Abstract
Background
Postherpetic neuralgia (PHN) is by far the most common complication of herpes zoster (HZ) and one of the most intractable pain disorders. Since PHN is seen most often in the elderly, the number of patients with this disorder is expected to increase in our ageing society. PHN may last for months to years and has a high impact on the quality of life. The results of PHN treatment are rather disappointing. Epidural injection of local anaesthetics and steroids in the acute phase of HZ is a promising therapy for the prevention of PHN. Since randomised trials on the effectiveness of this intervention are lacking, the PINE (Prevention by epidural Injection of postherpetic Neuralgia in the Elderly) study was set up. The PINE study compares the effectiveness and cost-effectiveness of a single epidural injection of local anaesthetics and steroids during the acute phase of HZ with that of care-as-usual (i.e. antivirals and analgesics) in preventing PHN in elderly patients.
Methods / design
The PINE study is an open, multicenter clinical trial in which 550 elderly (age ≥ 50 yr.) patients who consult their general practitioner in the acute phase of HZ (rash < 7 days) are randomised to one of the treatment groups. The primary clinical endpoint is the presence of HZ-related pain one month after the onset of the rash. Secondary endpoints include duration and severity of pain, re-interventions aiming to treat the existing pain, side effects, quality of life, and cost-effectiveness.
Conclusion
The PINE study is aimed to quantify the (cost-) effectiveness of a single epidural injection during the acute phase of HZ on the prevention of PHN.
Background
Herpes zoster (HZ) or shingles is a common disease, with a reported incidence varying from 2.2 to 3.4/1000 persons/year [1-4]. This incidence increases with age, rising from 2.4/1000 persons/year in younger subjects to 6.9/1000 persons/year in individuals older than 55 years of age [2,4,5]. The symptoms of HZ include a painful, vesicular rash with erythema.
It is a self-limiting disease for most patients, with healing of the skin and resolution of the pain generally occurring within three to four weeks. The most frequent complication of HZ is persistent pain that may last for several months or years, also called postherpetic neuralgia (PHN). PHN is commonly defined as the presence of HZ-related pain one month or more after the onset of the rash [5-7], whereas some use the threshold of three months [8,9]. This chronic pain has a potentially high impact on the quality of life. Many patients develop severe physical, occupational, and social disabilities as a consequence of their unceasing pain [8].
Depending on the applied definition, 9–34% of all HZ patients develop PHN. The risk of developing PHN is also age-dependent: the cumulative incidence of PHN is only 2% in HZ patients younger than 50 years of age, rising to about 20% in those older than 50 years and to 35% in those over 80 years [3,10-12]. Therefore, the number of patients with this disorder is expected to increase in our ageing society. Other important risk indicators for PHN include the severity of acute pain and inflammation during HZ.
Treatment of existing PHN has been rather disappointing. In fact, most treatment strategies such as tricyclic antidepressants [13,14], topical capsaicin [15,16], gabapentin [17], and controlled-release oxycodone [18], have demonstrated limited effects [19]. Intrathecal steroid injections appeared to be effective in one study [20]. Further evidence, however, of this invasive and potentially riskful intervention is lacking.
Much attention has therefore been given to strategies to prevent PHN [21]. Meta-analyses showed that antiviral medicines have no or limited effect on the incidence of PHN, although the duration of the HZ-related pain may be reduced [21,22]. One small randomised controlled trial showed that early medical treatment with amitriptyline caused a 50% reduction in the occurrence of PHN [23]. At present, there is no more evidence available.
Another preventive strategy proposed is epidural administration of steroids with or without local anaesthetics. These drugs can be administered either repeatedly by means of an epidural catheter for a certain number of days [24] or through a single epidural injection [25,26]. The latter is less burdensome for the patient and can be performed in an outpatient setting. Two retrospective, though uncontrolled, studies on this single epidural injection demonstrated a cumulative incidence of 2% of PHN when the injection was given during the acute phase of HZ, i.e. within one week from the onset of the rash [25,26]. This suggests that it is a promising strategy for preventing PHN. However, referring all patients with HZ to an anaesthesiologist for a preventive epidural injection will increase short-term medical costs. Part of these costs may be offset by savings due to a decrease in the costs related to the treatment of PHN. Whether these savings will outweigh the increased initial costs is subject of research.
