Abstract
Objectives
Varenicline was approved by the FDA in 2006. In 2009, based largely on case reports, the FDA issued a warning of possible adverse neuropsychiatric effects including depression and suicidal thoughts and behavior for varenicline and bupropion. Prospective trials of varenicline have not reported increased incidence of psychiatric adverse events other than sleep disturbance, but smokers with major mental illness have been excluded from large prospective trials of varenicline to date. We sought to evaluate the effect of a standard open-label 12-week varenicline trial on prospectively assessed safety and smoking outcomes in stable, treated adults with schizophrenia spectrum disorder and nicotine dependence.
Methods
One-hundred-and-twelve stable outpatients who smoked >10 cigarettes/day participated in a 12-week, open-label, smoking cessation trial of varenicline and weekly group cognitive behavioral therapy. Participants took varenicline for 4 weeks before attempting cessation. Trained raters collected safety and smoking outcome data weekly.
Results
Participants demonstrated improved psychotic symptoms, depressive symptoms and nicotine withdrawal symptoms from baseline to week 12 or early termination. At the end of 12 weeks open label treatment, the 14- and 28-day continuous abstinence rates were 47.3 and 34%, respectively. Expired CO declined significantly during treatment in those who did not achieve abstinence.
Conclusions
This prospective study suggests that varenicline may be well-tolerated and effective for smoking cessation in combination with group CBT in stable outpatients with schizophrenia, a group with high rates of smoking and smoking-attributable morbidity and mortality.
Keywords: Schizophrenia, smoking cessation, varenicline, CBT, open label, adverse events
INTRODUCTION
People with schizophrenia are up to 5 times more likely to smoke, smoke more cigarettes per day, self-administer higher doses of nicotine per cigarette, and have 2–6 times greater mortality from smoking-related diseases than those in the general population (de Leon & Diaz, 2005; Hennekens, Hennekens, Hollar, & Casey, 2005; Mauer, 2006; Olincy, Young, & Freedman, 1997; Williams, et al., 2010). While tobacco smoking is a critically important and modifiable risk factor (Bobes, Arango, Garcia-Garcia, & Rejas, 2010), nicotine dependence treatment often goes unaddressed for smokers with mental illness (Mauer, 2006; Prochaska, 2010).
Varenicline is a partial alpha4beta2 nicotine acetylcholine receptor (NAChR) agonist and full alpha7 NAChR agonist that is both effective and cost effective for smoking cessation (Aubin, et al., 2008; Gonzales, et al., 2006; Jorenby, et al., 2006; Keating & Lyseng-Williamson, 2010; Mihalak, Carroll, & Luetje, 2006; Nakamura, et al., 2007; Niaura, et al., 2008; Nides, et al., 2006; Oncken, et al., 2006; Rigotti, et al., 2010; Stapleton, et al., 2008; Tsai, et al., 2007; Wang, et al., 2009). Although varenicline has been well tolerated with respect to psychiatric adverse events in all controlled trials published to date (Gonzales, et al., 2006; Jorenby, et al., 2006; Nakamura, et al., 2007; Niaura, et al., 2008; Nides, et al., 2006; Oncken, et al., 2006; Rigotti, et al., 2010; Tsai, et al., 2007; Tsukahara, Noda, & Saku, 2010; Wang, et al., 2009), and a pooled analysis of placebo-controlled varenicline trials revealed no relative increase in psychiatric symptoms other than sleep disturbances in varenicline-treated subjects (Tonstad, Davies, Flammer, Russ, & Hughes, 2010), post-marketing case reports of psychiatric adverse events in smokers taking varenicline prompted an FDA mandated warning of neuropsychiatric symptoms, including hostility, agitation, depressed mood, and suicidal behavior to be included in prescribing information for varenicline. Large controlled trials to date have excluded smokers with current psychiatric illness.
In smokers with psychiatric illness, uncontrolled case reports have reported negative (Freedman, 2007; Kohen & Kremen, 2007; May & Rose, 2010; Purvis, Mambourg, Balvanz, Magallon, & Pham, 2009) and positive psychiatric tolerability effects (Anghelescu, 2009; Dutra, Stoeckel, Carlini, Pizzagalli, & Evins, 2011; Evins & Goff, 2008; Fatemi, 2008; Grosshans, Mutschler, Hermann, Mann, & Diehl, 2009; Weiner, et al., 2011). These reports have been followed by larger retrospective studies (McClure, et al., 2010; McClure, et al., 2009; Stapleton, et al., 2008), and small prospective open-label trials of varenicline in stable, treated outpatients with schizophrenia and depression that have reported generally good tolerability of varenicline with respect to psychiatric symptoms (Philip, Carpenter, Tyrka, Whiteley, & Price, 2009; Smith, et al., 2009). Because large, prospective trials of varenicline have excluded smokers with major psychiatric illness, they may have failed to detect common psychiatric adverse events in such smokers who may be more vulnerable to psychiatric adverse events. Although few data are available on safety of varenicline in those with schizophrenia, access to varenicline for smokers with schizophrenia is being commonly restricted in formularies around the United States due to safety concerns. We undertook an interim analysis of a large, 12-week, open-label trial of varenicline and weekly group cognitive behavioral therapy (CBT) in order to evaluate prospectively assessed psychiatric symptoms, adverse events and smoking outcomes in stable outpatient smokers with schizophrenia during treatment with varenicline and CBT (Culhane, et al., 2008).
