Summary
To cope with life in hypersaline environments, halophilic archaeal proteins are enriched in acidic amino acids. This strategy does not, however, offer a response to transient changes in salinity, as would post-translational modifications. To test this hypothesis, N-glycosylation of the Haloferax volcanii S-layer glycoprotein was compared in cells grown in high (3.4 M NaCl) and low (1.75 M NaCl) salt, as was the glycan bound to dolichol phosphate, the lipid upon which the N-linked glycan is assembled. In high salt, S-layer glycoprotein Asn-13 and Asn-83 are modified by a pentasaccharide, while dolichol phosphate is modified by a tetrasaccharide comprising the first four pentasaccharide residues. When the same targets were considered from cells grown in low salt, substantially less pentasaccharide was detected. At the same time, cells grown at low salinity contain dolichol phosphate modified by a distinct tetrasaccharide absent in cells grown at high salinity. The same tetrasaccharide modified S-layer glycoprotein Asn-498 in cells grown in low salt, whereas no glycan decorated this residue in cells grown in the high-salt medium. Thus, in response to changes in environmental salinity, Hfx. volcanii not only modulates the N-linked glycans decorating the S-layer glycoprotein but also the sites of such post-translational modification.
Introduction
In Haloferax volcanii, a halophilic archaeon first isolated from the Dead Sea (Mullakhanbhai and Larsen, 1975), the products of agl (archaeal glycosylation) genes are responsible for N-glycosylation of the surface (S)-layer glycoprotein, a robust reporter of this post-translational modification in this species. AglJ, AglG, AglI and AglE act sequentially to charge a common dolichol phosphate carrier with the first four sugar residues of the pentasaccharide attached to select S-layer glycoprotein Asn residues (Abu-Qarn et al., 2008; Yurist-Doutsch et al., 2008; Guan et al., 2010; Kaminski et al., 2010), while AglD adds the final pentasaccharide residue to a distinct dolichol phosphate (Abu-Qarn et al., 2007; Guan et al., 2010). In addition to these glycosyltransferases, the oligosaccharyl-transferase, AglB (Abu-Qarn et al., 2007), and the sugar-processing enzymes, AglF, AglM and AglP (Yurist-Doutsch et al., 2008; 2010; Magidovich et al., 2010), also contribute to the biogenesis of the pentasaccharide, comprising a hexose, two hexuronic acids, the methyl ester of hexuronic acid and mannose, N-linked to the S-layer glycoprotein (Abu-Qarn et al., 2007; Magidovich et al., 2010).
The fact that cells lacking AglB, and hence unable to perform N-glycosylation, are viable implies that in Hfx. volcanii, this post-translational modification is not essential for survival, at least under the conditions tested, namely growth in complete medium at 40°C (Abu-Qarn et al., 2007). Still, the absence or even perturbation of N-glycosylation compromises the ability of Hfx. volcanii to grow in high salt (Abu-Qarn et al., 2007), S-layer stability and architecture (Abu-Qarn et al., 2007) and S-layer resistance to added protease (Yurist-Doutsch et al., 2008; 2010; Kaminski et al., 2010). Thus, although not needed for viability, N-glycosylation is, nonetheless, advantageous to Hfx. volcanii in certain situations. Accordingly, quantitative PCR studies addressing a limited number of agl genes revealed the coordinated up- or downregulation of transcription of several (but not all) of these sequences in response to changes in the growth environment (namely, salinity and temperature) or growth phase, relative to the levels of transcription of the same genes in cells grown to mid-exponential phase under standard laboratory conditions (Yurist-Doutsch et al., 2008; 2010). This, in turn, raises the possibility that the N-linked glycan profile of Hfx. volcanii glycoproteins, such as the S-layer glycoprotein, is modified in response to changes in growth conditions.
In the original strain description (Mullakhanbhai and Larsen, 1975), Hfx. volcanii was reported to grow in media containing from 1 M to over 4 M NaCl. To date, our studies aimed at deciphering the Hfx. volcanii pathway responsible for the modification of the S-layer glycoprotein by an N-linked pentasaccharide have been conducted on cells grown in medium containing 3.4 M NaCl. In the present study, N-glycosylation of the S-layer glycoprotein was revisited in Hfx. volcanii cells grown in the presence of only 1.75 M NaCl. We report that in response to changes in the salinity of the growth medium, both dolichol phosphate and the S-layer glycoprotein are decorated by different glycans. Moreover, we reveal differences in the position of S-layer glycoprotein N-linked glycans as a function of medium salinity. These findings thus provide support for the concept that Hfx. volcanii can adapt to changes in its environment by modulating its N-glycosylation profile.
Results
Hfx. volcanii grown at different salinities present distinct glycan-charged dolichol phosphates
As a first step in determining whether the Hfx. volcanii S-layer glycoprotein undergoes differential N-glycosylation as a function of growth medium salinity, the glycan-charged dolichol phosphate pools from cells grown in the presence of either 3.4 or 1.75 M NaCl were examined by normal-phase liquid chromatography-electrospray ionization/mass spectrometry (LC-ESI/MS).
