Abstract
In the amphibian intestine during metamorphosis, stem cells appear and generate the adult absorptive epithelium, analogous to the mammalian one, under the control of thyroid hormone (TH). We have previously shown that the adult stem cells originate from differentiated larval epithelial cells in the Xenopus laevis intestine. To clarify whether TH signaling in the epithelium alone is sufficient for inducing the stem cells, we have now performed tissue recombinant culture experiments, using transgenic X. laevis tadpoles that express a dominant positive TH receptor (dpTR) under a control of heat shock promoter. Wild-type (Wt) or dpTR transgenic (Tg) larval epithelium (Ep) was isolated from the tadpole intestine, recombined with homologous or heterologous non-epithelial tissues (non-Ep), and then cultivated in the absence of TH with daily heat shocks to induce transgenic dpTR expression. Adult epithelial progenitor cells expressing sonic hedgehog became detectable on day 5 in both the recombinant intestine of Tg Ep and Tg non-Ep (Tg/Tg) and that of Tg Ep and Wt non-Ep (Tg/Wt). However, in Tg/Wt intestine, they did not express other stem cell markers such as Musashi-1 and never generated the adult epithelium expressing a marker for absorptive epithelial cells. Our results indicate that, while it is unclear why some larval epithelial cells dedifferentiate into adult progenitor/stem cells, TR-mediated gene expression in the surrounding tissues other than the epithelium is required for them to develop into adult stem cells, suggesting the importance of TH-inducible epithelial-connective tissue interactions in establishment of the stem cell niche in the amphibian intestine.
Keywords: thyroid hormone receptor, transgenic frog, organ culture, tissue interaction, intestinal remodeling
INTRODUCTION
In the mammalian digestive tract, the epithelium is continually renewed from stem cells throughout adulthood. Mechanisms regulating proliferation and differentiation of stem cells have attracted interest from the standpoint of stem cell biology and regenerative therapies. Although the importance of the microenvironment around the stem cells, called a “niche”, for regulating the stem cells has been well recognized [1–4], molecular bases for the stem cell niche are still poorly understood. Especially, it remains uncertain how the gut stem cell niche is established during development because of the difficulty of identifying and monitoring the stem cells in uterus.
During amphibian metamorphosis, the digestive tract undergoes extensive remodeling from larval to adult form, to transition from the aquatic herbivorous to terrestrial carnivorous life [5]. In the Xenopus laevis small intestine, a well-analyzed organ at the cellular level, a single layer of primary (larval) epithelium is surrounded by the immature connective tissue and thin muscles before metamorphosis. At the start of metamorphic climax (stage 60; [6]) when the level of thyroid hormone (TH) in the plasma becomes high [7], most of the larval epithelial cells begin to undergo apoptosis, whereas a small number of undifferentiated cells become detectable as small islets between the larval epithelium and the developing connective tissue [8–11]. These undifferentiated cells are stained strongly red with pyronin Y and, notably, express sonic hedgehog (Shh), Musashi-1 (Msi-1), and Akt [12–14], all of which are candidate markers for mammalian intestinal stem cells [15–18]. They gradually replace the degenerating larval epithelium through active proliferation and, as morphogenesis of multiple intestinal folds proceeds, differentiate into the secondary (adult) epithelium that consists of major absorptive cells expressing intestinal fatty acid-binding protein (IFABP), goblet cells, and enteroendocrine cells [19–21]. At the completion of metamorphosis the adult epithelium acquires a cell renewal system along the trough-crest axis of intestinal folds [8, 10, 11], analogous to the mammalian crypt-villus axis [22, 23]. These chronological observations indicate that multipotent stem cells analogous to those in the mammalian adult intestine appear at the start of metamorphic climax in the X. laevis intestine. Taken together with the fact that the X. laevis intestinal remodeling can be easily and experimentally induced by TH both in vivo and in vitro [24] and that a number of TH response genes have been identified in the X. laevis intestine [25, 26], this animal model offers an excellent opportunity to study molecular mechanisms regulating organ-specific adult stem cells common to various vertebrates.
Using transgenic (Tg) X. laevis tadpoles constitutively expressing GFP in tissue recombination experiments, we have recently shown that the adult stem cells originate exclusively from the larval epithelium [27]. Since all of the larval epithelial cells at this stage are essentially differentiated as larval-type by light and electron microscopy [8, 28] and are negative for stem cell markers such as Shh and Msi-1 [13, 14], this result implies that some of the larval epithelial cells dedifferentiate into the adult stem cells during amphibian metamorphosis, similar to “epithelial transit cells” which are known to dedifferentiate into the stem cells during regeneration of the adult mammalian intestine [3, 29]. Despite the biological and clinical importance of intestinal epithelial dedifferentiation, its molecular mechanisms have not yet been clarified. In the X. laevis intestine, the developmental transition from the larval to adult epithelium including the period when dedifferentiation occurs, is triggered by TH and requires the presence of the connective tissue [30]. Thus, the question arises whether TH must act directly on the larval epithelium, the connective tissue, or both in order for adult epithelial development to occur. In the present study, to address this question and investigate the molecular mechanisms underlying developmental of intestinal stem cells, we made use of Tg tadpoles which express a dominant positive thyroid hormone receptor (dpTR) under the control of a heat shock-inducible promoter [31]. In these Tg tadpoles, dpTR specifically binds to TH response elements within promoter regions of TH target genes and causes metamorphic changes in the absence of TH in vivo. The use of these Tg tadpoles in tissue recombinant cultures, established previously [30], paves the way to activate TH target genes in any tissue at any time by heat shocks. Here, we isolated intestinal tissues from dpTR Tg or wild-type (Wt) tadpoles and examined effects of tissue-specific expression of dpTR on the adult epithelial development. Our results indicate that, regardless how the cell fate is determined in the larval epithelium, TH target genes in the surrounding tissues other than the epithelium are required for the formation of the adult stem cells.
