter survival.2 Elucidation of the intrinsic or acquired factors that mediate chemoresistance remains of critical importance for the development of novel therapeutic strategies.
Interactions between leukemia cells and the bone marrow (BM) microenvironment are recognized to promote leukemia cell survival.3-5 BM-derived mesenchymal stem cells (MSC) were shown to prevent spontaneous or therapy-induced apoptosis in B-ALL cells,6 and the high recovery of leukemic blasts in stroma-supported cultures predicted a lower 4-year event-free survival rate in childhood B-ALL (50% vs. 91%).7 These findings indicate that protective signals arising from the stromal microenvironment maintain residual leukemic cells, potentially contributing to disease recurrence.
Recent data indicate that hypoxia, present primarily along endosteum at the bone-BM interface, is an integral feature of the normal bone marrow microenvironment.8 In a rat model of leukemogenesis, leukemic cells infiltrating the BM were shown to be markedly hypoxic compared with cells in the BM of healthy rats.9 We have recently shown that progression of leukemia is associated with vast expansion of the bone marrow hypoxic areas and that hypoxia contributes to chemoresistance of leukemic cells.10 Hypoxia-Inducible Factor α (HIF-1α), one of the best characterized markers of hypoxia, was shown to be overexpressed in clusters of BM-resident leukemic cells in pediatric ALL cases while absent in normal BM biopsies.11 In agreement with this, we found high levels of HIF-1α in 6 of the 9 BM biopsies obtained from ALL patients at the time of diagnosis that was reduced to low/undetectable levels in the paired BM samples obtained after patients have achieved complete remission.10 HIF-1 is a key regulator of the cellular response to hypoxia12 that is stabilized post-transcriptionally by levels of oxygen tension less than 2%.13 HIF-1α is a transcription factor that controls a vast array of gene products involved in energy metabolism, glycolysis, angiogenesis, apoptosis, cell cycle, and has become recognized as a strong promoter of tumor growth. From these, the switch to glycolysis and increased glucose metabolism can directly regulate the mitochondrial apoptotic pathway,14-16 thereby promoting chemoresistance through inhibiting the effectiveness of chemotherapeutic agents. Notably, genomic data have shown overexpression of the HIF-1α target gene, glucose transporter Glut-3 to correlate with poor outcomes in ALL.17
Although hypoxia is the best-characterized mechanism of HIF activation in tumors,18,19 HIF activity can also be induced in tumor cells through a variety of oncogenic stimuli and growth factors, primarily through activation of the AKT/m-TOR20 and MAPK pathways.21,22 Data in transgenic models demonstrated that AKT activation results in mTOR dependent transcriptional upregulation of the glycolytic enzyme HKII and glucose transporter Glut-1 via induction of HIF1-α.23 Several published reports suggest that the activation of mTOR is one of the central mechanisms of upregulation of the HIF-1α protein synthesis downstream of growth factors and PI3K/Akt signaling in mammalian and Drosophila cells.24,25 Of importance, combination of the mTOR inhibitor rapamycin with chemotherapy has been shown to reverse drug resistance in the preclinical models of AKT-expressing lymphomas and ALL.26,27 These findings suggest that the reliance of HIF-1α translation on mTOR can be exploited therapeutically in tumors with functional AKT/mTOR/HIF1α pathway.
The goal of this study was to investigate the molecular mechanisms of survival of leukemic cells growing under hypoxic conditions of the BM microenvironment. Our data demonstrate that BM stromal cells enhance HIF-1α expression under hypoxia, leading to HIF-1α-dependent upregulation of glucose transport and a switch to glycolytic metabolism in leukemic cells and primary ALL blasts. Downregulation of HIF-1α expression or blockade of mTOR signaling with everolimus promoted chemosensitivity. These findings, in conjunction with the observation of negative prognostic impact of HIF-1α expression in ALL, indicate new avenues of therapeutic targeting HIF-1α in leukemias.
Results
Examination of HIF-1α expression in primary ALL specimens
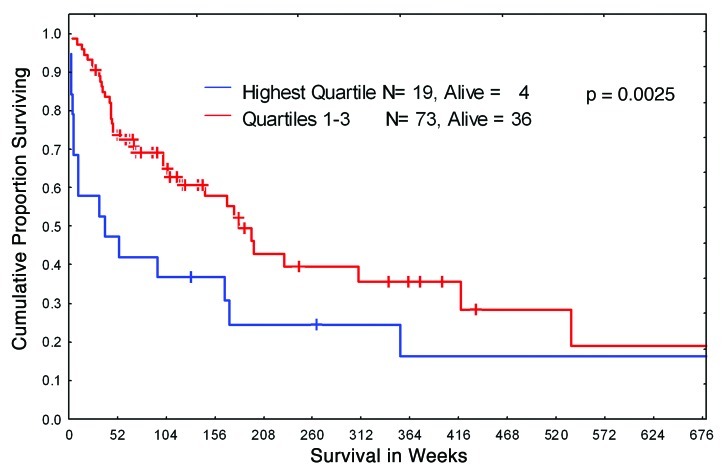
We elected to investigate the intensity of expression of HIF-1α, an established marker of hypoxic environment, in leukemic BM from 92 newly diagnosed patients with Ph-negative B-ALL. The protein levels of HIF were analyzed using the RPPA, a technique established by our group to provide an accurate and reproducible method for the study of protein expression levels in a large leukemia sample sets.32 The patient’s characteristics are summarized in Table 1. The majority of patients were treated with standard hyper-CVAD regimen, with a subset of CD20+ patients receiving rituximab. We stratified patients into four quartiles based on HIF-1α protein levels and analyzed for any correlation between HIF-1α expression and overall survival. As shown in Figure 1, the survival of patients with the highest (4th quartile) levels of HIF-1α was significantly worse compared with those who had lower HIF-1α levels. No association of HIF-1α overexpression with other clinical or laboratory parameters (age, WBC, BM blasts, PB blasts, FAB variants, cytogenetic variants, albumin, bilirubin, creatinin etc.) was found (data not shown). Notably, no significant differences in HIF-1 expression levels were seen in 140 paired blood-marrow samples (Fig. S1). A subset of protein lysates from 10 samples included in RPPA analysis was subjected to conventional western blotting, and HIF-1α protein was detectable in 50% of the samples validating the RPPA data (Fig. S2). These results indicate that hypoxia and its downstream effector HIF-1α may confer resistance to chemotherapeutic drugs commonly used in the therapy of ALL.
Table 1. Demographics and clinical characteristics of 92 newly diagnostic ALL patients.
| Variable category | Number or % |
|---|---|
| No of cases |
92 |
| male/female | 52.2/47.8% |
| Age, years | |
|---|---|
| mean |
39.9 |
| median |
39.2 |
| minimum |
1.7 |
| maximum | 80,6 |
| FAB* | |
|---|---|
| L1 |
32.6% |
| L2 |
42.4% |
| L3 |
8.7% |
| NA | 16.3% |
| Cytogenetics | |
|---|---|
| diploid |
32.6% |
| hyperdiploid |
19.6% |
| hypodiploid |
6.5% |
| pseudidiploid |
10.9% |
| miscellaneous |
7.6% |
| t4,11 |
7.6% |
| t8,14 |
2.1% |
| t8,22 |
1.1% |
| t11,14 |
1.1% |
| t11,19 |
1.1% |
| N/A | 9.8% |
| Immunophenotype | |
|---|---|
| CALLA |
60.9% |
| PRE B |
25% |
| B cell |
10.9% |
| T/CALLA/PRE B |
2.2% |
| NA | 1% |
| Treatment | |
|---|---|
| HCVAD |
63% |
| HCVAD+RITUXIMAB |
25% |
| VAD |
2.2% |
| AMSA+OAP ALONE |
1.1% |
| CCG00–1991 |
2.2% |
| AUG BFM |
5.4% |
| cytarabine+daunorubicin+vincristine | 1.1% |
| Response | |
|---|---|
| Complete Response |
84.8% |
| Resistant |
6.5% |
| Fail |
8.7% |
|
Relapse |
35.9% |
| Alive | 42.4% |
French-American-British (FAB) classification system of ALL.