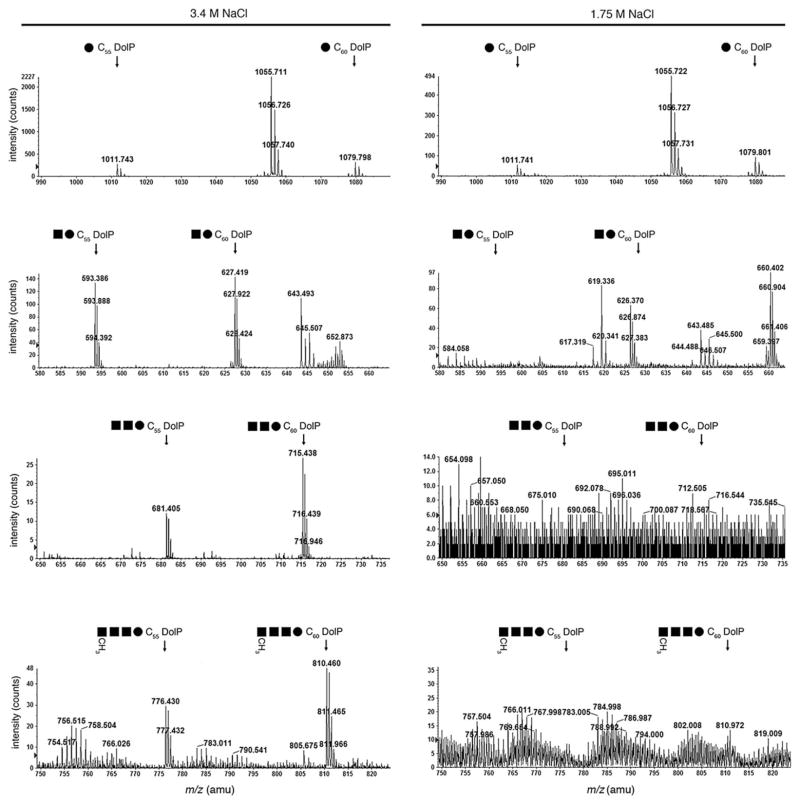
Previous efforts had shown that the pentasaccharide ultimately N-linked to the S-layer glycoprotein of Hfx. volcanii cells grown in 3.4 M NaCl-containing medium is initially assembled on two distinct (C55 and C60) dolichol phosphates (Guan et al., 2010). Specifically, the first four residues of the pentasaccharide (i.e. hexose, two hexu-ronic acids and the methyl ester of hexuronic acid) are sequentially added to a common dolichol phosphate, while the final pentasaccharide residue, mannose, is derived from a distinct dolichol phosphate carrier. Accordingly, in agreement with these earlier studies, LC-ESI/MS analysis of the total lipid extract of cells grown in 3.4 M NaCl-containing medium reveals monoisotopic [M-H]− ion peaks detected at m/z 1011.743 and 1079.798, respectively, corresponding to C55 and C60 dolichol phosphate modified by a hexose (Fig. 1, left column, top panel). In addition, monoisotopic [M-2H]2− ion peaks were detected at m/z 593.386 and 627.419 (Fig. 1, left column, second panel), at m/z 681.405 and 715.438 (third panel) and at m/z 776.430 and 810.460 (bottom panel), respectively, corresponding to C55 and C60 dolichol phosphate modified by a disaccharide (hexose-hexuronic acid), a trisac-charide (hexose-hexuronic acid-hexuronic acid) and a tetrasaccharide (hexose-hexuronic acid-hexuronic acid-methyl ester of hexuronic acid).
Fig. 1.
Dolichol phosphate is differentially modified by a previously described tetrasaccharide and its precursors in Hfx. volcanii cells grown in 3.4 or 1.75 M NaCl. The positions of hexose- (15.8–16.8 min retention time; top pair of panels), hexose-hexuronic acid- (20.5–21.5 min retention time; second pair of panels), hexose-dihexuronic acid- (26.0–27.0 min retention time; third pair of panels) and hexose-dihexuronic acid-methyl ester of hexuronic acid-modified (35.3–36.0 min retention time; bottom pair of panels) C55 and C60 dolichol phosphate from cells grown in medium containing 3.4 M NaCl (left column) or 1.75 M NaCl (right column), as revealed by LC-ESI/MS, are shown. In each case, the positions of the monoisotopic [M-2H]2− dolichol phosphate-based ion peaks are indicated. Symbols used: filled circles, hexose; filled squares, hexuronic acid.
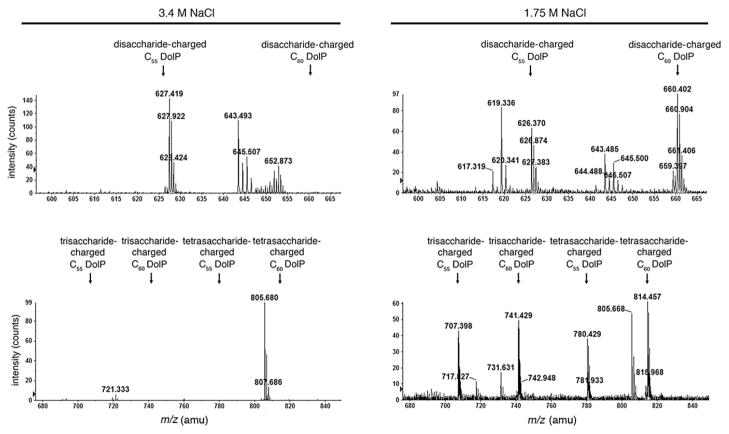
Although monoisotopic [M-H]− ion peaks corresponding to C55 and C60 dolichol phosphate modified by a hexose were also detected in the total lipid extract of cells grown in 1.75 M NaCl-containing medium (m/z 1011.7 and 1079.8 respectively; Fig. 1, right column, upper panel), the glycan-charged dolichol phosphate pool of cells grown in 1.75 M NaCl-containing medium differed from what was obtained from cells raised in 3.4 M NaCl-containing medium. Specifically, no hexose-hexuronic acid or hexose-hexuronic acid-hexuronic acid-modified C55 and C60 dolichol phosphate was obtained from cells grown in 1.75 M NaCl-containing medium (Fig. 1, right column, second and third panels respectively). A small amount of hexose-hexuronic acid-hexuronic acid-methyl ester of hexuronic acid-modified C60 dolichol phosphate was, however, detected at m/z 810.46 (Fig. 1, right column, bottom panel). At the same time, Hfx. volcanii cells grown in 1.75 M NaCl-containing medium presented monoiso-topic [M-2H]2− ion peaks at m/z 626.37 and 660.402 (Fig. 2, right column, upper panel), at m/z 707.398 and 741.429 and at m/z 780.429 and 814.457 (Fig. 2, right column, lower panel). None of these peaks was detected in the total lipid extract derived from cells grown in 3.4 M NaCl-containing medium (Fig. 2, left column).
