Summary
Bacterial c-type cytochrome maturation is dependent on a complex enzymic machinery. The key reaction is catalysed by cytochrome c haem lyase (CCHL) that usually forms two thioether bonds to attach haem b to the cysteine residues of a haem c binding motif (HBM) which is, in most cases, a CX2CH sequence. Here, the HBM specificity of three distinct CCHL isoenzymes (NrfI, CcsA1 and CcsA2) from the Epsilonproteobacterium Wolinella succinogenes was investigated using either W. succinogenes or Escherichia coli as host organism. Several reporter c-type cytochromes were employed including cytochrome c nitrite reductases (NrfA) from E. coli and Campylobacter jejuni that differ in their active site HBMs (CX2CK or CX2CH). W. succinogenes CcsA2 was found to attach haem to standard CX2CH motifs in various cytochromes whereas other HBMs were not recognized. NrfI was able to attach haem c to the active site CX2CK motif of both W. succinogenes and E. coli NrfA, but not to NrfA from C. jejuni. Different apo-cytochrome variants carrying the CX15CH motif, assumed to be recognized by CcsA1 during maturation of the octahaem cytochrome MccA, were not processed by CcsA1 in either W. succinogenes or E. coli. It is concluded that the dedicated CCHLs NrfI and CcsA1 attach haem to non-standard HBMs only in the presence of further, as yet uncharacterised structural features. Interestingly, it proved impossible to delete the ccsA2 gene from the W. succinogenes genome; a finding that is discussed in the light of the available genomic, proteomic and functional data on W. succinogenes c-type cytochromes.
Introduction
C-type cytochromes are a widespread class of proteins essential for the life of almost all organisms (Moore and Pettigrew, 1990; Scott and Mauk, 1995). They are characterised by the covalent attachment of haem (Fe-protoporphyrin IX) to a polypeptide chain via two (or rarely one) thioether bonds generated as a result of the reaction of thiol groups of reduced cysteine residues with haem vinyl groups. The two cysteine residues almost always occur in the amino acid sequence CX2CH, the so-called haem c binding motif (HBM). Mammalian and fungal mitochondrial cytochrome c is by far the best known member of this protein class but there are many different classes of bacterial c-type cytochromes, often with several haems attached to one polypeptide chain, that are structurally unrelated to mitochondrial cytochromes. In multihaem c-type cytochromes, covalent haem attachment appears to be a key factor to allow dense packing of haem groups, i.e. exhibiting a high ratio of haem c groups per polypeptide length (Allen et al., 2003; Stevens et al., 2004). Bacterial c-type cytochromes are located either at the outside of the cytoplasmic membrane, in the periplasm or in the outer membrane of Gram-negative bacteria. They typically function in aerobic and anaerobic electron transport chains, but haem c also resides in the active site of some enzymes. Consequently, growth of many aerobically or anaerobically respiring bacteria depends on the presence of at least one c-type cytochrome.
Two widespread bacterial cytochrome c biogenesis systems have been identified that differ considerably in their enzymic components. These systems were named Ccm (cytochrome c maturation, also known as system I) and Ccs (cytochrome c synthesis, system II) (for reviews see Kranz et al., 1998; 2009; Allen et al., 2003; Stevens et al., 2004; Ferguson et al., 2008; Hamel et al., 2009). The Ccm system usually consists of eight proteins (CcmA, -B, -C, -D, -E, -F, -G and -H) and is found in Alpha-, Gamma- and some Betaproteobacteria, Deinococcus and in plant and red algal mitochondria. The Ccs system involves at least four proteins (CcsA, CcsB, CcdA and CcsX) and occurs in Beta-, Delta- and Epsilonproteobacteria, Gram-positive bacteria, Aquificales, cyanobacteria and in plant and algal chloroplasts. In cytochrome c biogenesis the following tasks have to be accomplished: (a) transport of apo-cytochrome across the cytoplasmic membrane by the Sec apparatus and, in most cases, signal peptide cleavage; (b) transport of haem across the cytoplasmic membrane; (c) Transport of reductant (electrons) across the cytoplasmic membrane and provision of electrons to reduce the potential disulfide bond in the HBM and to generate the cysteine thiol groups (probably catalysed by CcdA/CcsX in system II); (d) Recognition of haem and the HBM and subsequent stereospecific covalent attachment of haem to apo-cytochrome catalysed by the enzyme cytochrome c haem lyase (CCHL). The system II CCHL is called CcsA, a membrane-bound protein of about 250 to 350 amino acid residues with six transmembrane domains (Goldman et al., 1998; Hamel et al., 2003). The ccsA gene is often located adjacent to ccsB encoding another essential membrane protein that possibly forms a complex with CcsA (Dreyfuss et al., 2003; Ahuja et al., 2009). Exceptionally, in the known genomes of Epsilonproteobacteria the ccsB and ccsA genes are fused resulting in genes coding for membrane-bound proteins of about 900 residues (Hartshorne et al., 2006; Frawley and Kranz, 2009). CcsA contains a short periplasmic tryptophan-rich domain (the “WWD domain”) that is also present in the system I proteins CcmC and CcmF. It is assumed that CcsB and CcmC enable transmembrane haem trafficking while CcsA and CcmF were proposed to catalyse the covalent haem attachment to the apo-cytochrome (Ahuja et al., 2009; Frawley and Kranz, 2009; Kranz et al., 2009; Richard-Fogal et al., 2009). Several conserved and functionally essential histidine residues present in either CcsB or CcsA were assigned to ligate haem b in order to facilitate its export across the membrane and to position the haem b group for attachment to the HBM in a haem binding pocket lined by the WWD domain.
In addition to the classical CX2CH HBM mentioned above, the sequences CX3CH, CX4CH, CX15CH, CX2CK and A/FX2CH have been described as HBMs in bacterial or eukaryotic proteins (Allen et al., 2009; Hartshorne et al., 2007; Fülöp et al., 2009). The latter three HBMs are apparently not processed by the components of the regular Ccm or Ccs systems but require dedicated CCHLs, thus raising the question as to how such enzymes are able to discriminate between distinct HBMs (Allen et al., 2004; Hartshorne et al., 2006, 2007). In accordance with this requirement, many bacterial genomes contain multiple copies of putative CCHL enzymes (Hartshorne et al., 2006). One of these organisms is the Epsilonproteobacterium Wolinella succinogenes, a close relative of pathogenic Campylobacter and Helicobacter species. The W. succinogenes genome predicts all components of a typical Ccs cytochrome c biogenesis system including three different CCHLs and CcdA (Baar et al., 2003). The three CCHLs (NrfI, CcsA1 and CcsA2) are CcsB-CcsA fusion proteins comprising 902 (NrfI), 897 (CcsA1) and 910 (CcsA2) residues and their primary structures exhibit between 40% and 43% of pairwise identity (Hartshorne et al., 2006). Recently, CcsB-CcsA fusion proteins from two different Helicobacter species were shown to be able to functionally replace the entire Ccm system in E. coli (Feissner et al., 2006; Richard-Fogal et al., 2007). Four conserved histidine residues have been shown to be involved in haem b binding and/or trafficking across the membrane using the CcsB-CcsA fusion protein of Helicobacter hepaticus produced in Eschericha coli (Frawley and Kranz, 2009).
The CCHL NrfI from W. succinogenes is encoded by the third gene of the nrfHAIJ cluster and was shown to be required for the covalent attachment of the active site haem c group to the unique CX2CK HBM of the pentahaem cytochrome c nitrite reductase NrfA (Pisa et al., 2002). NrfA is the terminal reductase of respiratory nitrite ammonification and forms a membrane-bound complex with the tetrahaem cytochrome c menaquinol dehydrogenase NrfH (Simon et al., 2000; Simon, 2002; Kern and Simon, 2009a). Residue K134 of the CX2CK HBM was shown to be essential for catalysis of electron transfer from formate to nitrite at physiologically relevant rates (Pisa et al., 2002). Cells producing a K134H variant of NrfA did not grow by nitrite respiration although NrfA was present and possessed all five haem c groups. A NrfI-deficient mutant did not catalyse nitrite reduction and lacked the active site haem group of NrfA whereas the other four haem groups were present. Mutant K134H/stopI which contained the K134H modification in addition to the inactivated nrfI gene had the same properties as strain K134H. Thus, NrfI was postulated to be specifically involved in haem attachment to the CX2CK HBM but not to any of the other four CX2CH motifs of NrfA (Pisa et al., 2002). As the nrfI gene proved dispensable for maturation of a modified NrfA protein with five CX2CH motifs, NrfI most likely functions as a dedicated CCHL whose catalytic activity depends on K134 of NrfA.
The ccsA1 gene of W. succinogenes is organised as part of a gene cluster that encodes MccA, an octahaem c-type cytochrome of unknown function, that only contains seven classical CX2CH HBMs (Hartshorne et al., 2006, 2007). It was shown recently that both cysteines of a conserved CX15CH motif serve in the covalent binding of the eighth haem c group to MccA, a finding that was further supported by the presence of a spectroscopically distinct haem c with a red-shifted absorption maximum (Hartshorne et al., 2007). A ccsA1 deletion mutant of W. succinogenes produced unstable MccA whereas all other detectable c-type cytochromes were found to be synthesized in wild-type amounts indicative of CcsA1 being a CCHL dedicated to MccA. Finally, the W. succinogenes ccsA2 gene encoding the third CCHL is located downstream of the hemH gene encoding ferrochelatase, but no cytochrome c-encoding gene is situated nearby (Hartshorne et al., 2006). Hence, the CcsA2 protein is likely to attach haem to conventional CX2CH HBMs.
In this study, the substrate specificity of the three W. succinogenes CCHLs was tested using different reporter c-type cytochromes with variable HBMs as substrates that were produced in either E. coli or W. succinogenes in combination with different CCHL isoenzymes. The experimental set-up addressed the question as to what structural components are actually recognized by standard as well as dedicated CCHLs. Furthermore, a genomic and functional survey on all c-type cytochromes from W. succinogenes is presented in order to explain the intriguing finding that growth by anaerobic respiration seems to generally depend on at least one cytochrome c.
Results
Growth of W. succinogenes cells by anaerobic respiration depends on the ccsA2 and ccdA genes and requires the presence of oxygen
Deletion of the ccsA2 gene on the genome of W. succinogenes was attempted using the approach that previously enabled deletion of any of the two other CCHL-encoding genes (nrfI and ccsA1; see above). However, transformation of W. succinogenes wild-type cells with a corresponding deletion plasmid (pΔccsA2; for details see Fig. S1 and Experimental procedures in Supporting Information) did not result in the desired mutant strains under both fumarate- and nitrate-respiring conditions. Using the same approach, attempts to delete the ccdA gene were also unsuccessful (Fig. S1). These findings suggest that both modes of anaerobic respiration depend on the synthesis of at least one essential cytochrome c. While it is known that respiratory nitrate ammonification relies on at least three c-type cytochromes (NrfA, NrfH and NapB; Kern and Simon, 2009a), the electron transport chain catalysing the oxidation of either formate or hydrogen gas by fumarate was demonstrated to be functional without any c-type cytochrome involved (Biel et al., 2002; Kröger et al., 2002). Therefore, the question was raised whether other c-type cytochromes were essential under the applied growth conditions.