METHOD
A 12-week, open-label, trial of varenicline and weekly group CBT for smoking cessation was conducted as part of a 40-week, double-blind, placebo-controlled, relapse-prevention trial in stable outpatient smokers with schizophrenia from April 2008 to July 2010 at Massachusetts General Hospital, Dartmouth Psychiatric Research Center, and Michigan State University in partnership with local mental health centers. The results of the relapse prevention phase of the trial will be reported elsewhere. This study was conducted in compliance with the ethical principles of the Declaration of Helsinki, the U.S. Food and Drug Administration guidelines and the International Conference on Harmonization Good Clinical Practices Guidelines. Human subjects review boards at each site approved the study, and participants provided written informed consent prior to initiation of study procedures.
Participants
Participants were women and men aged 18–70 with a DSM-IV-TR diagnosis of schizophrenia or schizoaffective disorder; smoked ≥10 cigarettes/day; were clinically stable on a stable dose of antipsychotic medication for ≥1 month; had expired carbon monoxide (CO) >9ppm; and expressed a desire to quit smoking. Exclusion criteria were unstable medical illness; diagnosis of dementia or substance use disorder other than nicotine or caffeine in the prior 6 months, or hospitalization for suicidal ideation in the prior 12 months.
Intervention
The intervention was varenicline 0.5 mg/day for 3 days, 0.5 mg bid for 4 days, then 1 mg twice daily for 11 weeks and weekly, one-hour, manualized, cognitive behavioral therapy smoking cessation groups based on the Freedom From Smoking program (American Lung Association), tailored for smokers with schizophrenia (Evins, et al., 2007; Evins, et al., 2005). In order to allow assessment of the effect of varenicline on psychiatric symptoms and treatment emergent adverse events (TEAE’s) in this population while minimizing confounding effects of nicotine withdrawal, the target quit date was set for week 4–5, and psychiatric symptoms are reported for the one-month period prior to the smoking cessation attempt and the two-month period of smoking abstinence or reduction following the smoking cessation attempt.
Assessments
Assessments included smoking and medical history, Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), laboratory assessment, including pregnancy test, cotinine and urine drug screen at baseline, self-report of smoking behavior, end-expiratory CO measurement, assessment of nicotine withdrawal symptoms with the Wisconsin Smoking Withdrawal Scale (WSWS) (Welsch, et al., 1999), of depressive symptoms with the Calgary Depression Scale for Schizophrenia (CDSS) (Addington, Addington, & Maticka-Tyndale, 1993) and adverse event (AE) inquiry, including general inquiry and specific query about common nicotine withdrawal symptoms and commonly reported AE’s with varenicline: headache, nausea, vomiting, tachycardia, excitement, agitation, anxiety, insomnia, and irritability. Clinician ratings of psychiatric symptoms with the Brief Psychiatric Rating Scale (BPRS) (Overall & Gorham, 1962) and Schedule for the Assessment of Negative Symptoms (SANS) (Andreasen, 1989) were performed at baseline and week 12 or early termination (ET) to assess general psychiatric symptoms. Participants who terminated the study early were asked to complete an early termination assessment of safety and efficacy outcomes. Seven-day point prevalence abstinence at each visit was defined as self-report of smoking no cigarettes for the prior 7 days and expired CO<9 ppm (Hughes, et al., 2003). Continuous abstinence was defined as consecutive weeks of biochemically verified 7-day point prevalence abstinence.
Analysis
The total sample to be enrolled is 324. We undertook an interim analysis when approximately 1/3 of the sample had been enrolled, when paired t-tests would have sufficient (≥ 0.80) statistical power to detect effects of d=0.30 or larger, assuming a medium correlation (0.50) between the pre- and post-treatment measures.
To test for change over time in safety outcomes measured at baseline and end of treatment only, we conducted paired t-tests. Only those with two assessments were included. Effects were considered significant if two-sided p<0.05.