MATERIALS AND METHODS
Animals
Adult South African clawed frogs (Xenopus laevis) were purchased from a commercial source. F0 Tg frogs expressing dpTR were generated by using the restriction enzyme mediated integration method as described previously [31]. F1 generation animals were produced by mating F0 Tg and Wt adult frogs. Tg F1 tadpoles were identified by the GFP expression in the lens of the eye [31] and reared until stage 57 [6]. Animal rearing and treatment were done according to the guidelines set by Nippon Medical School animal use and care committee.
Organ culture
Tubular fragments were isolated from the anterior part of the small intestine just behind the bile duct junction in Wt and Tg tadpoles at stage 57, when the small intestine is the longest during the larval period, and were split open lengthwise with scissors. Most of the intestines were treated with 1000 U/ml dispase (Godo, Tokyo, Japan) to separate the epithelium (Ep) from non-epithelial tissues (non-Ep), which consist mainly of the connective tissue and muscles. Each Ep was then recombined with homologous and heterologous non-Ep, or put on a matrigel (BD Biosciences, Bedford, MA, USA). They were then cultured at 26°C for 5 or 7 days as previously described [14, 30]. Briefly, they were placed on membrane filters (Millipore, Bedford, MA, USA) put on steel grids in culture dishes and cultured in 60% Leibovitz’s L-15 medium (Life technologies, Carlsbad, CA, USA) supplemented with 10% charcoal-treated FBS (Invitrogen), 100 IU/ml of penicillin, 100 μg/ml of streptomycin, 5 μg/ml of insulin (Sigma, St. Louis, MO, USA), and 0.5 μg/ml of hydrocortisone (Sigma). The culture medium was changed every other day. To induce metamorphic changes in the absence of TH, the intestines were heat shocked at 33°C for 1 h per day by moving the culture dish to a pre-warmed incubator. Since heat shocks after 5 days often caused abnormal differentiation of the adult epithelium (data not shown), the intestines were heat shocked until day 5. This abnormality may be caused by overexpression of dpTR-mediated genes such as Shh, whose expression normally decreases in Wt intestine after 5 days of TH treatments [13].
Immunohistochemistry
The cultured intestines were fixed with 95% ethanol at 4°C for 4 h, embedded in paraffin, and cut at 5 μm. Some sections were immunostained with the following antibodies at room temperature for 1 h: the mouse anti-FLAG M2 antibody (diluted 1:100; Stratagene, Cedar Creek, TX, USA) to detect cells expressing FLAG-dpTR, the mouse anti-PCNA antibody (1:100; Novacastra, Newcastle, UK) to detect proliferating cells, the rabbit anti-caspase 3 (Casp3) antibody (1:50; BD Pharminogen, San Diego, CA, USA) to detect apoptotic cells, the rabbit anti-sonic hedgehog (Shh) (1:500; [13]), anti-Musashi-1 (Msi-1) (1:50; Abcam, Cambridge, MA, USA), and anti-Akt antibodies (1:50; Signal Antibody Co., Pearland, TX, USA; Ishizuya-Oka and Shi, 2008) to identify stem cells, and the rabbit anti-IFABP antibody (1:500; [19] to identify differentiated absorptive cells. They were then incubated with Alexa Fluor 568-conjugated anti-rabbit IgG (1:500; Molecular Probes, Eugene, OR, USA), or with biotin-labeled anti-IgG and peroxidase-conjugated streptavidin (Nichirei, Tokyo, Japan) followed by 0.02% 3, 3′-diamino-benzidine-4HCl (DAB) and 0.006 % H2O2. There was no positive staining when the same concentration of pre-immune serum was applied as the specificity control (data not shown). In addition, to distinguish conventionally the adult progenitor cells from the larval epithelial cells during the larval-to-adult epithelial remodeling, other sections were stained with methyl green-pyronin Y (Muto, Tokyo, Japan) for 5 min [9], or immunostained for 1h with the mouse antibody against cytokeratin 19 (CK19) (1:50; Novacastra), which is a predominant cytokeratin in the mammalian intestinal crypt cells including stem cells [32]. Here, both the staining with pyronin-Y, which intensely stains RNA-rich cytoplasm [33], and the immunoreactivity for CK19 are stronger in the adult progenitor cells than in the other larval cells undergoing apoptosis. Furthermore, some other sections were double-immunostained at room temperature for 1 h with mixtures of anti-Shh and anti-PCNA antibodies, anti-Shh and anti-CK19 antibodies, anti-Msi-1 and anti-CK19 antibodies, or anti-Akt and anti-CK19 antibodies. They were then incubated with a mixture of Alexa Fluor 568-conjugated anti-rabbit IgG (1:500; Molecular Probes, Eugene, OR, USA) and Alexa Fluor 488-conjugated anti-mouse IgG antibodies (1:500; Molecular Probes) and analyzed by fluorescence microscopy.