Since randomised trials on the effectiveness and the cost-effectiveness of a single epidural injection of steroids and local anaesthetics are lacking, the PINE (Prevention by epidural Injection of postherpetic Neuralgia in the Elderly) study was set up. This paper describes the rationale and design of the study, preceded by a brief outline of the current hypotheses of PHN pathophysiology and the possible modes of action of the intervention. In the discussion we will elaborate on dilemmas encountered when designing the study.
Rationale for the efficacy of epidural administration of local anaesthetics and steroids
HZ is caused by a localised infection with the varicella zoster virus, which had been dormant in the sensory ganglia since the healing of a primary infection (chickenpox). Reactivation of the virus and its spread to the corresponding dermatome result in HZ. The concomitant inflammation of the peripheral nerve and skin damage are supposedly responsible for the acute pain [27].
The pain, hyperalgesia, and allodynia can best be explained by two different mechanisms: sensitisation and deafferentation. Sensitisation: nociceptors become sensitised following acute tissue injury, resulting in an ongoing discharge and hyperexcitability (peripheral sensitisation); prolonged nociceptor discharge enhances the response of dorsal horn neurones to afferent stimuli and expands the dorsal horn neurone's receptive field (central sensitisation), leading to allodynia without marked sensory loss. Deafferentation: reactivation of the varicella zoster virus in the dorsal root ganglion results in neural damage. In addition, the inflammatory response causes oedema. The ensuing increased intrafascicular pressure may result in impairment of endoneurial blood flow and, ultimately, neural destruction [28]. Loss of afferent neurones causes spontaneous activity in deafferented central neurones, generating constant pain in an area of marked sensory loss and minimal allodynia. Reactive sprouting of the spinal terminals of Aβ-mechanoreceptors, which contact receptors formerly occupied by C-fibres, leads to hyperalgesia and allodynia [29].
Interventions to prevent PHN should address these presumed underlying pathophysiological mechanisms. The provision of adequate analgesia in the acute phase might interrupt the process of sensitisation. Reduction of acute pain can be achieved by blocking the afferent transmission of the pain stimulus using local anaesthetics. Steroids might forestall deafferentation, as they inhibit inflammation and hinder swelling-induced neural ischemia, thus preventing persistent neural damage. The application of local anaesthetics and steroids at the level of the sensory ganglion, therefore, might be effective.
Methods / design
Study objectives
The aim of this trial is twofold: first, to quantify the effect of a single epidural injection of local anaesthetics and corticosteroids during the acute phase of HZ in preventing PHN and, second, to estimate the cost-effectiveness of this intervention as compared to care-as-usual.
Design
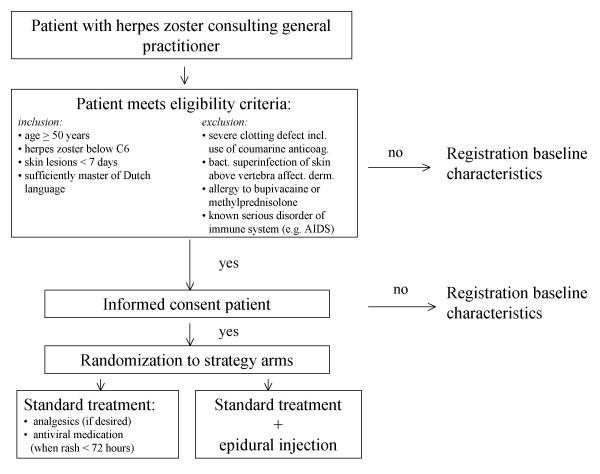
The PINE study is a pragmatic, randomised, multicenter clinical trial. Its design and timing of the investigations are presented in Figure 1 and Table 1, respectively.
Figure 1.
Flow diagram of the PINE randomised trial
Table 1.