To test for temporal trends in assessments performed weekly, we conducted repeated measures analyses, fitting an intercept and a linear trend. Statistically significant trends over time were interpreted as evidence of change in the dependent variable over time. To address the problem of multiple significance testing (i.e., we were interested in the temporal trends of 14 variables that were assessed on a weekly basis), we adjusted p-values by controlling the expected proportion of falsely rejected hypotheses, that is, the false discovery rate, as described by Benjamini and Hochberg (1995). We used mixed effects models for normally distributed outcome variables, and generalized estimating equations (GEE) (Liang & Zeger, 1986; Zeger & Liang, 1986) for Poisson-distributed and binary outcome variables. An AR1 structure was used to model serial dependence, allowing different correlation matrices per site, and using robust (i.e., “sandwich”) standard error estimates
Prior to the repeated measures analyses, we assessed retention biases using GEE analyses to predict retention, coded as a binary outcome (retained vs. not retained), across the 13 weekly assessments. As predictors of retention, we used baseline demographic descriptors, biological descriptors, and indices of nicotine dependence. Significant predictors of retention were included as covariates in all repeated measures analyses to adjust for retention biases (Graham, 2009) We also tested whether the outcome variables of interest were confounded with retention by using their week 1 values as predictors of retention. Two baseline descriptors were related to retention over time: weight and “age started smoking”. The main effect on retention was not significant for either variable, but the interaction effect of “age started smoking” with time was significant, and retention patterns diverged towards the end of the study such that participants who started smoking earlier were increasingly less likely to be retained. We thus included “age started smoking” as a control variable in all analyses. Of the week 1 measures, only the WSWS subscale score “Difficulty Concentrating” was related to retention over time, however, participants with greater difficulty concentrating were initially retained at a higher rate but had a higher rate of study drop-out over the span of the 12 weeks, such that these two effects cancelled each other out. Thus, analyses were not controlled for difficulty concentrating at week 1. Finally, because dropout from smoking cessation trials among those unable to quit smoking can be high, we evaluated safety outcomes first in the entire cohort, and then separately among those who dropped out vs. those who were retained in order to look for predictors of dropout other than failure to attain abstinence. For analyses of two- and four-week continuous abstinence rates at the end of treatment, participants who were not available to provide a CO measurement at a given timepoint were considered to be smokers at that timepoint.
RESULTS
Participants
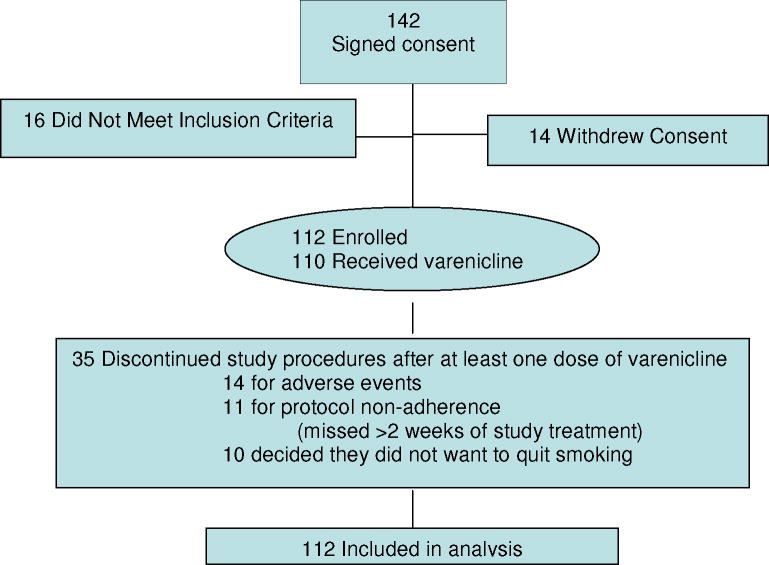
Of 142 participants who completed the informed consent process, 16 did not meet inclusion criteria, and 14 withdrew consent prior to initiation. One hundred and twelve participants initiated study procedures and were included in this analysis. See Figure 1. Participants were, on average, 47 years of age with 12 years of education, smoked over 20 cigarettes per day and had moderate to severe nicotine dependence (FTND = 6.1 (1.9)). See Table 1. Thirty-seven participants (33%) discontinued treatment prior to week 12. For those who discontinued, the mean time point of discontinuation was 5.1 weeks, (range 1 –12 weeks) just after the quit date. Twenty-eight of the 37 participants who dropped out (77%) completed early termination assessments.
Figure 1.
Enrollment
Table 1.