Figure 1. Kaplan-Meier curves of overall survival for B-ALL patients. Newly diagnosed B-ALL patients were stratified in two groups according to HIF-1α protein expression level: highest level (quartile 4) and lower level (quartile 1–3).
Role of HIF-1α in the sensitivity of leukemic cells to chemotherapy under hypoxia
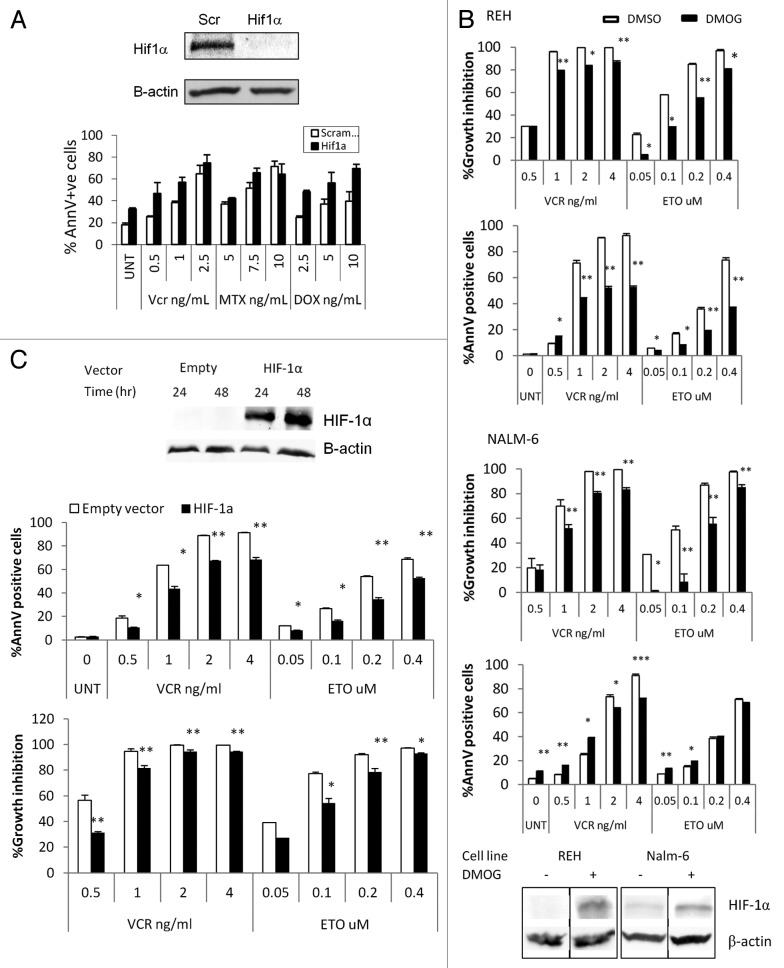
We have recently reported that culture of REH and NALM6 pre-B-ALL cells under hypoxic (1% O2) conditions significantly reduces the growth-inhibitory effects of vincristine, methotrexate, and etoposide.10 Curiously, only minor differences were seen in the sensitivity to doxorubicin, which is known to inhibit HIF-1α.33 Given the well-known function of HIF-1α as a master mediator of hypoxia responses, we next evaluated whether the protective effect of hypoxia was mediated by this transcription factor. To this end, the above experiments were repeated using REH cells in which HIF-1α expression had been silenced by HIF-1α locked messenger ribonucleic acid antagonist (LNA) EZN-2968.34 REH cells transfected with EZN-2968 had undetectable levels of HIF-1α when compared with the scramble transfected controls (Fig. 2A). When these cells were treated with vincristine or methotrexate at 1% O2, they had higher cell death levels than the controls, indicating that the hypoxia-induced chemoprotective effect is mediated at least in part by HIF-1α.
Figure 2. HIF-1α and cytotoxic effects of chemotherapy at 1% O2. (A) REH cells were transfected with a scramble or HIF-1a LNA, treated with the indicated chemotherapy agent, and incubated at 1% O2 for 48 h. Western blot showing HIF-1α knockdown achieved with HIF-1α LNA but not with the scramble LNA. UNT, untreated; Vnc, vincristine; MTX, methotrexate; DOX, doxorubicin; AnnV, Annexin V; Scr, scrambled LNA. (B, C) REH cells or NALM6 cells were treated with 100µM DMOG (B) or infected with empty vector or HIF-1α lentivirus and induced with 1µg/ml Doxocycline (REH, C). Western blots show expression of HIF-1α in DMOG (96hr treatment) or Doxocyline treated cells. After inducing HIF-1α expression at 21%O2, cells were treated with chemotherapy (VCR or ETO). After 72 h, effects on cell growth and apoptosis induction were determined by FACS. Growth inhibition was calculated for each group (DMSO, DMOG, Scr or HIF-1α) as the percentage relative to the untreated control. *p < 0.05; ** p < 0.01.
To further discern the role of HIF-1α in chemoresistance, we next overexpressed HIF-1α under normoxic conditions using two different approaches. First, REH cells were treated with dimethyloxalylglycine (DMOG), an α-ketoglutarate antagonist that inhibits prolyl hydroxylases leading to HIF-1α stabilization in the presence of oxygen.35 As shown in Figure 2B, treatment of REH cells with DMOG indeed resulted in prolonged stabilization of HIF-1α under normoxic conditions. Forced expression of HIF-1α significantly diminished apoptosis induced by Vcr in both, REH and NALM6 (Fig. 2B) cells, and by Etoposide (ETO) in NALM6 cells.
This translated into marked differences in the degree of growth inhibition, with higher number of viable (non-apoptotic) cells in DMOG-treated cells. Similarly, when an inducible lentivirus was used to express a non-degradable mutant HIF-1α, Vcr or ETO under normoxic conditions induced less apoptosis in REH cells overexpressing HIF-1α compared with the empty vector-infected control cells (Fig. 2C and Supplementary Figures). As shown in Fig. S3, both, DMOG and lentivirus induced expression of HIF-1α led to a transcriptionally functional form of the protein as indicated by induction of HIF downstream genes Glut-1, CXCR4 and VEGF-A. Notably, stabilization of HIF-1α under normoxic conditions prompted drastic inhibition of proliferation of ALL cell lines (Fig. S4) without significant induction of cell death (Fig. 2B). Taken together, these results are consistent with the notion that under hypoxia HIF-1α promotes resistance of leukemic cells to chemotherapeutic agents.
