Abstract
Background
Transfection agents comprised of cationic lipid preparations are widely used to transfect cell lines in culture with specific recombinant complementary DNA molecules. We have found that cells in culture are often resistant to stimulation with insulin subsequent to treatment with transfection agents such as LipofectAMINE 2000™ and FuGENE-6™. This is seen with a variety of different readouts, including insulin receptor signalling, glucose uptake into muscle cells, phosphorylation of protein kinase B and reporter gene activity in a variety of different cell types
Results
We now show that this is due in part to the fact that cationic lipid agents activate the insulin receptor fully during typical transfection experiments, which is then down-regulated. In attempts to circumvent this problem, we investigated the effects of increasing concentrations of LipofectAMINE 2000™ on insulin receptor phosphorylation in Chinese hamster ovary cells expressing the human insulin receptor. In addition, the efficiency of transfection that is supported by the same concentrations of transfection reagent was studied by using a green fluorescent protein construct. Our data indicate that considerably lower concentrations of LipofectAMINE 2000™ can be used than are recommended by the manufacturers. This is without sacrificing transfection efficiency markedly and avoids the problem of reducing insulin receptor expression in the cells.
Conclusion
Widely-used cationic lipid transfection reagents cause a state of insulin unresponsiveness in cells in culture due to fully activating and subsequently reducing the expression of the receptor in cells. This phenomenon can be avoided by reducing the concentration of reagent used in the transfection process.
Background
The cell membrane represents a major barrier to the intracellular delivery of macromolecules such as plasmids, oligonucleotides and therapeutic proteins from outside the cell. Cationic lipids, such as LipofectAMINE 2000™ and FuGENE-6™ therefore provide a useful tool for the introduction of polynucleic acids into cells. Such lipids perform at least three functions. First, by electrostatic association with the plasmid, the cationic lipid coats and partially condenses the plasmid. Second, the presence of cationic lipid at levels that give rise to an overall positive charge leads to enhanced association of the cationic lipid/plasmid complex with negatively charged cell surfaces, leading to cellular uptake via endocytosis [1-3]. Third, following uptake, the cationic lipid plays a role in destabilizing the endosomal membrane, thus facilitating cytoplasmic delivery of the plasmid. This is achieved partly by destabilizing endosomal or plasma membranes by inducing non-bilayer lipid structures in them [4,5]. In general, the liposomes used for complex formation contain at least two kinds of lipid molecules. The key component is cationic lipid (CL) (e.g. DOTAP), which serves as the condensing agents of the negatively charged DNA strands. The neutral helper lipids (HL) (e.g. DOPC) are also important, as they play a crucial role in determining the structure of the lipid phases [6,7].
The molecular actions of insulin in cells are mediated by the cognate insulin receptor. The insulin receptor (IR) is a heterodimeric protein tyrosine kinase whose kinase activity is activated upon binding of insulin [8]. The kinase activity is directed against a variety of intracellular protein substrates, the first of which is the receptor's intracellular domains themselves, which become phosphorylated through autophosphorylation. As such, phosphorylation of the IR on tyrosine residues represents the earliest stimulated reaction in the insulin signal transduction cascade. Early characterisation of the IR catalytic activity in vitro showed that mixtures of cationic and zwitterionic lipids activated the kinase fully [9]. Furthermore, it was observed that lipids that tend to induce hexagonal phases in cell membranes and liposomes increase signal transduction, including IR-dependent signalling [10,11]. In our work, we have observed that after treatment with cationic lipid transfection reagents, cells in culture are often resistant to stimulation with insulin (unpublished observations). Thus, glucose uptake into muscle cells, activation of protein kinase B (a key mediator of insulin signalling) and reporter gene activity in a variety of cell lines is often no longer responsive to insulin stimulation. We now show that this effect is in part due to the fact that these cationic lipid reagents markedly activate the kinase activity of the IR, leading to its subsequent down-regulation. In light of the foregoing discussion, we speculate that this is due to the formation of hexagonal phases in cell membranes, since transfection reagents such as LipofectAMINE 2000™ have been shown to do this in cells in culture [12]. Furthermore, we show that these phenomena can be avoided if the concentration of reagent used to transfect the cells is reduced to below that recommended by the manufacturers, whilst maintaining acceptable levels of cell transfection.