Demographics and Smoking History and Psychiatric and Nicotine Withdrawal Ratings
| Baseline | Endpoint | t | |
|---|---|---|---|
| Age | 47.2 (10) years | - | - |
| Sex | 44 (39.3%) female | - | - |
| Education | 12.2 (2.8) years | - | - |
| Race | |||
| African American | 16 (14.3%) | - | - |
| Caucasian | 84 (75%) | - | - |
| Other | 12 (10.7%) | - | - |
| Marital Status | |||
| Single | 73 (65%) | - | - |
| Married | 9 (8%) | - | - |
| Divorced | 23 (21%) | - | - |
| Widowed | 6 (5.4%) | - | - |
| Employment Status | |||
| Disabled | 67 (59.8%) | - | - |
| Unemployed | 13 (11.6%) | - | - |
| Working part-time | 27 (24.1%) | - | - |
| Working full-time | 4 (4.0%) | - | - |
| FTND | 6.1 (1.9) | ||
| Age began daily smoking | 17.5 (5.8) years | - | - |
| Years of regular smoking | 27.7 (11.2) | - | - |
| Cigarettes per day | 25.2 (13.5) | - | - |
| BPRS Total | 53.6 (14.6) | 51.76 (14.0) | 1.643 |
| BPRS -Psychosis | 14.21 (6.95) | 12.87 (6.39) | 2.815* |
| SANS Total | 39.92 (15.03) | 40.92 (15.90) | −0.914 |
| CDSS Total | 4.23 (3.13) | 3.65 (3.99) | 1.511 |
| WSWS Total | 59.09 (11.54) | 50.77 (12.90) | 6.808* |
| WSWS -Urge to Smoke | 11.85 (2.104) | 8.2 (4.02) | 8.718* |
| WSWS -Irritability | 5.62 (2.45) | 4.46 (2.92) | 4.392* |
| WSWS -Depression | 6.61 (3.14) | 5.8 (2.99) | 2.677* |
| WSWS -Increased Appetite | 11.79 (3.46) | 11.88 (3.56) | −0.245 |
| WSWS -Difficulty | 5.86 (2.58) | 5.15 (2.39) | 3.529* |
| Concentrating | |||
| WSWS -Insomnia | 8.52 (3.66) | 8.25 (3.87) | 0.706 |
| WSWS -Anxiety | 8.84 (2.82) | 7.04 (2.69) | 6.194* |
| Weight (lbs) | 202.59 (44.35) | 207.6 (45.4) | −5.374 * |
| CO | 23.04 (15.52) | 9.03 (12.74) | 8.872* |
p < 0.01
Psychiatric Symptoms and Nicotine Withdrawal Symptoms
No ratings of psychiatric or nicotine withdrawal symptoms worsened from baseline to week 12 or ET. Significant improvement was observed from baseline to Week 12 or ET in ratings of psychosis (BPRS psychosis subscale), nicotine withdrawal symptoms (WSWS total score and individual WSWS subscales: urge to smoke, irritability, depression, concentration and anxiety subscale scores. See Table 1. Exploratory subgroup analyses, splitting the sample by retention status and by abstinence status (data not shown) revealed the same pattern of stable or improved psychiatric and withdrawal symptoms in subgroups who were abstinent at the end of the trial (≥ 14 day point prevalence abstinence at week 12) and in those who terminated treatment early. The results of the repeated measures analyses of assessments conducted weekly are summarized in Table 2. For all measures of smoking outcomes and for 6 out of 9 clinical variables, a statistically significant trend was observed, always in the expected direction. That is, withdrawal symptoms decreased over time, while abstinence rates increased (data for 24 hour and 14 day point prevalence abstinence not shown). Linear trends were not significant for two subscales of the WSWS (i.e., depression, increased appetite), and for a third subscale (Difficulty Concentrating) after correction for multiple tests. Parameter estimates for the covariate, age started smoking, are not shown, because this covariate was used only to statistically control for retention biases, not for substantive reasons of its own. It should be noted though, that age started smoking was statistically significantly related to urge to smoke (t(1)=−3.11, p<0.01) and the total WSWS score (t(1)=−2.29, p<0.05). These data demonstrate that 7-day point prevalence abstinence rates increased progressively over the course of the 12 week trial and that CO, WSWS total scores and irritability, concentration, insomnia, anxiety and Calgary depression scale scores all decreased over the course of the trial. These data are more fine-grained than those assessed at baseline and week 12 only and indicate that symptoms are unlikely to have worsened early during treatment then recovered by week 12.
Table 2.
Parameter estimates for outcomes measured weekly over 12 weeks
| Outcome Variable | Intercept | Slope | Model | ||||
|---|---|---|---|---|---|---|---|
| Est | SE | t or z | Est | SE | t or z | ||
| CO | 1.22 | 0.33 | 3.7 ** | −0.03 | 0.01 | −2.8 ** | Poisson |
| Abstinent for at least the prior7 days | −3.54 | 0.60 | −5.9 ** | 0.34 | 0.03 | 12.7 ** | Binary |
| WSWS -Total | 60.92 | 2.54 | 24.0 ** | −0.65 | 0.15 | −4.3 ** | Normal |
| Urge to Smoke -WSWS | 12.39 | 0.66 | 18.7 ** | −0.29 | 0.04 | −7.0 ** | Normal |
| Irritability -WSWS | 5.24 | 0.44 | 11.9 ** | −0.06 | 0.03 | −2.1 * | Normal |
| Depression -WSWS | 6.64 | 0.52 | 12.9 ** | −0.04 | 0.03 | −1.2 | Normal |
| Increased Appetite -WSWS | 12.22 | 0.65 | 18.7 ** | 0.01 | 0.04 | 0.2 | Normal |
| Diff. Concentrating -WSWS | 6.45 | 0.50 | 12.9 ** | −0.06 | 0.03 | −2.0 | Normal |
| Insomnia -WSWS | 8.25 | 0.61 | 13.5 ** | −0.09 | 0.04 | −2.4 * | Normal |
| Anxiety -WSWS | 8.67 | 0.52 | 16.7 ** | −0.10 | 0.03 | −3.1 ** | Normal |
| Calgary Depression Scale -Total
|
3.53 | 0.20 | 17.9 ** | −0.14 | 0.01 | −9.5 ** | Poisson |
Note: All models include “age started smoking” as a covariate, which was a statistically significant predictor of retention across the 12 weeks.
p < 0.05,
p < 0.01
Adverse Events
Varenicline was administered for a one month lead-in period prior to the target quit date in order to assess potential adverse effects of varenicline on psychiatric symptoms prior to the possible confounding effects of nicotine withdrawal syndrome on psychiatric symptom ratings and TEAE’s. Table 3 shows psychiatric TEAE’s during the 4-week period prior to the target-quit-date and the 8-week period following the target-quit-date. Nausea was the most common adverse event reported and was generally described as mild or moderate and transient.