The timing of the investigations.
| 0 | 1w | 2w | 3w | 4w | 2m | 3m | 6m | (+6m) | |
| Diary + VAS | x | x | x | x | x | x | x | x | x |
| CES-D | x | x | |||||||
| STAI, | x | ||||||||
| EuroQol, SF-36 | x | x | x | x | x | ||||
| Health & Labor | x | x | x | x | |||||
| GP-record analysis | x | x | |||||||
| Side effects | x |
Abbreviations: 0 = enrolment; 1w, 2w, 3w, and 4w = 1, 2, 3, and 4 weeks after enrolment; 2m, 3m, and 6m = 2, 3, and 6 months after enrolment; (+6m) = every 6 months; VAS: Visual Analogue Scale; STAI: State-Trait Anxiety Inventory; CES-D: Center for Epidemiological Studies Depression scale, a screening instrument for depression in the elderly; EuroQol: five-dimensional questionnaire for assessment of the health-related quality of life; SF-36: short form-36 Health Survey, composed of 36 questions and standardised response choices; Health and Labor: questionnaire consisting of four modules, to collect data on absence from work, reduced productivity, unpaid labor production, and labor-related problems; GP-record analysis: report from general practitioners about re-interventions aiming to treat the HZ-related pain; Side effects: report from patients about side effects of treatment.
Patients
Patients who consult their general practitioner (GP) during the acute phase of HZ are potentially eligible for the PINE study. Inclusion predominantly depends on the location of the HZ rash and the age of the patient. Since the index treatment (see below) is performed by local anaesthesiologists and fluoroscopy is not routinely available in all participating clinics, the PINE study is confined to patients who present with HZ below the sixth cervical dermatome. From this level downwards an epidural injection can be performed without fluoroscopy. The inclusion criteria for the PINE study are: 1. age ≥ 50 years; 2. HZ below dermatome C6; 3. skin rash < 7 days; and 4. sufficiently mastery of the Dutch language. The exclusion for the PINE study are: 1. severe clotting defect, including the use of coumarine anticoagulants; 2. bacterial superinfection of the skin above the vertebra of the affected dermatome; 3. allergy to bupivacaine or methylprednisolone; and 4. a known serious disorder of the immune system (e.g. AIDS).
A standard checklist encompassing the inclusion and exclusion criteria of the study will be used to evaluate the eligibility of those patients with acute-phase HZ, who consult their GP, to participate in the study. Data (including severity of pain) of all (in- and excluded) patients older than 50 years of age, who consulted their GP within the first week of the HZ rash, will be used afterwards to determine whether the study included a selected group of HZ patients and to address generalizibility of the results.
Interventions
Standard treatment (control group)
Patients randomised to the control group receive the current standard treatment for patients in the acute phase of HZ. This consists of analgesics (if desired by the patient) and antiviral medication if the rash exists less than 72 hours [9]. The choice of the antiviral drug is left to the general practitioner and is either aciclovir (500 mg five times daily), famciclovir (500 mg three times daily), or valaciclovir (1000 mg three times daily). All three are administered orally for seven days. No antiviral drugs are prescribed if the patient has had the rash for more than 72 hours. All patients, irrespective of the duration of symptoms, may be prescribed any analgesic.
Epidural injection (index group)
Patients randomised to the epidural injection group will receive the same treatment regimens as the control group using the same criteria for the prescription of antiviral medication. In addition, they will be given an epidural injection of 80-mg slow-release methylprednisolone-acetate and 10 mg bupivacaine. The injection will be carried out by an anaesthesiologist in one of the local hospitals within one working day after randomisation. The injection will be performed at the level of the affected dermatome using the widely used "loss of resistance" or "hanging drop" technique [30].
Endpoints
Primary endpoint
In accordance with the commonly used definition of PHN [5-7], the primary endpoint is the presence of HZ-related pain four weeks after enrolment in the trial. The occurrence of pain will be determined by asking patients whether the pain related to HZ is (still) present, following the recommendations of the Herpes Zoster Clinical Trial Consensus Group [6].