Fig. 2.
Dolichol phosphate is modified by a putative distinct tetrasaccharide in Hfx. volcanii cells grown in 1.75 M NaCl. The positions of putative disaccharide- (20.5–21.5 min retention time; top pair of panels), trisaccharide- and tetrasaccharide-modified (23.5–24.5 min retention time; bottom pair of panels) C55 and C60 dolichol phosphate from cells grown in medium containing 3.4 M NaCl (left column) or 1.75 M NaCl (right column), as revealed by LC-ESI/MS, are shown. In each case, the positions of the monoisotopic [M-2H]2− dolichol phosphate-based ion peaks are indicated.
Dolichol phosphate in Hfx. volcanii cultured in low-salt medium is modified by a distinct tetrasaccharide
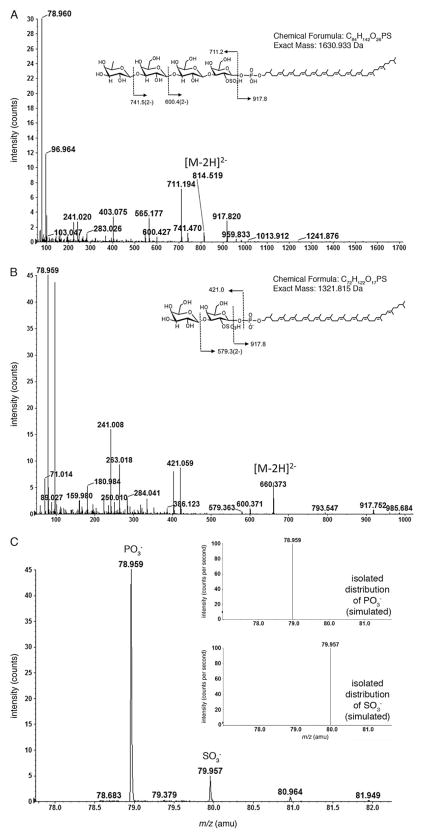
In an earlier analysis of dolichol phosphate in Hfx. volcanii cells grown in medium containing 1.25 M NaCl, Kuntz and colleagues (1997) described C55 and C60 dolichol phosphate modified by a tetrasaccharide comprising a sulfated or phosphorylated hexose, two hexoses and the deoxy-hexose, rhamnose. To determine whether decoration of dolichol phosphate by this tetrasaccharide and its precursors in cells could account for the dolichol phosphate-based species found in cells grown in medium containing 1.75 M NaCl observed in the present study, MS/MS was performed. As shown in Fig. 3A, fragmentation of the [M-2H]2− ion peak at m/z 814.457 yielded a product ion peak at m/z 917.8, corresponding to dolichol phosphate, doubly charged− ion peaks at m/z 741.5, corresponding to the dolichol phosphate modified by the tetrasaccharide reported by Kuntz and colleagues (1997) but lacking a deoxyhexose (146 Da) and at m/z 600.4, corresponding to the tetrasaccharide-modified dolichol phosphate lacking a deoxyhexose and a hexose (308 Da). The fragmentation pattern also included a product ion peak at m/z 711.194, corresponding to a sulfated or phosphorylated hexose, two hexoses and deoxyhexose. Thus, the monoisotopic [M-2H]2− ion peak detected at m/z 814.457 (monoisotopic mass of 1630.930 Da; Fig. 2, right column, lower panel) is in good agreement with calculated mass of C60 dolichol phosphate modified by a tetrasaccharide comprising a sulfated or phosphorylated hexose, two hexoses and rhamnose (1630.933 Da).
Fig. 3. MS/MS confirms that cells grown in 1.75 M NaCl modify dolichol phosphate with a novel tetrasaccharide.
A. Fragmentation of the [M-2H]2− ion peak at m/z 814.457 corresponding to C60 dolichol phosphate modified by a 711.2 Da tetrasaccharide comprising deoxyhexose, two hexoses and a sulfated or phosphorylated hexose.
B. Fragmentation of the [M-2H]2− ion peak at m/z 660.373 corresponding to C60 dolichol phosphate modified by the disaccharide precursor of the tetrasaccharide-modified species examined in (A).
In (A) and (B), the insets show the chemical structure of the glycan-charged C60 dolichol phosphate and the MS/MS fragmentation scheme of the [M-2H]2− ion. In addition, the chemical formula and exact mass are provided.
C. Product ion spectra showing SO3− and PO3− groups released from disaccharide-modified C60 dolichol phosphate from cells grown in 1.75 M NaCl. The inset shows the predicted isotopic distribution of SO3− (upper panel) and PO3− (lower panel) ions.
Similarly, fragmentation of the [M-2H]2− ion peak at m/z 660.373 thought to correspond to C60 dolichol phosphate modified by a disaccharide also yielded a product ion peak at m/z 917.8, corresponding to dolichol phosphate, as well as a [M-2H]2− ion peak at m/z 579.363, corresponding to the product ion resulted from the neutral loss of hexose (162 Da) and a [M-H]− ion peak at m/z 421.059, corresponding to the released disaccharide comprising a hexose and a sulfated or phosphorylated hexose (Fig. 3B). Thus, the monoisotopic [M-2H]2− ion peak detected at m/z 660.402 (i.e. 1320.84 Da; Fig. 2, right column, upper panel) is in good agreement with calculated mass of C60 dolichol phosphate modified by such a disaccharide (1321.815 Da), a precursor of the tetrasaccharide-modified species presented in Fig. 2 (right column, lower panel).