A comprehensive list of 23 known and putative c-type cytochromes encoded on the W. succinogenes genome is presented in Table 1. These cytochromes contain either a typical Sec-dependent signal peptide or at least one transmembrane segment and all of them possess at least one standard HBM (CX2CH) that is predicted to reside in the periplasmic space after apo-cytochrome export or membrane insertion. Out of these proteins, 11 have been experimentally detected and/or biochemically characterized (Table 1). The formation of c-type cytochromes in W. succinogenes cells grown by respiratory nitrate ammonification was examined using cell homogenates and cellular fractions subjected to denaturing polyacrylamide gel electrophoresis and subsequent in-gel staining for peroxidase activity catalysed by covalently bound haem (Fig. 1). Improvement of the resolution of this procedure and evaluation of the W. succinogenes genome sequence allowed to assign several additional cytochromes c as compared with previous reports (Simon et al., 2000, 2001). Newly detected cytochromes in nitrate-grown cells most likely include subunits of the cytochrome bc1 complex (PetC) and cytochrome cbb3 oxidase (CcoO and CcoP) implying that a (micro)aerobic respiratory chain is present under the experimental conditions. The same bands were found to be present in fumarate-grown cells although the cytochrome c content was somewhat lower under these conditions (not shown).
Table 1.
Compilation of W. succinogenes known and putative c-type cytochromes containing one or more CX2CH motifs.
| Cytochrome c designation | Total residues a | Molecular mass of mature holo- cytochrome c b | Number of CX2CH HBMs | Function/similarity and location | Detectable in haem staining c | Reference |
|---|---|---|---|---|---|---|
| cNosZ (Ws0914) | 864 (E, 24) | 95424 | 1 | Cytochrome c nitrous oxide reductase (periplasm) | N, N2O | Simon et al., 2004 |
| MccA (Ws0379) | 690 (E, 27) | 79684 | 7 and CX15CH | Octahaem cytochrome c (periplasm) | OE | Hartshorne et al., 2007 |
| NrfA (Ws0969) | 507 (E, 22) | 58331 | 4 and CX2CK | Pentahaem cytochrome c nitrite reductase (periplasm and membrane-attached by NrfH) | N, F, P | Einsle et al., 2000 |
| MccS (Ws0378) | 481 (2 TMS) | 55861 | 1 | Histidine kinase of two component regulatory system | ND | Hartshorne et al., 2006 |
| Ws0749 | 445 (8 TMS) | 50617 | 1 | No data available | ND | - |
| Ws0705 | 380 (4 or 5 TMS) | 45434 | 2 | N-terminal membrane-integral cytochrome b domain fused to a C-terminal dihaem cytochrome c domain | ND | - |
| CytA (Ws0009) | 371 (E, 21) | 40028 | 2 | Membrane-attached cytochrome of unknown function | P, N | Arnold, 1999 |
| Ccp (Ws1491) | 347 (P, 20) | 36513 | 2 | Dihaem cytochrome c peroxidase (periplasm) | OE | M. Kern and J. Simon, unpublished |
| PetC (Ws2154) | 285 (2 TMS) | 32955 | 2 | Dihaem subunit of cytochrome bc1 complex; typical for Epsilonproteobacteria (membrane) | N, F | Baymann et al., 2004 |
| CcoP (Ws0181) | 285 (1 or 2 TMS) | 32166 | 2 | Subunit III of cytochrome cbb3 oxidase (membrane) | N, F | - |
| FccC (Ws0121) | 213 (1 TMS) | 26776 | 4 | Member of the NapC/NrfH family. Possibly involved in electron transport to periplasmic methacrylate reductase FccA | ND | Simon et al., 1998b |
| CcoO (Ws0179) | 227 (1 TMS) | 26704 | 1 | Subunit II of cytochrome cbb3 oxidase (membrane) | N, F | - |
| NrfH (Ws0970) | 177 (1 TMS) | 22131 | 4 | Member of the NapC/NrfH family. Menaquinol dehydrogenase and membrane anchor of the cytochrome c nitrite reducase complex NrfHA. | N, F | Simon et al., 2001, 2002; Gross et al., 2005 |
| NapB (Ws1174) | 186 (P, 29) | 18886 | 2 | Redox partner of periplasmic nitrate reductase NapA (periplasm) | N | Simon et al., 2003, Kern et al., 2007 |
| NosC1 (Ws0921) | 184 (1 TMS) | 21469 | 1 | Possibly involved in electron transport to cNosZ (membrane) | ND | Simon et al., 2004 |
| TorC (Ws1850) | 188 (P, 20 or 1 TMS) | 19005, 21230 | 1 | Possibly involved in electron transport to TMAO/DSMO reductase TorA (Ws1849) | ND | Gross et al., 2005 |
| FccB (Ws0122) | 146 (P, 26) | 16073 | 4 | Possible redox partner of periplasmic methacrylate reductase FccA (flavocytochrome c complex) | ND | Simon et al., 1998b |
| NosC2 (Ws0922) | 154 (P, 34 or 1 TMS) | 13757, 17117 | 1 | Possibly involved in electron transport to cNosZ | ND | Simon et al., 2004 |
| Ws0710 | 117 (17, P) | 13186 | 4 | No data available | NA | - |
| Ws1344 | 123 (17, P) | 12946 | 1 | No data available | NA | - |
| Ws0706 | 120 (20, P) | 12068 | 1 | No data available | NA | - |
| Ws1182 | 101 (19, P) | 9926 | 1 | Cytochrome c553 | NA | - |
| Ws0700 | 98 (17, E) | 9213 | 1 | Cytochrome c553 (periplasm) | NA | Moura et al., 1988; Zhang and Hollocher, 1993 |
Cytochromes are arranged according to the size of the mature protein in descending order. The molecular mass of a covalently bound haem c group is 616.
E, experimentally determined signal peptide of denoted length, P, predicted signal peptide (SignalP 3.0) of denoted length, TMS, predicted transmembrane segment (TMpred)
If two masses are given, the smaller value refers to the protein with the putative signal peptide cleaved off.
Capital letters designate growth by anaerobic respiration with fumarate (F), nitrate (N), nitrous oxide (N2O) or polysulfide (P) as sole electron acceptor. ND, not detectable under any of these conditions; NA, no assignment possible due to insufficient gel resolution; OE, detected after over-expression of the corresponding gene.
Fig. 1.
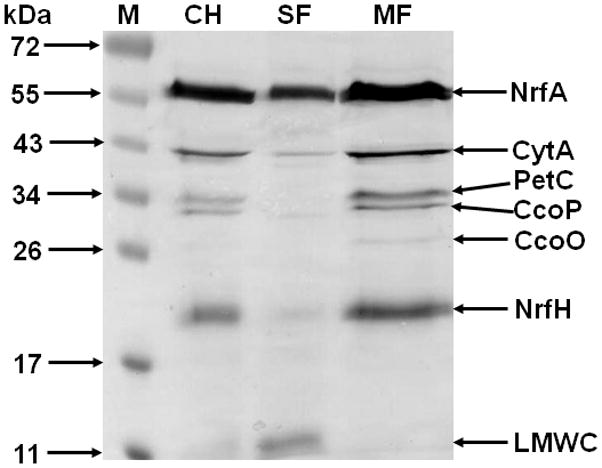
Detection of W. succinogenes c-type cytochromes in cell homogenates and cellular fractions of nitrate-grown cells. 100 μg protein per lane were separated on an SDS polyacrylamide gel (12.5%) and the gel was subjected to haem staining. The size marker (M) comprises pre-stained proteins. NrfA and NrfH have been experimentally identified (Simon et al., 2000, 2001). Assignment of other cytochromes is based on data shown in Table 1. CH, cell homogenate; SF, soluble fraction; MF, membrane fraction; LMWC, low molecular weight cytochromes.
Similar to other Epsilonproteobacteria, the W. succinogenes genome predicts the presence of a complete (micro)aerobic respiratory chain that comprises an NADH dehydrogenase (complex I; proteins Ws0472-Ws0488), a cytochrome bc1 complex (Ws2152-2154) and terminal reductases of the cbb3- (Ws0178-0181) and bd-types (Ws1852-1853) (Baar et al., 2003). In addition, several monohaem low-molecular weight c-type cytochromes are encoded that might act as redox partners of the cytochrome bc1 complex (Table 1). One of these, Ws0700, was previously purified and characterized as cytochrome c553 (Moura et al., 1988; Zhang and Hollocher, 1993). In line with the prediction of a (micro)aerobic respiratory chain, growth of W. succinogenes cells in the presence of 2% oxygen in the gas phase was reported previously (Schumacher et al., 1992). On the other hand however, cell growth in completely aerobic medium was never observed. Here, it was tested whether growth by anaerobic respiration occurs under fully anaerobic conditions (see Experimental procedures for medium preparation). It turned out that W. succinogenes cells were unable to grow in fumarate- or nitrate-containing batch cultures that were rigorously freed from oxygen. However, growth set in immediately after the injection of ambient air suggesting that low concentrations of oxygen are required to allow growth by anaerobic respiration (not shown).
Interestingly, it also proved impossible to delete the gene encoding the cytochrome c553 Ws0700 (Fig. S1 and Experimental procedures in Supporting Information) implying that this cytochrome is required under both fumarate- and nitrate-respiring conditions. In contrast, the cytochrome c peroxidase is dispensable under the described growth conditions of anaerobic respiration as the ccp gene could be successfully deleted from the W. succinogenes genome (M. Kern and J. Simon, unpublished data). This result suggests that the assumed generation of hydrogen peroxide due to the presence of oxygen and formate did not adversely affect the cells.
Taken together, it seems likely that CcsA2 and CcdA (but not NrfI or CcsA1) are essential for the synthesis of c-type cytochromes of the predicted (micro)aerobic respiratory chain that involves the cytochromes CcoO, CcoP, PetC and probably Ws0700. As these proteins contain at least one canonical CX2CH HBM, the resultssupport the idea that CcsA2 is responsible for haem c attachment to this sequenc.
Heterologous production of W. succinogenes CCHL isoenzymes in E. coli
Recently, two different W. succinogenes-type CCHLs, namely the CcsB-CcsA fusion proteins from Helicobacter pylori and H. hepaticus were reported to catalyse haem c attachment to a Bordetella pertussis dihaem cytochrome c4 (CycC) in E. coli RK103 cells that lack the genuine cytochrome c biogenesis system I (Feissner et al., 2006; Richard-Fogal et al., 2007). Here, this model system was adopted to test the functionality of the three different CCHLs (CcsA2, NrfI, CcsA1) from W. succinogenes. Similar to H. hepaticus CcsB-CcsA, the CcsA2 protein from W. succinogenes enabled E. coli RK103 to produce mature B. pertussis CycC (Fig. 2A). In contrast, holo-CycC was not detectable in E. coli RK103 cells when CcsA2 was replaced by either NrfI or CcsA1 (Fig. 2A). The formation of any of the three CCHLs from W. succinogenes was confirmed by immunoblot detection of the respective N-terminal glutathione S-transferase (GST)-tag (Fig. 2B). The detected proteins of 70 kDa most likely represented proteolytic CCHL fragments comprising the GST tag and the CcsB part of the CcsBA fusion protein whereas the full-length GST-tagged CCHLs (expected size of about 120 kDa) were not detected. Using the same genetic system, an equivalent truncated version of H. hepaticus CcsBA was also detected after enrichment from E. coli membranes (Frawley and Kranz, 2009). Notably, the amounts of the NrfI and CcsA2 fragments as shown in Fig. 2B were lower than that of CcsA1, but these amounts were found to vary in different E. coli cultures. The results demonstrate that CcsA2 is a functional CCHL that catalyses haem c attachment to the conventional CX2CH haem c binding motif. Assuming that the more specialized CCHLs (NrfI and CcsA1) were also formed in a functional form, it appears that these two proteins did not accept the CX2CH motif.
