The emergence of BioMEMS (Biological MicroElectroMechanical Systems) technology has been spurred by the development of precise microscale fabrication techniques across a wide range of biomaterials substrates. BioMEMS devices are now finding application in areas ranging from genomics and proteomics to clinical diagnostics and implantable drug delivery systems. The design principles of early lab-on-a-chip devices for point-of-care diagnostics are now being extended to platforms for tissue engineering.
The simplicity, ease, and low cost of BioMEMS fabrication techniques have been principal drivers in advancing the technology in applications ranging from the development of in vitro tissue models for drug discovery and toxicity screening to artificial organ assist devices and implantable tissue constructs. However, it is the capability of BioMEMS processing techniques to work with an enormous range of biomaterial substrates that has enabled the technology to advance rapidly in many tissue-engineering applications. BioMEMS devices can be built to mimic the mechanical and biochemical microenvironment of tissues and organs, and this is easily achieved by controlling the scaffold properties: elastic modulus, porosity, internal architecture, and the rates of mass transport and material biodegradation.
In this review, we describe four interesting applications of BioMEMS technology in tissue engineering: the formation of vascular networks, engineering vascular beds for (whole organs liver, kidney and lung), microfluidic bioreactors for screening stem cells, and the establishment of patterned tissue interfaces. These techniques may enable replication of the tissue and organ microenvironment on a small scale.
Engineering Vascular Networks
One of the most significant challenges in the field of tissue engineering has been to provide an intrinsic vascular supply necessary to maintain the viability and well-being of tissues at a meaningful scale for clinical use [1]. Early efforts to generate tissue constructs for highly metabolic organs such as the heart, or organs with very low oxygen diffusion coefficients such as the liver, resulted in the formation of necrotic regions as the thickness of the engineered tissue increased above just a few hundred micrometers. Therefore strenuous efforts have been focused on methods to replicate the highly efficient oxygen and nutrient transfer of physiologic tissues using a host of microfabrication techniques.
Microfluidic approaches for vascularization
Vascular networks for engineered tissues have been produced using growth factors, either singly or in combination, as functionalized moieties in a variety of scaffold materials. Early work by Richardson et al. [2] demonstrated that spatiotemporal control of growth factors introduced in a stepwise fashion in a poly(lactic co-glycolic acid) (PLGA) scaffold resulted in the formation of stable microvascular networks. A functional vasculature formed from human embryonic stem cells (hESC) in a poly(lactic acid) (PLA) based scaffold was demonstrated by Levenberg et al. for generation of vascularized skeletal muscle tissue [3].
More recently, the focus has shifted towards hydrogel matrices, where co-culture of endothelial cells with tissue-specific progenitor cells has produced microvascular networks. For example, micromolded collagen gels have been seeded with endothelial cells with establishment of barrier function and resistance to leukocyte adhesion [4].
One of the principal challenges associated with BioMEMS-based microvascular networks is the inherently planar nature of MEMS fabrication techniques, leading to networks comprised of stacks of two-dimensional capillary beds connected only by large conduits in the vertical dimension. This shortcoming has been addressed by two interesting and potentially very powerful techniques capable of generating branching networks of small channels in all three dimensions. In one case, a sacrificial spun sugar (cotton candy) technique has been developed where a polymer scaffold such as PDMS is cast around the cotton candy fiber network [5], followed by removal of the sacrificial material to create a network of capillary-sized branching channels oriented in all directions. Ugaz [6] has reported the use of electrical discharge in polymer scaffolds to generate a similar vascular-like network architecture.
BioMEMS-based approaches for vascularization
BioMEMS and microfluidic techniques are readily applicable towards the formation of microchannel networks on size scales suitable for engineered vasculature. About 85% of the cross-section of all human vasculature is at the capillary scale, with vessel diameters in the range of 10 µm. These dimensions are easily accessible using conventional photolithographic approaches, while solid freeform methods and conventional precision machining are typically limited to an order of magnitude larger size scales. BioMEMS approaches typically utilize silicon wafer molds as masters to provide multiple replicas in an array of structural biomaterials including the common microfluidic substrate PolyDiMethylSiloxane (PDMS), polystyrene [7] and cyclic olefin copolymer (COC) [8]. The most common technique for patterning a silicon master mold utilizes SU-8 epoxy resin as a thick photolithographic patterning layer upon which the polymeric films can be cast.