Results
Down-Regulation of Insulin Receptors by Cationic Lipid Reagents
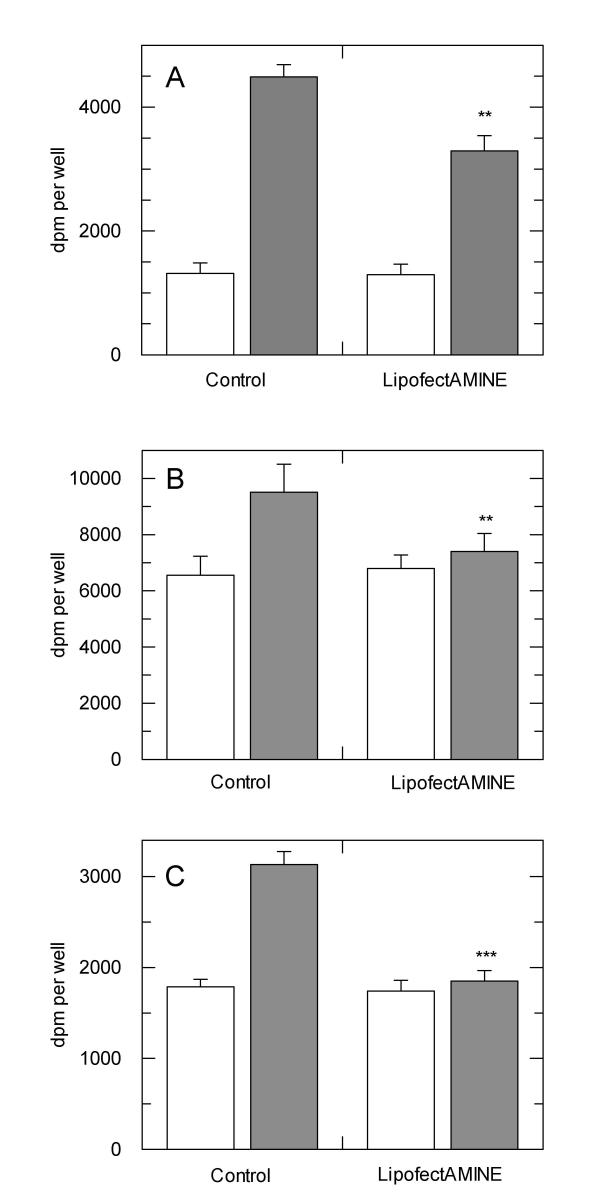
We have performed various analyses in our research work which investigate the effects of over-expression of a variety of different proteins on insulin-mediated events in cells in culture. Our results show that subsequent to transfection, cells are often less responsive to insulin, irrespective of whether the recombinant protein was expressed or not. For example, in experiments using Chinese hamster ovary (CHO) cells over-expressing the human insulin receptor, protein kinase B was significantly phosphorylated on threonine-308, which represents activation of the kinase, in cells that were transfected with a recombinant cDNA but left otherwise unstimulated (data not shown) We have also examined insulin-stimulated glucose transport in cells which express the insulin receptor endogenously at lower levels. In these experiments (Figure 1), cells were treated with and without LF2000 for four hours in mock transfections, cultured a further two days and then examined for insulin-stimulated glucose transport. The data show that in 3T3L1 mouse adipocytes, rat L6 myotubes and SHSY5Y human neuroblastoma cells, insulin is less able or unable to induce an increase in glucose transport in cells that received treatment with LF2000. Thus, LF2000 treatment of cells results in a partial or complete reduction in insulin sensitivity that may be dependent upon the cell type in question.
Figure 1.
Effects of LipofectAMINE 2000 reagent on insulin-stimulated glucose transport in adipocytes and muscle cells. A) 3T3L1 preadipocytes were differentiated to adipocytes and treated with and without LF2000 as described in the methods section. Insulin-stimulated glucose transport was measured in replicates of six for each treatment. Data are from a single experiment which is representative of five such experiments that gave similar results. B) Rat L6 myocytes were differentiated to myotubes as described in the methods section and treated with LF2000 as above. Glucose transport was measured in a similar manner to that for adipocytes. Data are from one experiment that has been repeated at least four times with similar results. C) SHSY5Y neuroblastoma cells were cultured and stimulated with insulin as described in the methods section and glucose transport measured as above. Data are from one experiment performed in sextuplet, which has been repeated two further times. **p < 0.005 with respect to no-LF2000 control; ***p < 0.0005 with respect to no-LF2000 control.