Activation of HIF-1α, pAKT and pERK expression in ALL cells co-cultured with BM-derived MSC under hypoxia
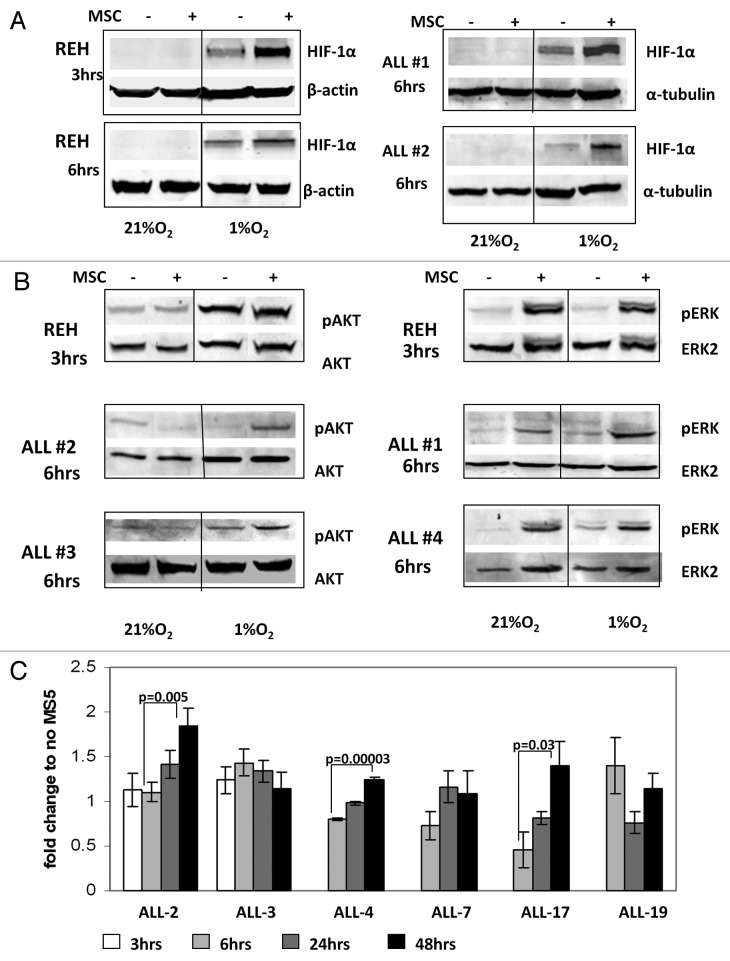
Growth of leukemic cells in vivo requires direct contact with BM-derived stromal cells, which in turn prevent apoptosis of B-ALL cells.36 To examine the molecular mechanisms of survival of leukemic cells growing under hypoxic conditions of the BM microenvironment, we established a co-culture system of B-ALL cells with BM-derived mesenchymal stem cells (MSC). REH and primary ALL cells (samples #1 and #2, Table 2) were pre-incubated overnight with or without MSC and then cultured under hypoxic conditions (1% O2) for the indicated time intervals. While hypoxia alone resulted in stabilization of HIF-1α protein, co-culture with MSC enhanced HIF-1α protein levels in both, pre-B REH cells and in the primary ALL samples tested (Fig. 3A). Since MSC are known to produce a variety of growth factors and cytokines that sustain leukemia cell growth in part via activation of AKT/mTOR and MAPK signaling pathways, we examined expression and phosphorylation of AKT Ser473 and ERK Thr202/Tyr204 in ALL cells. Analysis of signaling events demonstrated that culture of REH in hypoxic conditions stimulated AKT phosphorylation, and this was further increased by MSC co-cultures in primary ALL samples (Fig. 3B). In turn, ERK activation in leukemic cells was induced exclusively by MSC co-cultures independent of oxygen concentration (Fig. 3B). These data illustrate coordinate regulation of signaling and HIF-1α levels under conditions mimicking hypoxic BM microenvironment, and suggest that HIF-1α levels in leukemic cells are coordinately regulated by BM stromal cells and hypoxia.
Table 2. Clinical data for ALL patients whose samples were used for in vitro studies.
| Pt # | Source | % blasts | Cytogenetic | Immunophenotype | Status | Assay |
|---|---|---|---|---|---|---|
| ALL (#1) |
BM |
93 |
pseudodiploid |
Precursor B-ALL |
relapsed |
WB |
| ALL (#2) |
BM |
90 |
hypodiploid |
Precursor B-ALL |
diagnosis |
WB |
| ALL (#3) |
PB |
98 |
Ph-positive |
Precursor B-ALL |
relapsed |
WB |
| ALL (#4) |
PB |
50 |
Ph-positive |
Precursor B-ALL |
relapsed |
WB |
| ALL (#5) |
PB |
90 |
t4,11 |
Precursor B-ALL |
relapsed |
WB |
| ALL (#6) |
PB |
80 |
hyperdiploid |
Precursor T-ALL |
relapsed |
ATP |
| ALL (#7) |
PB |
50 |
diploid |
Precursor B-ALL |
relapsed |
ATP |
| ALL (#8) |
PB |
44 |
diploid |
Precursor B-ALL |
relapsed |
ATP |
| ALL (#9 |
PB |
80 |
diploid |
Precursor T-ALL |
diagnosis |
ATP |
| ALL (#10) |
PB |
92 |
pseudodiploid |
Precursor B-ALL |
relapsed |
ATP |
| ALL (#11) |
PB |
82 |
t4,11 |
Precursor B-ALL |
diagnosis |
ATP |
| ALL (#12) |
PB |
74 |
hypodiploid |
Precursor B-ALL |
relapsed |
LA |
| ALL (#13) |
BM |
90 |
Ph-positive |
Precursor B-ALL |
diagnosis |
LA |
| ALL (#14) |
PB |
61 |
Hypertriploid |
Precursor B-ALL |
relapsed |
LA |
| ALL (#15) | PB | 80 | Ph-positive | Precursor B-ALL | diagnosis | LA |
WB, western blot; ATP, adenosine triphosphate; LA, lactic acid
Figure 3. A and B. Activation of HIF-1α, pAKT and pERK protein expression in ALL cells co-cultured with bone marrow-derived MSC under hypoxia. REH cells and primary ALL blasts were cultured under normoxic (21% O2) or hypoxic (1% O2) conditions for the indicated time periods (with or without MSCs). Expression of HIF-1α (A), pAKT Ser473 and pERK proteins (B) was analyzed by immunoblotting. (C)Time-course of HIF-1α expression for six ALL xenograft lines. Cells were cultured with and without MS-5 and harvested at the indicated time points. EF1α was used as an internal control as described in the methods. Each fold change in relative HIF-1α mRNA expression levels was calculated based on their non-MS5-cultured counterpart. Results are the mean ± SE of at least three separate experiments.
Examination of HIF-1α mRNA level and functional activity in childhood ALL-xenograft cells co-cultured with murine MS5 cells
While hypoxia regulates HIF-1α levels primarily through blockade of its degradation, PI3K/mTOR-mediated signals were reported to affect both the synthesis and stabilization of HIF-1α. To this end, microarray analysis of differential gene expression in ALL-xenograft cells grown with and without murine MS-5 stromal cells under normoxia demonstrated that HIF-1α was the second highest ranked gene by both the t-test and SAM analysis, although it only displayed a 1.2-fold upregulation with the addition of MS5 culture (prepared for publication elsewhere, data not shown). These results were further assessed by real-time RT-PCR of the mRNA levels of HIF-1α in six different ALL-xenograft lines (Table 3) when compared with their non-MS5 treated counterparts. While the levels fluctuated somewhat over time HIF-1α mRNA was upregulated between 6 and 48 h after co-culture in 3 of 6 xenograft cell samples examined (Fig. 3C). Collectively, these results indicate that co-culture of ALL cells with stromal cells induces HIF-1α mRNA, which may contribute to increased levels of protein under hypoxia.