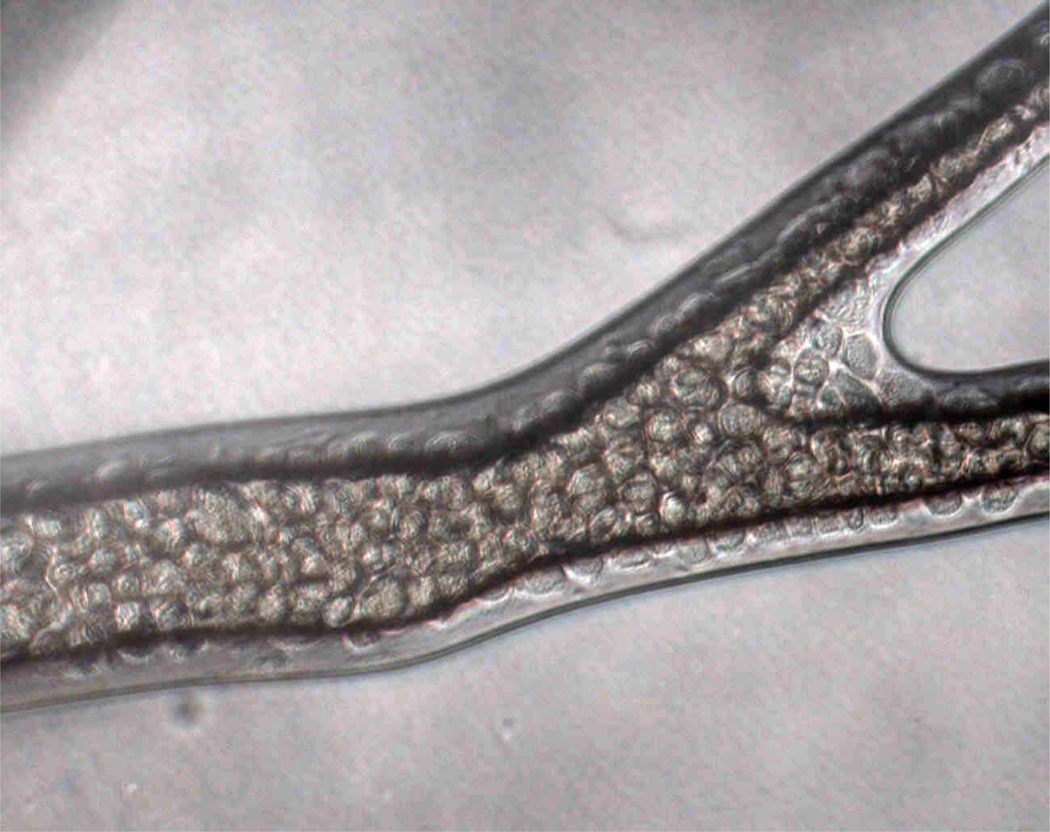
The first endothelialized networks produced using BioMEMS fabrication techniques utilized PDMS as the substrate material [9]. More recently, endothelialized networks have been produced in bioresorbable polymers for implantable applications, such as PolyGlycerol Sebacate (PGS). Figure 1 illustrates an endothelialized network created in a PGS scaffold [10].
Figure 1. Microvascular network.
Endothelialized microvascular network formed in PolyGlycerol Sebacate microfluidic scaffold, showing the formation of a confluent endothelium on the inner luminal surface of the channels.
Mimicking the structure and function of the vasculature
Conventional photolithographic approaches are widely used for lab-on-a-chip applications, but geometric considerations limit their applicability for vascularized tissues. Rectangular walls and sharp corners in microchannel networks lead to difficulties in cell seeding and attachment [11], and result in highly non-uniform circumferential wall shear stress profiles.
Several groups have therefore explored alternative microfabrication techniques capable of producing rounded or circular sidewalls in microchannel networks. One such approach utilizes special resist chemistries for thermally-driven reshaping of the rectangular sidewalls [12]. Etching techniques, typically using xenon difluoride (XeF2) to produce isotropic profiles, have also been used to generate semicircular trenches in silicon wafers for vascular network applications [13]. Modification of rectangular microchannels after replica molding has been recently demonstrated by a process involving liquid PDMS dispensed within the channels, followed by introduction of a pressurized air stream to create rounded sidewalls [14].
While each of these techniques is capable of producing nearly perfect circular sidewalls, a remaining challenge is to vary channel diameter smoothly and gradually when transitioning from one vessel size to another, and at vessel bifurcations. Conventional microfluidic fabrication techniques produce sudden transitions at intersections between vessels and in particular at junctions with varying diameter. One approach that has been explored to address this shortcoming is the use of electroplated molds with smoothly varying geometries [15]. These molds have been used to emboss polystyrene films which are assembled to form a closed microvascular network seeded with endothelial cells [7].
Vascularizing tissues and organs
Microfluidics and BioMEMS techniques for constructing tissue engineering scaffolds and bioreactors are particularly well-suited for applications in which the target organ comprises a highly vascularized structure housing intricate cell and fluid spaces that perform specific fluid processing functions. Therefore the liver, kidney and lung are among the organ systems that can benefit the most from engineered tissue strategies that utilize BioMEMS and microfluidic technologies. In the liver, hepatocytes are arranged with sinusoidal epithelium along fluid pathways in a cylindrical fashion, like spokes of a wheel. In the kidney, the roughly 4 million nephrons are structured along a pathway with fluid filtration and resorption functions integrated into a tubular construct with permeable membranes housing renal epithelial cells. In the lung, the alveoli comprise air spaces from the bronchial tree separated by microvasculature by a membrane only ~ 1 µm in thickness, resulting in enormous surface area and highly efficient gas transfer.
Fabricating Liver Tissue
The liver represents the earliest focus of efforts to apply BioMEMS fabrication technology to the challenge of tissue engineering vital organ constructs. Clinically, tissue engineering of liver has been driven by the fact that unlike the kidney and the lung, where maintenance therapies are available, the only sustainable approach for treating end stage liver failure is an organ transplant. The severe and constantly growing shortage of available donor organs has spurred strenuous efforts to develop tissue engineered liver replacement systems. In addition, there is a critical need for improved systems for assessing drug safety relative to hepatotoxicity, a predominant cause of post-market-approval failures of new compounds. Due to these urgent needs and the complexity of tissue architecture, the liver remains a principal target for BioMEMS technology.