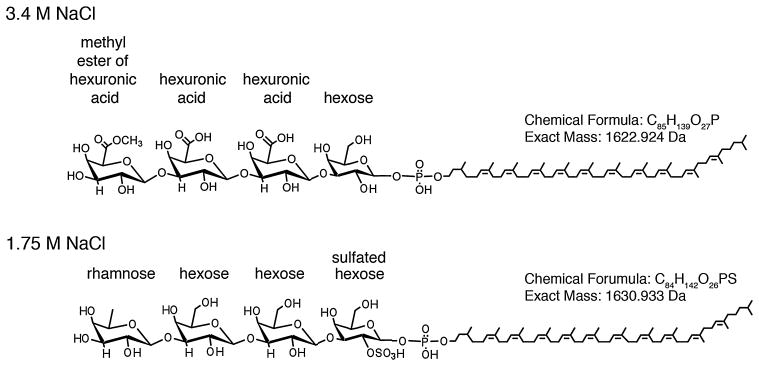
Closer examination of the low-mass ion regions supports the presence of sulfated hexose in the dolichol phosphate-linked glycans seen in the cells grown in 1.75 M NaCl-containing medium. As shown in the product ion spectrum of the C60 dolichol phosphate modified by the disaccharide, peaks at m/z 78.959 for PO3− and m/z 79.957 for SO3− are detected (Fig. 3C, inset, upper and lower panels respectively). The presence of peaks corresponding to both sulfate and phosphate groups in the cell-derived sample is consistent with the phosphorylated nature of the dolichol group and the sulfated nature of the first residue of the dolichol phosphate-linked glycan. Figure 4 shows the chemical structures of the dolichol phosphate-linked tetrasaccharides assembled in cells grown in medium containing 3.4 or 1.75 M NaCl.
Fig. 4.
Chemical structures of the dolichol phosphate-linked tetrasaccharides assembled in cells grown in medium containing 3.4 M NaCl (upper panel) or 1.75 M NaCl (lower panel). In each structure, the identity of each residue is provided, as is the chemical formula and exact mass. Only the tetrasaccharide-modified C60 dolichol phosphate species are depicted.
The Hfx. volcanii S-layer glycoprotein from cells grown at different salt levels is differentially N-glycosylated
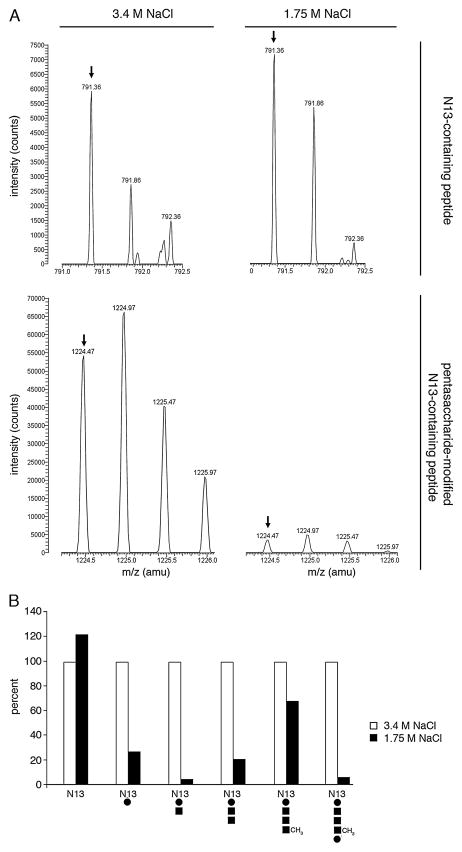
Next, efforts relying on nano LC/MS were directed at determining whether growth of Hfx. volcanii in surroundings of differing salinity also had an effect on S-layer glycoprotein N-glycosylation. Initially, the level of the pen-tasaccharide comprising a hexose, two hexuronic acids, a methyl ester of hexuronic acid and mannose, previously shown to decorate the Asn-13 residue of the trypsin-generated S-layer glycoprotein N-terminal 1ERGNLDAD-SESFNK14 fragment obtained from cells grow in 3.4 M NaCl-containing growth medium (Abu-Qarn et al., 2007), was considered in cells grown in 1.75 M NaCl. Although the samples prepared from cells grown in each medium contained similar levels of the unmodified peptide ([M+2H]2+ ion peak at m/z 791.36; Fig. 5A, upper panels), the pentasaccharide-modified N-terminal tryptic fragment ([M+2H]2+ ion peak at m/z 1224.47) generated from the S-layer glycoprotein of cells grown in 3.4 M NaCl-containing medium was present at a level 15-fold higher than that generated from the S-layer glycoprotein of cells grown in 1.75 M NaCl-containing medium (Fig. 5A, lower panels). Likewise, modification of the N-terminal tryptic fragment generated from the S-layer glycoprotein by the pentasaccharide precursor mono-, di-, tri- and tetrasac-charide glycans was much more pronounced in cells grown in 3.4 M NaCl-containing medium than in 1.75 M NaCl-containing medium (Fig. 5B). Examination of that S-layer glycoprotein fragment generated upon treatment with trypsin and Glu-C protease that includes the modified Asn-83 position (Abu-Qarn et al., 2007) revealed a similar pattern of differential glycosylation of the pentasaccharide and its precursor glycans as a function of medium salinity (not shown).
Fig. 5. The amount of pentasaccharide and its precursors linked to S-layer glycoprotein Asn-13 differs as a function of medium salinity.
A. Comparable amounts of the monoisotopic [M+2H]2+ ion peak (arrow) of a S-layer glycoprotein Asn-13-containing tryptic fragment in cells grown in medium containing 3.4 M NaCl (left column) or 1.75 M NaCl (right column) were detected by LC-ESI/MS (upper pair of panels). The lower pair of panels depicts the monoisotopic [M+2H]2+ ion peaks of the same peptides modified by a previously described pentasaccharide (Abu-Qarn et al., 2007; Magidovich et al., 2010).