RESULTS
Expression of dpTR causes larval-to-adult remodeling in intact Tg intestine in vitro
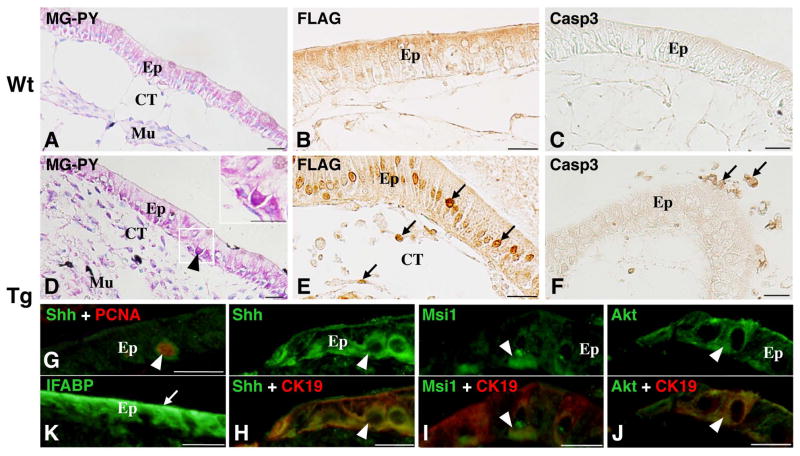
In the Tg tadpole intestine at stage 57 (before metamorphic climax) used for cultivation, a single layer of larval epithelium is surrounded by the immature connective tissue and thin muscular layers as in the Wt tadpole intestine at the same stage. To determine whether the larval-to-adult remodeling can be induced by the action of FLAG-dpTR in the Tg tadpole intestine, we immunohistochemically examined intact Tg and Wt intestines cultured in the absence of TH with daily heat shocks to induce FLAG-dpTR expression. In the intact Wt intestine, the epithelium remained larval-type (Fig. 1A), and FLAG immunoreactivity was not detected in any cells except for the weak staining at background levels throughout the cultivation (Fig. 1B). Neither apoptotic cells positive for Casp3 (Fig. 1C) nor cells positive for Shh (not shown) were observed in the entire larval epithelium. In contrast, in the intact Tg intestine, the epithelium underwent metamorphic changes during the cultivation (Fig. 1D–K). FLAG immunoreactivity became positive in nuclei of every tissue, although the number of positive nuclei tended to be larger in the epithelium than in the other tissues (Fig. 1E). Apoptotic cells positive for Casp3 became detectable in the epithelium on and after day 3 (Fig. 1F), similar to that observed in the Wt intestine cultured in the presence of TH [34]. On the other hand, the adult progenitor cells that are stained strongly red with pyronin-Y (Fig. 1D) and positive for CK19 became detectable on day 5 as small islets between the larval epithelium and the connective tissue. They mostly expressed Shh (Fig. 1H), and some of them also expressed Msi-1 (Fig. 1I) and Akt proteins (Fig. 1J), all of which are candidate markers for adult stem cells in the X. laevis intestine during spontaneous metamorphosis [24]. The immunoreactivities for Msi-1 and Akt were weaker than that for Shh, in agreement with our previous observations in the Wt intestine cultured in the presence of TH [27]. The adult progenitor cells expressing Shh actively proliferated (Fig. 1G), replaced the larval epithelial cells undergoing apoptosis, and then differentiated into the absorptive epithelium expressing IFABP on day 7 (Fig. 1K). These chronological changes in the epithelium of intact Tg intestine as induced by the expression of dpTR are essentially the same as those induced by TH treatment of Wt intestine in vitro [14, 19], although the number of adult progenitor and/or differentiated cells appeared to be smaller in the Tg intestine than in the TH-treated Wt intestine.
Figure 1.
Intact Wt (A–C) and Tg (D–K) tadpole intestines cultured with heat shocks in vitro. Cross sections were stained with methyl green-pyronin Y (MG-PY; A, D), immunostained with anti-FLAG (B, E), anti-Casp3 (C, F), and anti-IFABP antibodies (green;K), or double-immunostained with anti-Shh (green) and anti-PCNA (red) (G), anti-Shh (green) and anti-CK19 (red) (H), anti-Msi-1 (green) and anti-CK19 (red) (I), and anti-Akt (green) and anti-CK19 (red) antibodies (J). Wt intestine remains larval-type (A) and negative for FLAG (B) and Casp3 (C) throughout the cultivation. The weak staining for FLAG in cytoplasm of the epithelium (Ep) is non-specific. In contrast, Tg intestine expresses FLAG-tagged dpTR (arrows; E) and undergoes remodeling. Both apoptotic cells positive for Casp3 (arrows; F) and adult progenitor cells that are strongly stained red with pyronin Y (arrowhead and the inset; D) and positive for Shh (G, H) become detectable on day 5. The adult progenitor cells actively proliferate (arrowhead; G) and are also positive for CK19 (H–J), Msi-1 (I), and Akt (J). Thereafter, the absorptive epithelium expressing IFABP (arrow; K) is differentiated on day 7. CT, connective tissue; Mu, muscles. Bars, 20 μm.