Table 3. Clinical data from childhood ALL patients’ xenograft samples.
| Xenograft | Subtype | Status | Assay |
|---|---|---|---|
| ALL-2 |
c-ALL |
Relapse |
mRNA |
| ALL-3 |
Pre-B |
Diagnosis |
mRNA &ChIP |
| ALL-4 |
Ph+ c-ALL |
Diagnosis |
mRNA |
| ALL-7 |
Biphenotypic |
Diagnosis |
mRNA |
| ALL-17 |
c-ALL |
Diagnosis |
mRNA |
| ALL-19 | c-ALL | Relapse | mRNA |
Inhibition of mTOR downregulates HIF-1α, pS6K and pAKT expression in leukemic cells co-cultured with MSC under hypoxia
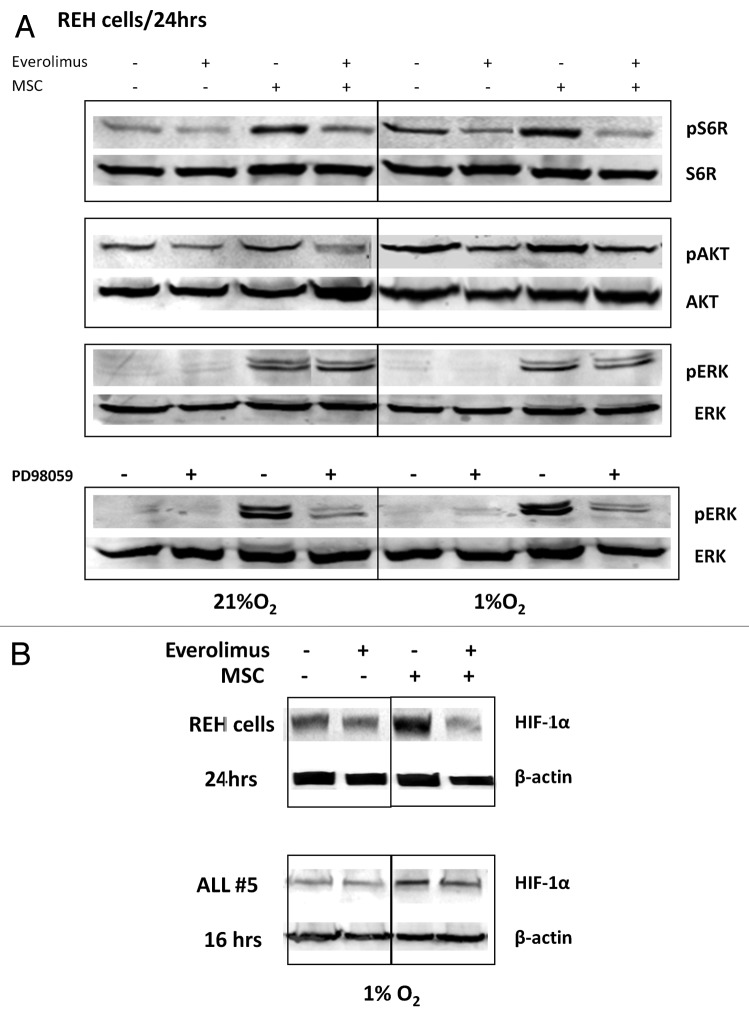
mTOR is known to regulate HIF-1α expression through increased rates of mRNA translation.37 There is also evidence that HIF-1α interacts directly with the mTORC1 complex component raptor through its mTOR signaling motif, and that this is important for HIF-1α transcriptional activity.38 To characterize mTOR-AKT-dependence of HIF-1α expression in stromal co-cultures, we utilized the rapamycin derivative everolimus. First, we examined AKT and MAPK phosphorylation following everolimus treatment. Consistent with activation of AKT/mTOR signaling, hypoxia alone—and to a greater extent MSC co-culture under hypoxic conditions - promoted phosphorylation of the mTOR target pS6K, and these effects were inhibited by everolimus (Fig. 4A). Further, 24 h exposure to everolimus decreased AKT phosphorylation in REH cells, likely through decreased stability of mTORC2 complex reported by us and others.29,39 In turn, everolimus did not affect pERK expression whereas MEK inhibitor PD98059 completely abrogated pERK expression induced by MSC (Fig. 4A). Examination of HIF-1α levels showed that everolimus diminished hypoxia- and MSC-induced protein expression in the REH cells and in primary ALL cells (Fig. 4B). These data indicate that mTOR is one of the regulators of HIF-1α expression in ALL cells within the hypoxic BM microenvironment.
Figure 4. Inhibition of mTOR downregulates HIF-1α, pS6K and pAKT expression in leukemic cells co-cultured with MSC under hypoxia. REH cells (A, B) or primary ALL cells (#5, B) were cultured under the indicated oxygen levels in the presence or absence of 20nM everolimus or PD98059 10µM (with or without MSCs). Expression of HIF-1α, pS6K/S6K, pAKT ser473/AKT and pERK/ERK2 proteins was determined by Western Blot.
Inhibition of mTOR decreases glucose uptake and diminishes glycolytic rate of leukemic cells
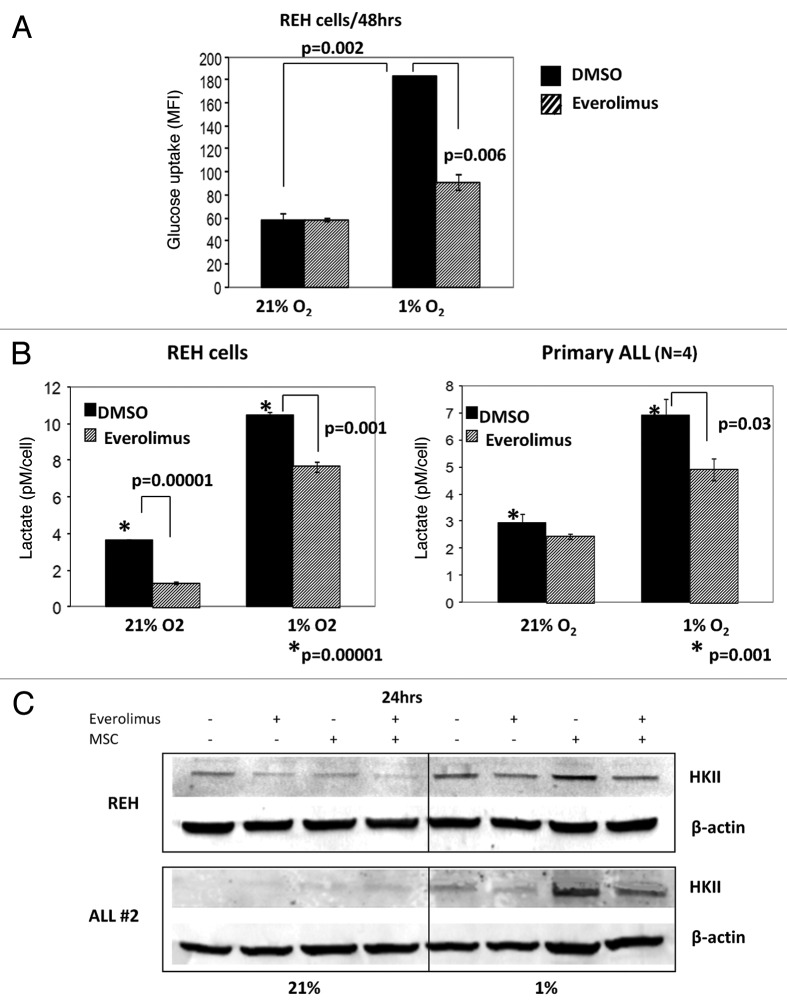
We next examined the effects of mTOR inhibition on the glycolytic rate of leukemic cells. As shown in Figure 5A, REH cells exhibited significantly higher glucose uptake when cultured in hypoxic compared with normoxic conditions (p = 0.002). In turn, incubation of REH cells with 20 nM everolimus for 48 h resulted in significant decrease of the glucose uptake under hypoxia (p = 0.006). To analyze the effects of mTOR inhibition on the glycolytic rate of leukemic cells we measured production of lactic acid (LA), an end product of glycolysis. LA accumulation was analyzed in the REH and primary ALL conditioned medium post 72 h of everolimus exposure. As illustrated in Figure 5B leukemic cells produced significantly more LA when cultured under hypoxia compared with normoxia. Consistent with its effects on HIF-1α and glucose uptake, everolimus significantly inhibited LA production in REH cells and in primary ALL blasts under hypoxic conditions (p = 0.001–0.03). Curiously, everolimus also diminished basal LA produced by REH cells under normoxia, indicating contribution of mTOR signaling to normoxic glycolytic activity of these cells.