Early efforts to apply BioMEMS technology to the liver focused on in vitro models for drug safety and evaluation of chemical and biological agents. One approach utilized micromachined silicon structures enabling perfusion of the liver at fluid flow and shear stress rates that mimic physiological levels [16]. A soft-lithography-based approach has been established as a screening platform for liver toxicity, where heterotypic interactions between hepatocytes and fibroblasts are governed by patterns on stamped substrates [17]. This approach has been integrated with a silicon micromachined “comb” to control spacing between the two cell populations [18]. Microfluidic PDMS constructs have been reported by Leclerc [19], while a membrane bilayer system has been shown to replicate liver function by Carraro [20].
The Shuler laboratory has developed a microfluidic in vitro model for the liver as part of a “human-on-a-chip” platform [21], while Lee et al. have utilized microfabrication technology to establish a perfusion barrier that mimics the sinusoidal structure in liver [22]. Efforts to develop tissue engineered liver replacements have explored the use of bioresorbable elastomers as a scaffolding material for implantable applications, where the use of nanopatterning to create an in-vivo -like topographic basement membrane has been shown to enhance the function of primary hepatocytes [23].
Microfabricated Devices for Artificial Lung
Pioneering efforts to develop microfabricated devices for artificial lung by utilizing arrays of microchannels constructed using conventional MEMS techniques were reported by Mockros [24] [and Federspiel [25]. Gas transport models for microfluidic constructs have been developed in order to determine whether BioMEMS approaches could achieve superior performance relative to the hollow fiber technology utilized over several decades for applications such as ExtraCorporeal Membrane Oxygenation (ECMO) and cardiopulmonary support for Coronary Artery Bypass Graft (CABG). Federspiel proposed the use of PDMS as a gas permeable membrane separating microchannels from an oxygen delivery compartment, and measured the efficiency of oxygen transfer as a function of membrane thickness.
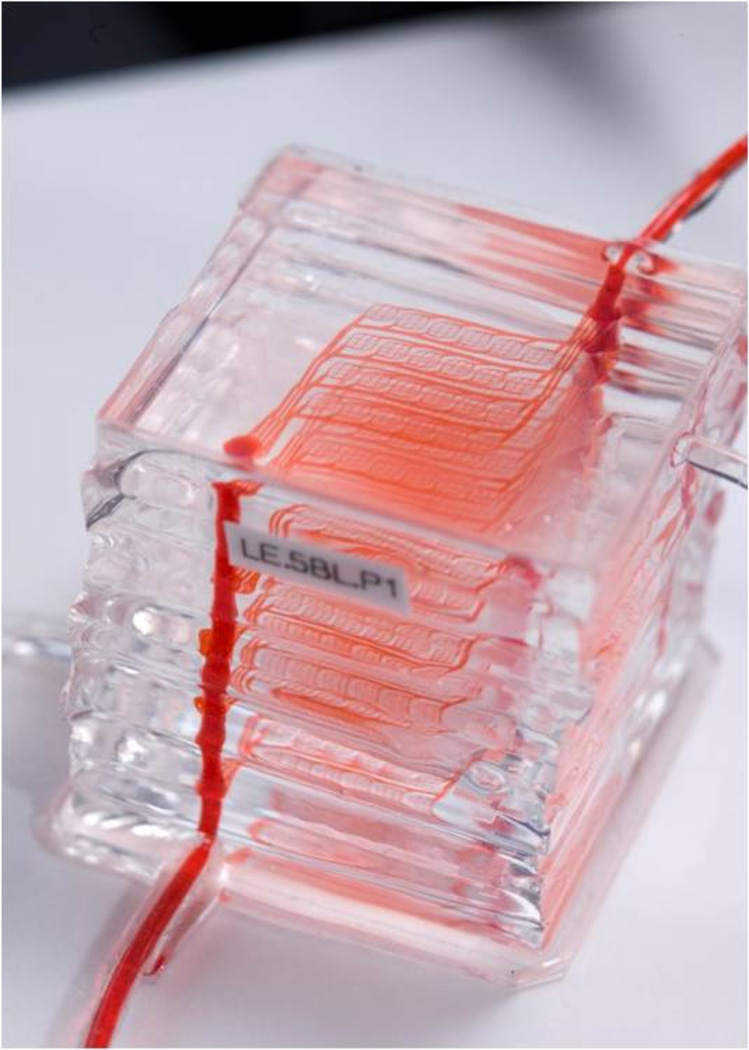
Recent efforts have focused on critical issues associated with the safety and convenience of membrane oxygenator technology. Hoganson et al. [26] have reported on the development of a network with physiological shear stress. Kniazeva [27] demonstrated a microfluidic oxygenator in PDMS comprising multiple layers in a design ultimately scalable for clinical use; one such device is shown in Figure 2. Potkay [28] has reported on a BioMEMS-based artificial lung that may be operated using room air rather than pure oxygen, a major step towards portable and ultimately wearable systems. In vitro models of artificial lung have been utilized to study a range of pulmonary diseases by the Takayama group [29], and Huh et al. have proposed an artificial lung unit device that may be coated with lung epithelial cells for drug discovery applications [30].
Figure 2. Multi-layer PDMS microfluidic oxygenator for artificial lung applications.