B. Histogram showing the relative levels of the S-layer glycoprotein Asn-13-containing tryptic fragment and the same fragment modified by the mono-, di-, tri- and tetrasaccharide pentasaccharide precursors, as well as by the complete pentasaccharide. In each case, the intensity of the peptide derived from the S-layer glycoprotein of cells grown in 3.4 M NaCl-containing medium is considered as 100%. Below each pair of bars, a schematic depiction of peptide N-glycosylation is depicted. Open bars, peptide derived from the S-layer glycoprotein of cells grown in 3.4 M NaCl-containing medium; full bars, peptide derived from the S-layer glycoprotein of cells grown in 1.75 M NaCl-containing medium. N13, Asn-13-containing peptide; full circles, hexose; full squares, hexuronic acid.
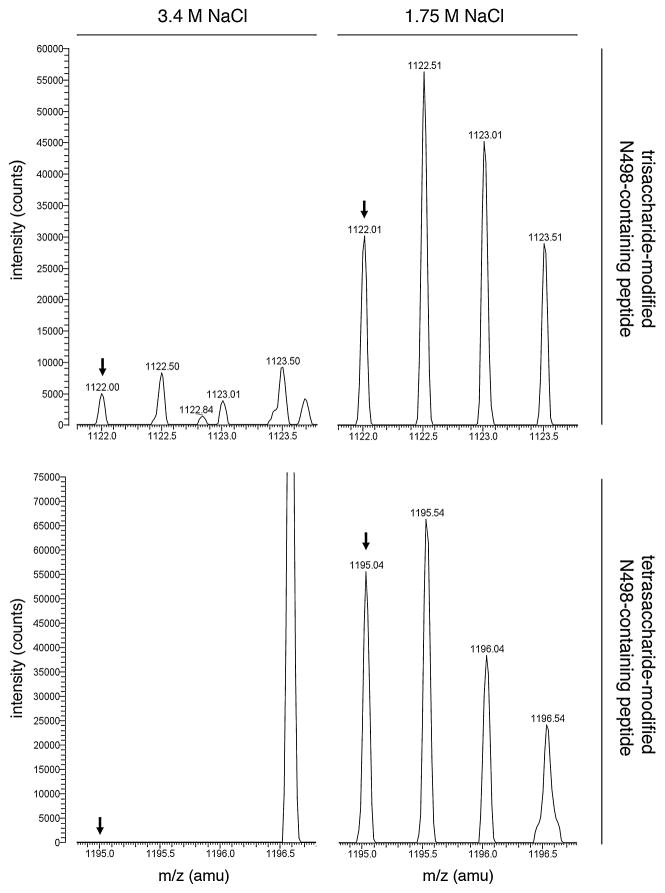
In contrast, LC-ESI/MS analysis of S-layer glycoprotein-derived fragments from cells grown in 1.75 M NaCl-containing medium and generated upon treatment with trypsin and Glu-C protease revealed [M+2H]2+ ion peaks at m/z 1122.01 and 1195.04. These positions of these peaks match those of the predicted doubly charged Asn-498-containing peptide modified by the first three residues of the tetrasaccharide (i.e. sulfated hexose-dihexose) (predicted [M+2H]2+ species, m/z 1121.94) and by the complete tetrasaccharide (predicted [M+2H]2+ species, m/z 1194.94) decorating dolichol phosphate in cells grown in low salinity (Fig. 6, right columns, top and bottom panels respectively). When the same positions were considered in cells grown in 3.4 M NaCl-containing medium, only a minor peak corresponding to trisaccharide-modified peptide was detected, while no peak corresponding to tetrasaccharide-modified peptide was detected (Fig. 6, left columns, top and bottom panels respectively). At the same time, no modification of this peptide by the N-linked pentasaccharide that predominates in the high-salt medium was detected in cells grown at either level of salinity (not shown).
Fig. 6.
Linkage of ‘low-salinity’ trisaccharide and tetrasaccharide to S-layer glycoprotein Asn-498 differs as a function of medium salinity. A S-layer glycoprotein Asn-498-containing tryptic/GluC-generated fragment in cells grown in medium containing 3.4 M NaCl (left column) or 1.75 M NaCl (right column) is differentially modified by sulfated hexose-dihexose (upper pair of panels) or by the same glycan capped with a rhamnose (lower pair of panels), as detected by LC-ESI/MS. The monoisotopic [M+2H]2+ ion peaks are shown (arrows).
Finally, no modification of S-layer glycoprotein Asn-370 by either glycan was detected either in 3.4 M NaCl-containing medium, as previously reported (Abu-Qarn et al., 2007), or in 1.75 M NaCl-containing medium (not shown).
Discussion
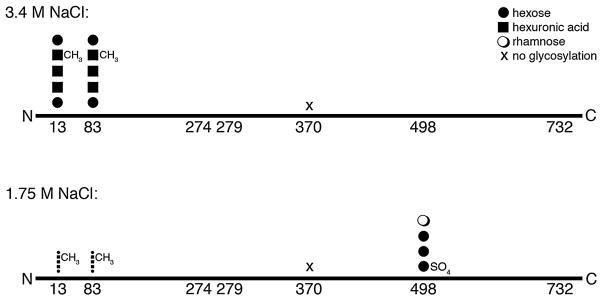
The proteins of extremophilic archaea are designed to remain properly folded, and hence functional, in the face of environmental challenges that would lead to the denaturation, loss of solubility and aggregation of homologous proteins from non-extremophilic organisms. In the case of halophilic archaea, proteins are generally enriched in negatively charged aspartic acids and glutamic acids and present reduced positively charged lysine and arginine content, relative to their non-halophilic counterparts (Lanyi, 1974; Fukuchi et al., 2003). In the folded conformation, haloarchaeal proteins localize the excess acidic residues to the protein surface so as to remain soluble in hypersaline surroundings (Madern et al., 2000; Tadeo et al., 2009). Such permanent changes in amino acid composition do not, however, allow haloarchaeal proteins to adapt to transient changes in environmental salt levels. In contrast, post-translational modification offers the possibility of rapid and reversible adaptation to such variability in surrounding salinity. Accordingly, the present study reports on variability in the glycosylation of dolichol phosphate, the lipid carrier used in Hfx. volcanii N-glycosylation (Guan et al., 2010), as well as on changes in the N-glycosylation of the Hfx. volcanii S-layer glycoprotein, as a function of changes in environmental salt levels. Specifically, it was shown that while dolichol phosphate from cells grown in the presence of 3.4 M NaCl are modified by a tetrasaccharide comprising a hexose, two hexuronic acids and a methyl ester of hexuronic acid, the same target is modified by a distinct tetrasaccharide composed of a sulfated hexose, two hexoses and a rhamnose in cells grown in the presence of 1.75 M NaCl (Fig. 7). This ‘low-salinity’ tetrasac-charide is likely the same glycan bound to dolichol phosphate partially characterized by Kuntz and colleagues (1997) in cells grown in medium containing 1.25 M NaCl. At the same time, this ‘low-salinity’ tet-rasaccharide is N-linked to the S-layer glycoprotein from cells grown in medium containing 1.75 M NaCl, in addition to the minor amounts of that pentasaccharide N-linked to the same protein in 3.4 M NaCl-containing medium. No such tetrasacharide decorates the protein in high-salt conditions.