Figure 5. Everolimus decreases glucose uptake and reduces lactic acid production in leukemic cells. REH and primary ALL cells were grown under normoxic (21% O2) or hypoxic (1% O2) conditions and treated with 20nM everolimus for the indicated time period. (A) Glucose uptake was measured by Flow Cytometry. The results are presented as mean fluorescent intensity (MFI). (B) Lactic acid (LA) concentration was measured in the aliquots collected from the medium using Accutrend Lactate device (Roche). The data was expressed as pM per cell. Left, REH; right, averaged data from 4 primary ALL samples (#12–15, Table S1). (C) REH and primary ALL cells (#2, Table S1) were cultured with or without MSC under normoxic (21% O2) or hypoxic (1% O2) conditions in the presence or absence of 20nM everolimus for 24hrs. HKII protein expression was examined by Western Blot. Expression of β-actin was used as a loading control.
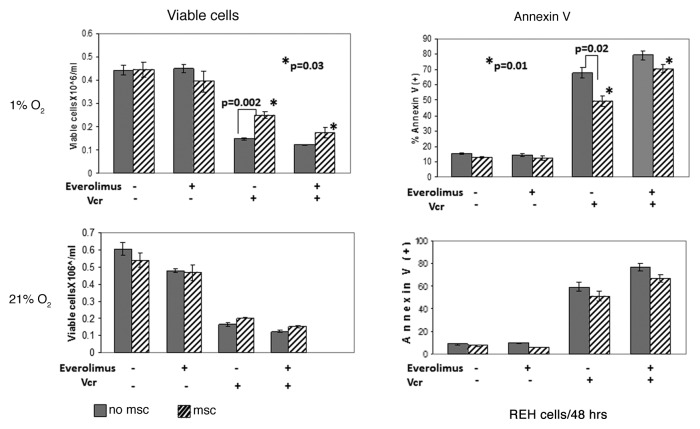
Building on the observation that culture of ALL cells under hypoxia induces a shift to glycolysis pathway, we sought to determine the expression of the key glycolytic enzyme hexokinase II (HKII), a known HIF-1α target. In both REH and primary ALL cells hypoxia alone induced expression of HKII protein, which was further upregulated in MSC co-cultures, in concert with the HIF-1α expression data above. In turn, everolimus treatment resulted in robust inhibition of HKII expression (Fig. 5C). Co-culture with MSC selectively diminished growth-inhibitory and pro-apoptotic effects of Vcr under hypoxia. Notably, this protective effect of MSC/hypoxia was reversed by inhibition of mTOR signaling with everolimus (Fig. 6). Everolimus by itself did not inhibit cell growth or induce apoptosis, and did not significantly affect Vcr-induced cell death in ALL monocultures. Similar findings were seen in another pre-B ALL cell line NALM-6, whereby everolimus enhanced growth inhibition caused by Vcr (Fig. S5).
Figure 6. Inhibition of mTOR signaling sensitized leukemic cells to chemotherapy under hypoxic conditions mimicking BM microenvironment. Exponentially growing REH cells were cultured with or without MSC for 48 h under normoxia (21% O2) and then were grown under normoxic (21% O2) or hypoxic (1% O2) conditions in the presence or absence of 20nM everolimus and/or 1ng/ml Vcr. After 48 h, effects on cell growth and apoptosis induction were determined by viable cell count and annexin V flow cytometry. *p = 0.01–0.03
Discussion
In this study, we investigated the role of hypoxia, a physiological component of BM microenvironment, in the acquisition of pro-survival properties and chemoresistance of ALL cells. Despite a well-recognized role of hypoxia and its major downstream mediator HIF-1α in solid tumors, the effects of hypoxia in leukemia cell survival and chemoresistance have not been completely elucidated, likely due to methodological limitations measuring bone marrow oxygen levels. Our published data showed reduced (6%) oxygen levels in BM of AML patients,40 however measuring O2 levels in BM aspirates mixed with venous blood likely overestimates the true oxygen content of BM niches. Our recent findings using the hypoxia marker Pimonidazole demonstrate vast expansion of the hypoxic niches in the BM from mice bearing ALL, AML and CML xenografts. These observations in the murine leukemia models were supported by histochemical detection of HIF-1α in BM biopsies of ALL patients at diagnosis, which reverted to low levels found in normal BM after patients achieved CR.10 In the current study, we have shown overexpression of HIF-1α in a large subset of newly diagnosed adult B-ALL cases, consistent with previously published data in pediatric ALL cases.11 It should be noted, however, that HIF-1α levels in cell lysates prepared after exposure of cells to ambient O2 levels may be reduced from the physiological conditions, given the very short (5–8 min) half-life of HIF-1α under normoxic conditions. Notably, HIF-1α was detectable at similar levels in paired peripheral blood and bone marrow samples from leukemia patients, possibly due to egress of hypoxic bone marrow blasts into the circulation, reported recently in a murine myeloma model.41 The observation of inferior outcomes of patients with the highest expression of HIF-1α prompted us to investigate the functional role of HIF-1α in ALL cells and mechanisms of its stabilization.
To validate the relevance of these findings to chemoresistance, we first tested the sensitivity of ALL cells to chemotherapy under conditions mimicking a hypoxic BM microenvironment. We observed that culture of ALL cells under hypoxia promoted resistance to several chemotherapeutic agents used in the frontline therapy of ALL. In turn, silencing of HIF-1α with EZN-2968 (LNA) enhanced the cytotoxic effects of chemotherapy. We have further demonstrated that forced expression of HIF-1α under normoxic conditions likewise conferred resistance of leukemic cells to chemotherapy. These findings indicate that HIF-1α and its downstream targets are key mediators of protective effects of hypoxia. Notably, culture under hypoxia or forced stabilization of HIF-1α expression under normoxia resulted in noticeable inhibition of leukemia cell growth without induction of cell death. While the mechanisms underlying this growth suppression require further studies, recent data in normal hematopoietic stem cells (HSC) indicate the important role of HIF-1α in the maintenance of quiescence and stemness of HSCs.42,43 Of further importance, HIF-1α was recently shown to be activated in the stem cells of mouse lymphoma and human acute myeloid leukemia at normoxic conditions.44 These findings clearly warrant further studies aimed at understanding of the role and mechanisms of stabilization of HIF transcription factors in leukemic stem cells.