Fig. 2.
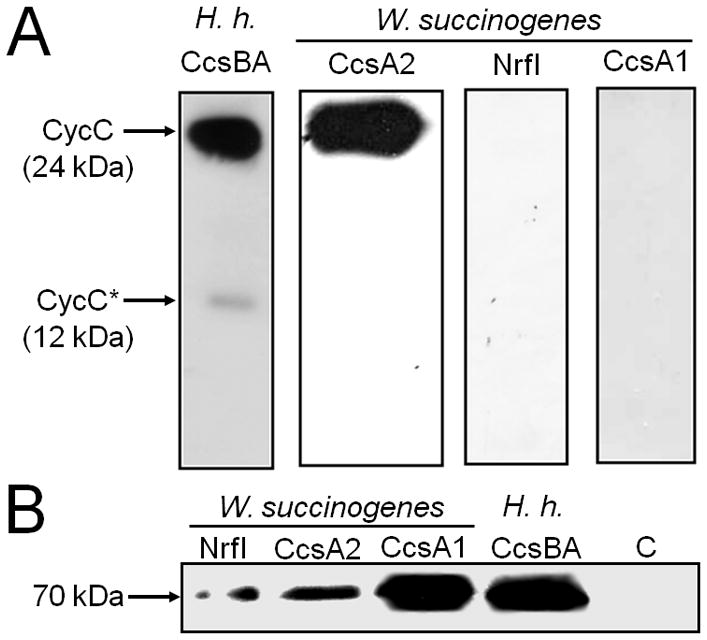
Production of CCHL isoenzymes from W. succinogenes in E. coli RK103 and their functionality in haem c attachment to a reporter cytochrome with two CX2CH haem c binding motifs. E. coli strains 21–24 (Table 4) were used.
A. Chemiluminescent haem stain detection of B. pertussis CycC in periplasmic extracts (35 μg per lane) of E. coli RK103 producing the indicated CCHL. The cells contained a plasmid that enabled production of the indicated CCHL. The previously observed proteolytic degradation product of CycC (denoted CycC*) was only observed in cells harbouring the H. hepaticus (H.h.) CCHL (Richard-Fogal et al., 2007).
B. Immunoblot detection of indicated CCHLs by Western blot analysis using an anti-GST serum. 50 μg of total cell protein were applied to each lane. The detected proteins of around 70 kDa most likely represent proteolytic CCHL fragments, each comprising the GST tag and the CcsB part of the CcsBA fusion protein. C refers to a control experiment using E. coli RK103 cells that contained the empty vectors pRGK330 and pGEX-4T-1 (Feissner et al., 2006). H.h., H. hepaticus.
A variety of B. pertussis CycC variants with altered haem c binding motifs was produced in E. coli RK103 cells that formed CcsA2 as sole CCHL (Table 2). No holo-CycC was detected when both haem c binding motifs were changed to AX2CH suggesting that CcsA2 was unable to covalently attach haem to a mono-cysteine CH signature (Fig. 3, lane 1). The same result was obtained after replacing both CycC CX2CH motifs by CX2CK (Fig. 3B, lane 2). Therefore, the histidine residue of the CX2CH motif is apparently indispensable for CcsA2 function. In contrast, modification of only the N-terminal haem c binding motif to either AX2CH or CX2CK did not abolish haem c attachment to CycC (Fig. 3, lanes 3 and 4). In these cases, however, a 12-kDa degradation product was present as a second prominent band in haem stain analysis. A similar band was shown previously to be a proteolytic cleavage product of CycC that was present in minor amounts when H. hepaticus CcsB-CcsA was employed to mature wild-type CycC (Fig. 2A; Feissner et al., 2006; Richard-Fogal et al., 2007). When the C-terminal haem c binding motif of CycC was replaced by AX2CH or CX2CK, only small amounts of holo-CycC were detected suggesting that CycC might be more susceptible to degradation when the C-terminal haem c was not incorporated (Fig. 3, lanes 5 and 6). Taken together, the results indicate that both haem c binding motifs were recognized by CcsA2 independently of each other and that the absence of one haem c destabilizes the respective monohaem CycC variant. Results similar to those described above were obtained when each of the six CycC variants was produced in E. coli RK103 cells that contained H. hepaticus CcsB-CcsA as their single CCHL, implying that H. hepaticus CcsB-CcsA is functionally equivalent to W. succinogenes CcsA2 (not shown).
Table 2.
Variants of B. pertussis dihaem cytochromes c4 (CycC) used in this study.
| Variant | Amino acid sequence of the N-terminal and C-terminal HBM (wild-type: CASCH and CAACH) a | Formation of holo-CycC in the corresponding periplasmic extract of E. coli RK103 producing the indicated single W. succinogenes CCHL b |
|---|---|---|
| 1 | AASCH and AAACH | CcsA2; CycC not detectable |
| 2 | CASCK and CAACK | CcsA2; CycC not detectable |
| 3 | AASCH and CAACH | CcsA2; CycC formed; susceptible for proteolytic degradation |
| 4 | CASCK and CAACH | CcsA2; CycC formed; susceptible for proteolytic degradation |
| 5 | CASCH and AAACH | CcsA2; Only small amounts of CycC detectable |
| 6 | CASCH and CAACK | CcsA2; Only small amounts of CycC detectable |
| 7 | AASCH and CAACK | NrfI; CycC not detectable |
| 8 | CX15CH and CX15CH | CcsA1 or NrfI; CycC not detectable |
| 9 | CASCH and CX15CH | CcsA1; CycC not detectable |
Wild-type haem c binding motifs are underlined. The introduced N- and C-terminal CX15CH motifs are 44-CYDQGDASRGVIAAASCH and 153-CWRGGLADRNVPAAAACH, respectively, with numbers referring to the amino acid position in pre-CycC.
See Fig. 3 for chemiluminescent detection of CycC variants 1–6.
Fig. 3.
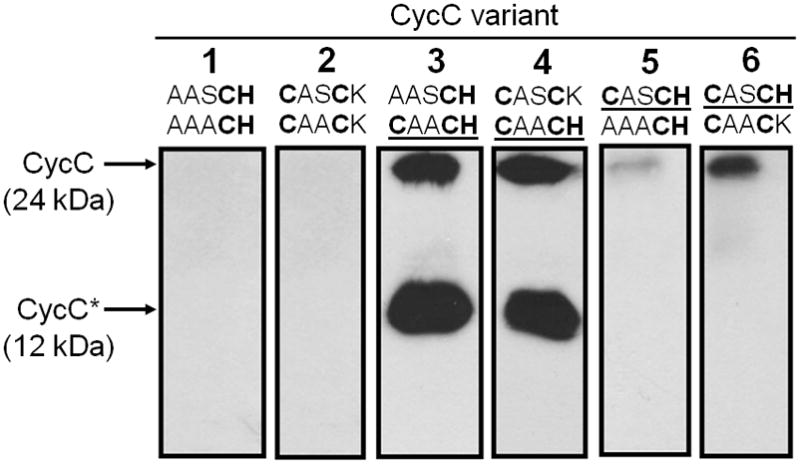
Chemiluminescent haem stain detection of B. pertussis CycC variants in periplasmic extracts (30 μg per lane) of E. coli RK103 cells that produced W. succinogenes CcsA2 as sole CCHL (strains 25–30 in Table 4). The two sequences denote the primary structures of the original haem c binding motifs (top, N-terminal motif, bottom, C-terminal motif). Wild-type haem c binding motifs are underlined.
To examine the haem c binding motif specificity of NrfI and CcsA1, variants of CycC containing CX2CK or CX15CH motifs were produced in appropriate E. coli RK103 strains (Table 2). Although several combinations were used, covalent haem attachment to CX2CK or CX15CH motifs was not observed (Table 2). Under the assumption made above, these data suggest that the mere presence of unconventional haem c binding motifs in CycC is not sufficient for haem c attachment catalysed by either NrfI or CcsA1. In an approach to produce an alternative reporter cytochrome, several monohaem c-type cytochrome genes (for example those encoding W. succinogenes Ws0700 or Ws1182, cf. Table 1) were cloned under the control of the araB promoter in pRGK330 derivatives (see Additional Experimental procedures in Supporting Information for details). Unfortunately, none of the corresponding holo-cytochromes was detected in CcsA2-producing E. coli RK103 cells.
W. succinogenes NrfI is functional in assembling NrfA from E. coli
The experiments described above raised the possibility that W. succinogenes NrfI is able to recognize the CX2CK haem c binding motif only when embedded in the active site of a cytochrome c nitrite reductase NrfA. To test this hypothesis, the E. coli nrfA gene was expressed in place of the genuine nrfA gene on the genome of strain W. succinogenes EcNrfA (Fig. 4A). E. coli NrfA was produced with the signal peptide from W. succinogenes NrfA and carried two consecutive C-terminal Strep-tag sequences. In addition, a second nrf promoter was introduced downstream of the kan gene to ensure transcription of the nrfI and -J genes (Fig. 4A). Cells of W. succinogenes EcNrfA were found to produce active E. coli NrfA indicating that the active site haem c group was attached (Table 3). The E. coli NrfA protein had the expected size of 57 kDa in SDS polyacrylamide gel electrophoresis but, according to haem stain analysis, its amount was less than that of the NrfA in W. succinogenes wild-type cells (Fig. 4B). Nitrite reductase activity staining in a native polyacrylamide gel indicated the formation of a homodimer with a molecular mass in the range of 134 kDa (Fig 4C). Cells of W. succinogenes EcNrfA did not grow by nitrite respiration and the E. coli NrfA was found almost exclusively in the soluble fraction (Table 3). This indicates that E. coli NrfA did not form a complex with the endogenous NrfH from W. succinogenes, thus preventing NrfA reduction by menaquinol.
Fig. 4.
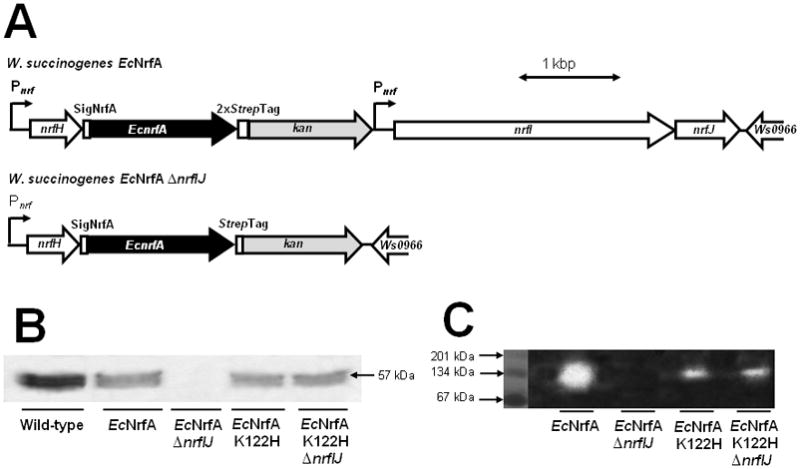
Heterologous production of catalytically active E. coli NrfA in W. succinogenes.