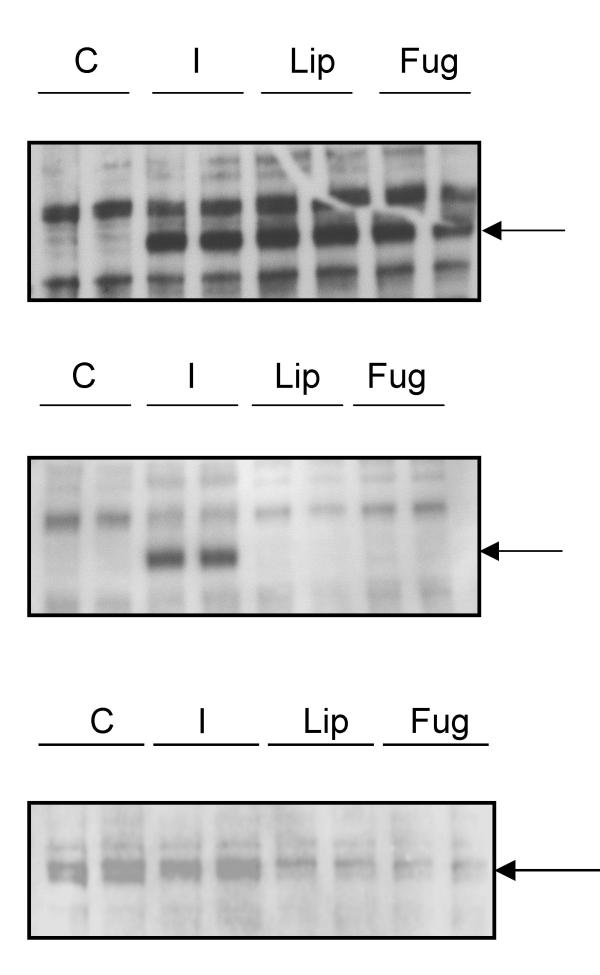
To examine the possible effects of the LF2000 transfection reagent on IR expression and function, CHO cells expressing the human insulin receptor (CHO.hIR) were treated with LF2000 for 4 hours and 24 hours and insulin receptor tyrosine-phosphorylation and expression levels were assessed. The transfection reagent FuGENE was used as a cationic liposome control. Results showed that after 4 hours treatment with LF2000 or FuGENE, the insulin receptor was maximally phosphorylated on tyrosine residues, equivalent to that induced by stimulation with 100nM insulin for 10 minutes (Figure 2 upper panel). After 24 hours treatment, no receptor phosphorylation could be detected with either LF2000 or FuGENE (Figure 2 middle panel). Furthermore when insulin receptor expression was analysed after 24 hour treatment, we found that IR concentrations were significantly reduced in cells treated with either LF2000 or FuGENE compared to resting and insulin-stimulated controls (Figure 2 lower panel). Thus, it appears that cationic liposome transfection reagents induce a state of insulin insensitivity in cultured cells through the maximal activation of the receptor followed by its subsequent down-regulation.
Figure 2.
Cationic lipid reagents activate and down-regulate the insulin receptor. Upper panel: Cho.hIR cells were incubated with LF2000 (Lip) or FuGENE (Fug) as described in the methods section or were treated with insulin (10mins) or medium (C). Cell lysates were blotted for phosphotyrosine and the position 97kDa molecular weight marker (which coincides with the insulin receptor beta chain) is indicated by the arrow. Middle panel: Cho.hIR cells were treated as above except that treatment with LF2000 and FuGENE lasted 24 hours and blotted for phosphotyrosine. Lysates labelled "I" are from cells incubated for 24 hours as control cells and subsequently stimulated with insulin for 10 minutes to confirm that cells are otherwise responsive to insulin after the incubation period. The position of the 97kDa marker is indicated by the arrow. Lower panel: The same cells as in the middle panel were blotted for the presence of the insulin receptor, which co-migrates with the 97kDa marker, indicated by the arrow. The blots are from a representative experiment repeated three times.