Each of the 10 layers of the device comprises a microvascular network and an oxygen delivery compartment separated by a thin (11 µm) membrane.
Developing Artificial Kidneys
Progress in renal replacement systems has been more limited than for liver and lung, in part because of the staggering complexity of the kidney in terms of structure and cellular phenotype. Many efforts to develop artificial kidneys for short-term applications have focused on the use of hollow fibers, the gold standard for kidney dialysis in the clinic and for nocturnal use in the home. Recently, the Suh laboratory [31] developed an in vitro microfluidic model to investigate renal cell attachment, proliferation and glucose uptake. A microfluidic bioreactor for assessment of renal epithelium has been reported [32], and efforts are being made to mimic the slit-like pores in the nephron using silicon microfabrication technology [33].
Microfluidic bioreactors
Human stem cells are central to the development of tissue engineering modalities for regenerative medicine, as well as models for fundamental research, study of disease and drug development. It is well known that, both in vitro and in vivo the cells respond to the entire context of their environment, with spatial and temporal cascades of molecular and physical regulatory factors [34]. Therefore, culture systems that faithfully replicate the “cell niche” found in vivo would radically improve our ability to utilize the full potential of stem cells in tissue engineering [35].
Microtechnologies, and in particular those based on microfluidic platforms, are being increasingly used to precisely manipulate the cellular microenvironment and study cellular responses in real time and in a quantitative fashion. In these applications, the small scale of the device allows for high-throughput studies within a large experimental space with only minimal amounts of cells and materials, as well as precise environmental control due to efficient mass transport to and from the cells.
Microfluidic techniques based on soft lithography, were pioneered by Whitesides and colleagues [36] and have found widespread application in micropatterning of cells and biomaterials [37] and cell culture. For example, in cultures of hepatocytes microscale features of these devices were used to mimic some aspects of the microstructure of liver [38]. In another set of applications, microfluidic platforms of interconnected chambers have been proposed as “living cell arrays” for studying gene expression [39] and cell co-culture [40].
An elegant microfluidic device was developed to culture murine embryonic stem cells (mESCs) with logarithmically scaled perfusion of medium, with more than a 3000-fold variation in flow rates across the array, and study the effects of the associated hydrodynamic shear on the size of cell colonies [41]. Subsequently, a microfluidic platform was developed to study self-renewal and differentiation of hESCs over a large parameter matrix and in a semi-automated way [42].
Microfluidic systems can also be used to spatially and temporally investigate the many factors that regulate cell differentiation in high throughput experiments. Conventional multiwell plates are certainly easy to use, but their limitation is that the composition of medium is constantly changing between medium replacements, thus limiting the control of environmental levels of nutrients and metabolites. On the other had, perfusion bioreactors enable tight control of concentration levels in cell environment, but generally require large amounts of medium and regulatory factors making screening studies impractical and expensive [43]. Microarray bioreactors with medium perfusion were developed to combine small scale with medium perfusion and thereby provide versatile and tightly controlled culture environments for screening of stem cells and factors regulating their differentiation [44,45,46].
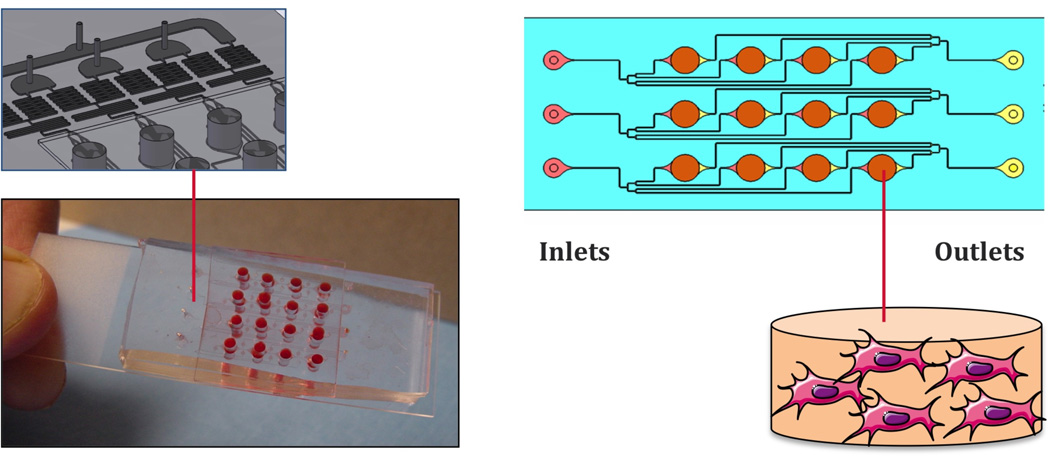
In one set of recent studies [43,47], a simple and practical device was developed by coupling a microfluidic platform with an array of culture wells, to enable systematic and precise variation of mass transport and hydrodynamic shear in cultures of human embryonic stem cells (hESCs) (Figure 3). This microarray bioreactor with twelve culture wells on a standard microscope slide format was fabricated using soft lithography and designed to accommodate stem cells attached to a 2D substrate, and cells encapsulated in 3D hydrogel. Both culture formats allow for controlled perfusion of medium, and tight control of medium composition and hydrodynamic shear. Using this microfluidic platform, hESCs were systematically studied for their cardiovascular differentiation potential, by automated image analysis of the expression of cell differentiation markers. Cell differentiation correlated to the level of hydrodynamic shear and transport rates of oxygen and growth factors, with the aid of computational fluid dynamic (CFD) modeling [47].
