Abstract
Several factors have been proposed to account for poor motor recovery after prolonged denervation, including motor neuron cell death and incomplete or poor regeneration of motor fibers into the muscle. Both may result from failure of the muscle and the distal motor nerve stump to continue expression of neurotrophic factors following delayed muscle reinnervation. This study investigated whether regenerating motor or sensory axons modulate distal nerve neurotrophic factor expression. We found that transected distal tibial nerve up-regulated brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) mRNA, down-regulated neuro-trophin-3 and ciliary neurotrophic factor mRNA, and that although these levels returned to normal with regeneration, the chronically denervated distal nerve stump continued to express these neurotrophic factors for at least 6 months following injury. A sensory nerve (the cutaneous saphenous nerve) sutured to distal tibial nerve lowered injury-induced BDNF and GDNF mRNA levels in distal stump, but repair with a mixed nerve (peroneal, containing muscle and cutaneous axons) was more effective. Repair with sensory or mixed nerves did not affect nerve growth factor or neurotrophin-3 expression. Thus, distal nerve contributed to a neurotrophic environment for nerve regeneration for at least 6 months, and sensory nerve repair helped normalize distal nerve neurotrophic factor mRNA expression following denervation. Furthermore, as BDNF and GDNF levels in distal stump increased following denervation and returned to control levels following reinnervation, their levels serve as markers for the status of regeneration by either motor or sensory nerve.
Keywords: mRNA, nerve injury, neurotrophins, rat, sciatic nerve
Following peripheral nerve injury, the freshly denervated nerve stump plays a role in providing the appropriate environment for axonal regeneration. Denervated muscle and Schwann cells of the distal nerve stump rapidly up-regulate many growth associated proteins, including neurotrophic factors, which are believed to aid regeneration and recovery (Fu and Gordon 1995; Raivich and Makwana 2007). However, if nerve regeneration does not occur promptly, the skeletal muscle and distal nerve stump gradually atrophy and lose receptivity to the regenerating axon over a period of several months (Irintchev et al. 1990; Schmalbruch et al. 1991; Fu and Gordon 1995; Bain et al. 2001; Borisov et al. 2001). This results in poor functional recovery, which is a serious clinical problem.
Following proximal peripheral nerve injury where poor motor recovery is predicted, most surgeons will perform a distal motor nerve transfer to hasten muscle reinnervation. In many situations a motor donor nerve is not available. We have previously shown that a sensory nerve sutured to the distal nerve stump during prolonged denervation (3–6 months) significantly improves distal nerve stump and skeletal muscle morphology and functional recovery of muscle (Hynes et al. 1997; Bain et al. 2001; Veltri et al. 2005). The sensory nerve also modulates neurotrophic factor expression in denervated muscle (Zhao et al. 2004). Thus, both distal stump and muscle benefit and respond to whatever category of nerve fiber reaches the distal target.
The distal nerve stump contributes to a neurotrophic environment immediately following injury, but this role is thought to be transitory. Most previous studies have followed the time course of neurotrophic factor expression in distal nerve stump in the short term, for days or weeks following injury. In sciatic nerve, brain-derived neurotrophic factor (BDNF) is up-regulated 3–7 days after transection, and levels remain highly increased for up to 4 weeks (Meyer et al. 1992; Funakoshi et al. 1993; Omura et al. 2005). Nerve growth factor (NGF) mRNA up-regulation after nerve transection exhibits two peaks, one at 6 h and another at 3 days, and this elevation lasts for up to 2 weeks (Heumann et al. 1987a,b). Glial cell line-derived neurotrophic factor (GDNF) mRNA levels are elevated after sciatic nerve crush or transection, peaking between days 2 and 7 and staying elevated for at least 5 months (Trupp et al. 1995; Hammarberg et al. 1996 and Naveilhan et al. 1997; Höke et al. 2000, 2002). In contrast, neurotrophin-3 (NT-3) mRNA decreases in distal stump as early as 6 h following injury, returning to normal levels by 1–2 weeks (Funakoshi et al.1993; Omura et al. 2005). Ciliary neurotrophic factor (CNTF) mRNA is highly expressed in intact nerve and is drastically reduced by 1 week after nerve transection (Sendtner et al. 1992; Smith et al. 1993; Ito et al. 1998), remaining at low levels for at least 1 year following crush injury (Sendtner et al. 1992). However, few studies have followed neurotrophic factor expression in distal nerve stump for the longer times typical of irreversible muscle atrophy. The objective of this study was to investigate the molecular changes in distal segment of injured nerve over a period of 6 months, with emphasis on the long-term contribution of sensory protection.