A. Partial physical maps of the genomes of W. succinogenes EcNrfA and W. succinogenes EcNrfA ΔnrfIJ. Pnrf, W. succinogenes cytochrome c nitrite reductase promoter element; SigNrfA, DNA stretch encoding the signal peptide of W. succinogenes NrfA; 2xStrep-Tag, DNA region encoding two consecutive Strep-tags.
B. Haem stain analysis of cell homogenates (100 μg protein per lane) from the indicated W. succinogenes strains.
C. Visualisation of nitrite reductase activity in cell homogenates of the indicated W. succinogenes strains. Proteins from the soluble cell fraction (50 μg per lane) were subjected to native gel electrophoresis and the gel was stained for activity as described in Experimental procedures. The marker proteins represent monomers, homodimers and homotrimers of bovine serum albumin.
Table 3.
Properties of W. succinogenes strains. Average values from three independent cultures are shown (individual values deviated less than 10%).
| Strain | Growth by nitrite respiration a | Specific nitrite reductase activity (BVred oxidation by nitrite) [U mg protein−1]
|
||
|---|---|---|---|---|
| Cell homogenate | Membrane fraction | Soluble fraction | ||
| Wild-type | + | 38 | 44 | 36 |
| ΔnrfHA | − | <0.1 | <0.1 | <0.1 |
| EcNrfA | − | 15 | 2.6 | 34 |
| EcNrfA ΔnrfIJ | − | <0.1 | <0.1 | <0.1 |
| EcNrfA K122H | − | 1.5 | 0.16 | 2.6 |
| EcNrfA K122H ΔnrfIJ | − | 1.5 | 0.26 | 3.6 |
| CjNrfA | − | 112 | 33 | 188 |
| CjNrfA ΔnrfIJ | − | 115 | 34 | 182 |
The cells were grown in a medium containing formate (50 mM) and nitrate (10 mM) as energy substrates in the presence of 10 mM NH4+. Growth by nitrate reduction to nitrite was similar in all strains. In the absence of nitrite respiration, however, cell growth ceased after exhaustion of nitrate.
When the nrfI and -J genes were deleted from the genome of W. succinogenes EcNrfA, the nitrite reductase activity corresponded to that of a W. succinogenes mutant that lacked the nrfHA genes (Table 3). In strain W. succinogenes EcNrfA ΔnrfIJ, NrfA was not detectable in haem staining (Fig. 4B) nor anti-Strep-tag immunoblot analysis (not shown) suggesting that NrfI is required for the formation of stable E. coli NrfA in W. succinogenes. It is quite likely that this protein instability is caused by the absence of the active site haem c group from NrfA, similar to what was found previously for W. succinogenes NrfA after purification from mutant W. succinogenesΔnrfIJ (Pisa et al., 2002). Notably, individual deletion of nrfJ did not affect nitrite respiration or nitrite reductase activity (Simon et al., 2000).
Two more W. succinogenes mutants were constructed that produced an E. coli NrfA variant in which the lysine residue of the CX2CK motif was replaced by histidine either in the presence or absence of the nrfIJ genes (W. succinogenes EcNrfA K122H and W. succinogenes EcNrfA K122H ΔnrfIJ). As judged from haem staining, both mutants produced stable NrfA in about the same amount as in strain W. succinogenes EcNrfA (Fig. 4B), a result that was also confirmed by anti-Strep-tag immunoblot analysis (not shown). However, the specific nitrite reductase activity was reduced in both strains to about 10% of that of W. succinogenes EcNrfA (Table 3; Fig. 4C). Reduced activity was also observed previously when the NrfA K122H variant was produced in E. coli (Eaves et al., 1998). The results suggest that the E. coli NrfA K122H variant produced in W. succinogenes contained a CX2CH-ligated active site haem c group whose covalent attachment was catalysed independently of NrfI, similar to what was found previously for the corresponding W. succinogenes NrfA K134H variant (Pisa et al., 2002). Most likely, in the Lys→His variants of both E. coli and W. succinogenes NrfA all five haem c groups were attached by CcsA2.
Heterologous production of catalytically active Campylobacter jejuni NrfA in W. succinogenes is independent of NrfI
Several Epsilonproteobacteria are predicted to produce NrfA proteins that contain five CX2CH motifs (see Fig. S2 in Supporting Information for an alignment of the primary structure region around the crucial first HBM). In these cases, the absence of the CX2CK motif is always accompanied by the lack of a nrfI homologue in the vicinity of the respective nrfA genes (Kern and Simon, 2009a). As there are no reports available on enzyme activity or maturation of such NrfA proteins, it was aimed to produce C. jejuni NrfA (CjNrfA) using W. succinogenes as host. Similar to the strategy described above, a chimeric nrf operon was constructed on the W. succinogenes genome (Fig. 5A). A codon-optimised version of the C. jejuni nrfA gene was synthesized and fused to nucleotide sequences encoding the W. succinogenes NrfA signal peptide and a C-terminal Strep-tag. The resulting nrfA gene was incorporated into the W. succinogenes genome immediately downstream of the genuine nrfH gene (Fig. 5A). Cells of the resulting strain (W. succinogenes CjNrfA) grew by nitrate respiration similar to the wild-type but growth by nitrite respiration was not observed (Table 3). The cells produced large amounts of highly active cytochrome c nitrite reductase during growth by nitrate respiration (Table 3; Fig. 5B). The enzyme had the expected monomeric size of 73 kDa and was located mainly in the soluble fraction indicating the absence of a stable complex between C. jejuni NrfA and W. succinogenes NrfH (Fig. 5B). Another mutant was constructed that produced CjNrfA in the absence of the nrfIJ genes (W. succinogenes CjNrfA ΔnrfIJ; Fig. 5A). The phenotype of this mutant was indistinguishable from that of strain W. succinogenes CjNrfA demonstrating that NrfI is dispensable for the maturation of a NrfA protein that naturally contains five CX2CH motifs (Table 3; Fig. 5B).
Fig. 5.
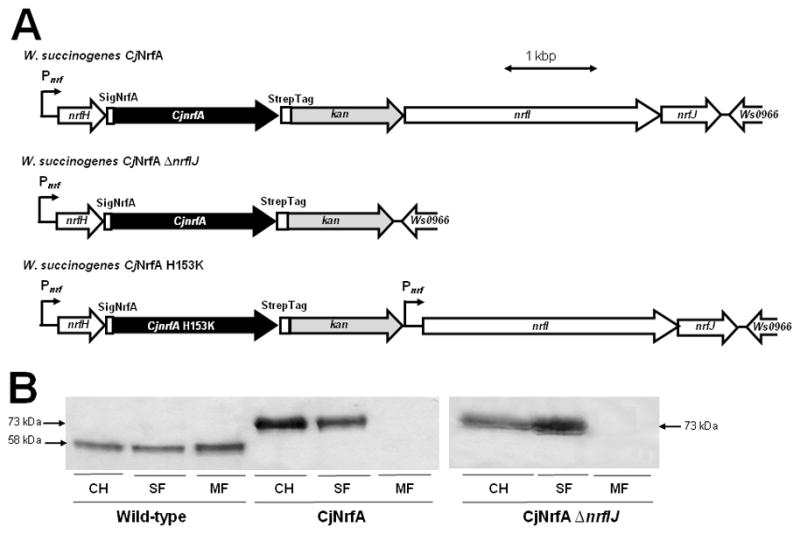
Heterologous production of C. jejuni NrfA in W. succinogenes.
A. Partial physical maps of the genomes of W. succinogenes CjNrfA, W. succinogenes CjNrfA ΔnrfIJ and W. succinogenes CjNrfA H153K. See legend of Fig. 4A for further explanations.
B. Haem stain analysis of cell homogenates and cell fractions (100 μg protein per lane) from the indicated W. succinogenes strains. CH, cell homogenate; SF, soluble cell fraction; MF, membrane fraction.
To test whether a CjNrfA variant in which the histidine residue of the active site HBM was replaced by lysine (His153→Lys) became dependent on NrfI, a mutant was generated that contained a second nrf promoter upstream of nrfI in addition to the indicated mutation in the C. jejuni nrfA gene (Fig. 5A). This strain (W. succinogenes CjNrfA H153K), however, was unable to produce any NrfA detectable by haem stain analysis (not shown). The most likely explanation for this finding is that NrfI was unable to attach haem to the introduced CX2CK site of C. jejuni NrfA. Consequently, NrfI might recognize an extended amino acid sequence in addition to the CX2CK motif. An alignment of available NrfA sequences indeed suggests that CX2CK motifs are part of a A-C-W-T/S-C-K-S-P-D-V consensus sequence (Fig. S2). As most of these residues are absent from the surrounding of the active site haem c motif in NrfA proteins with five CX2CH motifs, mutants were constructed in which residues near the H153K variation in C. jejuni NrfA (ACMNCKSGWT) were adjusted to the above mentioned consensus sequence. However, neither of the constructed variants (containing ACWNCKSGWT, ACWTCKSGWT or ACWTCKSPDV sequences) resulted in the production of a detectable CjNrfA variant (not shown).
NrfI and CcsA1 are unable to attach haem to variants of C. jejuni NrfA
Employment of the C. jejuni NrfA protein in a test system for active site haem c group attachment was further investigated by changing the corresponding haem c binding motif CMNCH to either AMNCH, SMNCH or CMNSH. Again, the three corresponding mutants did not produce detectable CjNrfA variants. In the next step, AMNCH- and CX15CH-encoding sequences were introduced in the C. jejuni nrfA gene and expressed in a mutant that contained the ccsA1 CCHL gene under the control of the nrf promoter. These mutants were also unable to produce stable holo-CjNrfA variants suggesting that CcsA1 recognizes not just the special CX15CH motif but other features in MccA (Hartshorne et al., 2007). In contrast, a control mutant in which the active site haem c binding motif of C. jejuni NrfA was altered to CX12CX2CH produced NrfA in wild-type amounts (not shown). Taken together, NrfI and CcsA1 seemingly do not recognize the HBM variants of CjNrfA as substrates for haem attachment. These findings strengthen the hypothesis that non-standard CCHLs attach haem to unconventional haem c binding motifs only in the presence of as yet unknown structural features that are present in addition to the CX2CK or CX15CH motifs.