Table 3.
Treatment emergent adverse events
| Weeks 1 – 4 | Weeks 5 – 12 | |
|---|---|---|
| Before quit date | After quit date | |
| Excitement | ||
| Mild | 13 (12%) | 10 (9%) |
| Moderate | 1 (1%) | 11 (10%) |
| Severe | 2 (2%) | 4 (4%) |
| Agitation | ||
| Mild | 9 (8%) | 7 (6%) |
| Moderate | 5 (4%) | 11 (10%) |
| Severe | 1 (1%) | 4 (4%) |
| Anxiety | ||
| Mild | 11 (10%) | 9 (8%) |
| Moderate | 11 (10%) | 10 (9%) |
| Severe | 2 (2%) | 9 (8%) |
| Insomnia | ||
| Mild | 10 (9%) | 8 (7%) |
| Moderate | 11 (10%) | 14 (12%) |
| Severe | 2 (2%) | 2 (2%) |
| Irritability | ||
| Mild | 11 (10%) | 9 (8%) |
| Moderate | 6 (5%) | 11 (10%) |
| Severe | 2 (2%) | 3 (3%) |
| Headache | ||
| Mild | 9 (8%) | 10 (9%) |
| Moderate | 6 (5%) | 15 (3%) |
| Severe | 0 | 5 (4%) |
| Nausea | ||
| Mild | 21 (18%) | 16 (14%) |
| Moderate | 7 (6%) | 9 (8%) |
| Severe | 1 (1%) | 7 (6%) |
| Vomiting | ||
| Mild | 11 (10%) | 12 (11%) |
| Moderate | 5 (4%) | 7 (6%) |
| Severe | 2 (2%) | 3 (3%) |
| Tachycardia | ||
| Mild | 9 (8%) | 9 (8%) |
| Moderate | 0 | 8 (7%) |
| Severe | 0 | 0 |
Three serious AE’s were reported, all voluntary psychiatric hospitalizations. One participant was hospitalized for dysphoric mood associated with exacerbation of chronic psychosocial stressors and considered unrelated to study procedures. This participant had not attained abstinence and was discontinued from the study at week 6. The second participant was hospitalized for dysphoric mood following 7 weeks abstinence. While this AE was considered to be possibly related to study interventions, this participant remained on varenicline and remained abstinent during the hospitalization and completed the study upon discharge. The third participant was hospitalized in study week 3 for paranoia and suicidal ideation. She was discontinued from the study. This was thought by investigators to be precipitated by heavy cocaine use immediately prior to the hospitalization, which could possibly have been related to study interventions. In addition, 12 participants experienced AEs that led to treatment discontinuation: nausea (5), anxiety (2), weight gain (1), depressed mood (1), paranoia (1), suicidal ideation (1), and substance use (1). Weight gain of approximately 5 pounds from baseline to end of treatment was significant. (Table 1)
Smoking Outcomes
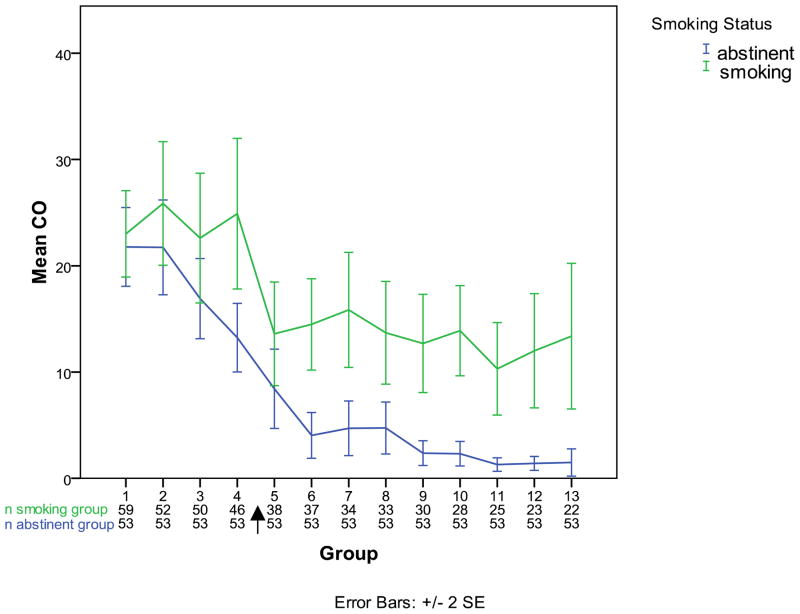
Fifty-three participants (47.3%) achieved ≥2 consecutive weeks biochemically-verified continuous tobacco abstinence at week 12, and 38 participants (34%) achieved ≥4 consecutive weeks of continuous abstinence at week 12. A significant temporal trend for reduction in expired CO and increase in 7-day point prevalence abstinence over time was noted. See Table 2. Expired CO was 22.6 (14.2) ppm at baseline and 9.0 (12.7) ppm at week 12 or ET. Participants who completed the 12-week trial achieved greater reduction in CO (baseline: 22.8 (16.3) ppm, week 12: 5.3 (10.1) ppm than those who terminated early (baseline: 23.7 (13.5) ppm, ET visit 19.2 (13.7) ppm). See Figure 2.