Materials and methods
Animals and surgical procedures
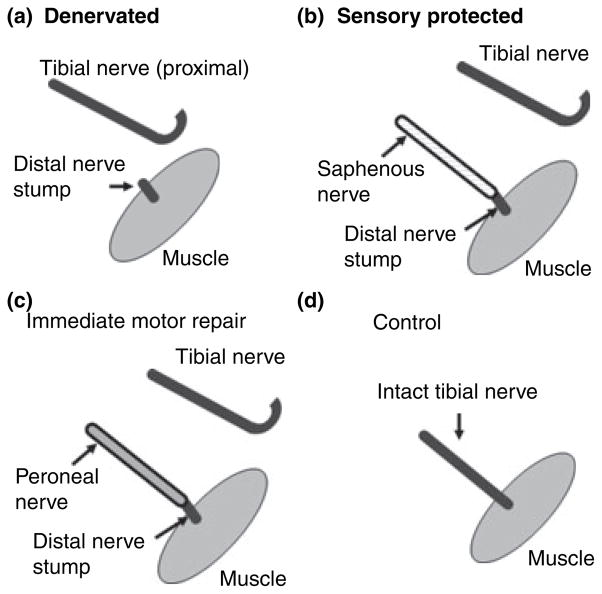
All procedures were carried out in accordance with Canadian Council on Animal Care guidelines and were approved by the Animal Research Ethics Board of McMaster University, ON, Canada. A total of 115 male Lewis rats weighing 200–250 g (Charles River, Saint-Constant, QC, Canada) were used in this study (see Table 1 for the number of animals in each group). Surgical procedures were carried out as previously described (Bain et al. 2001; Zhao et al. 2004). Briefly, the right gastrocnemius muscle of rat was denervated by transecting the tibial branch of the sciatic nerve (Fig. 1), and either (a) the proximal nerve stump was buried in the biceps femoris muscle to prevent regeneration (denervated group), (b) the saphenous nerve (a purely cutaneous sensory nerve) was sutured to the distal nerve stump (sensory protected group), or (c) the peroneal nerve (mixed motor and sensory nerve containing both muscle and cutaneous axons) was sutured to the distal nerve stump (immediate motor repair group). The contralateral unoperated tibial nerves and tibial nerves from naïve animals were used as controls (Fig. 1d). At 1–2 weeks and at 1, 2–3, and 6 months after transection, animals were killed by anesthetic overdose. The distal nerve in the denervated group was harvested from the tibial nerve transection site just proximal to the popliteal fossa to the entry zone of the gastrocnemius muscle. The intact tibial nerve on the contralateral side was harvested as a control. The distal stump from the sensory protected and immediate motor repair groups were similarly harvested, distal to the suture line with the saphenous or peroneal nerve to the muscle insertion site. The distal nerve stumps (~1 cm) were immediately snap-frozen in liquid nitrogen prior to storage at −80°C. The number of animals in each group was equivalent to the number of distal stumps as shown in Table 1.
Table 1.
Number of distal nerve stumps (shown in parentheses) and RNA pools in each group at different time points
| Group | Time
|
||||
|---|---|---|---|---|---|
| 1 week | 2 weeks | 1 month | 2–3 months | 6 months | |
| Control | 2 (7) | 2 (6) | 4 (14) | 4 (11) | 7 (23) |
| Immediate repair | 2 (4) | 2 (7) | 2 (7) | 5 (15) | 4 (8) |
| Denervated | 2 (7) | 2 (6) | 2 (8) | 4 (15) | 4 (8) |
| Sensory protected | 2 (4) | 2a (6) | 2 (8) | 4 (13) | 4 (8) |
One RNA extraction performed on a single distal stump. The number of animals (n) in each group is equivalent to the number of distal stumps. Distal stumps were pooled to obtain sufficient RNA for analysis.
Fig. 1.
The right gastrocnemius muscle of rat was denervated by transecting the tibial nerve, and either (a) the proximal nerve stump was buried in the biceps femoris muscle to prevent regeneration (denervated group), (b) the saphenous nerve was sutured to the distal nerve stump (sensory protected group), or (c) the peroneal nerve was sutured to the distal nerve stump (immediate repair group). Unoperated tibial nerves were used as controls (d).
Generation of real-time PCR standards
cDNA standards were generated by regular RT-PCR performed on randomly chosen rat RNA samples. Each 50 μL PCR reaction contained 300 or 150 nM primers, cDNA derived from 0.25 μg of rat RNA, 1.5 mM MgCl2, 0.2 mM deoxynucleotide triphosphates, and 1 U of platinum Taq polymerase (Invitrogen, Burlington, ON, Canada). The PCR reaction began with a hot start for 2 min at 94°C and then 40 cycles as described below for real-time PCR. For each target, four PCR reactions were pooled and separated in a preparative 1.8% agarose gel. The bands were excised and cDNA extracted with a Qiagen MinElute Gel extraction kit™ (Invitrogen). The concentration and purity of the cDNA was determined by absorbance at 260 and 280 nm. Aliquots of 1 ng/μL cDNA were stored at −80°C. Standards were diluted fresh for each experiment.
RNA extraction
For isolation of RNA, two to five distal stumps were pooled (with the exception of one isolation where one distal stump was used, as indicated). The total number of distal stumps used for real-time RT-PCR and the number pooled for RNA isolation at the different time points are shown in Table 1. Six additional RNA pools (not shown) were used for analysis of RNA yield. Tissue was homogenized on ice in a Duall type all-glass tissue grinder (Kimble/Kontes, Vineland, NJ, USA) in the presence of Trizol™ (Invitrogen). The manufacturer’s protocol was followed through the collection of the RNA-containing aqueous phase. The RNA was further purified using an RNeasy™ Mini Kit (Qiagen, Mississauga, ON, Canada). RNA eluted from the column was concentrated to ~9 μL in a SpeedVac® concentrator (Savant Instruments, Holbrook, NY, USA). Concentration and purity of RNA were determined by absorbance at 260 and 280 nm. All samples exhibited absorbance ratios (260/280) > 1.7.