Figure 3. Microarray bioreactor for stem cell screening.
A simple microarray bioreactor with twelve culture wells on a standard microscope slide format was fabricated using soft lithography and designed to accommodate human stem cells attached to a 2D substrate or encapsulated in 3D hydrogel. Each individual culture well is independently perfused with culture medium. Cell responses can be analyzed in real time using automated image processing [60, 68].
Patterning tissue interfaces
Another important use of microfluidic technologies is for the formation of concentration gradients of cytokines in cell cultures. In developing tissues, signaling molecules present themselves in form of concentration gradients, which determine the migration, fate specification and maturation of the sensing cells. Spatial gradients of diffusible signaling molecules are therefore of paramount interest both for identifying regulatory pathways and for engineering complex tissue structures starting from undifferentiated cells.
A major advantage of microfluidic devices over other culture systems is in they capability to generate complex and well defined concentration patterns, via tight control of fluid dynamics on a cell level scale. Examples of gradient makers include microfluidic - photopolymerization systems for the formation of graded hydrogels [47], microfluidic devices with substrate-bound molecules [48] and the membrane-based diffusion chips [49,50].
A simple microfluidic platform was designed to generate stable concentration gradients in cell culture space by a combination of osmotic and capillary forces [53]. This system was successfully used to investigate the effects of gradients of Shh, FGF8, and BMP4 on hESC-derived neural progenitors for as long as 8 days. Neural progenitors differentiated into neurons, and connected into a neural network.
Another microfluidic device was designed to generate stable concentration gradients over a field of cells at low hydrodynamic shear, to allow long term culture of adherent cells [51]. To this end, a gradient of Wnt3 was established over a field of cells cultured between two parallel laminar flow streams of culture medium. Wnt3a regulation of β-catenin signaling was chosen as a case study, because of its major role in stem cell proliferation, differentiation and assembly into tissues. The activation of the β-catenin pathway in response to a gradient of Wnt3a was assessed in real time using the expression of GFP at a reporter gene. The exact algorithm for defining the concentration gradients was established with the aid of mathematical modeling of flow and mass transport. This simple and versatile microfluidic platform offers high level of control over single and multiple gradients under cell-protective flow conditions.
Microfluidic platforms are now also used to pattern cell differentiation. In general, the development of transitional interfacial zones between adjacent tissues remains a significant challenge for engineering functional tissue grafts. For example, the interface between bone and soft tissue facilitates transmission of mechanical loads and minimizes stress concentration in junctions. Without adequate tissue interaction in such junctions, conventional soft tissue grafts fail at the bone insertion site. An important goal in tissue engineering is therefore to recapitulate tissue interfaces if the organization of an engineered tissue is to reflect that of the parent native tissue.
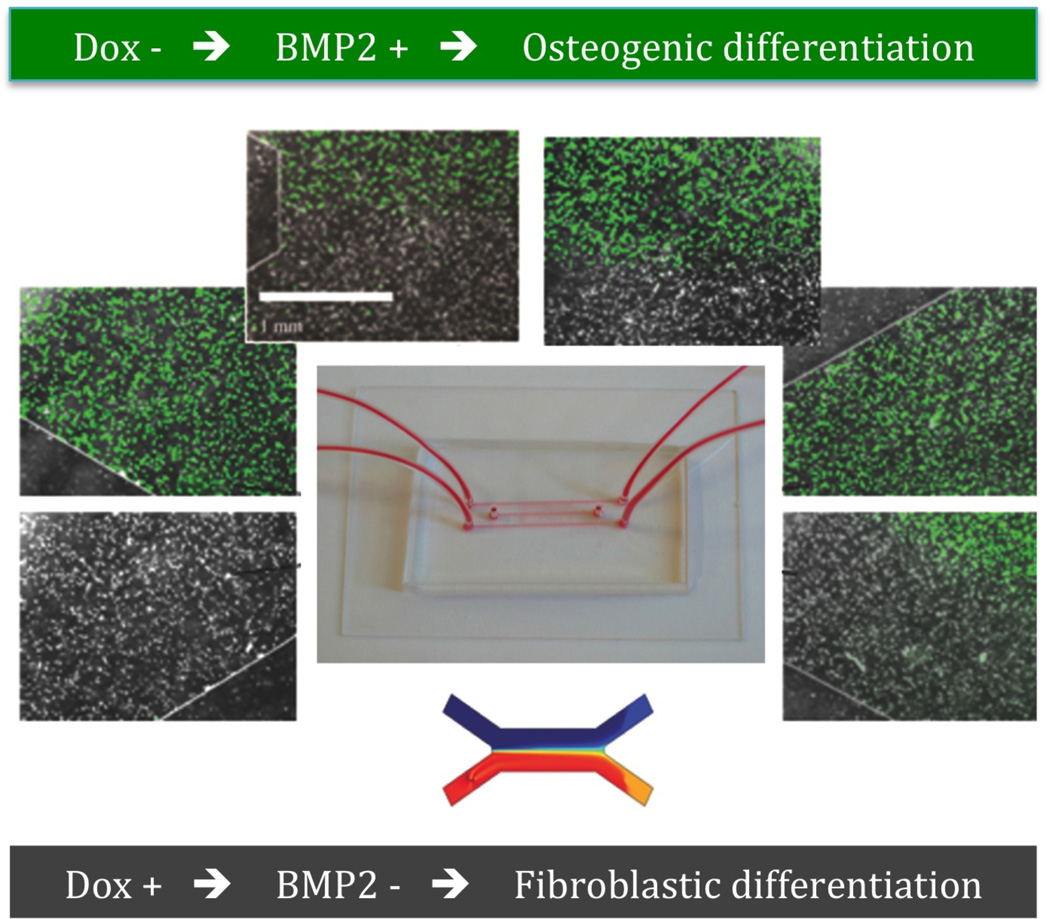