Epithelial changes dependent on tissue-specific dpTR expression in recombinant intestines in vitro
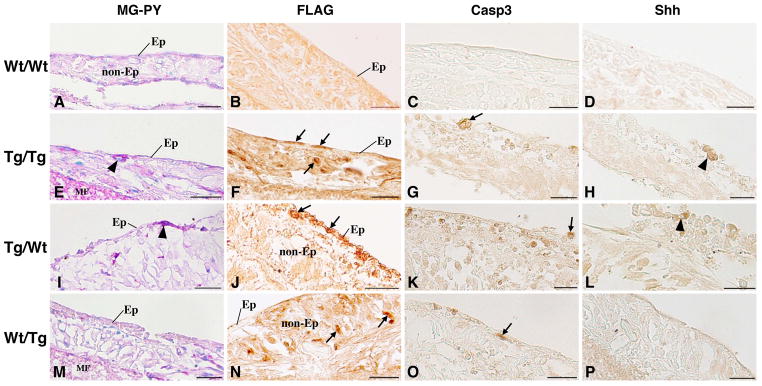
To clarify the effects of tissue-specific expression of dpTR on the larval-to-adult epithelial remodeling, we then performed tissue recombination experiments by using Tg and Wt intestines and immunohistochemically examined four kinds of recombinant intestines cultured with daily heat shocks (Fig. 2). The epithelium in any recombinant intestine comprised smaller number of cells and was lower in cell height than that in the intact intestine as described previously [27, 30]. This is possibly due to partial degeneration of the epithelial cells when they are separated from the connective tissue and/or when they fail to properly contact the recombined connective tissue. Nevertheless, FLAG-dpTR was expressed in the recombinant intestine after heat shock treatment, as expected. In the recombinant intestine made of Wt epithelium (Ep) and Wt non-epithelial tissues (non-Ep) (Wt/Wt), both Ep and non-Ep remained negative for FLAG throughout the cultivation (Fig. 2B). In contrast, both Ep and non-Ep became positive for FLAG in the recombinant intestine of Tg Ep and Tg non-Ep (Tg/Tg), although the intensity of FLAG immunoreactivity varied among different cell-types (Fig. 2F). In the recombinant intestine of Tg Ep and Wt non-Ep (Tg/Wt), cells positive for FLAG were localized in Ep (Fig. 2J), while they were localized in non-Ep in the recombinant intestine of Wt Ep and Tg non-Ep (Wt/Tg) (Fig. 2N). Therefore, as in intact intestinal cultures, FLAG-dpTR was successfully induced in recombinant cultures but only in the intestinal tissues derived from Tg tadpoles.
Figure 2.
Recombinant intestines cultured for 5 days with heat shocks in vitro. Cross sections were stained with MG-PY (A, E, I, M), or immunostained with anti-FLAG (B, F, J, N), anti-Casp3 (C, G, K, O), and anti-Shh antibodies (D, H, L, P). In recombinant intestines made of Wt epithelium (Ep) and Wt non-epithelial tissues (non-Ep) (Wt/Wt; A–D), Tg Ep and Tg non-Ep (Tg/Tg; E–H), Tg Ep and Wt non-Ep (Tg/Wt; I–L), and Wt Ep and Tg non-Ep (Wt/Tg; M–P), nuclei positive for FLAG-tagged dpTR are localized in tissues derived from Tg intestine (arrows; F, J, N). Apoptotic cells positive for Casp3 are detected in Tg/Tg (arrow; G), Tg/Wt (K), and Wt/Tg intestines (O). On the other hand, adult progenitor cells strongly stained red with pyronin-Y (arrowhead; E, I) and positive for Shh (H, L) become detectable only in Tg/Tg and Tg/Wt intestines. MF; membrane filter. Bars, 20 μm.
Development of the recombinant intestines after heat shock treatment depended on the source tissues. In Wt/Wt intestine, the epithelium remained larval-type. Neither apoptotic cells (Fig. 2C) nor adult progenitor cells (Fig. 2A, D) were detected throughout the cultivation, just like in the intact Wt intestine. In contrast, in Tg/Tg intestine, the larval apoptotic cells positive for Casp3 were detected in the epithelium on and after day 3 (Fig. 2G). Then, on day 5, the adult progenitor cells stained red with pyronin-Y (Fig. 2E) and expressing Shh (Fig. 2H) became detectable (Table 1), just like in the intact Tg intestine. In Tg/Wt intestine, similar to Tg/Tg intestine, both apoptotic larval cells positive for Casp3 (Fig. 2K) and adult progenitor cells stained red with pyronin-Y (Fig. 2I) and expressing Shh (Fig. 2L) were detected. On the other hand, in Wt/Tg intestine, similar to Wt/Wt intestine, the epithelium mostly remained larval-type (Fig. 2M), and cells positive for Shh were never observed (Fig. 2P). However, unlike in Wt/Wt intestine, apoptotic cells positive for Casp3 were occasionally detected in the larval epithelium (Fig. 2O), although they were fewer than that in Tg/Tg and Tg/Wt intestines.
TABLE 1.
Larval-to-adult epithelial remodeling in intact and recombinant intestines cultured with heat shocks in vitro
| Epithelial cell-type (positive for) | Day | Intact intestine | Recombinant intestine | ||||
|---|---|---|---|---|---|---|---|
| Wt | Tg | Wt/Wt * | Tg/Tg | Tg/Wt | Wt/Tg | ||
| Larval apoptotic cell (Casp3) | 5 | 0/8 # | 8/8 | 0/6 | 6/6 | 7/7 | 6/7 |
| Adult progenitor cell (Shh) | 5 | 0/5 | 4/4 | 0/8 | 5/5 | 7/9 | 0/7 |
| Adult stem cell (Shh, Msi-1, Akt) | 5 | 0/5 | 4/4 | 0/8 | 5/5 | 0/9 | 0/7 |
| Adult differentiated cell (IFABP) | 7 | – | 4/4 | – | 6/6 | 0/9 | – |
type of Ep/type of non-Ep;
number of intestines including positive cells/total number of intestines; – not determined because adult progenitor cells were not detected.