Figure 2.
Expired carbon monoxide among participants who attained at least 14-day continuous abstinence at the end of treatment and those who did not, observed samples.
Arrow indicates target-quit date.
DISCUSSION
This relatively large, prospective, open study suggests that varenicline combined with CBT may be well-tolerated and effective for smoking cessation in stable, treated outpatient smokers with schizophrenia and nicotine dependence. Over 12 weeks, participants demonstrated increased abstinence rates, and decreased withdrawal symptoms, depressive symptoms and psychosis. The most common AE was transient nausea. The observed improvement in psychiatric symptoms is consistent with other early evidence from prospective trials that varenicline improves mood and cognition in smokers with diagnosis of major depressive disorder, depression or schizophrenia as well as those with no identified psychiatric illness (Patterson, et al., 2009; Philip, et al., 2009; Smith, et al., 2009). There were, however, during both the one-month varenicline lead-in period prior to the quit date, and during the 8-week period following the target quit date, episodic complaints of psychiatric symptoms that are both common nicotine withdrawal symptoms and common symptoms for those with schizophrenia. However, these were generally mild to moderate in severity and were seldom reported on more than one occasion.
The strengths of this study include the prospective nature of the evaluations, structured AE and symptom ratings, and the relatively large number of smokers with schizophrenia assessed. An important implication of the findings are that a medication that is highly effective for smoking cessation in the general population may be well tolerated and effective for smokers with schizophrenia and thus have the potential to reduce a major public health burden in this population with major mental illness and comorbid nicotine dependence. An important limitation is the absence of a placebo control. Because affective components of the nicotine withdrawal syndrome, irritability, anxiety, insomnia and depressed mood, are also common psychiatric symptoms of concern in this population, assessment of psychiatric TEAEs for 4 weeks prior to the target quit date provides information about the effect of varenicline on psychiatric AEs in this population prior to nicotine withdrawal. As expected, there was a small increase in complaints of moderate excitement, agitation, and irritability after the quit date. These were generally time-limited, did not require clinical intervention, and were largely resolved by the week-12 assessment. Study with a placebo comparison group will be critical to allow separation of the effect of medication from that of the nicotine withdrawal syndrome, on the incidence of psychiatric AEs during the course of smoking cessation treatment. Such placebo-controlled studies would also control for the strong association between a diagnosis of nicotine dependence and suicidal behavior among smokers with (Ostacher, et al., 2009; Ostacher, et al., 2006) and without (Bolton & Robinson, 2010) major mental illness.
This study was also limited by the sample size of 112, which confers limited statistical power to detect rare events such as suicidal ideation and attempts. The paired t-tests had sufficient (≥ 0.80) statistical power to detect effects of d=0.30 or larger, but not small effects (Cohen’s d=0.20), assuming a medium correlation (0.50) between the pre and post measures. Further, to the degree that symptoms worsened after participants dropped out, the study could only capture an attenuated effect, since no data were available following the ET assessment. The fact that the mean symptom scores were generally lower after baseline, and that temporal trends for symptom ratings all decreased during the study suggests that such a worsening did not take place, but the possibility cannot be ruled out.
Another potential limitation is early termination. Thirty-seven participants (33% of the sample) dropped out. While we obtained ET evaluations on 28 of these participants and while generally drop-out appeared to be associated with failure to quit smoking, no subjects were abstinent at the visit prior to drop out, and ET did not appear to be confounded with safety outcomes, drop-out does limit the generalizability of results. The trial included stable, antipsychotic-treated outpatient smokers with schizophrenia, and results are generalizable to this subgroup, who may have been relatively protected from psychiatric AE’s by stable antipsychotic treatment. It is also possible that improvement in psychiatric symptoms observed over time in this study could be due to abstinence-associated increases in antipsychotic drug levels due to reduced hepatic metabolic rate for some antipsychotic medications. While improvement in psychosis, irritability and anxiety was observed in those who did not quit smoking, this group reduced smoking, which could have increased serum concentration of some antipsychotic medications. Also, because participants in this study received twelve weekly one hour sessions of CBT for smoking cessation that included careful safety monitoring, these results may not generalize to the context of minimal counseling support and less frequent follow-up common in many health care settings.
Conclusion
The results of this prospective study suggest that 12 weeks of varenicline treatment may be well tolerated and effective for smoking cessation in stable outpatient smokers with schizophrenia. Based on these and previously published results, limitation of varenicline prescribing in this population requires closer scrutiny in order to achieve a proper balance between potential health benefits and potential psychiatric adverse events.