Secondary endpoints
Secondary endpoints include presence of HZ-related pain after 3 and 6 months, duration of pain (time to resolution of pain), severity of pain as determined by visual analogue scale (VAS) [31], re-interventions aiming to treat the HZ-related pain (such as drug prescription, physiotherapy, and anaesthesiological interventions), side effects, quality of life as determined by SF-36 [32,33] and EuroQol [34,35], costs, and the cost-effectiveness. Data will be collected about units of resource utilisation with an explicit clause asking whether it can be related to the PHN. Cost-effectiveness will be addressed in terms of the incremental costs per additional patient year free of PHN and in terms of incremental costs per QALY gained after one and three years. Additional estimates will be formulated for remaining life expectancy. The points of time when the secondary endpoints will be measured are shown in Table 1.
The measurement of the study endpoints will take place using written questionnaires. The Data Co-ordinating Center will send the questionnaires, together with self-addressed envelopes, to the patients. In case of a non-response, the patient will be encouraged by phone to fill out the questionnaire. Mailing of questionnaire and endpoint measurements will take place according to the time schedule presented in Table 1.
Statistical power
The sample size calculation has been based on a clinically relevant reduction in the cumulative incidence of PHN one-month after the onset of the HZ rash. Many earlier studies showed an incidence of PHN of 20% in standardly treated HZ patients older than 50 years [3,11,12]. However, based on data from a pilot study [4], we used a cumulative incidence of 12% to be expected in the control group. This could guarantee adequate numbers of patients to perform proper analyses. Based on a two-sided alpha error of 0.05 and 80% power, we calculated that 250 patients are needed in each treatment arm in order to detect a reduction in the cumulative incidence from 12% to 5%. This means that, allowing for a 10% loss of follow-up, at least 550 patients must participate in the study.
Data analysis
Primary endpoint
The primary data analysis will include estimation of the relative risk with 95% confidence intervals (intention-to-treat analysis). An additional analysis will be performed to evaluate the preventive efficacy of the intervention with regard to the actual treatment received. To account for the fact that the data derive from repeated measurements, we will use Generalised Linear Mixed Models to analyse the difference in the incidence of PHN at later moments (2, 3, and 6 months).
Secondary endpoints
The difference in time to pain resolution between the two treatment groups will be evaluated using Kaplan-Meyer analyses. Difference in severity of pain and quality of life will be compared using difference of means with 95% confidence intervals. Difference in re-interventions and side effects will be compared using relative risk estimates with 95% confidence intervals. In addition, the incremental cost-effectiveness of the treatment with epidural injection versus standard treatment will be assessed. Costs are estimated per patient by multiplying units of resource utilisation with Dutch estimates of unit costs. QALY's are estimated per patient by interpolating the valuations at randomisation, 1 month, 3 months, 6 months, and 1 year. Difference between both treatments is tested by Generalised Linear Mixed Models. The uncertainty surrounding the cost effectiveness in terms of costs per additional patient without PHN will be addressed by probability ellipses and acceptability curves. The uncertainty surrounding the cost effectiveness in terms of costs per QALY is addressed by multivariate sensitivity analysis using a simple model distinguishing between patients with and without PHN.
The following planned subgroup analyses will be performed: analysis according to patients' age, analysis according to severity of pain at study entry, and analysis according to severity of skin rash at study entry.
No interim analysis will be performed.
Ethical aspects
This study will be conducted in accordance with the principles of the Declaration of Helsinki and GCP guidelines. The study protocol has been approved by the Medical Ethical Committee of the University Medical Center Utrecht, Utrecht, The Netherlands. Written informed consent will be obtained from each participating patient.
Implementation of study results
The results of the PINE study, which is supported by the Dutch Society of Anaesthesiology and the Dutch College of General Practitioners, will be implemented in guidelines for treatment of acute HZ for both anaesthesiologists and GPs. If the intervention appears not to be effective, anaesthesiologists and GPs will be advised to refrain from this rather expensive and invasive procedure. In case the effectiveness of the intervention has been demonstrated, guidelines will indicate which patients may profit from the epidural injection. Finally, the cost-effectiveness of the procedure (i.e. the cost per QALY) may determine whether this intervention will be covered by the Health Care Insurances. It is expected, however, that any outcome of the PINE study will contribute to a more efficient utilisation of the available resources for health care.
Discussion
Some aspects of the design of the PINE study, however, must be addressed in order to appreciate its importance for primary care.