Real-time RT-PCR
One microgram of each RNA pool was DNase treated (Ambion, Austin, TX, USA) and used for RT with Superscript II according to the manufacturer’s protocol (Invitrogen). Randomly chosen RNA samples were verified as free of DNA contamination by running an RT-PCR negative control lacking reverse transcriptase. RT was carried out in 20-μL reactions in the GeneAmp PCR system 2400 thermal cycler (Applied Biosystems, Streetsville, ON, Canada) at 25°C for 10 min, 42°C for 50 min, and 70°C for 15 min. Each 20-μL RT product was subjected in different reactions to real-time PCR for BDNF, GDNF, CNTF, NGF, and NT-3. All primers were generated with PRIMER3 software (freeware program online, http://www.frodo.wi.mit.edu/, Rozen and Skaletsky 2000) and were synthesized at the Central Facility of the Institute for Molecular Biology and Biotechnology at McMaster University. Primers, their sequences and product sizes are shown in Table 2. For all reactions, 300 nM of the forward and reverse primers were used. In addition, each 20-μL real-time PCR reaction contained 10 μL of SYBR Green qPCR Supermix UDG™ (Invitrogen), 30 nM of reference dye ROX (Stratagene, La Jolla, CA, USA or Invitrogen) and cDNA derived from 50 ng RNA sample (25 ng for CNTF) or standards from 1 pg to 10 ag (5 ag for BDNF and GDNF). Amplifications were carried out in triplicate for samples and standards and in duplicate for controls lacking template and lacking RT, using the MX3000P real-time PCR system (Stratagene) and the following thermal profile: 2 min at 50°C, 2 min at 95°C followed by 40 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 45 s. Standard curve R2 values were ≥ 0.998 and efficiencies were between 88% and 110%, with the exception of one out of five experiments with NGF primers where the efficiency was 84% with R2 = 1. Following PCR, a dissociation curve was added to determine if any secondary products had formed. For NT-3 and GDNF, the dissociation curves revealed the presence of small amounts of secondary products. To eliminate fluorescence readings caused by formation of the secondary products, the thermal profile for GDNF and NT-3 contained a segment of 83°C for 15 s at the end of each cycle. Data were collected and analyzed both at 72°C and 83°C, with no significant differences in the results. Data collected at 72°C are reported here.
Table 2.
Sequences and product sizes of PCR primers
| BDNF (product size 238 bp) | |
| Forward primer | 5′-GCGGCAGATAAAAAGACTGC |
| Reverse primer | 5′-GCCAGCCAATTCTCTTTTTG |
| GDNF (product size 242 bp) | |
| Forward primer | 5′-CCCGAAGATTATCCTGACCA |
| Reverse primer | 5′-TAGCCCAAACCCAAGTCAGT |
| CNTF (product size189 bp) | |
| Forward primer | 5′-CACCCCAACTGAAGGTGACT |
| Reverse primer | 5′-ACCTTCAAGCCCCATAGCTT |
| NGF (product size 168 bp) | |
| Forward primer | 5′-ACCTCTTCGGACACTCTGGA |
| Reverse primer | 5′-GTCCGTGGCTGTGGTCTTAT |
| NT-3 (product size 182 bp) | |
| Forward primer | 5′-GATCCAGGCGGATATCTTGA |
| Reverse primer | 5′-AGCGTCTCTGTTGCCGTAGT |
BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; NGF, nerve growth factor; NT-3, neurotrophin-3; GDNF, glial cell line-derived neurotrophic factor.
Axon number and morphometry
To evaluate the nerve fiber population regenerating into the distal stump, three distal segments were procured from each experimental group at 3 months after surgery and fixed in Karnowski’s fixative (4% p-formaldehyde and 5% glutaraldehyde in 0.1 M phosphate buffer), serially dehydrated in ethanol, and embedded in epon resin. Semi-thin sections (1 μm) were cut, stained with toluidine blue, and examined under light microscopy. For each segment, number of axons per nerve area, mean diameter of the axons, mean area of the axons, and mean area of the myelin were determined using Metamorph software (Molecular Devices Corporation, Downing-town, PA, USA).
Statistical analysis
Statistical analyses were performed using SPSS 15.0 software (SPSS, Chicago, IL, USA). The mean of triplicate samples was expressed as number of mRNA copies/50 ng RNA (25 ng for CNTF). A one-way ANOVA followed by post hoc Tukey test was used to analyze differences in total RNA yield for the mean of all operated groups at different time points and to determine the significance of differences in mRNA copy numbers between groups at each time point. Differences were considered significant at p < 0.05.