Fig. 7.
Schematic depiction of the differential N-glycosylation of the Hfx. volcanii S-layer glycoprotein as a function of environmental salinity. The N-glycosylation of S-layer glycoprotein Asn-13, Asn-83 and Asn-498 in 3.4 M and 1.75 M NaCl-containing media are portrayed. Asn-370 is not modified in either case. In the low-salt medium, Asn-13 and Asn-83 are modified with the same glycan as in the high-salt medium, albeit 15-fold less. The possible modification of Asn-274, Asn-279 and Asn-732 was not detected. The identities of the different sugar residues are given in the upper right corner. The N- and C-termini of the protein are also indicated.
In addition to revealing differences in the composition of the N-linked glycans generated by Hfx. volcanii grown at different salt concentrations, the present study also revealed differences in sites of S-layer glycoprotein N-glycosylation at the different levels of salinity considered. Whereas Asn-13 and Asn-83 were both modified by a pentasaccharide at both salt levels tested, approximately 15-fold more pentasaccharide was detected on the S-layer glycoprotein from cells grown in the presence of 3.4 M NaCl than in 1.75 M NaCl-containing medium. More strikingly, however, was the observation that although not modified by the pentasaccharide in high-salt conditions, Asn-498 was modified by the ‘low-salinity’ tetrasaccharide in 1.75 M NaCl-containing medium. As such, it appears that the various S-layer glycoprotein sequons are differentially accessible or selected for N-glycosylation as a function of environmental salinity. Moreover, these results point to the promiscuous nature of AglB, the archaeal oligosaccharyltransferase (Abu-Qarn and Eichler, 2006; Chaban et al., 2006; Igura et al., 2008), given its ability to deliver two very different tetrasaccharides, containing different linking sugars, to distinct sequons of the S-layer glycoprotein.
At present, those enzymes comprising the pathway responsible for biogenesis of the N-linked tetrasaccha-ride decorating the S-layer glycoprotein of cells grown in 1.75 M NaCl-containing medium remain to be identified. It is attractive to speculate that these enzymes are encoded by a set of clustered genes, much like the agl gene cluster responsible for N-glycosylation at higher salinity (Yurist-Doutsch and Eichler, 2009). Indeed, examination of the Hfx. volcanii genome reveals the proximity of several putative protein glycosylation genes, although the involvement of the products of these genes in protein glycosylation remains to be shown. It also remains possible that some Agl proteins are involved in the generation of both glycans N-linked to the S-layer glycoprotein in cells raised at the two levels of salinity tested here. Accordingly, previous studies reported that the transcription of certain agl genes is upregulated in cells grown at the lower salinity (Yurist-Doutsch et al., 2008; 2010). In particular, the finding that transcription of aglM, encoding a UDP-glucose dehydrogenase and shown to produce glucuronic acid in vitro, was not enhanced at the lower level of salinity (Yurist-Doutsch et al., 2010), correlates with the absence of hexuronic acid in the ‘low-salinity’ tetrasaccharide. Detailed qPCR studies designed to identify genes involved in N-glycosylation at the low-salt conditions are in progress. It is also of note that a mixture of the two N-glycosylation profiles did not decorate the S-layer gly-coprotein when cells were raised at ‘intermediate salinity’, namely in medium containing 2.6 M NaCl. Further studies addressing N-glycosylation of the S-layer glyco-protein grown in the range between 1.75 and 2.6 M NaCl will serve to determine whether those genes involved in generating the different N-glycosylation pro-files reported here can be simultaneously activated at intermediate salinity. Finally, the differential glycosylation observed here is apparently not related to growth phase, as comparable results as reported here for cells in logarithmic phase were also observed for cells in stationary phase (not shown).
Whereas N-glycosylated proteins have been identified in a variety of Archaea living across a broad range of environmental niches, the functions of the archaeal version of this post-translational modification remain largely unknown. Indeed, as aglB can be deleted from both Hfx. volcanii and Methanococcus voltae (Abu-Qarn and Eichler, 2006; Chaban et al., 2006), it would seem that N-glycosylation is not essential for archaeal viability, at least under the conditions tested. Still, N-glycosylation may contribute to the ability of Archaea to survive or adapt to the harsh environments in which they exist. Compromised M. voltae flagellin N-glycosylation reduced flagella numbers, concomitant with defects in motility (Chaban et al., 2006). In addition to these permanent roles, transient roles for N-glycosylation had been proposed in the past. In Methanothermus sociabilis, a role for N-glycosylation in cell aggregation has been suggested (Kärcher et al., 1993), while glycosylation of Methanospir-illum hungatei flagellins was reported to only occur in low phosphate media (Southam et al., 1990). In Hfx. volcanii, previous efforts had shown N-glycosylation to regulate S-layer architecture and stability (Abu-Qarn et al., 2007), the ability to grow in elevated salt concentrations (Abu-Qarn et al., 2007) and to resist proteolytic attack (Yurist-Doutsch et al., 2008; 2010). The finding that N-glycosylation offers Hfx. volcanii a means with which to respond to changes in the salinity of its environment now offers direct proof for the adaptive role of this post-translational modification in Archaea.