Insulin Receptor Down-Regulation is Dose-Dependent
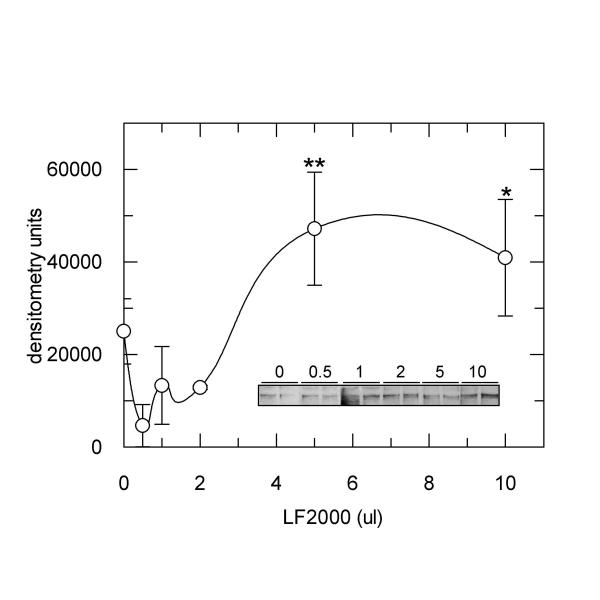
To investigate the dose-dependency of this effect, various dilutions of LF2000 were made in culture medium lacking serum, simulating the preparation of the reagent for cell transfection, except that no recombinant cDNA was included. Cho.hIR cells starved of serum were subsequently treated with the different dilutions for 4 hours and phosphorylation of the IR was measured as described above. Data showed that the phosphotyrosine content of the IR increased above a threshold volume of LF2000 of 2 μl, with maximal receptor phosphorylation observed when cells were treated with 5 μl or greater volumes of LF2000 in medium (Figure 3). The presence of plasmid DNA in the LF2000 dilutions did not affect the dose-response relationship, since in the presence of pGREEN LANTERN cDNA (9 μg), IR phosphorylation was seen to increase in a similar fashion to that shown in Figure 3 (results not shown). Thus, the activation of the IR by LF2000 appeared to be dependent on the dose of the transfection reagent to which the cells were exposed.
Figure 3.
LF2000 increases IR tyrosine phosphorylation in a dose-dependent fashion. Cho.hIR cells were treated for four hours with the indicated volumes of LF2000 which were previously diluted as described in the methods section. Lysates were blotted for phosphotyrosine and bands corresponding to the phosphorylated IR were quantified by densitometric scanning. Data are from one experiment performed in duplicate which was repeated with similar results three times. *p < 0.05; **p < 0.01 compared to control. Insert: shows a typical western blot of phosphotyrosine on the insulin receptor in cells treated with the indicated volumes of LF2000.
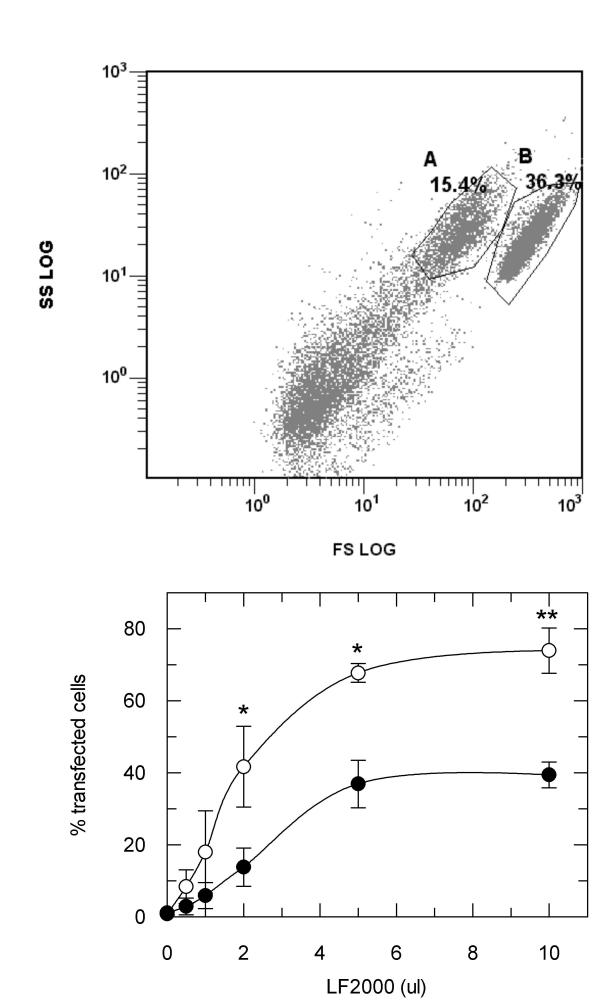
Transfection Efficiency with Lower Doses of Cationic Lipid Reagent
According to the manufacturer's instructions, a recommended ratio of LF2000 to cDNA is 3 μl to 1 μg. Thus, when transfecting cells with 3 μg of any given cDNA, the recommended amount of LF2000 to be used lies within the range where the IR will be maximally activated. We investigated the efficiency of transfection of Cho.hIR cells with pGREEN LANTERN green fluorescent protein DNA using the same range of volumes of LF2000 as were used in the IR phosphorylation experiments. The proportion of transfected cells was quantified using flow cytometry. The data showed that two subpopulations of cells, which we have termed A and B, could be distinguished in the flow cytometer (Figure 4 upper panel), an effect that has been reported by others previously [13,14]. These two subpopulations behaved differently, with population B apparently being more resistant to transfection than population A. Nevertheless, the proportion of transfected cells increased for both cell populations as the volume of LF2000 used in the transfection increased (Figure 4 lower panel). Maximal transfection of each population was observed when volumes of LF2000 of 5 μl or greater were used. This represents a ratio of approximately 0.6 μl LF2000 to 1 μg cDNA, five-fold lower than that recommended by the manufacturers. Furthermore, significant rates of transfection of both cell populations were observed when the ratio of LF2000 to cDNA was even lower (2 μl total volume of LF2000, a ratio of 0.24 μl/μg cDNA), where there is unlikely to be any simultaneous activation and down-regulation of the IR.