Results
Yield of total RNA
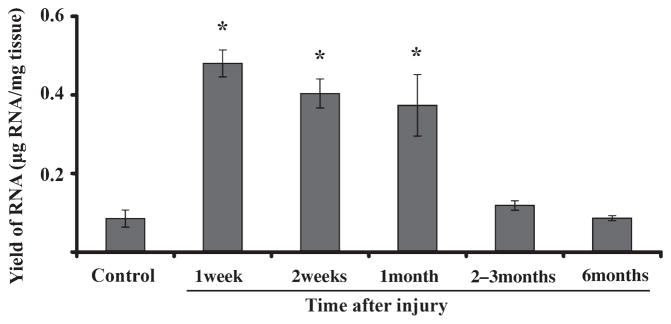
A one-way ANOVA (p < 0.001) followed by post hoc Tukey test revealed a highly significant increase in mean yield of RNA from distal nerve stumps for all operated groups combined compared with unoperated controls at 1 and 2 weeks and 1 month (Fig. 2, p < 0.001). The increased amount of RNA per mg tissue returned to normal by 2–3 months following surgery, a time coinciding with the change from reversible to irreversible atrophy.
Fig. 2.
Increased RNA in distal nerve stumps of operated groups at 1 and 2 weeks and 1 month compared with controls (*p < 0.001). Control represents mean of all intact tibial nerves from all time points. Each time point represents the mean of all operated groups from that time. Error bars represent SEM.
Controls
For each time point, analysis of mRNA copy number for each target was performed on 6–23 control unoperated nerves (from 6 to 23 animals) processed in two to seven RNA pools (Table 1). Control nerves were obtained from naïve rats and from contralateral unoperated limbs of experimental animals. At the 1 month time point, eight distal stumps from naïve, unoperated rats were compared with six distal stumps from contralateral, unoperated limbs of tested animals. A two-tailed Student’s t-test revealed there were no differences in copy number for any of the mRNA targets between those two groups, and therefore they were combined for further analysis.
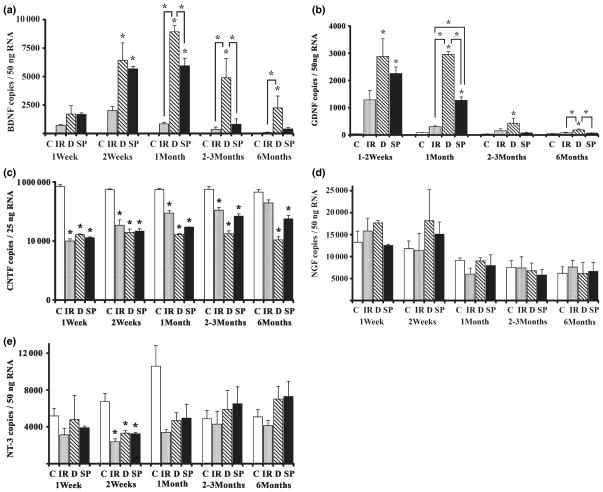
Brain-derived neurotrophic factor
There were significant differences in BDNF mRNA levels between groups at each time point from 2 weeks to 6 months (p < 0.05; Fig. 3a). BDNF was barely detectable only in a subset of control, unoperated nerves. BDNF mRNA levels were not significantly different from controls at any of the time points for the immediate repair group (regeneration with an ‘appropriate’ nerve). BDNF mRNA copy numbers for denervated and sensory protected groups were highly increased and similar for both groups at 2 weeks (p < 0.05 between each group and controls), reaching a peak at 1 month (p < 0.001) and declining thereafter in both groups. In the denervated group, BDNF mRNA remained significantly elevated above control levels for at least 6 months (p < 0.01), whereas BDNF mRNA copy numbers in sensory protected samples returned gradually to control levels. In sensory protected animals, BDNF mRNA levels were significantly lower than in denervated samples beginning at 1 month and returned to control levels beginning at 2–3 months (p > 0.05).
Fig. 3.
Temporal mRNA expression of BDNF (a), GDNF (b), CNTF (c), NGF (d), and NT-3 (e) in distal nerve stump after tibial nerve transection. C, control; IR, immediate repair; D, denervated, SP, sensory protected. Asterisks (*) placed directly above standard error bars indicate a significant difference between a particular operated group and control at this time point (p < 0.05). Significant differences between other groups are indicated by bars. Error bars represent SEM.
Glial cell line-derived neurotrophic factor
Glial cell line-derived neurotrophic factor mRNA levels at 1 and 2 weeks were not significantly different from each other [two-way ANOVA (group × time)]; therefore, these two time points were combined for further analysis. Like BDNF, GDNF mRNA copy numbers were low in intact control nerve and in the immediate repair group (regeneration with an ‘appropriate’ nerve; p > 0.05 for immediate repair compared with control at all time points). Also like BDNF, GDNF mRNA levels were highly elevated in sensory protected and denervated groups 1–2 weeks after transection (Fig. 3b). In the denervated group, GDNF levels remained elevated at 1 month (p < 0.001), declining thereafter but remaining elevated above controls. In the sensory protected group, GDNF levels peaked earlier than in the denervated group, at 1–2 weeks, and began to decline earlier than in the denervated group (p < 0.05). At 1 month, GDNF mRNA copies differed dramatically among all four groups, with sensory protected samples still significantly increased over control and immediate repair (p < 0.001) groups, but significantly lower than in denervated (p < 0.001). GDNF levels in the sensory protected group returned to control levels by 2–3 months, whereas at 6 months GDNF mRNA in the denervated group was still significantly greater than in immediate repair (p < 0.01) and sensory protected groups (p < 0.01).