Still, it is unclear how such differential N-glycosylation of the S-layer glycoprotein allows Hfx. volcanii to adapt to these changes in its surroundings. Earlier studies comparing the N-linked glycan profiles of the S-layer glyco-proteins from Hfx. volcanii and Halobacterium salinarum attributed the ability of Hbt. salinarum to grow at higher salt concentrations to the more highly sulfated, and hence more negatively charged nature of the N-linked glycans decorating the protein, relative to Hfx. volcanii (Mengele and Sumper, 1992). The findings reported here, namely that the N-linked tetrasaccharide decorating the Hfx. vol-canii S-layer glycoprotein in low salt is sulfated, whereas the pentasaccharide N-linked to the same protein in high salt is not, does not agree with the earlier hypothesis. Instead, the accessibility of the Hfx. volcanii S-layer gly-coprotein Asn-498 residue for N-glycosylation in low but not high-salt surroundings suggests that differential salinity affects the folding of the protein. Since the relation between protein folding and N-glycosylation in Archaea has yet to be addressed, the interplay between these post-translational modifications and environmental salinity remains an open question.
Experimental procedures
Strains and growth conditions
Haloferax volcanii WR536 (H53) cells (Allers et al., 2004) were grown in medium containing 3.4 M (high-salt medium) or 1.75 M (low-salt medium) NaCl, 0.15 M MgSO4•7H2O, 1 mM MnCl2, 4 mM KCl, 3 mM CaCl2, 0.3% (w/v) yeast extract, 0.5% (w/v) tryptone, 50 mM Tris-HCl, pH 7.2, at 42°C (Mevarech and Werczberger, 1985).
Liquid chromatography/mass spectrometry (LC/MS) and tandem mass spectrometry (MS/MS)
LC-ESI/MS/MS analysis of a total Hfx. volcanii lipid extract was performed as described previously (Kaminski et al., 2010)
For LC-ESI/MS analysis of Hfx. volcanii S-layer glycoprotein, the protein contents of Hfx. volcanii cells grown in 3.4 M or 1.75 M NaCl-containing medium were separated on 7.5% polyacrylamide gels and stained with Coomassie R-250 (Fluka). For in-gel digestion of the S-layer glycoprotein from each strain, the relevant bands (identified via the unique SDS-PAGE migration and staining pattern of the protein) were excised, destained in 400 μl of 50% (v/v) acetonitrile (Sigma) in 40 mM NH4HCO3, pH 8.4, dehydrated with 100% acetonitrile and dried using a SpeedVac drying apparatus. The S-layer glycoprotein was reduced with 10 mM dithiothreitol (Sigma) in 40 mM NH4HCO3 at 56°C for 60 min and then alkylated for 45 min at room temperature with 55 mM iodoacetamide in 40 mM NH4HCO3. The gel pieces were washed with 40 mM NH4HCO3 for 15 min, dehydrated with 100% acetonitrile, and SpeedVac dried. The gel slices were rehydrated with 12.5 ng μl−1 of mass spectrometry (MS)-grade Trypsin Gold (Promega) in 40 mM NH4HCO3. The peptides were extracted with 0.1% (v/v) formic acid in 20 mM NH4HCO3, followed by sonication for 20 min at room temperature, dehydration with 50% (v/v) acetonitrile and additional sonication. After three rounds of extraction, the gel pieces were dehydrated with 100% acetonitrile and dried completely with a SpeedVac. In some cases, further digestion was carried upon addition of 12.5 ng μl−1 Glu-C (V8) protease (Promega, sequencing-grade). Following incubation, the samples were dried with a SpeedVac. The digested peptide mixtures were resuspended in 5% (v/v) acetonitrile containing 1% formic acid (v/v) and infused into the mass spectrometer using static nanospray Econotips (New Objective, Woburn, MA). The protein digests were separated on line by nano-flow reverse-phase liquid chromatography (LC) by loading onto a 150 mm by 75 μm (internal diameter) by 365 μm (external diameter) Jupifer prepacked fused silica 5 μm C18 300 Å reverse-phase column (Thermo Fisher Scientific, Bremen, Germany). The sample was eluted into the LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific) using a 60 min linear gradient of 0.1% formic acid (v/v) in acetonitrile/0.1% formic acid (1:19, by volume) to 0.1% formic acid in acetonitrile/0.1% formic acid (4:1, by volume) at a flow rate of 300 nl min−1. The full MS range was between m/z 400 and 2000.
Acknowledgments
J.E. is supported by the Israel Science Foundation (Grant 30/07). The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z.G. are supported by the LIPID MAPS Large Scale Collaborative Grant No. GM-069338 from NIH. S.N. is the recipient of a Negev-Zin Associates Scholarship.