Discussion
The role of cytochromes c in W. succinogenes during growth by anaerobic respiration under microoxic conditions
Epsilonproteobacteria form a class of cytochrome c-producing bacteria that usually grow by microaerobic or anaerobic respiration, thereby using a variety of electron donor and acceptor substrates (Schumacher et al., 1992; Simon et al., 2008; Kern and Simon, 2009a). The results presented in this study provide evidence that the prototypic Epsilonproteobacterium W. succinogenes is unable to grow by fumarate or nitrate respiration in the absence of either c-type cytochromes or of small amounts of oxygen. This finding was rather surprising as fumarate respiration was expected to work independently of c-type cytochromes and oxygen. Therefore, it is conceivable that W. succinogenes cells require oxygen in an anabolic metabolic pathway. For example, oxygen-dependent ribonucleotide reductase might be an essential enzyme in nucleotide metabolism, as discussed previously for C. jejuni by Sellars et al. (2002). Since the presence of oxygen or formate usually causes oxidative stress due to the generation of reactive oxygen species, the c-type cytochrome-containing aerobic respiratory chain might be essential in order to detoxify excess oxygen, even in the presence of sufficient amounts of a terminal electron acceptor suitable for anaerobic respiration. Furthermore, one or more of the functionally uncharacterised c-type cytochromes might also be involved in an anabolic process. Viable cytochrome c-deficient mutants have been reported for many bacterial species like E. coli, B. pertussis, Rhodobacter capsulatus or Paracoccus pantotrophus while in others, for example Synechocystis sp. PCC 6803, the ccsA gene proved essential, similar to what was found for W. succinogenes (Grove et al., 1996; Hübschmann et al., 1997; Beckett et al., 2000; Deshmukh et al., 2000; Bardischewsky and Friedrich, 2001; Feissner et al., 2005). Interestingly, although Bacillus subtilis is known to grow aerobically in the absence of c-type cytochromes, the genes encoding the CcsB/CcsA homologs ResB/ResC could not be inactivated (LeBrun et al., 2000). To the best of our knowledge, there is no report available describing a cytochrome c-deficient mutant of any Epsilonproteobacterium.
The fact that CcsA2 proved essential for growth of W. succinogenes cells might be also explained by a pleiotropic function of this membrane protein. As the ccsA2 gene seems to form a transcriptional unit with the ferrochelatase-encoding hemH gene (Fig. S1), it could be possible that both proteins serve in iron homoeostasis aiming to minimize the concentration of free cytoplasmic Fe2+/Fe3+ ions through haem synthesis and export. Although the hemH gene is situated upstream of ccsA2 (and hence, hemH transcription should not be impaired by ccsA2 deletion), it cannot be excluded that HemH and CcsA2 need to interact at the cytoplasmic side of the membrane in order to drive haem synthesis by ferrochelatase. Therefore, the absence of CcsA2 might lead to the accumulation of toxic iron in W. succinogenes. Fe2+ ions (1.5 μM are present in standard W. succinogenes minimal growth media) were found to be readily replaceable by either haemin (0.5 to 2 μM) or by the addition of brain-heart-infusion broth [0.6 – 1.5% (w/v)] without affecting growth; however, the desired ccsA2 deletion mutant was also not obtained when haemin was present instead of Fe2+ (M. Kern and J. Simon, unpublished data). A comparable pleiotropic function was also assigned to CcmC of Pseudomonas fluorescens, a protein which was postulated to function as a haem exporter in the cytochrome c maturation system I (Baysse et al., 2003; Cianciotto et al., 2005; Baert et al., 2008). In this case, CcmC was found to play a role in haem biosynthesis and iron acquisition via siderophores. Accumulation of a haem precursor in a ccmC-negative mutant was reported (Baysse et al., 2003).
Function and specificity of the three CCHLs in W. succinogenes
It was demonstrated here for the first time that CcsA2 recognizes the standard CX2CH HBM within a reporter cytochrome c in a heterologous system, suggesting that CcsA2 genuinely recognizes CX2CH motifs in W. succinogenes in a wide range of apo-cytochromes (Table 1). Recently, the functionally similar CcsB-CcsA fusion protein from H. hepaticus produced in E. coli was purified from membranes and several essential histidine residues were identified that could be involved in haem b binding on opposite sides of the membrane (Frawley and Kranz, 2009). These residues are generally conserved in epsilonproteobacterial CCHLs suggesting a conserved mechanism of CCHL-catalysed cytochrome c biogenesis (Ahuja et al., 2009; Kranz et al., 2009).
The NrfI CCHL from W. succinogenes was shown to recognize the CX2CK HBM not only in the NrfA protein from W. succinogenes but also in E. coli NrfA whereas it did not seem to attach haem to the CX2CK sequence in CycC. Furthermore, NrfI did not recognize an introduced CX2CK site in NrfA from C. jejuni. These findings indicate that NrfI needs more structural features than the lysine residue to recognize an NrfA active site and the consensus motif around the NrfA CX2CK site (see above and Fig. S2) seems to be a good candidate to be involved in NrfA-NrfI interaction. Allen et al. (2005) came to a similar conclusion when they found that the NrfA-dedicated E. coli system I machinery (NrfEFG) was unable to attach haem to an artificial CWSCK site in a cytochrome b562 variant; in contrast to a CWSCH HBM which was matured by the regular Ccm system.
Similarly to NrfI, CcsA1 also seems to be dedicated to the formation of a single c-type cytochrome (i.e. MccA) which carries a unique CX15CH HBM (Hartshorne et al., 2007). Artificial CX15CH motifs were not recognized by CcsA1 in W. succinogenes or E. coli suggesting that this motif may need to fold in an appropriate manner for recognition of the two cysteines during CcsA1-catalysed haem attachment. Interestingly, the CX15CH region is part of a conserved region (consensus sequence D-L-M-W-X1-C-A-R-T-X3-D-X1-D/N-X3-A/N-X1-G-C-H-S) in various MccA proteins. The function of CcsA1 will be further elucidated in the future when a genetic system has been established allowing site-directed modification of MccA in W. succinogenes.
With regard to the heterologous CCHL production system in E. coli, it cannot be excluded that HBMs other than CX2CH are not recognized by the redox system that has to reduce both cysteines residues of the HBM prior to CCHL-catalysed haem attachment. To date, it is not known which thioredoxin(s) recognize HBMs in the ccmA-H deletion mutant E. coli RK103 (thus replacing CcmG and CcmH) and it is possible that more than one such alternative system exists. Furthermore, the fact that the tested W. succinogenes monohaem c-type cytochromes were not matured in E. coli RK103 might also be due to unsuitable thiol-disulfide conversion enzymes.
It is not yet known how a HBM might be recognized by a CCHL enzyme but future large-scale purification and crystallization of a CcsB-CcsA fusion protein might pave the way towards a more detailed understanding of CCHL function. The production of CCHLs from Epsilonproteobacteria as described here and previously by Frawley and Kranz (2009) is an important prerequisite to elucidate the structure-function relationship and the molecular basis of HBM specificity of this intriguing enzyme class.
Heterologous production of NrfA cytochromes in W. succinogenes
Currently, E. coli is the only bacterium that is commonly used as host for the heterologous production of c-type cytochromes. Naturally, this system I organism produces c-type cytochromes only under anaerobic conditions but aerobic cytochrome c synthesis was achieved upon co-expression of the ccm genes from plasmid pEC86 (Arslan et al., 1998). This procedure has been used successfully for the production of reasonable amounts of various monohaem and some multihaem cytochromes c but, for unknown reasons, proved unsuccessful in other cases. This study shows that the system II organism W. succinogenes can be used as an alternative host for heterologous multihaem c-type cytochrome production.
The available structural and functional data on NrfA enzymes clearly show that the lysine residue in the active site CX2CK HBM is required for efficient nitrite turnover (Pisa et al., 2002; Einsle et al., 2002). Therefore, it was rather surprising to learn that NrfA from C. jejuni is highly active despite lacking the CX2CK motif. The Campylobacter NrfA enzymes seem to form a separate subset within the NrfA family that also differs in length and conserved regions in the primary structure (Pittman et al., 2007). It will be attempted in the future to purify the C. jejuni NrfA from W. succinogenes and to determine its high-resolution structure. Especially interesting will be the active site of nitrite reduction, the active site haem c group and the distance of the axial histidine ligand to the central haem c iron atom. This distance was found to be increased in an active site CX2CH variant of W. succinogenes NrfA arguing that the genuine lysine residue plays a role in proper haem c ligation, a feature that cannot be accomplished by histidine for spatial reasons (M. Rudolf, R. Pisa, A. Messerschmidt, J. Simon, and P.M.H. Kroneck, unpublished data).
Experimental procedures
Cell growth of W. succinogenes and E. coli
Bacterial strains used in this study are listed in Table 4. W. succinogenes cells were grown in minimal medium by either fumarate or nitrate respiration as described previously (Kröger et al., 1994; Kern and Simon, 2009b). Brain-Heart-Infusion broth (0.25% or 0.5%, w/v) was added to the medium where appropriate. Routinely, the growth medium was degassed and flushed with dinitrogen several times in order to reduce the oxygen content. To generate strictly anoxic growth conditions, freshly prepared medium was boiled for 10 min while gassed with dinitrogen gas. Subsequently, reducing agents cysteine hydrochloride (0.5 g l−1) and sodium sulfide (5 mg l−1) were added to the cold medium in an anaerobic chamber and the medium was sterilized by autoclaving at 121°C for 15 min. Filter-sterilized ambient air was added with a syringe if appropriate. E. coli cells were grown aerobically at 37°C in LB medium. Antibiotics were used at the following concentrations: ampicillin (100 μg ml−1), kanamycin (50 μg ml−1) and chloramphenicol (25 μg ml−1).
Table 4.
Strains of W. succinogenes and E. coli used in this study.