Ciliary neurotrophic factor
Ciliary neurotrophic factor mRNA was highly expressed in intact control nerve (Fig. 3c). Its levels were dramatically decreased at 1 week after surgery in all operated groups (p < 0.001) and remained at significantly lower levels than controls for 6 months, with the exception of the immediate repair group. CNTF mRNA in the immediate repair group (regeneration with an ‘appropriate’ nerve) began to rise towards normal levels by 1 month and by 6 months did not differ significantly from controls (p > 0.05), while the sensory protected and denervated groups continued to be depressed. CNTF expression in the sensory protected group was not significantly different from either denervated or immediate repair groups at any time point (p > 0.05). However, at longer time points there was a trend towards normal levels of CNTF mRNA in the sensory protected group, with this group exhibiting CNTF levels intermediate between immediate repair and denervated groups.
Nerve growth factor and neurotrophin-3
There were no significant differences in NGF mRNA between groups at any time point (Fig. 3d). NT-3 mRNA was down-regulated in all operated groups compared with controls, but only at the 2-week time point (Fig. 3e, p < 0.05 at 2 weeks, p > 0.05 at all other times). At 2 weeks, a post hoc Tukey test revealed no differences between sensory protected, immediate repair, and denervated groups. NT-3 mRNA returned to control levels in all operated groups by 1 month following transection.
Axon numbers and morphometry in distal segments
There were no regenerating axons found at 3 months after surgery in the distal stumps of the denervated group. The number of regenerating axons in sensory protected and immediate repair groups were significantly different from the denervated group (Fig. 4a, p < 0.05), but there was no significant difference in fiber numbers between the sensory protected and the immediate repair groups (Fig. 4a, p > 0.05). Mean diameter of the regenerating axons of the sensory protected and immediate repair groups were also not different (Fig. 4b, p > 0.05). Regenerating groups did not differ in mean area of the regenerating axons or mean area of the myelin, although there was trend towards smaller axon and myelin area in the regenerating saphenous nerve compared with the peroneal nerve (Fig. 4c, p > 0.05).
Fig. 4.
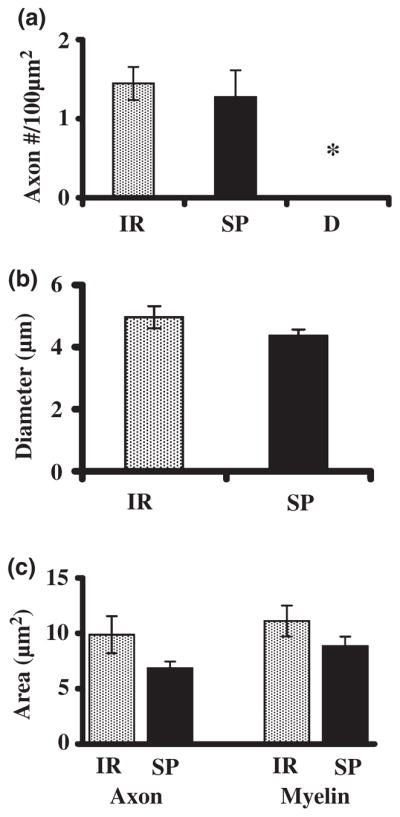
No difference between sensory and mixed motor nerves in number of regenerating axons, axon diameter, axon area, or myelin area in distal stump (p > 0.05). (a) number of regenerating axons per 100 μm2 of nerve area, (b) mean axon diameter, (c) mean axon and myelin area. Asterisk (*) indicates significant difference between denervated group (D) and immediate repair (IR) or sensory protected (SP), post hoc Tukey test p < 0.05. Number of animals: IR n = 5, SP n = 5, and D n = 4. Error bars represent SEM.
Discussion
Following nerve injury, regeneration and recovery of function can occur if reinnervation is not delayed. However, at 2–3 months post-injury and longer, in the absence of successful nerve regeneration, the muscle suffers irreversible atrophy that cannot be overcome by the regenerating nerve (Fu and Gordon 1995; Kobayashi et al. 1997; Bain et al. 2001). Changes in the profile of proteins synthesized by muscle 2–3 months after nerve injury contribute substantially to irreversible muscle atrophy (Batt et al. 2006). It has been proposed that the collagenization of the distal stump with concomitant blockage of the pathway for regenerating axons is an additional barrier to recovery (Fu and Gordon 1995; Veltri et al. 2005). Another suggested mechanism is the failure of Schwann cells in the distal stump to continue secreting nerve growth-promoting trophic factors (Fu and Gordon 1995). We show in this study that the denervated distal nerve stump can, in fact, continue to express neurotrophic factor mRNA, specifically elevated levels of BDNF and GDNF and control levels of NGF and NT-3, for at least 6 months, suggesting that loss of distal stump neurotrophic factor expression is not a mechanism of irreversible muscle atrophy.