First, we specifically opted for a pragmatic trial instead of a placebo-controlled trial. An epidural injection is quite burdensome and not without risks. An epidural haematoma, for example, although rare, can occur and we feel that the risk of such severe complication makes it unethical to perform a sham injection. Besides, the addition of local anaesthetics offers immediate pain relief, which can not be realised by a placebo. The most important reason for a pragmatic approach, however, is that we aim to quantify the effect of an epidural injection as might be expected in future practice. Hence, we chose to evaluate the addition of the epidural injection to care-as-usual. Consequently, the placebo effects in this trial must be considered as part of the overall treatment effect that would also be encountered during care-as-usual in future HZ patients.
Second, the treatment in the control group was not standardised. Meta-analyses have shown that antiviral drugs, when given within 72 hours after the onset of the rash, have some effect on the duration of HZ-associated pain [21]. Hence, all patients participating in the PINE study who consult their GP within this time limit will be prescribed antiviral medicines. Since there is no clear proof of a difference in efficacy between the three antiviral drugs (aciclovir, famciclovir, and valaciclovir) on the development or duration of PHN, the choice of drug is left to the GP. However, the prescribed antiviral drug of each patient will be registered on the data forms. One consequence of not standardising the treatment protocol is that GPs may prescribe therapies in addition to the antiviral medication more often to those patients randomised to the care-as-usual (control) group. An additional therapy, for example, includes amitriptyline, which has been reported to potentially prevent the development of PHN [23]. Such therapies could reduce the observed difference in outcome between the two groups. Although the pre-emptive prescription of amitriptyline is unusual in The Netherlands, the careful registration of all prescribed drugs in relation to HZ will enable us to correct for the possible effect of any additional drug.
Third, epidural administration of steroids and local anaesthetics can be performed either repeatedly by means of an epidural catheter for a certain number of days [24] or through a single epidural injection [25,26]. As the latter is less burdensome for the patients, can be performed in an outpatient setting, and is advocated by the Dutch Society of Anaesthesiology, we decided to evaluate the single rather than the multiple injection therapy.
Finally, we chose for the presence of HZ-related pain four weeks after enrolment in the trial as the primary outcome of the PINE study, because it is the most widely advocated definition of PHN [5,6]. There is some controversy, however, about the definition of PHN with respect to the severity and the duration of pain after the onset of the rash [5-7,36-38]. Some researchers define PHN as HZ-related pain that occurs after the rash has healed, while others describe it as HZ-related pain one [5,6] or three months [13] after the onset of the rash. Hence, we chose as secondary outcomes the severity of pain at one month, the presence of HZ-related pain after three and six months since rash onset, and the duration of pain. To minimise bias that is inherent to a subjective endpoint in an open-label trial, we will systematically document endpoints using structured written questionnaires.
In summary, the PINE study is the first randomised trial to quantify the effectiveness and cost-effectiveness of an epidural injection of local anaesthetics and steroids on the prevention of postherpetic neuralgia in elderly patients with acute-phase herpes zoster. The results of this study may also facilitate the selection of HZ patients who are at risk of developing PHN.
Competing interests
None declared.
Authors' contributions
EB, GAvE, CJK, KGMM, WO, ThJMV and AJMvW conceived the trial design. AAAB contributed to adaptations in the trial design. WO and AJMvW drafted the manuscript.
All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The PINE Study is funded by the Netherlands Organisation for Scientific Research (NWO nr 945-02-009).
Contributor Information
Wim Opstelten, Email: w.opstelten@med.uu.nl.
Albert JM van Wijck, Email: A.vanWijck@azu.nl.
Gerrit A van Essen, Email: gavessen@knmg.nl.
Erik Buskens, Email: e.buskens@jc.azu.nl.
Annette AA Bak, Email: a.a.a.bak@azu.nl.
Cornelis J Kalkman, Email: c.j.kalkman@azu.nl.
Theo JM Verheij, Email: t.j.m.verheij@med.uu.nl.
Karel GM Moons, Email: k.g.m.moons@azu.nl.