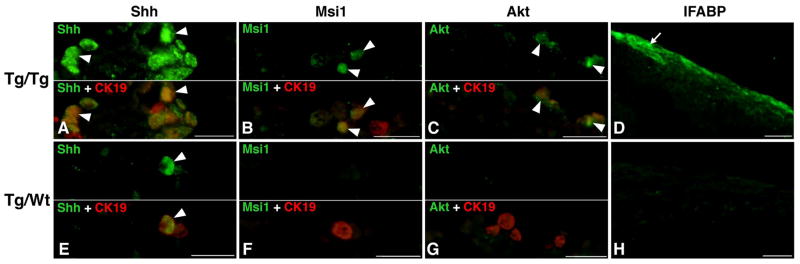
To analyze the development of the adult stem cells in the Tg/Tg and Tg/Wt intestines, we next examined stem cell markers by using double immunofluorescence labeling. In Tg/Tg intestine, the adult progenitor cells positive for CK19 mostly expressed Shh (Fig. 3A), and some of them also expressed Msi-1 (Fig. 3B) and Akt (Fig. 3C). The immunoreactivity for Msi-1 and that for Akt were weaker than that for Shh in Tg/Tg intestine, similarly to that observed in the intact Tg intestine. On the other hand, in Tg/Wt intestine, while the epithelial cells positive for CK19 mostly expressed Shh (Fig. 3E), they never expressed Msi-1 nor Akt (Fig. 3F, G). Thereafter, on day 7, absorptive epithelial cells expressing IFABP were detected in Tg/Tg intestine (Fig. 3D), but not in Tg/Wt intestine (Fig. 3H). These results indicate that adult stem cells that will give rise to the adult absorptive epithelium were formed only in Tg/Tg intestine but not in Tg/Wt intestine. In Tg/Wt intestine, although dpTR expression in the epithelium also induces the cells strongly stained with pyronin-Y and expressing Shh, these cells lack properties characteristic of adult stem cells and fail to generate the adult epithelium due to the lack of TR-mediated gene expression in non-Ep tissues.
Figure 3.
Adult epithelial development in recombinant Tg/Tg (A–D) and Tg/Wt (B–H) intestines in vitro. Cross sections were double-immunostained with anti-Shh (green) and anti-CK19 (red) (A, E), anti-Msi-1 (green) and anti-CK19 (red) (B, F), and anti-Akt (green) and anti-CK19 (red) antibodies (C, G), or immunostained with anti-IFABP antibody (green; D, H). In both Tg/Tg (arrowheads; A) and Tg/Wt intestines (E), cells positive for Shh become detectable on day 5 among cells expressing CK19. Cells positive for Msi1 and Akt are also detected among CK19-immunoreactive cells in Tg/Tg intestine (arrowheads; B, C) but not in Tg/Wt intestine (F, G). Thereafter, on day 7, absorptive cells expressing IFABP are differentiated in Tg/Tg intestine (arrow; D) but not in Tg/Wt intestine (H). Bars, 20 μm.
TR-mediated epithelial changes in the absence of non-epithelial tissues in vitro
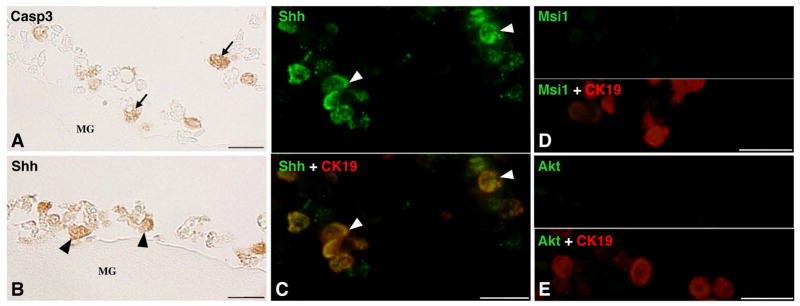
Finally, to determine whether dpTR up-regulates Shh expression in the epithelium even in the absence of non-epithelial tissues, we isolated the epithelium from the Tg intestine and cultured it alone on a matrigel, which mimics the basal lamina underneath the epithelium, in the absence of TH with heat shocks. In the Tg epithelium, similar to Tg/Wt intestine, both apoptotic cells positive for Casp3 (Fig. 4A) and adult progenitor-like cells double positive for Shh and CK19 (Fig. 4B, C) were detected on day 5. However, these cells did not express Msi-1 (Fig. 4D) or Akt (Fig. 4E). Thereafter, the epithelial cells gradually decreased in cell number and never differentiated into the absorptive epithelium expressing IFABP, similar to the case of the Wt epithelium cultured alone in the presence of TH [14]. Thus, TR-mediated changes in both the epithelium and non-epithelial tissues are essential for proper cell-cell interactions to facilitate the development of the adult epithelial stem cells.
Figure 4.