HIF-1α stability is regulated at different levels, most prominently through oxygen-dependent hydroxylation of the protein. Additional factors include mutations of the ubiquitin-ligase VHL that degrades hydroxylated HIF-1α protein, prevalent in renal cell carcinoma; modulation of expression of prolyl hydroxylases; and oncogenic mutations in tyrosine kinase receptors or their downstream signaling mediators in the PI3K/AKT/mTOR and MAPK signaling pathways. Recent genomic data demonstrate frequent perturbation of PI3K/mTOR pathway in acute lymphocytic leukemia,45,46 and blockade of mTOR signaling was recently reported to induce apoptosis in ALL blasts.27 While genomic alterations in a subset of ALL patients will likely result in mTOR-dependent stabilization of HIF-1α, we and others have recently proposed the major role of BM-derived MSC in activation of the intracellular signaling cascades in leukemic cells even in the absence of mutations.47 Since stromal cells comprise a major component of the BM microenvironment, we investigated the contribution of leukemia-stroma interactions under hypoxic conditions. Consistent with our previous observations in AML cells, MSC induced MAPK activation in ALL cells independent of oxygen levels. On the contrary, hypoxia played an important role in activation of AKT/mTOR signaling: culture of ALL cells under hypoxia resulted in phosphorylation of AKT and the mTOR target S6R, and these effects were significantly enhanced in MSC co-cultures. Studies performed with human ALL xenograft cells indicate that co-culture with stromal cells upregulated HIF-1α mRNA levels even under ambient oxygen levels. Under hypoxic conditions, co-culture of ALL cells and stromal cells led to increased levels of HIF-1α protein expression. These data suggest that stroma-inducible activation of mTOR signaling enhances HIF-1α stabilization under the hypoxic conditions of leukemic BM. We observed in pre-B REH cells and in primary ALL samples, that inhibition of mTOR signaling with the rapamycin analog everolimus reversed several phenotypic attributes of hypoxia, such as upregulated HIF-1α protein, facilitated glucose uptake, increased expression of HKII and accelerated glycolytic rate of leukemic cells. These findings indicate that ALL cells utilize the glycolytic pathway for energy generation under the hypoxic conditions present in the leukemic BM microenvironment and that these mechanisms are largely controlled by mTOR signaling. Further studies are needed to elucidate the role of MAPK in these processes, taking into consideration robust phosphorylation of ERK in MSC co-cultures. Notably, inhibition of mTOR signaling with everolimus selectively sensitized ALL cells to pro-apoptotic effects of Vcr under hypoxic conditions, indicating the contribution of AKT/mTOR signaling to MSC-driven HIF-1α activation. It is, however, plausible that HIF-1α and/or mTOR targets other than HKII may contribute to enhanced survival of ALL cells. For instance, HIF-1α not only stimulates the influx and utilization of glucose in tumor cells but also stabilizes mitochondria through multiple pathways (reviewed in ref. 48). In addition, our results have demonstrated the ability of everolimus to decrease levels of Mcl-1, one of the major anti-apoptotic proteins, irrespective of oxygen or glucose levels (data not shown). A phase I/II study of everolimus conducted at MD Anderson Center in patients with relapsed/refractory hematological malignancies has demonstrated the safety of this agent and most importantly attainment of the biologic endpoints of inhibition of mTOR targets eIF4E-BP1 and/or p70S6 kinase in six of the nine patient samples.49 Notably, downregulation of Glut-1 mRNA (> 40%) was observed in 3 of 6 samples, in which everolimus inhibited mTOR signaling, providing in vivo conformation of the findings reported in this manuscript.
In summary, our observations indicate that HIF-1α, known to be upregulated in a variety of solid tumor types, constitutes an important factor in the survival of ALL blasts within the BM microenvironment, likely contributing to chemoresistance. We propose that targeting HIF-1α itself,50 its upstream mediators such as mTOR, or the glycolytic pathways impaired by the HIF-1α metabolic switch51 will enhance the efficacy of the therapeutic regiments in ALL. The Phase I clinical trial of everolimus in combination with hyper-CVAD chemotherapy is actively accruing patients at MD Anderson cancer center.
Materials and Methods
Chemicals and reagents
The mTOR inhibitor everolimus was provided by Novartis Pharmaceuticals (East Hanover, NJ). The compound was dissolved in dimethyl sulfoxide (DMSO) in the dark immediately before use in cell culture. Vincristine (Vcr) and Etoposide (ETO) were purchased at MD Anderson Cancer Center pharmacy; 2-deoxy-D-glucose (2-DG) and dimethyloxalylglycine (DMOG) were purchased from Sigma-Aldrich Corp.
Cell lines and primary ALL samples
Human leukemia pre-B ALL REH and NALM-6 cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and 50 μg/ml penicillin/streptomycin at 37°C in a humidified atmosphere with 5% CO2. These cell lines have been tested and authenticated by STR DNA fingerprinting performed by The Characterized Cell Line Core at MD Anderson Cancer Center. Bone marrow or peripheral blood samples were obtained from patients with newly diagnosed, relapsed or refractory acute lymphocytic leukemia (ALL) after informed consent under IRB-approved laboratory protocol (LAB01–473) at M. D. Anderson Cancer Center and all experiments were performed under IRB approved protocol (LAB02–372). The clinical characteristics of patients whose samples were used for in vitro experiments are presented in Table 1. Mononuclear cells were purified by Ficoll-Hypaque (Sigma Chemical) density-gradient centrifugation. Primary ALL mononuclear cells were seeded at 1–2 x 106 cells/mL in QBSF-60 medium (Quality Biological, Inc.) containing 15% FBS, 10 ng/ml Flt-3 ligand (R&D Systems). For the experiments performed under hypoxic conditions the hypoxic Workstation INVIVO2 400 from Ruskinn Technology was used. Cells were incubated in 1% O2, 5%CO2 and 37°C. Cells were exposed to everolimus (20 nM), 2-DG (10 mM), Vincristine (Vcr; 0.25–2 ng/ml), ETO (0.025–0.2 μM), DMOG (100 μM) for 24, 48 and 72 h. MSC obtained from normal BM donors were cultured at a density of 5000 to 6000 cells/cm2 in minimum essential medium α supplemented with 20% FBS, 1% L-glutamine and 1% penicillin-streptomycin as described.4 Passage 3 or 4 MSC were used for the co-culture experiments.
Childhood ALL xenografts were derived from patient biopsies and continuous xenografts were established by harvesting human leukemia cells from the spleens of engrafted mice as described previously.28 Preparations of childhood ALL cells used to establish continuous childhood ALL xenografts from mouse spleens routinely consisted of more than 3 × 108 cells per spleen at more than 85% purity. These experimental studies were approved by the Human Research Ethics Committee and the Animal Care and Ethics Committee of the University of New South Wales. Clinical characteristics of patients whose samples were used to establish xenografts are presented in Table 2. Cells were maintained in QBSF-60 medium supplemented with penicillin/streptomycin/L-glutamine, and used for all subsequent experiments. The murine stromal cell line MS5 was maintained in MEM-α with 10% FBS and penicillin/streptomycin/L-glutamine.
Western blot
Analysis was performed as previously described.29 The following antibodies were used: HKII (CHEMICON International, Inc.), HIF-1α (BD Biosciences), pS6R/S6R, pAKT Ser473/AKT, pERK (Cell Signaling Technology, Inc.), ERK 2 (Santa Cruz Biotechnology), α-tubulin and β-actin (Sigma-Aldrich Corp.).
Reverse phase protein arrays (RPPA)
RPPA assay BM and peripheral blood samples were collected for the Leukemia Sample Bank at the University of Texas M. D. Anderson Cancer Center on institutional review board (IRB)–approved protocol Lab01–473, and consent was obtained in accordance with the Declaration of Helsinki. Samples were analyzed under an IRB-approved laboratory protocol (Lab05–0654). RPPA was performed as previously described.30 HIF-1α (BD Biosciences) was used as primary antibody. All analyses were performed using the R Statistical Programming Environment, version 2.4.0.
HIF-1α downregulation
HIF-1α gene expression knockdown in leukemic cells was achieved by locked messenger ribonucleic acid antagonist (LNA). Scramble and human HIF-1α LNA were obtained from Enzon Pharmaceuticals. Transfection of leukemic cells was performed by electroporation using the Nucleofection System, according to the manufacturer protocol.
RNA isolation, cDNA synthesis and real-time reverse RT-PCR
After treatment, total cellular RNA was isolated from childhood ALL xenograft cell suspensions. Cell suspensions were collected, washed in ice-cold PBS, and pelleted (500 xg; 5 min; 4°C). Cell pellets were resuspended in 1 ml Trizol® reagent. Conversely, adherent cells were washed in ice-cold PBS after which 1 ml Trizol® reagent was added. The Trizol protocol was followed to the separation of the aqueous layer.