A novel approach to the formation of tissue interfaces was recently proposed, where cell differentiation in patterned by patterning gene expression using a microfluidic platform [52]. The concentration gradients were verified by computational simulation and dye separation experiments. An inducible BMP-2 expressing cell line under the control of Tet-off gene expression system was investigated, because of the efficient control of BMP-2 gene expression achieved by modulating the concentration of BMP-2 expression modulator doxycycline (Dox) (Figure 4). As for the gradient makers described above, the cells (human mesenchymal stem cells, hMSC) were cultured between two laminar streams of culture medium containing different levels of the regulatory molecule, in this case Dox. The regulation of hMCS differentiation is rather straightforward. In the areas exposed to high concentrations of Dox, BMP2 was inactivated and the cells became fibrolastic; in the areas exposed to low concentration of Dox, BMP2 is activated and the cells undergo osteogenic differentiation. A tissue interface in the microfluidic device depended on the gradient of Dox. Therefore, by patterning the delivery of Dox to the cultured cells in laminar flow system, one can effectively pattern the expression of BMP-2 and thereby modulate osteogenic differentiation.
Figure 4. Microfluidic system for patterning a tissue interface.
hMSCs with BMP-2 expression under the control of Tet-off gene were cultured between two laminar streams of culture medium, one of which contained Dox. In the areas exposed to high concentrations of Dox, BMP2 was inactivated and the cells became fibroblastic; in the areas exposed to low concentration of Dox, BMP2 was activated and the cells underwent osteogenic differentiation [73].
Summary
In summary, microfluidic-BioMEMS platforms are increasingly contributing to tissue engineering in many different ways. First, the accurate control of the cell environment, in settings suitable for cell screening, and with imaging compatibility, are greatly advancing our ability to optimize cell sources for a variety of tissue engineering applications. Second, the microfluidic technology is ideal for the formation of perfusable networks, either to study their stability and maturation, or to use these networks as templates for engineering vascularized tissues. Third, the approaches based on microfluidic and BioMEMS devices enable engineering and study of minimally functional modules of complex tissues, such as liver sinusoid, kidney nephron, and lung bronchiole. This mini-review highlighted some of the unique advantages of this elegant technology, using representative examples of tissue engineering research. We focused on some of the universal needs of the area of tissue engineering: tissue vascularization, faithful recapitulation in vitro of functional units of our tissues and organs, and predictable selection and differentiation of stem cells, that are being addressed using the power and versatility of microfluidic-BioMEMS platforms.
Acknowledgments
The authors are grateful for the funding support provided by the NIH (grants HL076485, DE016525, EB002520, and RR026244 to GVN, and grants HL106585-01 and R21RR031253 to JTB).
Contributor Information
Jeffrey T Borenstein, Email: jborenstein@draper.com, Biomedical Engineering Center, Draper Laboratory, Cambridge, MA.
Gordana Vunjak-Novakovic, Email: gv2131@columbia.edu, Biomedical Engineering Department, Columbia University, New York, NY..