Figure 4.
Quantification of the rates of transfection efficiency in the presence of increasing concentrations of LF2000. Upper panel: Flow cytometric analysis showing forward scatter against side scatter of Cho.hIR cells transfected with pGREEN LANTERN using 10 μl LF2000 reagent (ratio of LF2000 to cDNA of 1.1). The separation of intact cells into two subpopulations is indicated by the gates. Percentage values show the relative proportions of the two cell populations compared to the total number of events measured. Lower panel: The proportion of transfected cells in each subpopulation of Cho.hIR cells plotted against the volume of LF2000 reagent used. Data are the average ± S.D. from three experiments which were performed in duplicate for each LF2000 concentration. Filled circles, population A, open circles, population B. *p < 0.01 transfection rate in A> B; **p < 0.001 transfection rate in A > B.
Discussion
In this work, we have described a phenomenon of membrane receptor activation by cationic lipid transfection reagents in cells in culture, and a method by which to avoid this, whilst retaining significant rates of cell transfection. It was described in early experiments with the insulin receptor that its kinase activity could be affected by the phospholipid milieu in which it resided, where zwitterionic lipids in particular (such as phosphatidylethanolamine, PE) were able to activate the kinase. A common property of zwitterionic lipids, such as PE, is their tendency to form hexagonal phase (HCII) structures in membranes rather than lamellar structures (Lαc). Thus, experiments reported by Lewis and Czech [9] in which the insulin receptor kinase was fully activated in PE/PC-containing liposomes, may have been due to hexagonal phases in the vesicle membrane increasing the kinase activity. Interestingly, Koltover and co-workers [12] recently reported that cationic liposomes used as transfection agents comprising PE amongst others form hexagonal phases when complexed with DNA. This is in contrast to liposomes containing PC where lamellar phases are formed. Furthermore, both in prokaryotes and eukaryotes, there are experimental results connecting lipids that form hexagonal phases with altered enzyme activity in different membrane models, including cells over-expressing the human insulin receptor and E. coli [10,11,15,16]. Thus, apart from the insulin receptor kinase, PC and PE have been shown to be involved in activation of PKC isoforms and activation of prokaryotic diacylgycerol kinase. Furthermore, diacylglycerol, which is also associated with increased hexagonal phase separations in membranes, exerts stimulatory effects on enzymes such as PKC and phospholipase C.