Primers and probes were from Applied Biosystems: Elongation factor (EF)1α probe, gene expression assays for all genes of interest (HIF1α; Hs00153153_m1), 2X Taqman mastermix, as well as real-time PCR plates, optical caps and plate seals. The reactions were performed on either the ABI Prism 7000 or ABI Prism 7500 (Applied Biosystems). The sequences: forward: 5′- CTG AAC CAT CCA GGC CAA AT-3′; reverse: 5′- GCA GTG TGG CAA TCC AAT-3′; probe: 5′VIC - AGC GCC GGC TAT GCC CCTG - TAMRA 3′. cDNA was prepared based on a previously published protocol.31
Lentiviral-induced HIF-1α overexpression
A Tet-ON inducible system based on two lentiviral vectors was developed. The first lentiviral vector (pCD510-rtTA) expresses the reverse tetracycline-controlled transactivator (rtTA) under the CMV promoter and Puromycin selection marker under a second promoter (EF-1). The second vector (pCDH-TRE) expresses the desired ORF under the control of a tetracycline inducible promoter (TRE) followed by the minimal CMV promoter and CopGFP, as selection marker, under the control of the EF-1 promoter. pCD510-rtTA was generated by excising the rtTA coding sequence from pSLIK-Venus-TmiR-Luc (ATCC ID: MBA-239) with BamHI and BstBI and cloning the resulting fragment into NotI and BstBI restriction sites of pCD510-B1 (SystemBio). To generate HIF-1α-ODD (ODD, Oxygen Degradation Domain) mutant we first mutated Prolines 402 and 564 to Alanines in the ODD of HIF-1α by site directed mutagenesis. Forward primers were designed as follows: P402A 5′ gctttaactttgctggccgcagccgctggagacac and P564A 5′Gacttggagatgttagctgcctatatcccaatggatgatgacttcc. Reverse primers had the exact complementary sequence of each forward primer respectively. Mutated codons in the forward primers are underlined. Mutated HIF-1α ORF was then excised from HA-HIF1alpha-pcDNA3 (Addgene) and cloned into pCDH-CMV-MCS-EF1-copGFP (SystemBio) in which the CMV promoter has been replaced by the Tetracyclin Responsive Element (TRE). The resulting lentiviral vector was designated pCDH-TRE- HIF-1α-ODD mut.
Lentiviral infections were performed according to standard procedures. Briefly, 293T cells were co-transfected with pMD2.G and psPAX2 (Addgene Inc.) along with either pCDH-TRE- HIF-1α-ODD mut or pCDH-TRE empty vector using JetPrime transfection reagent (Polyplus) according to the manufacturer protocol. The transfection medium was replaced after 12 h with fresh DMEM medium with 10% FBS and 48 h later the viral supernatants were collected and concentrated by using Centricon Plus-70 filter units (Millipore). REH cells were infected overnight with viral supernatants supplemented with 8 µg/ml of Polybrene (Sigma). Two days after infection stably transduced REH cells were selected with 2 µg/mL Puromycin for 2 weeks resulting in a homogeneous population of 100% Puromycin-resistant CopGFP-positive cells.
Real-time reverse RT-PCR
For detailed analysis on HIF-1α downstream target genes, RNA expression normalized to the housekeeping gene Abl was measured. Real time-PCR monitoring was performed by adding the double-stranded DNA dye SYBR Green. Gene-specific primers were designed using the Primer Premier software and are as follows: CXCR4-F- 5′-CAGTGGCCGACCTCCTCTT-3′, CXCR4-R- 5′-CAGTTTGCCACGGCATCA-3′; Glut-1-F 5′-TGCCATTGCCGTTGCA-3′, Glut-1-R 5′- GACCACACAGTTGCTCCACATAC-3′; VEGF α-F 5′-CTTGCCTTGCTGCTCTACC-3′, VEGF α-R- 5′-CACACAGGATGGCTTGAAG-3′.
Cell viability and apoptosis
Cell viability was assessed by cell counts with Trypan blue exclusion test. Apoptosis induction was determined by AnnexinV flow cytometry. Analysis was performed using FACScalibur flow cytometer (Becton Dickinson) operated at 488 nm and Cell QuestPro Software (Beckman-Coulter). The induction of apoptosis was quantified as the percentage of Annexin V-positive cells.
Lactic acid (LA) production measurements
LA concentration was measured in the medium using Accutrend Lactate device (Roche) which quantifies lactate by reflectance photometry using BM-Lactate test strips. Lactate is determined at a wavelength of 657 nm via a colorimetric lactate-oxidase mediator reaction. The data were expressed as pM per cell.
Glucose uptake measurement
100 μM of 2-NBDG (Invitrogen Corp.) was added to cultures 30 min prior to harvesting and incubated at 37°C under 1% or 21% O2. Samples were washed twice with ice-cold PBS, and analyzed by flow cytometry using a 488 nm argon excitation laser. The 2-NBDG uptake was expressed as mean fluorescence intensity (MFI).
Statistical analysis
All experiments were conducted at least three times unless specified otherwise. The two-sided Student’s t-test was used to evaluate the statistical significance between groups. Results are expressed as the mean ± standard error (SE). The Kaplan-Meier method was used to estimate overall survival (OS), and p-value was obtained with log-rank test.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Marina Konopleva holds a patent pending on rapamycin WIBL-P03–057.
Acknowledgments
Supported in part by NIH (AML-P01) CA55164 and the Paul and Mary Haas Chair in Genetics (to M. Andreeff); Multidisciplinary Research Program: Diabetes and Impaired Glucose Tolerance in Cancer Patients, The University of Texas M. D. Anderson Cancer; by the American Cancer Society grant #RSG-06–054–01-LIB; and by 1R01CA155056–01 and by CDP-01 from Leukemia and Lymphoma Society (to M.K.) by The Cancer Council New South Wales grant RG 49/03 (to R.L.).
Note
Dr. Frolova is now deceased.