| Strain | Construction and/or relevant properties a | Reference |
|---|---|---|
| W. succinogenes wild-type and mutants: | ||
| 1. Wild-type | Type strain DSMZ 1740. | DSMZ |
| 2. ΔnrfHA | Deletion mutant lacking the cytochrome c nitrite reductase genes nrfHA; CmR. | Simon et al., 2003 |
| 3. EcNrfA | The W. succinogenes nrfA gene was replaced by nrfA from E. coli and a second W. succinogenes nrf promoter element was introduced upstream of nrfI (Fig. 4A); KmR. | This work |
| 4. EcNrfA ΔnrfIJ | Deletion of the nrfI and -J genes in the genome of strain 3 (Fig. 4A); KmR. | This work |
| 5. EcNrfA K122H | Similar to strain 3 but encoding a K122H variant of NrfA; KmR. | This work |
| 6. EcNrfA K122H ΔnrfIJ | Deletion of the nrfI and -J genes in the genome of strain 5 (Fig. 4A); KmR. | This work |
| 7. CjNrfA | The W. succinogenes nrfA gene was replaced by nrfA from C. jejuni (Fig. 5A); KmR. | This work |
| 8. CjNrfA ΔnrfIJ | Deletion of the nrfI and -J genes in the genome of strain 7 (Fig. 5A); KmR. | This work |
| 9. CjNrfA H153K | Similar to strain 7 but encoding a H153K variant of NrfA and a second W. succinogenes nrf promoter element introduced upstream of nrfI (Fig. 5A); KmR. | This work |
| 10. CjNrfA H153K ΔnrfIJ | Deletion of the nrfI and -J genes in the genome of strain 9; KmR. | This work |
| 11. CjNrfA CWNCK | Similar to strain 9 but with a CWNCK active site HBM; KmR. | This work |
| 12. CjNrfA CWTCK | Similar to strain 9 but with a CWTCK active site HBM; KmR. | This work |
| 13. CjNrfA CWTCKSPDV | Similar to strain 9 but with a CWTCKSPDV active site HBM; KmR. | This work |
| 14. CjNrfA AMNCH | Similar to strain 7 but with a AMNCH active site HBM; KmR. | This work |
| 15. CjNrfA SMNCH | Similar to strain 7 but with a SMNCH active site HBM; KmR. | This work |
| 16. CjNrfA CMNSH | Similar to strain 7 but with a CMNSH active site HBM; KmR. | This work |
| 17. CjNrfA CX12CX2CH | Similar to strain 7 but with a CX12CX2CH active site HBM; KmR, CmR. | This work |
| 18. CjNrfA AMNCH pnrf-ccsA1 | Similar to strain 14 but with a W. succinogenes nrf promoter element introduced upstream of ccsA1; KmR, CmR. | This work |
| 19. CjNrfA CX15CH pnrf-ccsA1 | Similar to strain 18 but with a CX15CH active site HBM; KmR, CmR. | This work |
| E. coli strains: | ||
| 20. RK103 | Derivative of E. coli MG1655 with the entire ccm gene cluster replaced by kan; KmR. | Feissner et al., 2006 |
| 21. RK103 pRGK332 pRGK368 | Strain 20 containing two plasmids encoding B. pertussis CycC (pRGK332) and the CcsBA fusion protein from H. hepaticus (pRGK368). | Richard-Fogal et al., 2007 |
| 22. RK103 pRGK332 pWsCcsA2 | Similar to strain 21 but with pRGK368 replaced by a plasmid encoding W. succinogenes CcsA2. | This work |
| 23. RK103 pRGK332 pWsNrfI | Similar to strain 21 but with pRGK368 replaced by a plasmid encoding W. succinogenes NrfI. | This work |
| 24. RK103 pRGK332 pWsCcsA1 | Similar to strain 21 but with pRGK368 replaced by a plasmid encoding W. succinogenes CcsA1. | This work |
| 25. RK103 pRGK332-1 pWsCcsA2 | Similar to strain 22 but with a derivative of pRGK332 encoding CycC with AASCH and AAACH HBMs. | This work |
| 26. RK103 pRGK332-2 pWsCcsA2 | Similar to strain 22 but with a derivative of pRGK332 encoding CycC with CASCK and CAACK HBMs. | This work |
| 27. RK103 pRGK332-3 pWsCcsA2 | Similar to strain 22 but with a derivative of pRGK332 encoding CycC with AASCH and CAACH HBMs. | This work |
| 28. RK103 pRGK332-4 pWsCcsA2 | Similar to strain 22 but with a derivative of pRGK332 encoding CycC with CASCK and CAACH HBMs. | This work |
| 29. RK103 pRGK332-5 pWsCcsA2 | Similar to strain 22 but with a derivative of pRGK332 encoding CycC with CASCH and AAACH HBMs. | This work |
| 30. RK103 pRGK332-6 pWsCcsA2 | Similar to strain 22 but with a derivative of pRGK332 encoding CycC with CASCH and CAACK HBMs. | This work |
| 31. RK103 pRGK332-7 pWsNrfI | Similar to strain 23 but with a derivative of pRGK332 encoding CycC with AASCH and CAACK HBMs. | This work |
| 32. RK103 pRGK332-8 pWsCcsA1 | Similar to strain 24 but with a derivative of pRGK332 encoding CycC with two CX15CH HBMs. | This work |
| 33. RK103 pRGK332-8 pWsNrfI | Similar to strain 23 but with a derivative of pRGK332 encoding CycC with two CX15CH HBMs. | This work |
| 34. RK103 pRGK332-9 pWsCcsA1 | Similar to strain 24 but with a derivative of pRGK332 encoding CycC with CASCH and CX15CH HBMs. | This work |
See Experimental procedures for details of mutant construction.
CmR and KmR denote resistance to chloramphenicol or kanamycin. Cells of strains 21-34 are resistant to ampicillin, kanamycin and chloramphenicol.
Cell fractionation and determination of protein concentrations
To separate soluble and membrane fractions, W. succinogenes cells harvested in the exponential growth phase were suspended (10 g cell protein l−1) in an anoxic buffer (pH 8.0) containing 50 mM Tris-HCl. The suspension was passed through a French press at 130 MPa and at 10 ml min−1 flow rate. The resulting cell homogenate was centrifuged for 45 min at 100,000 g to yield the membrane fraction (sediment) and the soluble fraction. The periplasmic protein fraction from harvested E. coli cells was obtained as described previously (Feissner et al., 2006). Protein was measured using the Biuret method with KCN (Bode et al., 1968) or the Bradford assay.
Determination of nitrite reductase activity
Nitrite reductase activity was measured under anoxic conditions at 37°C by photometrically recording the oxidation of benzyl viologen radical (BV) by nitrite at 546 nm. The test solution contained 50 mM potassium phosphate (pH 7.0), 1 mM BV and 10 mM sodium nitrite. BV was reduced by sodium dithionite and the reaction was started by addition of nitrite. Specific activities were calculated using the protein concentration and an extinction coefficient of η = 19.5 mM−1 cm−1. 1 unit of enzyme activity is defined as the oxidation of 2 μmol BV per min.
To assess in-gel nitrite reductase activity, soluble cell proteins were separated by non-denaturing polyacrylamide gel electrophoresis and stained for nitrite reductase activity. The stacking and separating polyacrylamide gels contained 4% and 7.5% polyacrylamide, respectively. After electrophoresis, the gel was transferred into a transparent polyethylene bag and immersed in a degassed reaction mixture to create limited oxygen conditions for the detection of BV-dependent nitrite reductase activity. The reaction mixture contained 50 mM phosphate buffer, pH 7.5, 10 mM sodium nitrite, 1 mM benzyl viologen and 10 mM freshly prepared sodium dithionite.
Cytochrome c and GST detection
Haem staining of SDS polyacrylamide gels containing protein samples from W. succinogenes was performed using 3,3′-dimethoxybenzidine according to the method of Francis and Becker (1984). Samples from E. coli were subjected to SDS PAGE using a non-reducing loading buffer (Roth) and transferred to PVDF membrane by Western blotting. Cytochromes c were detected using the SuperSignal West Pico chemiluminescence substrate (Thermo Scientific). X-ray films (CL-Xposure film, Thermo Scientific) were exposed for 5 min. GST fusion protein were detected using polyclonal GST antibodies (New England Biolabs).
Construction of plasmids for cytochrome c production in E. coli
E. coli XL-1 Blue was used as host for cloning steps. Plasmids encoding GST fused to the N-terminus of a W. succinogenes CCHL (pWsCcsA2, pWsNrfI and pWsCcsA1) are derivatives of pGEX-4T-1 (GE Healthcare). CCHL-encoding DNA fragments were amplified by PCR using genomic DNA from W. succinogenes as template. Each primer carried a suitable restriction site at the 5′-end to facilitate cloning of the PCR product (primers 1–6 in Table S1). In each of the three plasmids, the gene encoding the respective GST-CCHL fusion protein is transcribed from an IPTG-inducible tac promoter. For the production of CycC variants with altered HBMs, plasmid pRGK332 (Feissner et al., 2006) was modified using the QuikChange Site-directed Mutagenesis Kit (Stratagene) with a suitable plasmid template and specifically synthesized primers (primers 7–12 in Table S1). The constructed nine plasmids were named pRGK332-1 to pRGK332-9 (see Table 2 for details on CycC encoded by each plasmid).
Production of CycC (variants) in E. coli RK103
E. coli strains containing the plasmids encoding B. pertussis CycC (or a CycC variant) and a CCHL from W. succinogenes or H. hepaticus (strains 21–34 in Table 4) were inoculated (1%, v/v) with a fresh overnight culture and grown aerobically in the presence of 0.9 mM aminolevulinic acid for 3 h at 37°C. IPTG (1 mM) was added to induce transcription of the CCHL-encoding gene. After 1 h of incubation, arabinose (0.2%, w/v) was added to induce the synthesis of apo-CycC. Cells were harvested after an additional 3 h of growth.
Construction of W. succinogenes mutants
Standard genetic procedures were used (Sambrook et al., 1989).DNA was isolated from W. succinogenes using the DNeasy Tissuekit (Qiagen). PCR was carried out using Biotaq Red DNA polymerase(Bioline) withstandard amplification protocols. Site-directed mutagenesis of plasmid DNA was performed using either the QuikChange Site-directed Mutagenesis Kit XL (Stratagene) or the Phusion Site-directed Mutagenesis Kit (Finnzymes).
W. succinogenes CjNrfA (Fig. 5A) was constructed through double homologous recombination by replacing the genomic nrfA by a custom-made codon-optimised nrfA from C. jejuni (synthesized by GENEART, Regensburg, Germany). The recombination plasmid (pCjNrfA) contained the kanamycin resistance gene cartridge (kan) and the C. jejuni nrfA gene flanked by two PCR fragments identical to regions comprising partial nrfH and nrfI sequences in the W. succinogenes genome. The flanking regions were amplified by PCR using the following primer pairs (EcoRI, BamHI and NcoI restriction sites underlined): 5′-GCGAATTCAATTCCTAGTGTATGACTCGCTGG and 5′-ATTGCTCGCGAGTAACCCCATAGAGACG (upstream flanking fragment including the DNA encoding the W. succinogenes NrfA signal peptide) and 5′-GCGGATCCCCTGAGGCGCCCTCCATC and 5′-CGCCATGGCTTCTCTTTGTGGAGTAGCTCCAAATC (downstream flanking fragment). A C. jejuni nrfA fragment was amplified that lacked the signal peptide-encoding DNA at the 5′-end but contained a nucleotide stretch coding for a C-terminal Strep-tag II using the primer pair: 5′-GATATCAATCAAAAAAGGAGGATGAGGCC and 5′-CCGGATCCTTATTACTTCTCAAATTGGGGATGG. After phosphorylation, the upstream fragment was ligated to the C. jejuni nrfA fragment and the resulting fragment was inserted into plasmid pPR-IBA1 (IBA BioTAGnology) using EcoRI and BamHI restriction. Subsequently, the downstream fragment was introduced via the BamHI and NcoI sites and finally a kan fragment (excised with BamHI from pUC4K) was placed between the C. jejuni nrfA sequence and the downstream fragment. PCR analysis confirmed that kan had the same orientation as C. jejuni nrfA. The His153 codon in C. jejuni nrfA was replaced by a lysine codon by site-directed mutagenesis (QuikChange Kit) using a pair of complementary primers of which the forward primer is 5′-GCCTGCATGAATTGCAAGAGCGGATGGACCCCC (altered nucleotides printed in bold; introduced lysine codon underlined). To ensure transcription of the nrfI and -J genes, a second nrf promoter element (Pnrf) comprising 204 bp immediately upstream of the nrfA start codon (including the nrfA ribosome binding site) was incorporated downstream of kan in the plasmid that carried the H153K mutation in C. jejuni nrfA. Pnrf was amplified using the 5′-phosphorylated primer pair 5′-TCATTAGAATTTACAGCGTGGTCTCCCC and 5′-ATTCCCTCCTTCAGATGGTAAAACCTTAC and ligated with the linear plasmid amplified using the following divergent primer pair; 5′-GTGCTATACAAACTCTTAAGCTCC and 5′-GGATCCCGCTGAGGTCTGCCTC.
W. succinogenes CjNrfA ΔnrfIJ (Fig. 5B) was constructed using a modified pCjNrfA. To delete the nrfIJ genes, the downstream fragment from pCjNrfA was excised and replaced by an alternative fragment that is identical to a region on the W. succinogenes genome located downstream of nrfJ. The latter fragment was amplified using the primer pair 5′-GCGGATCCTCATTGCCTAGGCCAAAAAGTC and 5′-CGCCATGGATTGGCAGGCGCTGATG (BamHI and NcoI restrictionsites underlined). For the construction of W. succinogenes CjNrfA H153K ΔnrfIJ, the plasmid was subjected to nrfA mutagenesis as described above.