References
- 1.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;vol. 354(Suppl 1):SI32–SI34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 2.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;vol. 19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 3.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;vol. 99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;vol. 71:185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Bellan LM, Singh SP, Henderson PW, Porri TJ, Craighead HG, Spector JA. Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter. 2009;vol. 5:1354–1357. [Google Scholar]
- 6.Huang J-H, Kim J, et al. Rapid Fabrication of Bio-inspired 3D Microfluidic Vascular Networks. Advanced Materials. 2009;vol. 21:3567–3571. [Google Scholar]
- 7.Borenstein J, Tupper M, et al. Functional endothelialized microvascular networks with circular cross-sections in a tissue culture substrate. Biomedical Microdevices. 2010;vol. 12:71–79. doi: 10.1007/s10544-009-9361-1. [DOI] [PubMed] [Google Scholar]
- 8.Jeon J, Chung S, Kamm R, Charest J. Hot embossing for fabrication of a microfluidic 3D cell culture platform. Biomedical Microdevices. 2010;vol. 13:325–333. doi: 10.1007/s10544-010-9496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borenstein JT, Terai H, King KR, Weinberg EJ, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication Technology for Vascularized Tissue Engineering. Biomedical Microdevices. 2002;vol. 4:167–175. [Google Scholar]
- 10.Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, Wang Y. Endothelialized Microvasculature Based on a Biodegradable Elastomer. Tissue Engineering. 2005;vol. 11:302–309. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 11.Green JV, Kniazeva T, Abedi M, Sokhey DS, Taslim ME, Murthy SK. Effect of channel geometry on cell adhesion in microfluidic devices. Lab on a Chip. 2009;vol. 9:677–685. doi: 10.1039/b813516a. [DOI] [PubMed] [Google Scholar]
- 12.Wang GJ, Hsueh C-C, Hsu S-H, Hung H-S. Fabrication of PLGA microvessel scaffolds with circular microchannels using soft lithography. J. Micromech. Microeng. 2007;vol. 17:2000–2005. [Google Scholar]
- 13.Camp JP, Stokol T, Shuler ML. Fabrication of a multiple-diameter branched network of microvascular channels with semi-circular cross-sections using xenon difluoride etching. Biomedical Microdevices. 2008;vol. 10:179–186. doi: 10.1007/s10544-007-9123-x. [DOI] [PubMed] [Google Scholar]
- 14.Abdelgawad M, Wu C, Chien WY, Geddie WR, Jewett MA, Sun Y. A fast and simple method to fabricate circular microchannels in polydimethylsiloxane (PDMS) Lab Chip. vol. 11:545–551. doi: 10.1039/c0lc00093k. [DOI] [PubMed] [Google Scholar]
- 15.LaVan DA, George PM, Langer R. Simple, Three-Dimensional Microfabrication of Electrodeposited Structures. Angew. Chem. Int. Ed. 2003;vol. 42:1262–1265. doi: 10.1002/anie.200390323. [DOI] [PubMed] [Google Scholar]
- 16.Powers MJ, Domansky K, et al. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnology and Bioengineering. 2002;vol. 78:257–269. doi: 10.1002/bit.10143. [DOI] [PubMed] [Google Scholar]
- 17.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;vol. 26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 18.Hui EE, Bhatia SN. Micromechanical control of cell-cell interactions. Proc Natl Acad Sci U S A. 2007;vol. 104:5722–5766. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclerc E, Sakai Y, Fujii T. Cell culture in 3-dimensional microfluidic structure of PDMS. Biomed Microdevices. 2003;vol. 5:109–114. [Google Scholar]
- 20.Carraro A, Hsu WM, et al. In vitro analysis of a hepatic device with intrinsic microvascular-based channels. Biomed Microdevices. 2008;vol. 10:795–805. doi: 10.1007/s10544-008-9194-3. [DOI] [PubMed] [Google Scholar]
- 21.Sung JH, Choi JR, Kim D, Shuler ML. Fluorescence optical detection in situ for real-time monitoring of cytochrome P450 enzymatic activity of liver cells in multiple microfluidic devices. Biotechnol Bioeng. 2009;vol. 104:516–525. doi: 10.1002/bit.22413. [DOI] [PubMed] [Google Scholar]
- 22.Lee PJ, Hung PJ, Lee LP. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnology and Bioengineering. 2007;vol. 97:1340–1346. doi: 10.1002/bit.21360. [DOI] [PubMed] [Google Scholar]
- 23.Bettinger CJ, Kulig KM, Vacanti JP, Langer R, Borenstein JT. Nanofabricated collagen-inspired synthetic elastomers for primary rat hepatocyte culture. Tissue Eng Part A. 2009;vol. 15:1321–1329. doi: 10.1089/ten.tea.2008.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JK, Kung MC, Kung HH, Mockros LF. Microchannel Technologies for Artificial Lungs: (3) Open Rectangular Channels. ASAIO Journal. 2008;vol. 54:390–395. doi: 10.1097/MAT.0b013e31817eda02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess K, Hu H-H, Wagner W, Federspiel W. Towards microfabricated biohybrid artificial lung modules for chronic respiratory support. Biomedical Microdevices. 2009;vol. 11:117–127. doi: 10.1007/s10544-008-9215-2. [DOI] [PubMed] [Google Scholar]
- 26.Hoganson DM, Pryor HI, 2nd, Bassett EK, Spool ID, Vacanti JP. Lung assist device technology with physiologic blood flow developed on a tissue engineered scaffold platform. Lab Chip. vol. 11:700–707. doi: 10.1039/c0lc00158a. [DOI] [PubMed] [Google Scholar]
- 27.Kniazeva T, Hsiao JC, Charest JL, Borenstein JT. A microfluidic respiratory assist device with high gas permeance for artificial lung applications. Biomed Microdevices. vol. 13:315–323. doi: 10.1007/s10544-010-9495-1. [DOI] [PubMed] [Google Scholar]