We have previously shown that cross-repair of a sensory nerve to denervated muscle (sensory protection) protects the muscle from irreversible atrophy and allows partial recovery of function after 3–6 months (Hynes et al. 1997; Bain et al. 2001). We also previously showed that the sensory nerve modulates GDNF mRNA in muscle (Zhao et al. 2004). Furthermore, the sensory nerve reduces collagenization of the distal stump, allowing improved regeneration of the motor axons through this structure (Veltri et al. 2005). In this study, we investigated whether the beneficial effects of sensory protection could also be mediated by the promotion of higher levels of neurotrophic factors in the injured distal nerve stump. On the contrary, we found that sensory protection reduces denervation-induced changes in neurotrophic factor levels. Repair with an appropriate mixed motor and sensory nerve (peroneal nerve, containing both muscle and cutaneous axons) reduces denervation-induced BDNF and GDNF expression, while sensory protection with the purely cutaneous, sensory, saphenous nerve also causes a reduction, albeit somewhat more slowly. Nevertheless, the reduction is much greater than if the distal nerve stump remains denervated.
The poorer efficacy of the sensory nerve compared with repair with an appropriate mixed nerve is not because of a smaller number of regenerating axons in the distal stump of the sensory protected group. As expected, there were significantly greater numbers of axons regenerating into the distal stumps of the immediate repair and sensory protected groups compared with the denervated group. However, the number of axons in the distal stumps 3 months after surgery did not differ between immediate repair and sensory protected groups. Furthermore, there were no significant differences in mean axon diameter, mean axon area, or mean myelin area between regenerating groups. Thus, the tibial distal stump supports regeneration of both types of nerve, sensory saphenous nerve and mixed peroneal nerve. Another possible reason for differential expression of growth factors in response to sensory versus motor nerves is that Schwann cells express motor and sensory phenotypes (Höke et al. 2006). It is therefore possible that sensory protection is not as effective in normalizing trophic factor expression as ‘appropriate’ innervation because of the absence of the motor component in the regenerating saphenous nerve.
Sensory protection modulates expression of BDNF and GDNF mRNA in distal nerve stump
Our findings of robust up-regulation of BDNF mRNA in distal stump for up to 1 month after nerve transection are in agreement with previous reports (Meyer et al. 1992; Funakoshi et al. 1993; Omura et al. 2005). Furthermore, we show here that levels of BDNF mRNA in denervated distal stump remain significantly increased above control levels for at least 6 months, a time when irreversible muscle atrophy has occurred (Irintchev et al. 1990; Schmalbruch et al. 1991; Bain et al. 2001; Borisov et al. 2001; Veltri et al. 2005). Both mixed and sensory nerves sutured to the distal nerve stump during prolonged denervation significantly lower BDNF mRNA expression in distal nerve stump compared with the denervated condition. At 2–3 and 6 months following transection and repair, the levels of BDNF mRNA in sensory protected distal stump are not significantly different from immediate repair or control nerves, demonstrating that sensory protection returns the BDNF mRNA expression profile to normal levels.
We confirmed the previously reported up-regulation of GDNF mRNA in the few weeks after nerve transection and the slow decline towards control levels by 6 months in denervated distal stump (Hammarberg et al. 1996; Naveilhan et al. 1997; Höke et al. 2000, 2002). In our study, however, GDNF levels remain significantly above control levels (fourfold up-regulation) at 6 months. Sensory protection, similar to BDNF, ameliorates increased GDNF mRNA levels following denervation, although this difference is statistically significant only at 1 and 6 months. Unlike BDNF, sensory protection also shortens the duration of peak GDNF expression from 1 month in denervated animals to 2 weeks, suggesting that sensory protection accelerates the return of GDNF expression to control levels. Immediate repair with an appropriate mixed motor and sensory rather than a sensory nerve further reduces peak GDNF expression towards control levels at all points, implicating regenerating motor axons in further normalization of GDNF levels.
Sensory protection does not affect expression of CNTF, NGF, or NT-3 mRNA in distal nerve stump
We found little to no influence of sensory protection on CNTF, NGF, or NT-3 mRNA levels. We confirmed in our study the drastic reduction of CNTF mRNA after denervation (Sendtner et al. 1992; Smith et al. 1993; Ito et al. 1998). The decrease is sustained for at least 6 months in denervated and sensory protected groups, in agreement with data demonstrating a lack of full recovery for up to 1 year (Sendtner et al. 1992). In the immediate repair group, CNTF mRNA copy numbers gradually return towards normal levels and are not significantly different from controls by 6 months. There was also a trend for CNTF mRNA to return towards normal levels in the sensory protected group, albeit more slowly than in the immediate repair group, consistent with sensory protection’s ability to ameliorate the effects of denervation on other neurotrophic factors.