Supplemental Materials
Supplemental materials may be downloaded at:
Glossary
Abbreviations:
- 2-DG
2-deoxy-D-glucose
- ALL
Acute Lymphocytic Leukemia
- BM
bone marrow
- CR
complete remission
- DMOG
dimethyloxalylglycine
- DMSO
dimethyl sulfoxide
- ETO
etoposide
- FBS
fetal bovine serum
- HIF-1α
Hypoxia-inducible factor-1α
- LA
lactic acid
- LNA
locked mRNA antagonist
- MFI
mean fluorescent intensity
- MRD
minimal residual disease
- MSC
mesenchymal stem cells
- mTOR
mammalian Target Of Rapamycin
- OS
overall survival
- RPPA
reverse phase protein array
- SE
standard error
- Vcr
vincristine
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/20838
References
- 1.Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–61. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 2.Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL) Blood. 2009;113:4153–62. doi: 10.1182/blood-2008-11-185132. [DOI] [PubMed] [Google Scholar]
- 3.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–24. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 4.Tabe Y, Konopleva M, Munsell MF, Marini FC, Zompetta C, McQueen T, et al. PML-RARalpha is associated with leptin-receptor induction: the role of mesenchymal stem cell-derived adipocytes in APL cell survival. Blood. 2004;103:1815–22. doi: 10.1182/blood-2003-03-0802. [DOI] [PubMed] [Google Scholar]
- 5.Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. Clin Cancer Res. 2008;•••:14. doi: 10.1172/JCI30235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manabe A, Coustan-Smith E, Behm FG, Raimondi SC, Campana D. Bone marrow-derived stromal cells prevent apoptotic cell death in B-lineage acute lymphoblastic leukemia. Blood. 1992;79:2370–7. [PubMed] [Google Scholar]
- 7.Kumagai MA, Manabe A, Pui CH, Behm FG, Raimondi SC, Hancock ML, et al. Stroma-supported culture in childhood B-lineage acute lymphoblastic leukemia cells predicts treatment outcome. J Clin Invest. 1996;97:755–60. doi: 10.1172/JCI118474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–6. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortensen BT, Jensen PO, Helledie N, Iversen PO, Ralfkiaer E, Larsen JK, et al. Changing bone marrow micro-environment during development of acute myeloid leukaemia in rats. Br J Haematol. 1998;102:458–64. doi: 10.1046/j.1365-2141.1998.00801.x. [DOI] [PubMed] [Google Scholar]
- 10.Benito J, Shi YX, Szymanska B, Carol H, Boehm I, Lu HB, et al. Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. PLoS One. 2011;6:e23108. doi: 10.1371/journal.pone.0023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wellmann S, Guschmann M, Griethe W, Eckert C, von Stackelberg A, Lottaz C, et al. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia. 2004;18:926–33. doi: 10.1038/sj.leu.2403332. [DOI] [PubMed] [Google Scholar]
- 12.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–80. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb E, Armour SM, Thompson CB. Mitochondrial respiratory control is lost during growth factor deprivation. Proc Natl Acad Sci U S A. 2002;99:12801–6. doi: 10.1073/pnas.202477599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6:683–92. doi: 10.1016/S1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 16.Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899–912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lugthart S, Cheok MH, den Boer ML, Yang W, Holleman A, Cheng C, et al. Identification of genes associated with chemotherapy crossresistance and treatment response in childhood acute lymphoblastic leukemia. Cancer Cell. 2005;7:375–86. doi: 10.1016/j.ccr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–43. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 19.Kallio PJ, Wilson WJ, O’Brien S, Makino Y, Poellinger L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J Biol Chem. 1999;274:6519–25. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- 20.Das F, Ghosh-Choudhury N, Dey N, Mandal CC, Mahimainathan L, Kasinath BS, et al. Unrestrained mammalian target of rapamycin complexes 1 and 2 increase expression of phosphatase and tensin homolog deleted on chromosome 10 to regulate phosphorylation of Akt kinase. J Biol Chem. 2012;287:3808–22. doi: 10.1074/jbc.M111.246397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, et al. Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem. 2003;278:31277–85. doi: 10.1074/jbc.M300763200. [DOI] [PubMed] [Google Scholar]
- 22.Stiehl DP, Jelkmann W, Wenger RH, Hellwig-Bürgel T. Normoxic induction of the hypoxia-inducible factor 1alpha by insulin and interleukin-1beta involves the phosphatidylinositol 3-kinase pathway. FEBS Lett. 2002;512:157–62. doi: 10.1016/S0014-5793(02)02247-0. [DOI] [PubMed] [Google Scholar]
- 23.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 24.Brugarolas J, Kaelin WG., Jr. Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell. 2004;6:7–10. doi: 10.1016/j.ccr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Dekanty A, Lavista-Llanos S, Irisarri M, Oldham S, Wappner P. The insulin-PI3K/TOR pathway induces a HIF-dependent transcriptional response in Drosophila by promoting nuclear localization of HIF-alpha/Sima. J Cell Sci. 2005;118:5431–41. doi: 10.1242/jcs.02648. [DOI] [PubMed] [Google Scholar]
- 26.Xu R-H, Pelicano H, Zhang H, Giles FJ, Keating MJ, Huang P. Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia. 2005;19:2153–8. doi: 10.1038/sj.leu.2403968. [DOI] [PubMed] [Google Scholar]
- 27.Teachey DT, Obzut DA, Cooperman J, Fang J, Carroll M, Choi JK, et al. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood. 2006;107:1149–55. doi: 10.1182/blood-2005-05-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lock RB, Liem N, Farnsworth ML, Milross CG, Xue C, Tajbakhsh M, et al. The nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of childhood acute lymphoblastic leukemia reveals intrinsic differences in biologic characteristics at diagnosis and relapse. Blood. 2002;99:4100–8. doi: 10.1182/blood.V99.11.4100. [DOI] [PubMed] [Google Scholar]
- 29.Zeng Z, Sarbassov D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–12. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–21. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 31.Noonan KE, Beck C, Holzmayer TA, Chin JE, Wunder JS, Andrulis IL, et al. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990;87:7160–4. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornblau SM, Tibes R, Qiu YH, Chen W, Kantarjian HM, Andreeff M, et al. Functional proteomic profiling of AML predicts response and survival. Blood. 2009;113:154–64. doi: 10.1182/blood-2007-10-119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K, Qian DZ, Rey S, Wei H, Liu JO, Semenza GL. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci U S A. 2009;106:2353–8. doi: 10.1073/pnas.0812801106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Greenberger LM, Horak ID, Filpula D, Sapra P, Westergaard M, Frydenlund HF, et al. A RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968, inhibits tumor cell growth. Mol Cancer Ther. 2008;7:3598–608. doi: 10.1158/1535-7163.MCT-08-0510. [DOI] [PubMed] [Google Scholar]
- 35.Rey S, Lee K, Wang CJ, Gupta K, Chen SP, McMillan A, et al. Synergistic effect of HIF-1alpha gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia. Proc Natl Acad Sci U S A. 2009;106:20399–404. doi: 10.1073/pnas.0911921106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manabe A, Coustan-Smith E, Behm FG, Raimondi SC, Campana D. Bone marrow-derived stromal cells prevent apoptotic cell death in B-lineage acute lymphoblastic leukemia. Blood. 1992;79:2370–7. [PubMed] [Google Scholar]
- 37.Land SC, Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem. 2007;282:20534–43. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 38.Görlach A, Camenisch G, Kvietikova I, Vogt L, Wenger RH, Gassmann M. Efficient translation of mouse hypoxia-inducible factor-1alpha under normoxic and hypoxic conditions. Biochim Biophys Acta. 2000;1493:125–34. doi: 10.1016/s0167-4781(00)00172-x. [DOI] [PubMed] [Google Scholar]
- 39.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 40.Fiegl M, Samudio I, Clise-Dwyer K, Burks JK, Mnjoyan Z, Andreeff M. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood. 2009;113:1504–12. doi: 10.1182/blood-2008-06-161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of endothelial to mesenchymal transition-like features. Blood. 2012 doi: 10.1182/blood-2011-09-380410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eliasson P, Rehn M, Hammar P, Larsson P, Sirenko O, Flippin LA, et al. Hypoxia mediates low cell-cycle activity and increases the proportion of long-term-reconstituting hematopoietic stem cells during in vitro culture. Exp Hematol. 2010;38:301–10, e2. doi: 10.1016/j.exphem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutierrez A, Sanda T, Grebliunaite R, Carracedo A, Salmena L, Ahn Y, et al. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009;114:647–50. doi: 10.1182/blood-2009-02-206722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Remke M, Pfister S, Kox C, Toedt G, Becker N, Benner A, et al. High-resolution genomic profiling of childhood T-ALL reveals frequent copy-number alterations affecting the TGF-beta and PI3K-AKT pathways and deletions at 6q15-16.1 as a genomic marker for unfavorable early treatment response. Blood. 2009;114:1053–62. doi: 10.1182/blood-2008-10-186536. [DOI] [PubMed] [Google Scholar]
- 47.Tabe Y, Jin L, Tsutsumi-Ishii Y, Xu Y, McQueen T, Priebe W, et al. Activation of integrin-linked kinase is a critical prosurvival pathway induced in leukemic cells by bone marrow-derived stromal cells. Cancer Res. 2007;67:684–94. doi: 10.1158/0008-5472.CAN-06-3166. [DOI] [PubMed] [Google Scholar]
- 48.Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–86. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yee KW, Zeng Z, Konopleva M, Verstovsek S, Ravandi F, Ferrajoli A, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–73. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 50.Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007;12:853–9. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol. 2004;53:116–22. doi: 10.1007/s00280-003-0724-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.