References
- Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–1609. doi: 10.1001/archinte.155.15.1605. [DOI] [PubMed] [Google Scholar]
- Galil K, Choo PW, Donahue JG, Platt R. The sequelae of herpes zoster. Arch Intern Med. 1997;157:1209–1213. doi: 10.1001/archinte.157.11.1209. [DOI] [PubMed] [Google Scholar]
- Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571–575. [PMC free article] [PubMed] [Google Scholar]
- Opstelten W, Mauritz JW, de Wit NJ, van Wijck AJ, Stalman WA, van Essen GA. Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Pract. 2002;19:471–475. doi: 10.1093/fampra/19.5.471. [DOI] [PubMed] [Google Scholar]
- Kost RG, Straus SE. Postherpetic neuralgia--pathogenesis, treatment, and prevention. N Engl J Med. 1996;335:32–42. doi: 10.1056/NEJM199607043350107. [DOI] [PubMed] [Google Scholar]
- Wood MJ, Balfour H, Beutner K, Bruxelle J, Fiddian P, Johnson R, Kay R, Cubed S, Portnoy J, Rentier B, et al. How should zoster trials be conducted? J Antimicrob Chemother. 1995;36:1089–1101. doi: 10.1093/jac/36.6.1089. [DOI] [PubMed] [Google Scholar]
- Watson CP. Postherpetic neuralgia. Neurol Clin. 1989;7:231–248. [PubMed] [Google Scholar]
- Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain. 1996;67:241–251. doi: 10.1016/0304-3959(96)03122-3. [DOI] [PubMed] [Google Scholar]
- Johnson R. Herpes zoster--predicting and minimizing the impact of post-herpetic neuralgia. J Antimicrob Chemother. 2001;47:1–8. doi: 10.1093/jac/47.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- Johnson RW. The future of predictors, prevention, and therapy in postherpetic neuralgia. Neurology. 1995;45:S70–2. doi: 10.1212/wnl.45.12_suppl_8.s70. [DOI] [PubMed] [Google Scholar]
- Ragozzino MW, Melton L. J., 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61:310–316. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ. 2000;321:794–796. doi: 10.1136/bmj.321.7264.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max MB, Schafer SC, Culnane M, Smoller B, Dubner R, Gracely RH. Amitriptyline, but not lorazepam, relieves postherpetic neuralgia. Neurology. 1988;38:1427–1432. doi: 10.1212/wnl.38.9.1427. [DOI] [PubMed] [Google Scholar]
- Watson CP, Chipman M, Reed K, Evans RJ, Birkett N. Amitriptyline versus maprotiline in postherpetic neuralgia: a randomized, double-blind, crossover trial. Pain. 1992;48:29–36. doi: 10.1016/0304-3959(92)90128-X. [DOI] [PubMed] [Google Scholar]
- Watson CP, Tyler KL, Bickers DR, Millikan LE, Smith S, Coleman E. A randomized vehicle-controlled trial of topical capsaicin in the treatment of postherpetic neuralgia. Clin Ther. 1993;15:510–526. [PubMed] [Google Scholar]
- Bernstein JE, Korman NJ, Bickers DR, Dahl MV, Millikan LE. Topical capsaicin treatment of chronic postherpetic neuralgia. J Am Acad Dermatol. 1989;21:265–270. doi: 10.1016/s0190-9622(89)70171-7. [DOI] [PubMed] [Google Scholar]
- Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. Jama. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- Watson CP, Babul N. Efficacy of oxycodone in neuropathic pain: a randomized trial in postherpetic neuralgia. Neurology. 1998;50:1837–1841. doi: 10.1212/wnl.50.6.1837. [DOI] [PubMed] [Google Scholar]
- Alper BS, Lewis PR. Treatment of postherpetic neuralgia: a systematic review of the literature. J Fam Pract. 2002;51:121–128. [PubMed] [Google Scholar]
- Kotani N, Kushikata T, Hashimoto H, Kimura F, Muraoka M, Yodono M, Asai M, Matsuki A. Intrathecal Methylprednisolone for Intractable Postherpetic Neuralgia. N Engl J Med. 2000;343:1514–1519. doi: 10.1056/NEJM200011233432102. [DOI] [PubMed] [Google Scholar]