Previous studies have shown that NGF mRNA is elevated in denervated distal stump for up to 2 weeks following injury (Heumann et al. 1987a). We did not detect a significant increase in NGF mRNA in denervated distal stump compared with controls at any time point, although we did find that the mean level of NGF in all groups combined at 1–2 weeks is elevated compared with later time points. The lack of increase in NGF mRNA at the 1–2 weeks time point is not surprising, given that we assayed the distal portion of the tibial nerve near the entry point of the gastrocnemius muscle. This segment most likely corresponds to segment E and possibly segment D in the Heumann study (Heumann et al. 1987a). Segment E in particular exhibited very little NGF up-regulation compared with more proximal segments, and the initial up-regulation at 6 h was barely detectable. Furthermore, there was little difference in NGF mRNA levels between denervated and regenerating groups in distal stump (Heumann et al. 1987b). In agreement with these data, we show that there is no significant effect of repair with either a mixed or sensory nerve on NGF levels at any time point in our study.
Previous studies have shown that NT-3 mRNA decreases in distal stump as early as 6 h following injury, returning to control levels by 1–2 weeks (Funakoshi et al.1993; Omura et al. 2005). Our NT-3 data are consistent with these observations. Down-regulation of NT-3 is significant for all groups only at 2 weeks, while at 1 week there is a trend towards NT-3 down-regulation. At 1 month and later times, NT-3 mRNA levels return to control levels in all operated groups. Similar to NGF, we saw no apparent effects of sensory protection on NT-3 levels in distal nerve stump.
RNA yield and protein expression
The observed increase in yield of total RNA from the distal stump 1 and 2 weeks and 1 month following nerve transection is in agreement with reported up-regulation of 18S ribosomal RNA in distal stump from 12 h to 14 days after sciatic nerve transection (Heumann et al. 1987a). This increase in RNA does not, however, reflect a global increase in all mRNAs, as we demonstrate here the specific up-regulation of BDNF and GDNF, down-regulation of CNTF and NT-3, and no significant change in NGF mRNA. Furthermore, these changes in neurotrophic factor mRNAs are entirely consistent with the literature demonstrating comparable changes in the proteins. BDNF protein following sciatic nerve transection is continuously up-regulated from day 4 following transection, reaching highest levels at day 28 (Omura et al. 2005). GDNF protein changes also parallel the expression of GDNF mRNA, with GDNF protein up-regulated at 1 month and back to control levels at 6 months (Höke et al. 2002). We have verified this data by ELISA in our own samples (data not shown). CNTF protein is present in the distal segment of the nerve and is decreased following injury (Sendtner et al. 1992). Similarly, NGF protein mirrors changes in mRNA in distal nerve after injury (Heumann et al. 1987a). Less is known about NT-3 protein. One study reported significant increases in NT-3 protein 14 days after crush and 28 days after sciatic nerve transection (Omura et al. 2005).
Neurotrophin receptor expression
Our study clearly shows that injury and reinnervation with either a mixed or a sensory nerve dramatically regulates neurotrophic factor levels in the distal stump. The distal stump also regulates co-receptors for these neurotrophic factors, which can aid in presentation of the neurotrophic factors to signaling receptors on the regenerating motor nerve (Naveilhan et al. 1997). The low affinity NT receptor, p75NTR, is strongly and consistently up-regulated in the distal stump from 36 h after nerve transection up to at least 4 months (Heumann et al. 1987a,b; Taniuchi et al. 1988; You et al. 1997). The truncated high-affinity BDNF receptor TrkB undergoes slight changes after nerve injury, although it is not clear in which direction (Frisen et al. 1993; Funakoshi et al. 1993). The truncated high-affinity NT-3 receptor TrkC is down-regulated hours after injury but recovers quickly and by 2 weeks is twofold increased (Funakoshi et al. 1993). Injury of sciatic nerve induces up-regulation of GDNF receptor-α mRNA, which presents increased GDNF to Ret receptors on the motor axon (Naveilhan et al. 1997). In contrast to CNTF mRNA, which is dramatically decreased in the distal stump, its receptors CNTFRα and gp130 gradually increase after injury (Ito et al. 1998), possibly as a compensatory mechanism.
Conclusions
In conclusion, we report that tibial nerve transection induces up-regulation of BDNF and GDNF mRNA and down-regulation of CNTF and NT-3 mRNA in the distal nerve stump in the weeks immediately following injury. At later time points (2–6 months in this study) corresponding to the appearance of irreversible muscle atrophy, BDNF and GDNF expression remains elevated above control values in the distal nerve stump, NT-3 levels return to normal, while CNTF mRNA remains depressed. These data demonstrate the ability of the distal nerve stump to contribute to a neurotrophic environment for nerve regeneration for at least 6 months following injury. In addition, we show that repair with either a mixed motor nerve or a sensory nerve normalizes BDNF and GDNF mRNA levels in distal nerve stump and accelerates the return of GDNF mRNA expression to control levels. We have previously shown that the sensory nerve normalizes GDNF expression in muscle. Therefore, BDNF and GDNF may serve as markers of the state of communication between a nerve and its target, independent of the type of nerve.
Acknowledgments
This work was supported by a grant from the Neuromuscular Research Partnership (NRP) to MF and JRB. The NRP is an alliance of the Amyotrophic Lateral Sclerosis Society of Canada (ALS Canada), Muscular Dystrophy Canada (MDC), and the Canadian Institutes of Health Research. We thank Marcia Reid for help with axon counts and morphometry.