Tg epithelium cultured on a matrigel (MG) for 5 days with heat shocks in vitro. Sections were immunostained with anti-Casp3 (A), anti-Shh antibodies (B), or double-immunostained with anti-Shh (green) and anti-CK19 (red) (C), anti-Msi1 (green) and anti-CK19 (red) (D), and anti-Akt (green) and anti-CK19 (red) antibodies (E). Apoptotic cells positive for Casp3 (arrows; A) are detected. Cells positive for Shh (arrowheads; B) are also detected among cells positive for CK19 (C), which remain negative for Msi-1 (D) and Akt (E). Bars, 20 μm.
DISCUSSION
The Xenopus tadpole intestine, in which dedifferentiation of the larval epithelium into stem cells can be induced by TH, provides us an excellent opportunity to study mechanisms regulating organ-specific adult stem cells during postembryonic development. In the present study, we examined effects of tissue-specific TH signaling on intestinal epithelial remodeling by using Tg tadpoles expressing dpTR and showed that TR-mediated gene expression in both the epithelium and the surrounding tissues is essential for dedifferentiation of the larval cells into the adult stem cells.
Two distinct epithelial responses to TH exist in the tadpole intestinal epithelium
Previous in vivo study with the Tg tadpoles showed that TR is sufficient to mediate metamorphic events including intestinal remodeling [31]. In the present study, we first demonstrated the usefulness of intestinal tissues of these Tg tadpoles for organ cultures in vitro. The number of adult progenitor and/or differentiated cells in the intact Tg intestine was small compared to Wt intestine cultured with TH, possibly because of effects of endogenous TR and insufficient FLAG-dpTR expression. However, the time course of larval-to-adult epithelial remodeling in the intact Tg intestine is basically the same as that in the Wt intestine cultured in the presence of TH [13, 14, 19]. This indicates that dpTR-induced expression of endogenous genes in the X. laevis intestine can reproduce the entire process of the epithelial remodeling in the absence of TH in vitro. In addition, our recombinant experiments showed that FLAG-dpTR can be induced by heat shock in a manner consistent with the source tissues.
One of important findings in our culture study is that adult progenitor cells expressing Shh become detectable in the intact Tg intestine and recombinant Tg/Tg and Tg/Wt intestines, that is, whenever the epithelium is derived from Tg tadpoles. It is worthy to note that the number of adult progenitor cells were limited and very few when they first appeared, although most if not all Tg epithelial cells expressed FLAG-dpTR. In addition, a small number of cells expressing Shh also became detectable even in the Tg epithelium cultured on an ECM but in the absence of connective tissue. These observations strongly suggest that by stage 57, the larval epithelium can respond to TH action in two distinct manners: (1) a few cells that can express Shh by the inductive action of TH and have a potency to become adult progenitor cells and (2) the rest of the larval-proper cells that undergo apoptosis under the action of TH. How apparently identical epithelial cells exhibit such different responses is unclear. One possibility is that homogeneous larval epithelial cells may be determined to become either the adult progenitor cells or the larval-proper cells after TH-induced cell-cell communications between the epithelium and non-epithelium. In other X. laevis organs, adult progenitor cells of the skin have been shown to originate from “basal skein cells” which are morphologically distinguished as early as stage 45 [35–37], when the circulating TH level is still low [7]. It is an interesting future problem to clarify how the adult stem cells are determined in these organs.
As for the larval-proper cells, their apoptosis was detected in Wt/Tg intestine as in the intact Tg and recombinant Tg/Tg intestines. This indicates that TR-mediated gene expression in the surrounding tissues (cell-cell and/or cell-ECM interaction-dependent pathway) can induce apoptosis of epithelial cells. Previously, stromelysin-3 (ST3), one of the matrix metalloproteinases (MMPs), has been identified as a direct TH response gene whose expression is connective tissue-specific and has been shown to induce larval epithelial apoptosis by altering cell-ECM interactions in the X. laevis intestine [34, 38, 39]. The present observation that apoptotic larval epithelial cells, but no adult progenitor cells, were detected in Wt/Tg intestine agrees well with ST3 functions demonstrated previously. On the other hand, in Tg tadpoles expressing a dominant negative TR under the control of ST3 promoter, TH appears to cause apoptosis irrespective of ST3 expression [40], which agrees with the present result that apoptosis was also detected in Tg/Wt intestine and the Tg epithelium alone and with the previous study showing apoptosis in epithelial cell cultures of the X. laevis intestine [41]. Taken together, either one of the pathways, the cell-autonomous or the ST3-mediated pathway, is sufficient to induce apoptosis of at least some of epithelial cells (Fig. 5), although their molecular mechanisms have thus far not been well identified.
Figure 5.
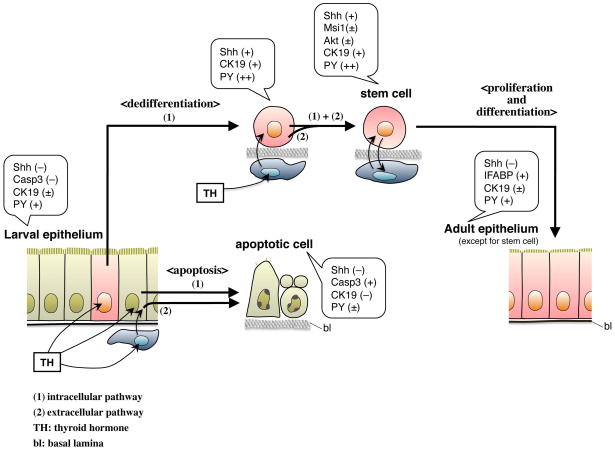
Schematic model showing larval-to-adult epithelial remodeling of the X. laevis intestine. The larval epithelium consists of a small number of cells that have a potency to become adult stem cells and the other larval-proper cells. The former cells express Shh by direct action of TH and begin to dedifferentiate (1). However, expression of TH target genes in the surrounding tissues is required for them to fully dedifferentiate into stem cells that can generate the adult absorptive epithelium (2). Thus, TH-induced tissue crosstalk is involved in the establishment of the stem cell niche. On the other hand, TH can induce apoptosis of the larval-proper cells through either intracellular (1) or extracellular pathways (2).