Therefore, the impaired insulin signalling we have observed in LF2000-treated cells may be coupled to the formation of HCII phase lipids in the cell membrane. Potential cellular mechanisms which lead to this are three-fold: increased autophosphorylation of the receptor which is subsequently down-regulated; reduced sensitivity of the receptor to insulin such that it is no longer stimulated by insulin and increased PKC activation, which is associated with insulin resistance. The membrane perturbations induced by LF2000, including the formation of HCII phases, may be involved in induction of insulin resistance.
By using lower concentrations of LF2000 than those recommended by the manufacturer, we have shown that activation of the IR can be kept to a minimum, thereby preserving the responsiveness of cells to insulin. Whilst reductions of LF2000 concentrations also reduce the efficiency of cell transfection, we found that over half of all cells can be transfected when the ratio of LF2000 to cDNA is 0.5 (volume:mass), which is considerably lower than the ratio of 3 recommended by the manufacturers. By reducing the ratio in this manner, the positive charge coating the cDNA is not in the same excess as is recommended for optimal transfection. It is therefore not known whether the rate of cell transfection we observed with lower concentrations of LF2000 is cell type specific. Thus, other commonly used cell lines in culture may require higher concentrations of LF2000 to retain efficient transfection rates. Such things must be worked out empirically on a case-by-case basis. Alternative modes of transfection can also be tested. We have recently tested polyamine-based reagents which give acceptable levels of transfection (Eva Danielsson, unpublished). However, it was demonstrated a long time ago that polyamines are able to exert insulin-like effects in fat cells through the production of hydrogen peroxide [17]. Hydrogen peroxide is now known to cause the reversible inhibition of protein tyrosine phosphatases in cells, thereby strengthening insulin signalling. We have also shown that production of hydrogen peroxide chemically in cells acts as an insulin mimetic (Liljebris et al, in the press). Thus, polyamine-based transfection reagents may also activate insulin signalling, albeit through an unrelated mechanism. It will thus be necessary to investigate this if one intends to use polyamine-based cell transfection in cells that are otherwise required to be unstimulated.
Conclusions
Cationic lipid preparations are commercially available transfection reagents that are used widely to affect the expression of recombinant or endogenous genes in cells in culture. Our work has shown that a common side-effect of treatment of cells with such reagents is likely to have been the reduced expression of the insulin receptor on the surface of such cells with concomitant reductions in insulin signalling. Apart from defining this phenomenon, our work also shows how this can be avoided by reducing the concentration of transfection reagent used without necessarily reducing transfection rates to unacceptably low levels.
Methods
Materials
All cell culture reagents were from Gibco. LipofectAMINE 2000™ and SDS-PAGE reagents were from Invitrogen and FuGENE-6™ was from Roche. Hybond™-C nitrocellulose membrane and enhanced chemiluminescence (ECL) was from Amersham Life Science. Anti-phosphotyrosine antibodies were from Santa Cruz. All other reagents were from Sigma and were analytical grade.
Cell Culture and Treatment with Transfection Reagents
Chinese hamster ovary cells over-expressing the human insulin receptor (CHO-hIR cells) were grown in F-12K Nutrient Mixture (Kaighn' Modification) supplemented with 10% heat inactivated fetal bovine serum (FBS) in 75 cm2 cell culture flasks. CHO-hIR cells were cultured at 37°C in a humidified 5% CO2 atmosphere. Two days before treatment with LipofectAMINE™ 2000 (LF2000), the cells were plated out in two 6-well plates. After 24 hours, cells were starved by culturing overnight in serum-free medium. Cells were then treated in duplicate with increasing dilutions of LF2000, which were prepared in advance and allowed to sit at room temperature for 30 minutes. (0.5, 1, 2, 5, 10 μl diluted in 1 ml F-12K Nutrient Mixture (Kaighn' Modification) lacking FBS). Cells were incubated for 4 hours in 37°C in a humidified 5% CO2 atmosphere. Alternatively, cells were incubated with single concentrations of LF2000 or FuGENE as indicated in the figure legends. After washing, cells were lysed in 1ml buffer comprising 20mM Hepes pH 7.6, 20% (v/v) Glycerol, 10mM NaCl, 1.5mM MgCl2, 0.2mM EDTA, 0.1% NP40 (v/v), 25mM NaF, 25mM β-glycerolphosphate, 1mM DTT, 1mM Na vanadate and protease inhibitors (Boehringer). The cells were flash frozen in liquid nitrogen and then stored at -70°C until protein concentration was determined (Bradford assay) and analysis by Western blot. Where indicated, cells were stimulated with 100nM insulin for the indicated periods as controls.