Abbreviations used
- BDNF
brain-derived neurotrophic factor
- CNTF
ciliary neurotrophic factor
- GDNF
glial cell line-derived neurotrophic factor
- NGF
nerve growth factor
- NT-3
neurotrophin-3
References
- Bain JR, Veltri KL, Chamberlain D, Fahnestock M. Improved functional recovery of denervated skeletal muscle after temporary sensory nerve innervation. Neuroscience. 2001;103:503–510. doi: 10.1016/s0306-4522(00)00577-7. [DOI] [PubMed] [Google Scholar]
- Batt J, Goncalves J, Michalski B, Bain J, Fahnestock M, Woodgett J. Gene expression profiling of long term denervated muscle. FASEB J. 2006;20:115–117. doi: 10.1096/fj.04-3640fje. [DOI] [PubMed] [Google Scholar]
- Borisov AB, Dedkov EL, Carlson BM. Interrelations of myogenic response, progressive atrophy of muscle fibers and cell death in denervated skeletal muscle. Anat Record. 2001;264:203–218. doi: 10.1002/ar.1155. [DOI] [PubMed] [Google Scholar]
- Frisen J, Verge VM, Fried K, Risling M, Persson H, Trotter J, Hokfelt T, Lindholm D. Characterization of glial trkB receptors: differential response to injury in the central and peripheral nervous systems. Proc Natl Acad Sci USA. 1993;90:4971–4975. doi: 10.1073/pnas.90.11.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg H, Piehl F, Cullheim S, Fjell J, Hokfelt T, Fried K. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7:857–860. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987a;104:1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R, Lindhom D, Bandtlow C, et al. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerves during development, degeneration and regeneration: role of macrophages. Proc Natl Acad Sci USA. 1987b;84:8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höke A, Cheng C, Zochodne DW. Expression of glial cell line-derived neurotrophic factor family of growth factors in peripheral nerve injury in rats. Neuroreport. 2000;11:1651–1654. doi: 10.1097/00001756-200006050-00011. [DOI] [PubMed] [Google Scholar]
- Höke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- Höke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes NM, Bain JR, Thoma A, Veltri K, Maguire JA. Preservation of denervated muscle by sensory protection in rats. J Reconstr Microsurg. 1997;13:337–343. doi: 10.1055/s-2007-1006413. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Draguhn A, Wernig A. Reinnervation and recovery of mouse soleus muscle after long-term denervation. Neuroscience. 1990;39:231–243. doi: 10.1016/0306-4522(90)90236-w. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yamamoto M, Li M, Doyu M, Tanaka F, Mutch T, Mitsuma T, Sobue G. Differential expression of mRNAs for ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), interleukin-6 (IL-6), and their receptors (CNTFRα, LIFRβ, IL-6Rα and gp130) in injured peripheral nerves. Brain Res. 1998;793:321–327. doi: 10.1016/s0006-8993(98)00242-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Mackinnon SE, Watanabe O, Ball DJ, Gu XM, Hunter DA, Kuzon WM., Jr The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve. 1997;20:858–866. doi: 10.1002/(sici)1097-4598(199707)20:7<858::aid-mus10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119:45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Omura T, Sano M, Omura K, Hasegawa T, Doi M, Sawada T, Nagano A. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J Peripher Nerv Syst. 2005;10:293–300. doi: 10.1111/j.1085-9489.2005.10307.x. [DOI] [PubMed] [Google Scholar]
- Raivich G, Makwana M. The making of successful axonal regeneration: genes, molecules and signal transduction pathways. Brain Res Rev. 2007;53:287–311. doi: 10.1016/j.brainresrev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa: 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H, Al-Amood WS, Lewis DM. Morphology of long-term denervated rat soleus muscle and the effect of chronic electrical stimulation. J Physiol. 1991;441:233–241. doi: 10.1113/jphysiol.1991.sp018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendtner M, Stockli KA, Thoenen H. Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration. J Cell Biol. 1992;118:139–148. doi: 10.1083/jcb.118.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Rabinovsky ED, McManaman JL, Shine HD. Temporal and spatial expression of ciliary neurotrophic factor after peripheral nerve injury. Exp Neurol. 1993;121:239–247. doi: 10.1006/exnr.1993.1091. [DOI] [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, Schwitzer JB, Johnson EM., Jr Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltri K, Kwiecien JM, Minet W, Fahnestock M, Bain JR. Contribution of the distal nerve sheath to nerve and muscle preservation following denervation and sensory protection. J Reconstr Microsurg. 2005;21:57–70. doi: 10.1055/s-2005-862783. discussion 71–74. [DOI] [PubMed] [Google Scholar]
- You S, Petrov T, Chung PH, Gordon T. The expression of the low affinity nerve growth factor receptor in long-term denervated Schwann cells. Glia. 1997;20:87–100. doi: 10.1002/(sici)1098-1136(199706)20:2<87::aid-glia1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Zhao C, Veltri K, Li S, Bain JR, Fahnestock M. NGF, BDNF, NT-3, and GDNF mRNA expression in rat skeletal muscle following denervation and sensory protection. J Neurotrauma. 2004;21:1468–1478. doi: 10.1089/neu.2004.21.1468. [DOI] [PubMed] [Google Scholar]