Development of adult epithelium requires TH signaling in the surrounding tissues other than the epithelium
Another important finding of the present study is that, despite the cell autonomous formation of the Shh-positive adult progenitor cells in the larval epithelium in response to TR activation, it should not be sufficient to call them authentic stem cells. Similar to Wt/Wt intestine cultured in the presence of TH [14, 30], the adult epithelium expressing IFABP was formed only in Tg/Tg intestine but not in Tg/Wt intestine or Tg epithelium alone upon dpTR expression. This implies that TR-mediated gene expression in non-epithelial tissues is required for the progenitor cells to become true stem cells that express all of the candidate stem cell markers such as Msi-1 and can generate the differentiated adult epithelium (Fig. 5). This is not inconsistent with the observation that TH failed to cause normal adult epithelial development in Tg tadpoles expressing of a dominant negative TR under the control of ST3, i.e., in the connective tissue [40], although this early study did not identify the adult epithelium nor analyze stem cells or stem cell markers. Among the stem cell markers examined in the present study, Shh is the only one known to be a direct TH response gene that is regulated at the transcriptional level by TR [42]. Previously, we have shown that Shh induces expression of bone morphogenetic protein-4 (BMP-4) only in the connective tissue and that Shh/BMP-4 signaling pathway is necessary for adult epithelial development [43]. Although functions of Shh in dedifferentiation have not yet been elucidated, it seems likely that TH-up-regulated expression of Shh plays a key role in this process and is an early step towards establishing the stem cells. In contrast, it is highly possible that the expression of Msi-1 and Akt is up-regulated indirectly by TH through the expression of TH response genes in the surrounding tissues and is involved in a subsequent step in stem cell formation. Future studies should be directed towards identification and functional analyses of such TH response genes, focusing on members of BMP, Wnt, and Notch signaling pathways, which are known to be expressed in the stem cell niche of the adult mammalian intestine [44, 45] and are proposed to be correlated with Msi-1 and Akt [46–48].
CONCLUSION
In summary, by using dpTR Tg frogs for organ cultures, we demonstrated that TR-mediated gene expression in both the epithelium and surrounding tissues is essential for the epithelial dedifferentiation into stem cells that generate the adult absorptive epithelium. This implies the importance of TR-mediated signaling through tissue crosstalk to establish the stem cell niche in the Xenopus intestine, from which a number of TH response genes have been isolated [25, 26, 49, 50]. Our amphibian intestinal remodeling system should pave a way to functionally characterize candidate TH-induced niche factors. Moreover, a growing number of TH response genes identified in the mammalian intestine [51] are homologous with genes expressed in the amphibian intestine during postembryonic development [52]. Among them there are signaling molecules such as β-catenin, secreted frizzled-related protein 2, Gli2, and Notch 1. This conservation of gene expression between these two animal classes may prove useful for elucidating the molecular basis of stem cell niche development.
Acknowledgments
We would like to thank Dr. Kosuke Kawamura for his kind care to Tg tadpoles and Mrs. Mitsuko Kajita for her technical help. This work was supported in part by the JSPS Grants-in-Aid for Scientific Research (C) (Grant number 20570060 to A. I.-O.) and in part by the Intramural Research Program of NICHD, NIH (to Y.-B. S.).
Brief acknowledgments: the JSPS Grants-in-Aid for Scientific Research (C) (to A. I.-O.) and the Intramural Research Program of NICHD, NIH (to Y.-B. S.).