L6 myocytes were maintained in minimum essential medium-alpha (α-MEM) supplemented with 10% foetal bovine serum (FBS) and 100 IU/ml penicillin-streptomycin at 37°C in 5% CO2. Cells were seeded into 96-well plates and the medium was replaced with α MEM containing 2% FCS to induce differentiation. The medium was changed every other day and cytidine (0.24 mg/ml medium) was added to the cultures after a week to suspend cycling cells. The cells were used in experiments after over night serum starvation after day 10.
3T3L1 preadipocytes were cultured in DMEM/F12 containing 10% serum until they became confluent. Differentiation to adipocytes was achieved by sequential culture in DMEM/F12/10% serum containing IBMX 0,5mM, IGF-I 20ng/ml, Dexamethasone 1 μM and with or without 1 μM Rosiglitazone for 4 days followed by DMEM/F12/10% serum and IGF-1 alone for four days. Medium was then reverted to culture medium and cells were used after 14 days once full differentiation was achieved.
Glucose Transport
3T3L1 cells, L6 cells and SHSY5Y cells were incubated 18 hours in serum free α-MEM supplemented with 0.25% (v/w) fat free albumin (50 ml DMEM + 125 mg albumin). The medium was aspirated, cells were washed with glucose-free α-MEM, and the cells were incubated for 30 minutes in glucose-free α-MEM supplemented with 100 nM insulin (when indicated) for 30 min. The medium was aspirated and glucose-free α-MEM containing the tracer was added. Glucose uptake rates were carried out for 8 minutes. The medium was aspirated and the cells were washed twice with cold PBS. Subsequently the cells were solubilized in 50 μl 0.5M NaOH over night at RT, scintillation liquid was added (100 μl/well), plates were shaken on the vortex at RT for 30 minutes and radioactivity was counted in μ-Beta counter.
SDS-PAGE analysis
Samples were matched for protein concentration and heated at 70°C for 10 minutes with 20 μl 4× concentrated sample buffer. Samples were resolved on 4–12% gradient gels and blotted onto nitrocellulose membranes. Membranes were probed for the presence of phosphotyrosine using a mouse monoclonal anti-phosphotyrosine antibody and developed using enhanced chemoluminescence. Phosphotyrosine content of the insulin receptor was then quantified by densitometric analysis.
Flow cytometric analysis of green fluorescent protein expression
Cells were transfected with pGREEN LANTERN as above and analysed using an EPICS® XL-MCL flow cytometer (Beckman Coulter). For each sample, 30 000 events were collected by list-mode data, which consisted of side scatter, forward scatter, and fluorescence emissions centered at 530 nm (FL1), 580 nm (FL2), and 610 nm (FL3). pGREEN LANTERN-1 fluorescence occurs primarily in FL1 but is also detectable in FL2 and FL3. However, we chose only to present FL1 in this work. The gates were drawn along a line of maximum detected pGREEN LANTERN-1 intensity for control samples. The gates were held unchanged through the analysis of all measurements in all experiments.
Abbreviations
IR, insulin receptor; LF2000, LipofectAMINE™ 2000; PC, phosphatidylcholine; PE, phosphatidylethanolamine;
Authors' contributions
CP and JL performed analyses of the insulin receptor in treated cells, MA performed flow cytometric analysis of transfected cells, CK, ED, I-MR and MW were involved in analyzing the dose-dependency of the phenomena and SRJ conceived of the study, designed experiments and played a coordinating role. All authors read and approved the final manuscript.
Contributor Information
Camilla Pramfalk, Email: Camilla.Pramfalk@biosci.ki.se.
Johanna Lanner, Email: Johanna.Lanner@fyfa.ki.se.
Monica Andersson, Email: monica.andersson@biovitrum.com.
Eva Danielsson, Email: eva.danielsson@biovitrum.com.
Christina Kaiser, Email: christina.kaiser@biovitrum.com.
Ing-Marie Renström, Email: ing-marie.renstrom@biovitrum.com.
Malin Warolén, Email: malin.warolen@biovitrum.com.