Acknowledgments
Funded by R01 DA021245 (Evins) and K24 DA030443 (Evins) Study medication was provided by Pfizer.
Supported by NIDA R01 DA021245, Smoking Cessation and Relapse Prevention in Patients with Schizophrenia (Evins) and NIDA K24 DA030443 (Evins). Study medication was provided by Pfizer, Inc.
Footnotes
Presented in part at the 2009 Annual meeting of the Society for Research on Nicotine and Tobacco, Dublin, Ireland.
DISCLOSURES
Dr. Evins has received research grant support from Pfizer, Janssen, GSK, EnVivo Pharmaceuticals, and Bowman Family Foundation; honoraria from Reed Medical Education and has Advisory/Consultant relationships with Euthymics, Pfizer, and Boehringer Ingelheim.
Dr. Rigotti has received research grant support from Pfizer and Nabi Biopharmaceuticals, honoraria from UptoDate and is an unpaid consultant for Pfizer and Free and Clear.
Dr. Goff has served as a consultant or advisor to: Merk, Bristol-Meyers Squibb, Wyeth, Organon, Xytis, XenoPort, Proteus, Vanda, Astra-Zeneca, Forest Labs, Pfizer, Indevus Pharmaceuticals, H. Lundbeck, Ortho-McNeil-Janssen, Schering-Plough, Eli Lilly, Takeda, Biovail, Solvay, Hoffman-La Roche, Cypress, Dainippon Sumitomo, Abbott Laboratories, Genentech and Endo Pharmaceuticals. He served on a Data Safety Monitoring Board for Otsuka and Weyth. He has received research grant funding from Pfizer, Novartis, Janssen, and GlaxoSmithKline.
Dr. Achtyes has received research support from: AssureRx, Eli Lilly, Novartis, and Ortho-McNeill-Janssen.
Dr.Cather has received compensation as a consultant for United Biosource Corporation and as a speaker for the MGH Academy.
Drs. Pachas, Nino-Gomez, Lando, Mueser, Pratt, Hoeppner and Ms. Carlini have no conflicts to disclose.
References
- Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl. 1993;22:39–44. [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;(7):49–58. [PubMed] [Google Scholar]
- Anghelescu I. Successful smoking cessation and improvement of negative symptoms with varenicline in a stable schizophrenia patient. J Neuropsychiatry Clin Neurosci. 2009;21(1):102–103. doi: 10.1176/jnp.2009.21.1.102. [DOI] [PubMed] [Google Scholar]
- Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB, Jr, Gong J, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63(8):717–724. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Bobes J, Arango C, Garcia-Garcia M, Rejas J. Healthy lifestyle habits and 10-year cardiovascular risk in schizophrenia spectrum disorders: an analysis of the impact of smoking tobacco in the CLAMORS schizophrenia cohort. Schizophr Res. 2010;119(1–3):101–109. doi: 10.1016/j.schres.2010.02.1030. [DOI] [PubMed] [Google Scholar]
- Bolton JM, Robinson J. Population-attributable fractions of Axis I and Axis II mental disorders for suicide attempts: findings from a representative sample of the adult, noninstitutionalized US population. Am J Public Health. 2010;100(12):2473–2480. doi: 10.2105/AJPH.2010.192252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhane MA, Schoenfeld DA, Barr RS, Cather C, Deckersbach T, Freudenreich O, et al. Predictors of early abstinence in smokers with schizophrenia. J Clin Psychiatry. 2008;69(11):1743–1750. doi: 10.4088/jcp.v69n1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76(2–3):135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Dutra SJ, Stoeckel LE, Carlini SV, Pizzagalli DA, Evins AE. Varenicline as a smoking cessation aid in schizophrenia: effects on smoking behavior and reward sensitivity. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, et al. A 12-week double-blind, placebo-controlled study of bupropion sr added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol. 2007;27(4):380–386. doi: 10.1097/01.jcp.0b013e3180ca86fa. [DOI] [PubMed] [Google Scholar]
- Evins AE, Cather C, Deckersbach T, Freudenreich O, Culhane MA, Olm-Shipman CM, et al. A double-blind placebo-controlled trial of bupropion sustained-release for smoking cessation in schizophrenia. J Clin Psychopharmacol. 2005;25(3):218–225. doi: 10.1097/01.jcp.0000162802.54076.18. [DOI] [PubMed] [Google Scholar]
- Evins AE, Goff DC. Varenicline treatment for smokers with schizophrenia: a case series. J Clin Psychiatry. 2008;69(6):1016. doi: 10.4088/jcp.v69n0620a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH. Varenicline efficacy and tolerability in a subject with schizophrenia. Schizophr Res. 2008;103(1–3):328–329. doi: 10.1016/j.schres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Freedman R. Exacerbation of schizophrenia by varenicline. Am J Psychiatry. 2007;164(8):1269. doi: 10.1176/appi.ajp.2007.07020326. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Grosshans M, Mutschler J, Hermann D, Mann K, Diehl A. Reduced affective symptoms during tobacco dependence treatment with varenicline. Addiction. 2009;104(5):859–861. doi: 10.1111/j.1360-0443.2009.02537.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Keating GM, Lyseng-Williamson KA. Varenicline: a pharmacoeconomic review of its use as an aid to smoking cessation. Pharmacoeconomics. 2010;28(3):231–254. doi: 10.2165/11204380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kohen I, Kremen N. Varenicline-induced manic episode in a patient with bipolar disorder. Am J Psychiatry. 2007;164(8):1269–1270. doi: 10.1176/appi.ajp.2007.07010173. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Mauer B. Morbidity and Mortality in People with Serious Mental Illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006. [Google Scholar]