- 28.Potkay JA, Magnetta M, Vinson A, Cmolik B. Bio-inspired, efficient, artificial lung employing air as the ventilating gas. Lab Chip. vol. doi: 10.1039/c1lc20020h. [DOI] [PubMed] [Google Scholar]
- 29.Huh D, Fujioka H, et al. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proceedings of the National Academy of Sciences. 2007;vol. 104:18886–18891. doi: 10.1073/pnas.0610868104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;vol. 328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang K-J, Suh K-Y. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab on a Chip. 2010;vol. 10:36–42. doi: 10.1039/b907515a. [DOI] [PubMed] [Google Scholar]
- 32.Ferrell N, Desai RR, Fleischman AJ, Roy S, Humes HD, Fissell WH. A microfluidic bioreactor with integrated transepithelial electrical resistance (TEER) measurement electrodes for evaluation of renal epithelial cells. Biotechnology and Bioengineering. 2010;vol. 107:707–716. doi: 10.1002/bit.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fissell WH, Dubnisheva A, Eldridge AN, Fleischman AJ, Zydney AL, Roy S. High-Performance Silicon Nanopore Hemofiltration Membranes. J Memb Sci. 2009;vol. 326:58–63. doi: 10.1016/j.memsci.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295:1009. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 35.Vunjak-Novakovic G, Scadden DT. Biomimetic platforms for human stem cell research. Cell Stem Cell. 2011;8:252–261. doi: 10.1016/j.stem.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annual Review of Biomedical Engineering. 2001;3:335. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 37.Bhatia SN, Yarmush ML, Toner M. Controlling cell interactions by micropatterning in co-cultures: hepatocytes and 3T3 fibroblasts. Journal of Biomedical Materials Research. 1997;34:189. doi: 10.1002/(sici)1097-4636(199702)34:2<189::aid-jbm8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 38.Park, Berthiaume F, Toner M, Yarmush ML, Tilles AW. Microfabricated grooved substrates as platforms for bioartificial liver reactors. Biotechnology and Bioengineering. 2005;90:632. doi: 10.1002/bit.20463. [DOI] [PubMed] [Google Scholar]
- 39.Thompson DM, King KR, Wieder KJ, Toner M, Yarmush ML, Jayaraman A. Dynamic gene expression profiling using a microfabricated living cell array. Anal. Chem. 2004;76:4098. doi: 10.1021/ac0354241. [DOI] [PubMed] [Google Scholar]
- 40.Khademhosseini, Yeh J, Eng G, Karp J, Kaji H, Borenstein J, Farokhzad OC, Langer R. Cell docking inside microwells within reversibly sealed microfluidic channels for fabricating multiphenotype cell arrays. Lab on a Chip. 2005;5:1380. doi: 10.1039/b508096g. [DOI] [PubMed] [Google Scholar]
- 41.Kim L, Vahey MD, Lee HY, Voldman J. Microfluidic arrays for logarithmically perfused embryonic stem cell culture. Lab Chip. 2006;6(3):394–406. doi: 10.1039/b511718f. [DOI] [PubMed] [Google Scholar]
- 42.Kamei K, Guo S, Yu ZT, Takahashi H, Gschweng E, Suh C, Wang X, Tang J, McLaughlin J, Witte ON, Lee KB, Tseng HR. An integrated microfluidic culture device for quantitative analysis of human embryonic stem cells. Lab Chip. 2009;9(4):555–563. doi: 10.1039/b809105f. [DOI] [PubMed] [Google Scholar]
- 43.Figallo E, Cannizzaro C, Gerecht-Nir S, Burdick J, Langer R, Elvassore N, Vunjak-Novakovic G. Micro-bioreactor array for controlling cellular environments. Lab on a Chip. 2007;7(6):710–719. doi: 10.1039/b700063d. [DOI] [PubMed] [Google Scholar]
- 44.Beebe DJ, Mensing GA, Walker GM. Physics and applications of microfluidics in biology. Annual Review of Biomedical Engineering. 2002;4:261. doi: 10.1146/annurev.bioeng.4.112601.125916. [DOI] [PubMed] [Google Scholar]
- 45.Chin V, Taupin P, Sanga S, Scheel J, Gage FH, Bhatia SN. Microfabricated platform for studying stem cell fates. Biotechnology and Bioengineering. 2004;88:399. doi: 10.1002/bit.20254. [DOI] [PubMed] [Google Scholar]
- 46.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotech. 2004;22:863. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 47.Cimetta E, Cannizzaro C, Elvasore N, Vunjak-Novakovic G. Microarray bioreactors for steady-state and transient studies of stem cells. Methods. 2009;47:81–89. doi: 10.1016/j.ymeth.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab on a Chip. 2009;9:269–275. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- 49.Diao, Young L, Kim S, Fogarty EA, Heilman SM, Zhou P, Shuler ML, Wu M, Delisa MP. A three-channel microfluidic device for generating static linear gradients and its application to the quantitative analysis of bacterial chemotaxis. Lab on a Chip. 2006;6:381–388. doi: 10.1039/b511958h. [DOI] [PubMed] [Google Scholar]
- 50.Toetsch S, Olwell P, Prina-Mello A, Volkov Y. The evolution of chemotaxis assays from static models to physiologically relevant platforms. Integrative Biology. 2009:170–181. doi: 10.1039/b814567a. [DOI] [PubMed] [Google Scholar]
- 51.Cimetta E, Cannizzaro C, James R, Biechele T, Moon R, Elvassore N, Vunjak-Novakovic G. Microfluidics-generated Wnt-3 gradients induce a proportionate response in b-catenin signalling. Lab on a Chip. 2010;10(23):3277–3283. doi: 10.1039/c0lc00033g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Gazit Z, Pelled G, Gazit D, Vunjak-Novakovic G. Engineering a tissue interface by inducible gene expression. Integrative Biology. 2011;3(1):39–47. doi: 10.1039/c0ib00053a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park JY, Kim SK, Woo DH, Lee EJ, Kim JH, Lee SH. Differentiation of neural progenitor cells in a icrofluidic chip-generated cytokine gradient. Stem Cells. 2009;27(11):2646–2654. doi: 10.1002/stem.202. [DOI] [PubMed] [Google Scholar]