Stephen R James, Email: stephen.james@biovitrum.com.
References
- Stamatatos L, Leventis R, Zuckermann M, Silvius J. Interactions of cationic lipid vesicles with negatively charged phospholipid vesicles and biological membranes. Biochemistry. 1988;27:3917–3925. doi: 10.1021/bi00411a005. [DOI] [PubMed] [Google Scholar]
- Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- Wrobel I, Collins D. Fusion of cationic liposomes with mammalian cells occurs after endocytosis. Biochim Biophys Acta. 1995;1235:296–304. doi: 10.1016/0005-2736(95)80017-A. [DOI] [PubMed] [Google Scholar]
- El Ouahabi A, Thiry M, Pector V, Fuks R, Ruysschaert J, Vandenbranden M. The role of endosome destabilizing activity in the gene transfer process mediated by cationic lipids. FEBS Lett. 1997;414:187–192. doi: 10.1016/S0014-5793(97)00973-3. [DOI] [PubMed] [Google Scholar]
- Wattiaux R, Jadot M, Warnier Pirotte M, Wattiauz De C. Cationic lipids destabilise liposomal membrane in vitro. FEBS Lett. 1997;417:199–202. doi: 10.1016/S0014-5793(97)01283-0. [DOI] [PubMed] [Google Scholar]
- Hui S, Langner M, Zhao Y-L, Patrick R, Hurley E, Chan K. The role of helper lipids in cationic liposome-mediated gene transfer. Biophys J. 1996;71:590–599. doi: 10.1016/S0006-3495(96)79309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidam N, Lerner D, Margulies S, Barenholz Y. Lamellarity of cationic liposomes and mode of perparation of lipocomplexes affect transfection efficiency. Biochim Biophys Acta. 1999;1419:207–220. doi: 10.1016/S0005-2736(99)00069-3. [DOI] [PubMed] [Google Scholar]
- Yip CC, Ottensmeyer P. Three-dimensional structural interactions of insulin and its receptor. J Biol Chem. 2003;278:27329–27332. doi: 10.1074/jbc.R300021200. [DOI] [PubMed] [Google Scholar]
- Lewis R, Czech M. Phospholipid environment alters hormone sensitivity of the purified insulin receptor kinase. Biochem J. 1987;248:829–836. doi: 10.1042/bj2480829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgione J, Huang Z, Epand R. Increased activation of protein kinase C with cubic phase lipid compared with liposomes. Biochemistry. 1998;37:2384–2392. doi: 10.1021/bi970873e. [DOI] [PubMed] [Google Scholar]
- McCallum C, Epand R. Insulin receptor autophosphorylation and signalling is altered by modulation of membrane physical properties. Biochemistry. 1995;34:1815–1824. doi: 10.1021/bi00006a001. [DOI] [PubMed] [Google Scholar]
- Koltover I, Salditt T, Radler J, Safinya C. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 1998;281:78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- Duda M, Gasinska A, Gregoraszczuk E. Flow cytometric cell cycle analysis of two subpopulations of porcine granulosa cells. Exp Clin Endocrinol Diabetes. 1999;17:203–207. doi: 10.1055/s-0029-1212099. [DOI] [PubMed] [Google Scholar]
- Tseng W, Haselton F, Giorgio T. Transfection by cationic liposomes using simultaneous single cell measurements of plasmid delivery and transgene expression. J Biol Chem. 1997;272:25641–25647. doi: 10.1074/jbc.272.41.25641. [DOI] [PubMed] [Google Scholar]
- Pilot J, East J, Lee A. Effects of phospholipid headgroup and phase on the activity of diacylglycerol kinase of Escherichia coli. Biochemistry. 2001;40:14891–14897. doi: 10.1021/bi011333r. [DOI] [PubMed] [Google Scholar]
- Sweet L, Dudley D, Pessin J, Spector A. Phospholipid activation of the insulin receptor kinase: regulation by phosphatidylinositol. FASEB J. 1987;1:55–59. doi: 10.1096/fasebj.1.1.3038645. [DOI] [PubMed] [Google Scholar]
- Livingston J, Gurny P, Lockwood D. Insulin-like effects of polyamines in fat cells. Mediation by H2O2 formation. J Biol Chem. 1977;252:560–562. [PubMed] [Google Scholar]