The mutants W. succinogenes EcNrfA and W. succinogenes EcNrfA ΔnrfIJ using a similar strategy as outlined above for the expression of C. jejuni nrfA. The E. coli nrfA gene (lacking the signal peptide-encoding 5′-end) was amplified using genomic DNA from E.coli XL-1 Blue (Stratagene) and the 5′-phosphorylated primer pair 5′-GAACAAACGGCTGCTCCCGCAAAACC and 5′-TTGGCTTAACAGACCGTTTTTACGTGCCTGTTC. Plasmids pEcNrfA and pEcNrfA ΔnrfIJ were constructed by amplifying pCjNrfA or pCjNrfA ΔnrfIJ (see above) with the primer pair 5′-AGCGCGTGGAGCCATCCCCAATTTGAG and 5′-CGCGAGTAACCCCATAGAGACGATCGCGAC followed by ligation with the E. coli nrfA fragment. In order to introduce a nucleotide sequence encoding a tandem arrangement of two Strep-tag II sequences (One-STrEP-tag, IBA BioTAGnology), the following divergent primer pair was used: 5′-TCAGCGTGGAGCCACCCTCAGTTCGAGAAATAATAAGGATCCAAAGCCACGTTGTG and 5′-ACCTCCCGATCCACCGCCAGAACCTCCACCCTTCTCAAATTGGGGATGGCTCC (linker region in bold, additional Strep-tag sequence underlined). Site-directed mutagenesis of the E. coli nrfA gene was performed using the QuikChange Kit with pEcNrfA or pEcNrfA ΔnrfIJ as template. A pair of complementary primers was used to replace histidine codon 122 by a lysine codon, of which the forward primer sequence is as follows; 5′-CCGATGGCATGCTGGAGTTGTCATAGCCCGGATGT (altered nucleotides are printed in bold and the modified codon is underlined).
Transformation of W. succinogenes cells was carried out by electroporation as described (Simon et al., 1998a). Transformants were selected in the presence of kanamycin (25 mg l−1) or chloramphenicol (12.5 mg l−1). The desired modification in the transformant genome was confirmed by PCR and the identity of DNA stretches involved in recombination events was confirmed by sequencing suitable PCR products.
Supplementary Material
Table S1. Nucleotide primers used for the construction of E. coli plasmids serving in the production of W. succinogenes CCHLs, cytochromes c (Ws0700; Ws1182) and CycC variants.
Table S2. Nucleotide primers used for the construction of plasmids for W. succinogenes transformation.
Fig. S1. Arrangement of the genes encoding CcsA2 (Ws2159), CcdA (Ws1481) and cytochrome c553 (Ws0700) on the genome of W. succinogenes.
Fig. S2. Partial primary structure alignment (CLUSTAL W) of available NrfA proteins with CX2CH active site HBMs and selected NrfA proteins carrying the typical CX2CK motif.
Acknowledgments
The authors are grateful to Elaine Frawley, James Allen and Stuart Ferguson for stimulating discussions and to Tamara Hes for excellent technical assistance. JS is supported by the Deutsche Forschungsgemeinschaft (project section P33 of SFB 472 and grants SI 848/1-1 and 2-1). RGK is supported by NIH grant GM 47909.
References
- Ahuja U, Kjelgaard P, Schulz BL, Thöny-Meyer L, Hederstedt L. Haem-delivery proteins in cytochrome c maturation system II. Mol Microbiol. 2009;73:1058–1071. doi: 10.1111/j.1365-2958.2009.06833.x. [DOI] [PubMed] [Google Scholar]
- Allen JWA, Daltrop O, Stevens JM, Ferguson SJ. C-type cytochromes: diverse structures and biogenesis systems pose evolutionary problems. Phil Trans R Soc Lond B Biol Sci. 2003;358:255–266. doi: 10.1098/rstb.2002.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JWA, Ginger ML, Ferguson SJ. Maturation of the unusual single-cysteine (XXXCH) mitochondrial c-type cytochromes in trypanosomatids must occur through a novel biogenesis pathway. Biochem J. 2004;383:537–542. doi: 10.1042/BJ20040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JWA, Leach N, Ferguson SJ. The histidine of the c-type cytochrome CXXCH haem-binding motif is essential for haem attachment by the Escherichia coli cytochrome c maturation (Ccm) apparatus. Biochem J. 2005;389:587–592. doi: 10.1042/BJ20041894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JWA, Sawyer EB, Ginger ML, Barker PD, Ferguson SJ. Variant c-type cytochromes as probes of the substrate specificity of the E. coli cytochrome c maturation (Ccm) apparatus. Biochem J. 2009;419:177–184. doi: 10.1042/BJ20081999. [DOI] [PubMed] [Google Scholar]
- Arnold B. Diploma Thesis. Department of Biology, University of Frankfurt am Main; Germany: 1999. [Google Scholar]
- Arslan E, Schulz H, Zufferey R, Künzler P, Thöny-Meyer L. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem Biophys Res Comm. 1998;251:744–747. doi: 10.1006/bbrc.1998.9549. [DOI] [PubMed] [Google Scholar]
- Baar C, Eppinger M, Raddatz G, Simon J, Lanz C, Klimmek O, et al. Complete genome sequence and analysis of Wolinella succinogenes. Proc Natl Acad Sci USA. 2003;100:11690–11695. doi: 10.1073/pnas.1932838100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert B, Baysse C, Matthijs S, Cornelis P. Multiple phenotypic alterations caused by a c-type cytochrome maturation ccmC gene mutation in Pseudomonas aeruginosa. Microbiology. 2008;154:127–138. doi: 10.1099/mic.0.2007/008268-0. [DOI] [PubMed] [Google Scholar]
- Bardischewsky F, Friedrich CG. Identification of ccdA in Paracoccus pantrotrophus GB17: disruption of ccdA causes complete deficiency in c-type cytochromes. J Bacteriol. 2001;183:257–263. doi: 10.1128/JB.183.1.257-263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baymann F, Lebrun E, Nitschke W. Mitochondrial cytochrome c1 is a collapsed di-heme cytochrome. Proc Natl Acad Sci USA. 2004;101:17737–17740. doi: 10.1073/pnas.0407442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysse C, Matthijs S, Schobert M, Layer G, Jahn D, Cornelis P. Co-ordination of iron acquisition, iron porphyrin chelation and iron-protoporphyrin export via the cytochrome c biogenesis protein CcmC in Pseudomonas fluorescens. Microbiology. 2003;149:3543–3552. doi: 10.1099/mic.0.26566-0. [DOI] [PubMed] [Google Scholar]
- Beckett CS, Loughman JA, Karberg KA, Donato GM, Goldman WE, Kranz RG. Four genes are required for the system II cytochrome c biogenesis pathway in Bordetella pertussis, a unique bacterial model. Mol Microbiol. 2000;38:465–481. doi: 10.1046/j.1365-2958.2000.02174.x. [DOI] [PubMed] [Google Scholar]
- Biel S, Simon J, Gross R, Ruiz T, Ruitenberg M, Kröger A. Reconstitution of coupled fumarate respiration in liposomes by incorporating the electron transport enzymes isolated from Wolinella succinogenes. Eur J Biochem. 2002;269:1974–1983. doi: 10.1046/j.1432-1033.2002.02842.x. [DOI] [PubMed] [Google Scholar]
- Bode C, Goebell H, Stähler E. Zur Eliminierung von Trübungsfehlern bei der Eiweiβsbestimmung mit der Biuretmethode. Z Klin Chem Klin Biochem. 1968;6:418–422. [PubMed] [Google Scholar]
- Cianciotto NP, Cornelis P, Basse C. Impact of the bacterial type I cytochrome c maturation system on different biological processes. Mol Microbiol. 2005;56:1408–1415. doi: 10.1111/j.1365-2958.2005.04650.x. [DOI] [PubMed] [Google Scholar]
- Deshmukh M, Brasseur G, Daldal F. Novel Rhodobacter capsulatus genes required for the biogenesis of various c-type cytochromes. Mol Microbiol. 2000;35:123–138. doi: 10.1046/j.1365-2958.2000.01683.x. [DOI] [PubMed] [Google Scholar]
- Dreyfuss BW, Hamel P, Nakamoto SS, Merchant S. Functional analysis of a divergent system II protein, Ccs1, involved in c-type cytochrome biogenesis. J Biol Chem. 2003;278:2604–2613. doi: 10.1074/jbc.M208652200. [DOI] [PubMed] [Google Scholar]
- Eaves DJ, Grove J, Staudenmann W, James P, Poole RK, White SA, Griffiths I, Cole JA. Involvement of products of the nrfEFG genes in the covalent attachment of haem c to a novel cysteine-lysine motif in the cytochrome c552 nitrite reductase from Escherichia coli. Mol Microbiol. 1998;28:205–216. doi: 10.1046/j.1365-2958.1998.00792.x. [DOI] [PubMed] [Google Scholar]
- Einsle O, Stach P, Messerschmidt A, Simon J, Kröger A, Huber R, Kroneck PMH. Cytochrome c nitrite reductase from Wolinella succinogenes: structure at 1.6 Å resolution, inhibitor binding, and heme-packing motifs. J Biol Chem. 2000;275:39608–39616. doi: 10.1074/jbc.M006188200. [DOI] [PubMed] [Google Scholar]
- Einsle O, Messerschmidt A, Huber R, Kroneck PMH, Neese F. Mechanism of the six-electron reduction of nitrite to ammonia by cytochrome c nitrite reductase. J Am Chem Soc. 2002;124:11737–11745. doi: 10.1021/ja0206487. [DOI] [PubMed] [Google Scholar]
- Feissner RE, Beckett CS, Loughman JA, Kranz RG. Mutations in cytochrome assembly and periplasmic redox pathways in Bordetella pertussis. J Bacteriol. 2005;187:3941–3949. doi: 10.1128/JB.187.12.3941-3949.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feissner RE, Richard-Fogal CL, Frawley ER, Loughman JA, Earley KW, Kranz RG. Recombinant cytochromes c biogenesis systems I and II and analysis of haem delivery pathways in Escherichia coli. Mol Microbiol. 2006;60:563–577. doi: 10.1111/j.1365-2958.2006.05132.x. [DOI] [PubMed] [Google Scholar]
- Ferguson SJ, Stevens JM, Allen JWA, Robertson IB. Cytochrome c assembly: a tale of ever increasing variation and mystery? Biochim Biophys Acta. 2008;1777:980–984. doi: 10.1016/j.bbabio.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Francis RT, Becker RR. Specific indication of hemoproteins in polyacrylamide gels using a double-staining process. Anal Biochem. 1984;136:509–514. doi: 10.1016/0003-2697(84)90253-7. [DOI] [PubMed] [Google Scholar]
- Frawley ER, Kranz RG. CcsBA is a cytochrome c synthetase that also functions in heme transport. Proc Natl Acad Sci USA. 2009;106:10201–10206. doi: 10.1073/pnas.0903132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fülöp V, Sam KA, Ferguson SJ, Ginger ML, Allen JWA. Structure of the trypanosomatid mitochondrial cytochrome c with heme attached via only one thioether bond and implications for the substrate recognition requirements of heme lyase. FEBS J. 2009;276:2822–2832. doi: 10.1111/j.1742-4658.2009.07005.x. [DOI] [PubMed] [Google Scholar]