References
- Abu-Qarn M, Eichler J. Protein N-glycosylation in Archaea: defining Haloferax volcanii genes involved in S-layer glycoprotein glycosylation. Mol Microbiol. 2006;61:511–525. doi: 10.1111/j.1365-2958.2006.05252.x. [DOI] [PubMed] [Google Scholar]
- Abu-Qarn M, Yurist-Doutsch S, Giordano A, Trauner A, Morris HR, Hitchen P, et al. Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. J Mol Biol. 2007;14:1224–1236. doi: 10.1016/j.jmb.2007.10.042. [DOI] [PubMed] [Google Scholar]
- Abu-Qarn M, Giordano A, Battaglia F, Trauner A, Morris HR, Hitchen P, et al. Identification of AglE, a second glycosyltransferase involved in N-glycosylation of the Haloferax volcanii S-layer glycoprotein. J Bacteriol. 2008;190:3140–3146. doi: 10.1128/JB.00056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers T, Ngo HP, Mevarech M, Lloyd RG. Development of additional selectable markers for the halo-philic archaeon Haloferax volcanii based on the leuB and trpA genes. Appl Environ Microbiol. 2004;70:943–953. doi: 10.1128/AEM.70.2.943-953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban B, Voisin S, Kelly J, Logan SM, Jarrell KF. Identification of genes involved in the biosynthesis and attachment of Methanococcus voltae N-linked glycans: insight into N-linked glycosylation pathways in Archaea. Mol Microbiol. 2006;61:259–268. doi: 10.1111/j.1365-2958.2006.05226.x. [DOI] [PubMed] [Google Scholar]
- Fukuchi S, Yoshimune K, Wakayama M, Moriguchi M, Nishikawa K. Unique amino acid composition of proteins in halophilic bacteria. J Mol Biol. 2003;327:347–357. doi: 10.1016/s0022-2836(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Guan Z, Naparstek S, Kaminski L, Konrad Z, Eichler J. Distinct glycan-charged phosphodolichol carriers are required for the assembly of the pentasaccha-ride N-linked to the Haloferax volcanii S-layer glycoprotein. Mol Microbiol. 2010;78:1294–1303. doi: 10.1111/j.1365-2958.2010.07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igura M, Maita N, Kamishikiryo J, Yamada M, Obita T, Maenaka K, Kohda D. Structure-guided iden-tification of a new catalytic motif of oligosaccharyltrans-ferase. EMBO J. 2008;27:234–243. doi: 10.1038/sj.emboj.7601940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski L, Abu-Qarn M, Guan Z, Naparstek S, Ventura VV, Raetz CRH, et al. AglJ adds the first sugar of the N-linked pentasaccharide decorating the Haloferax volcanii S-layer glycoprotein. J Bacteriol. 2010;192:5572–5579. doi: 10.1128/JB.00705-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärcher U, Schröder H, Haslinger E, Allmaier G, Schreiner R, Wieland F, et al. Primary structure of the heterosaccharide of the surface glycoprotein of Metha-nothermus fervidus. J Biol Chem. 1993;268:26821–26826. [PubMed] [Google Scholar]
- Kuntz C, Sonnenbichler J, Sonnenbichler I, Sumper M, Zeitler R. Isolation and characterization of dolichol-linked oligosaccharides from Haloferax volcanii. Glycobiology. 1997;7:897–904. doi: 10.1093/glycob/7.7.897. [DOI] [PubMed] [Google Scholar]
- Lanyi JK. Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriol Rev. 1974;38:272–290. doi: 10.1128/br.38.3.272-290.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madern D, Ebel C, Zaccai G. Halophilic adaptation of enzymes. Extremophiles. 2000;4:91–98. doi: 10.1007/s007920050142. [DOI] [PubMed] [Google Scholar]
- Magidovich H, Yurist-Doutsch S, Konrad Z, Ventura VV, Hitchen PG, Dell A, Eichler J. AglP is a S-adenosyl-L-methionine-dependent methyltransferase that participates in the N-glycosylation pathway of Halof-erax volcanii. Mol Microbiol. 2010;76:190–199. doi: 10.1111/j.1365-2958.2010.07090.x. [DOI] [PubMed] [Google Scholar]
- Mengele R, Sumper M. Drastic differences in glycosylation of related S-layer glycoproteins from moderate and extreme halophiles. J Biol Chem. 1992;267:8182–8185. [PubMed] [Google Scholar]
- Mevarech M, Werczberger R. Genetic transfer in Halobacterium volcanii. J Bacteriol. 1985;162:461–462. doi: 10.1128/jb.162.1.461-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullakhanbhai MF, Larsen H. Halobacterium volcanii spec. nov., a Dead Sea halobacterium with a moderate salt requirement. Arch Microbiol. 1975;104:207–214. doi: 10.1007/BF00447326. [DOI] [PubMed] [Google Scholar]
- Southam G, Kalmoko ML, Jarrell KF, Koval SF, Beveridge TJ. Isolation, characterization and cellular insertion of the flagella from two strains of the archae-bacterium Methanospirillum hungatei. J Bacteriol. 1990;172:3221–3228. doi: 10.1128/jb.172.6.3221-3228.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadeo X, López-Méndez B, Trigueros T, Laín A, Castaño D, Millet O. Structural basis for the aminoacid composition of proteins from halophilic archaea. PLoS Biol. 2009;12:e1000257. doi: 10.1371/journal.pbio.1000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurist-Doutsch S, Eichler J. Manual annotation, transcriptional analysis and protein expression studies reveal novel genes in the agl cluster responsible for N-glycosylation in the halophilic archaeon Haloferax volca-nii. J Bacteriol. 2009;191:3068–3075. doi: 10.1128/JB.01838-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurist-Doutsch S, Abu-Qarn M, Battaglia F, Morris HR, Hitchen PG, Dell A, Eichler J. aglF, aglG and aglI, novel members of a gene cluster involved in the N-glycosylation of the Haloferax volcanii S-layer glycopro-tein. Mol Microbiol. 2008;69:1234–1245. doi: 10.1111/j.1365-2958.2008.06352.x. [DOI] [PubMed] [Google Scholar]
- Yurist-Doutsch S, Magidovich H, Ventura VV, Hitchen PG, Dell A, Eichler J. N-glycosylation in Archaea: on the coordinated actions of Haloferax volcanii AglF and AglM. Mol Microbiol. 2010;75:1047–1058. doi: 10.1111/j.1365-2958.2009.07045.x. [DOI] [PubMed] [Google Scholar]