- Alper BS, Lewis PR. Does treatment of acute herpes zoster prevent or shorten postherpetic neuralgia? J Fam Pract. 2000;49:255–264. [PubMed] [Google Scholar]
- Lancaster T, Silagy C, Gray S. Primary care management of acute herpes zoster: systematic review of evidence from randomized controlled trials. Br J Gen Pract. 1995;45:39–45. [PMC free article] [PubMed] [Google Scholar]
- Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327–331. doi: 10.1016/S0885-3924(97)00077-8. [DOI] [PubMed] [Google Scholar]
- Pasqualucci A, Pasqualucci V, Galla F, De Angelis V, Marzocchi V, Colussi R, Paoletti F, Girardis M, Lugano M, Del Sindaco F. Prevention of post-herpetic neuralgia: acyclovir and prednisolone versus epidural local anesthetic and methylprednisolone. Acta Anaesthesiol Scand. 2000;44:910–918. doi: 10.1034/j.1399-6576.2000.440803.x. [DOI] [PubMed] [Google Scholar]
- Gomesz FAR, Wicks MA. The use of epidural blocks and trigeminal ganglion blocks in acute HZ to prevent PHN. 2nd Int Conf on the Varicella-Zoster Virus, Paris. 1994. p. D2.
- Moesker A, Boersma FP. The effect of extradural administration of corticosteroids as pain treatment of acute herpes zoster and to prevent postherpetic neuralgia. Proc The Pain Clinic 1. 1984. pp. 273–279.
- Haanpaa M, Dastidar P, Weinberg A, Levin M, Miettinen A, Lapinlampi A, Laippala P, Nurmikko T. CSF and MRI findings in patients with acute herpes zoster. Neurology. 1998;51:1405–1411. doi: 10.1212/wnl.51.5.1405. [DOI] [PubMed] [Google Scholar]
- Lundborg G. Structure and function of the intraneural microvessels as related to trauma, edema formation, and nerve function. J Bone Joint Surg Am. 1975;57:938–948. [PubMed] [Google Scholar]
- Rowbotham MC, Baron R, K.L. Petersen, Fields HL. Spectrum of pain mechanisms contributing to PHN. In: Watson CPN and Gershon AA, editor. Herpes zoster and postherpetic neuralgia. 2nd revised and enlarged. Amsterdam, Elsevier Science B.V.; 2001. pp. 183–195. [Google Scholar]
- Cousins MJ, Bridenbaugh PO. Neural blockade in clinical anesthesia and management of pain. Philadelphia, Lippencott - Raven; 1998. pp. 243–320. [Google Scholar]
- Sriwatanakul K, Kelvie W, Lasagna L, Calimlim JF, Weis OF, Mehta G. Studies with different types of visual analog scales for measurement of pain. Clin Pharmacol Ther. 1983;34:234–239. doi: 10.1038/clpt.1983.159. [DOI] [PubMed] [Google Scholar]
- Ware J. E., Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, Sprangers MA, te Velde A, Verrips E. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055–1068. doi: 10.1016/S0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Carrington D, Cunningham A, Kost RG, Levin MJ, McKendrick MW, Oxman MN, Rentier B, Schmader KE, Tappeiner G, Wassilew SW, Whitley RJ. Assessment of pain in herpes zoster: lessons learned from antiviral trials. Antiviral Res. 1997;33:73–85. doi: 10.1016/S0166-3542(96)01007-8. [DOI] [PubMed] [Google Scholar]
- Arani RB, Soong SJ, Weiss HL, Wood MJ, Fiddian PA, Gnann JW, Whitley R. Phase specific analysis of herpes zoster associated pain data: a new statistical approach. Stat Med. 2001;20:2429–2439. doi: 10.1002/sim.851.abs. [DOI] [PubMed] [Google Scholar]
- Desmond RA, Weiss HL, Arani RB, Soong SJ, Wood MJ, Fiddian PA, Gnann JW, Whitley RJ. Clinical applications for change-point analysis of herpes zoster pain. J Pain Symptom Manage. 2002;23:510–516. doi: 10.1016/S0885-3924(02)00393-7. [DOI] [PubMed] [Google Scholar]