- Goldman BS, Beck DL, Monika EM, Kranz RG. Transmembrane heme delivery systems. Proc Natl Acad Sci USA. 1998;95:5003–5008. doi: 10.1073/pnas.95.9.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R, Eichler R, Simon J. Site-directed modifications indicate differences in axial haem c iron ligation between the related NrfH and NapC families of multihaem c-type cytochromes. Biochem J. 2005;390:689–693. doi: 10.1042/BJ20050448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove J, Tanapongpipat S, Thomas G, Griffiths L, Crooke H, Cole J. Escherichia coli K-12 genes essential for the synthesis of c-type cytochromes and a third nitrate reductase located in the periplasm. Mol Microbiol. 1996;19:467–481. doi: 10.1046/j.1365-2958.1996.383914.x. [DOI] [PubMed] [Google Scholar]
- Hamel P, Dreyfuss BW, Xie Z, Gabilly ST, Merchant S. Essential histidine and tryptophan residues in CcsA, a system II polytopic cytochrome c biogenesis protein. J Biol Chem. 2003;278:2593–2603. doi: 10.1074/jbc.M208651200. [DOI] [PubMed] [Google Scholar]
- Hamel P, Corvest V, Giegé P, Bonnard G. Biochemical requirements for the maturation of mitochondrial c-type cytochromes. Biochim Biophys Acta. 2009;1793:125–138. doi: 10.1016/j.bbamcr.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Hartshorne RS, Richardson DJ, Simon J. Multiple haem lyase genes indicate substrate specificity in cytochrome c biogenesis. Biochem Soc Trans. 2006;34:146–149. doi: 10.1042/BST0340146. [DOI] [PubMed] [Google Scholar]
- Hartshorne RS, Kern M, Meyer B, Clarke TA, Karas M, Richardson DJ, Simon J. A dedicated haem lyase is required for the maturation of a novel bacterial cytochrome c with unconventional haem binding. Mol Microbiol. 2007;64:1049–1060. doi: 10.1111/j.1365-2958.2007.05712.x. [DOI] [PubMed] [Google Scholar]
- Hübschmann T, Wilde A, Elanskaya I, Shestakov SV, Börner T. A putative cytochrome c biogenesis gene in Synechocystis sp. PCC 6803. FEBS Lett. 1997;408:201–205. doi: 10.1016/s0014-5793(97)00421-3. [DOI] [PubMed] [Google Scholar]
- Kern M, Simon J. Electron transport chains and bioenergetics of respiratory nitrogen metabolism in Wolinella succinogenes and other Epsilonproteobacteria. Biochim Biophys Acta. 2009a;1787:646–656. doi: 10.1016/j.bbabio.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Kern M, Simon J. Periplasmic nitrate reduction in Wolinella succinogenes: cytoplasmic NapF facilitates NapA maturation and requires the menaquinol dehydrogenase NapH for membrane attachment. Microbiology. 2009b;155:2784–2794. doi: 10.1099/mic.0.029983-0. [DOI] [PubMed] [Google Scholar]
- Kern M, Mager AM, Simon J. Role of individual nap gene cluster products in NapC-independent nitrate respiration of Wolinella succinogenes. Microbiology. 2007;153:3739–3747. doi: 10.1099/mic.0.2007/009928-0. [DOI] [PubMed] [Google Scholar]
- Kranz RG, Lill R, Goldman B, Bonnard G, Merchant S. Molecular mechanisms of cytochrome c biogenesis: three distinct systems. Mol Microbiol. 1998;29:383–396. doi: 10.1046/j.1365-2958.1998.00869.x. [DOI] [PubMed] [Google Scholar]
- Kranz RG, Richard-Fogal C, Taylor JS, Frawley ER. Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol Mol Biol Rev. 2009;73:510–528. doi: 10.1128/MMBR.00001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A, Geisler V, Duchêne A. Isolation of Wolinella succinogenes hydrogenase, Chromatofocusing. In: von Jagow G, Schägger H, editors. A practical guide to membrane protein purification. London: Academic Press; 1994. pp. 141–147. [Google Scholar]
- Kröger A, Biel S, Simon J, Gross R, Unden G, Lancaster CRD. Fumarate respiration of Wolinella succinogenes: enzymology, energetics and coupling mechanism. Biochim Biophys Acta. 2002;1553:23–38. doi: 10.1016/s0005-2728(01)00234-1. [DOI] [PubMed] [Google Scholar]
- LeBrun N, Bengtsson J, Hederstedt L. Genes required for cytochrome c synthesis in Bacillus subtilis. Mol Microbiol. 2000;36:638–650. doi: 10.1046/j.1365-2958.2000.01883.x. [DOI] [PubMed] [Google Scholar]
- Moore GR, Pettigrew GW. Cytochromes c: evolutionary, structural, and physicochemical aspects. New York: Springer; 1990. [Google Scholar]
- Moura I, Liu MY, Costa C, Liu MC, Pai G, Xavier AV, LeGall J, Payne WJ, Moura JJG. Spectroscopic characterization of a high-potential monohaem cytochrome from Wolinella succinogenes, a nitrate-respiring organism. Redox and spin equilibria studies. Eur J Biochem. 1988;177:673–682. doi: 10.1111/j.1432-1033.1988.tb14422.x. [DOI] [PubMed] [Google Scholar]
- Pisa R, Stein T, Eichler R, Gross R, Simon J. The nrfI gene is essential for the attachment of the active site haem group of Wolinella succinogenes cytochrome c nitrite reductase. Mol Microbiol. 2002;43:763–770. doi: 10.1046/j.1365-2958.2002.02784.x. [DOI] [PubMed] [Google Scholar]
- Pittman MS, Elvers KT, Lee L, Jones MA, Poole RK, Park SF, Kelly DJ. Growth of Campylobacter jejuni on nitrate and nitrite: electron transport to NapA and NrfA via NrfH and distinct roles for NrfA and the globin Cgb in protection against nitrosative stress. Mol Microbiol. 2007;63:575–590. doi: 10.1111/j.1365-2958.2006.05532.x. [DOI] [PubMed] [Google Scholar]
- Richard-Fogal CL, Frawley ER, Feissner RE, Kranz RG. Heme concentration dependence and metalloprotein inhibition of the system I and II cytochrome c assembly pathways. J Bacteriol. 2007;189:455–463. doi: 10.1128/JB.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard-Fogal CL, Frawley ER, Bonner ER, Zhu H, San Francisco B, Kranz RG. A conserved haem redox and trafficking pathway for cofactor attachment. EMBO J. 2009;28:2349–2359. doi: 10.1038/emboj.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a Laboratory Manual. 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Schumacher W, Kroneck PMH, Pfennig N. Comparative systematic study on “Spirillum“ 5175, Campylobacter and Wolinella species. Arch Microbiol. 1992;158:287–293. [Google Scholar]
- Scott RA, Mauk AG. Cytochrome c: a multidisciplinary approach. Mill Valley, CA: University Science Books; 1995. [Google Scholar]
- Sellars MJ, Hall SJ, Kelly DJ. Growth of Campylobacter jejuni supported by respiration of fumarate, nitrate, nitrite, trimethylamine-N-oxide, or dimethyl sulfoxide requires oxygen. J Bacteriol. 2002;184:4187–4196. doi: 10.1128/JB.184.15.4187-4196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. Enzymology and bioenergetics of respiratory nitrite ammonification. FEMS Microbiol Rev. 2002;26:285–309. doi: 10.1111/j.1574-6976.2002.tb00616.x. [DOI] [PubMed] [Google Scholar]
- Simon J, Gross R, Ringel M, Schmidt E, Kröger A. Deletion and site-directed mutagenesis of the Wolinella succinogenes fumarate reductase operon. Eur J Biochem. 1998a;251:418–426. doi: 10.1046/j.1432-1327.1998.2510418.x. [DOI] [PubMed] [Google Scholar]
- Simon J, Gross R, Klimmek O, Ringel M, Kröger A. A periplasmic flavoprotein in Wolinella succinogenes that resembles the fumarate reductase of Shewanella putrefaciens. Arch Microbiol. 1998b;169:424–433. doi: 10.1007/s002030050593. [DOI] [PubMed] [Google Scholar]
- Simon J, Gross R, Einsle O, Kroneck PMH, Kröger A, Klimmek O. A NapC/NirT-type cytochrome c (NrfH) is the mediator between the quinone pool and the cytochrome c nitrite reductase of Wolinella succinogenes. Mol Microbiol. 2000;35:686–696. doi: 10.1046/j.1365-2958.2000.01742.x. [DOI] [PubMed] [Google Scholar]
- Simon J, Pisa R, Stein T, Eichler R, Klimmek O, Gross R. The tetraheme cytochrome c NrfH is required to anchor the cytochrome c nitrite reductase (NrfA) in the membrane of Wolinella succinogenes. Eur J Biochem. 2001;268:5776–5782. doi: 10.1046/j.0014-2956.2001.02520.x. [DOI] [PubMed] [Google Scholar]
- Simon J, Eichler R, Pisa R, Biel S, Gross R. Modification of heme c binding motifs in the small subunit (NrfH) of the Wolinella succinogenes cytochrome c nitrite reductase complex. FEBS Lett. 2002;522:83–87. doi: 10.1016/s0014-5793(02)02885-5. [DOI] [PubMed] [Google Scholar]
- Simon J, Sänger M, Schuster SC, Gross R. Electron transport to periplasmic nitrate reductase (NapA) of Wolinella succinogenes is independent of a NapC protein. Mol Microbiol. 2003;49:69–79. doi: 10.1046/j.1365-2958.2003.03544.x. [DOI] [PubMed] [Google Scholar]
- Simon J, Einsle O, Kroneck PMH, Zumft WG. The unprecedented nos gene cluster of Wolinella succinogenes encodes a novel respiratory electron transfer pathway to cytochrome c nitrous oxide reductase. FEBS Lett. 2004;569:7–12. doi: 10.1016/j.febslet.2004.05.060. [DOI] [PubMed] [Google Scholar]
- Simon J, van Spanning RJM, Richardson DJ. The organisation of proton motive and non-proton motive redox loops in prokaryotic respiratory systems. Biochim Biophys Acta. 2008;1777:1480–1490. doi: 10.1016/j.bbabio.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Stevens JM, Daltrop O, Allen JWA, Ferguson SJ. C-type cytochrome formation: chemical and biological enigmas. Acc Chem Res. 2004;37:999–1007. doi: 10.1021/ar030266l. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hollocher TC. The reaction of reduced cytochromes c with nitrous oxide reductase of Wolinella succinogenes. Biochim Biophys Acta. 1993;1142:253–261. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Nucleotide primers used for the construction of E. coli plasmids serving in the production of W. succinogenes CCHLs, cytochromes c (Ws0700; Ws1182) and CycC variants.
Table S2. Nucleotide primers used for the construction of plasmids for W. succinogenes transformation.
Fig. S1. Arrangement of the genes encoding CcsA2 (Ws2159), CcdA (Ws1481) and cytochrome c553 (Ws0700) on the genome of W. succinogenes.
Fig. S2. Partial primary structure alignment (CLUSTAL W) of available NrfA proteins with CX2CH active site HBMs and selected NrfA proteins carrying the typical CX2CK motif.