- May AC, Rose D. Varenicline withdrawal-induced delirium with psychosis. Am J Psychiatry. 2010;167(6):720–721. doi: 10.1176/appi.ajp.2010.09081194. [DOI] [PubMed] [Google Scholar]
- McClure JB, Swan GE, Catz SL, Jack L, Javitz H, McAfee T, et al. Smoking outcome by psychiatric history after behavioral and varenicline treatment. J Subst Abuse Treat. 2010;38(4):394–402. doi: 10.1016/j.jsat.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB, Swan GE, Jack L, Catz SL, Zbikowski SM, McAfee TA, et al. Mood, side-effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline. J Gen Intern Med. 2009;24(5):563–569. doi: 10.1007/s11606-009-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther. 2007;29(6):1040–1056. doi: 10.1016/j.clinthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Niaura R, Hays JT, Jorenby DE, Leone FT, Pappas JE, Reeves KR, et al. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial. Curr Med Res Opin. 2008;24(7):1931–1941. doi: 10.1185/03007990802177523. [DOI] [PubMed] [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166(15):1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry. 1997;42(1):1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166(15):1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Ostacher MJ, Lebeau RT, Perlis RH, Nierenberg AA, Lund HG, Moshier SJ, et al. Cigarette smoking is associated with suicidality in bipolar disorder. Bipolar Disord. 2009;11(7):766–771. doi: 10.1111/j.1399-5618.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostacher MJ, Nierenberg AA, Perlis RH, Eidelman P, Borrelli DJ, Tran TB, et al. The relationship between smoking and suicidal behavior, comorbidity, and course of illness in bipolar disorder. J Clin Psychiatry. 2006;67(12):1907–1911. doi: 10.4088/jcp.v67n1210. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale (BPRS) Psychological Reports. 1962;10:799–812. [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65(2):144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Carpenter LL, Tyrka AR, Whiteley LB, Price LH. Varenicline augmentation in depressed smokers: an 8-week, open-label study. J Clin Psychiatry. 2009;70(7):1026–1031. doi: 10.4088/jcp.08m04441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ. Failure to treat tobacco use in mental health and addiction treatment settings: a form of harm reduction? Drug Alcohol Depend. 2010;110(3):177–182. doi: 10.1016/j.drugalcdep.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis TL, Mambourg SE, Balvanz TM, Magallon HE, Pham RH. Safety and effectiveness of varenicline in a veteran population with a high prevalence of mental illness. Ann Pharmacother. 2009;43(5):862–867. doi: 10.1345/aph.1L661. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121(2):221–229. doi: 10.1161/CIRCULATIONAHA.109.869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Lindenmayer JP, Davis JM, Cornwell J, Noth K, Gupta S, et al. Cognitive and antismoking effects of varenicline in patients with schizophrenia or schizoaffective disorder. Schizophr Res. 2009;110(1–3):149–155. doi: 10.1016/j.schres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, et al. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103(1):146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Davies S, Flammer M, Russ C, Hughes J. Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: a pooled analysis. Drug Saf. 2010;33(4):289–301. doi: 10.2165/11319180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Tsai ST, Cho HJ, Cheng HS, Kim CH, Hsueh KC, Billing CB, Jr, et al. A randomized, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther. 2007;29(6):1027–1039. doi: 10.1016/j.clinthera.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Tsukahara H, Noda K, Saku K. A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers: efficacy, safety and withdrawal symptoms (the VN-SEESAW study) Circ J. 2010;74(4):771–778. doi: 10.1253/circj.cj-09-0803. [DOI] [PubMed] [Google Scholar]
- Wang C, Xiao D, Chan KP, Pothirat C, Garza D, Davies S. Varenicline for smoking cessation: a placebo-controlled, randomized study. Respirology. 2009;14(3):384–392. doi: 10.1111/j.1440-1843.2008.01476.x. [DOI] [PubMed] [Google Scholar]
- Weiner E, Buchholz A, Coffay A, Liu F, McMahon RP, Buchanan RW, et al. Varenicline for smoking cessation in people with schizophrenia: a double blind randomized pilot study. Schizophr Res. 2011;129(1):94–95. doi: 10.1016/j.schres.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Lu SE, Kumar S, Shen J, Foulds J, et al. Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine Tob Res. 2010;12(8):855–859. doi: 10.1093/ntr/ntq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]