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
Author contributions: T.H.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; D.R.B.: provision of study material or patients, data analysis and interpretation; Y-B.S.: financial support, provision of study material or patients, data analysis and interpretation; A.I-O.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
References
- 1.Mills JC, Gordon JI. The intestinal stem cell niche: there grows the neighborhood. Proc Natl Acad Sci USA. 2001;98:12334–12336. doi: 10.1073/pnas.231487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 3.Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997;78:219–243. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 5.Shi YB, editor. Amphibian Metamorphosis: From morphology to molecular biology. New York: John Wiley & Sons, Inc; 1999. [Google Scholar]
- 6.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: North-Holland Pub; 1967. [Google Scholar]
- 7.Leloup J, Buscaglia M. La triiodothryronine: hormone de la metamorphose des amphibians. C R Acad Sci. 1977;284:2261–2263. [Google Scholar]
- 8.Hourdry J, Dauca M. Cytological and cytochemical changes in the intestinal epithelium during anuran metamorphosis. Int Rev Cytol (Supple) 1977;5:337–385. [Google Scholar]
- 9.Ishizuya-Oka A, Ueda S. Apoptosis and cell proliferation in the Xenopus small intestine during metamorphosis. Cell Tissue Res. 1996;286:467–476. doi: 10.1007/s004410050716. [DOI] [PubMed] [Google Scholar]
- 10.McAvoy JW, Dixon KE. Cell proliferation and renewal in the small intestinal epithelium of metamorphosing and adult Xenopus laevis. J Exp Zool. 1977;202:129–138. [Google Scholar]
- 11.Shi Y-B, Ishizuya-Oka A. Biphasic intestinal development in amphibians: embryogenesis and remodeling during metamorphosis. Curr Top Dev Biol. 1996;32:205–235. doi: 10.1016/s0070-2153(08)60429-9. [DOI] [PubMed] [Google Scholar]
- 12.Hasebe T, Kajita M, Shi Y-B, et al. Thyroid hormone-up-regulated hedgehog interacting protein is involved in larval-to-adult intestinal remodeling by regulating sonic hedgehog signaling pathway in Xenopus laevis. Dev Dyn. 2008;237:3006–3015. doi: 10.1002/dvdy.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishizuya-Oka A, Ueda S, Inokuchi T, et al. Thyroid hormone-induced expression of sonic hedgehog correlates with adult epithelial development during remodeling of the Xenopus stomach and intestine. Differentiation. 2001;69:27–37. doi: 10.1046/j.1432-0436.2001.690103.x. [DOI] [PubMed] [Google Scholar]
- 14.Ishizuya-Oka A, Shimizu K, Sakakibara S, et al. Thyroid hormone-upregulated expression of Musashi-1 is specific for progenitor cells of the adult epithelium during amphibian gastrointestinal remodeling. J Cell Sci. 2003;116:3157–3164. doi: 10.1242/jcs.00616. [DOI] [PubMed] [Google Scholar]
- 15.de Santa Barbara P, van den Brink GR, Roberts DJ. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci. 2003;60:1322–1332. doi: 10.1007/s00018-003-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He XC, Zhang J, Tong WG, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 17.Kayahara T, Sawada M, Takaishi S, et al. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535:131–135. doi: 10.1016/s0014-5793(02)03896-6. [DOI] [PubMed] [Google Scholar]
- 18.Potten CS, Booth C, Tudor GL, et al. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71:28–41. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- 19.Ishizuya-Oka A, Ueda S, Damjanovski S, et al. Anteroposterior gradient of epithelial transformation during amphibian intestinal remodeling: immunohistochemical detection of intestinal fatty acid-binding protein. Dev Biol. 1997;192:149–161. doi: 10.1006/dbio.1997.8749. [DOI] [PubMed] [Google Scholar]
- 20.McAvoy JW, Dixon KE. Cell specialization in the small intestinal epithelium of adult Xenopus laevis: structural aspects. J Anat. 1978;125:155–169. [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y-B, Hayes WP. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev Biol. 1994;161:48–58. doi: 10.1006/dbio.1994.1006. [DOI] [PubMed] [Google Scholar]
- 22.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat. 1981;160:77–91. doi: 10.1002/aja.1001600107. [DOI] [PubMed] [Google Scholar]
- 23.Cheng H, Bjerknes M. Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat Rec. 1985;211:420–426. doi: 10.1002/ar.1092110408. [DOI] [PubMed] [Google Scholar]
- 24.Ishizuya-Oka A, Shi Y-B. Thyroid hormone regulation of stem cell development during intestinal remodeling. Mol Cell Endocrinol. 2008;288:71–78. doi: 10.1016/j.mce.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Buchholz DR, Heimeier RA, Das B, et al. Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol. 2007;303:576–590. doi: 10.1016/j.ydbio.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y-B, Brown DD. The earliest changes in gene expression in tadpole intestine induced by thyroid hormone. J Biol Chem. 1993;268:20312–20317. [PubMed] [Google Scholar]
- 27.Ishizuya-Oka A, Hasebe T, Buchholz DR, et al. Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. FASEB J. 2009;23:2568–2575. doi: 10.1096/fj.08-128124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall JA, Dixon KE. Cell specialization in the epithelium of the small intestine of feeding Xenopus laevis tadpoles. J Anat. 1978;126:133–144. [PMC free article] [PubMed] [Google Scholar]
- 29.Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci. 1998;353:821–830. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishizuya-Oka A, Shimozawa A. Connective tissue is involved in adult epithelial development of the small intestine during anuran metamorphosis in vitro. Roux’s Arch Dev Biol. 1992;201:322–329. doi: 10.1007/BF00592113. [DOI] [PubMed] [Google Scholar]
- 31.Buchholz DR, Tomita A, Fu L, et al. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol. 2004;24:9026–9037. doi: 10.1128/MCB.24.20.9026-9037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quaroni A, Calnek D, Quaroni E, et al. Keratin expression in rat intestinal crypt and villus cells. Analysis with a panel of monoclonal antibodies. J Biol Chem. 1991;266:11923–11931. [PubMed] [Google Scholar]
- 33.Schulte EK, Lyon HO, Hoyer PE. Simultaneous quantification of DNA and RNA in tissue sections. A comparative analysis of the methyl green-pyronin technique with the gallocyanin chromalum and Feulgen procedures using image cytometry. Histochem J. 1992;24:305–310. doi: 10.1007/BF01046161. [DOI] [PubMed] [Google Scholar]
- 34.Ishizuya-Oka A, Li Q, Amano T, et al. Requirement for matrix metalloproteinase stromelysin-3 in cell migration and apoptosis during tissue remodeling in Xenopus laevis. J Cell Biol. 2000;150:1177–1188. doi: 10.1083/jcb.150.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki K, Utoh R, Kotani K, et al. Lineage of anuran epidermal basal cells and their differentiation potential in relation to metamorphic skin remodeling. Dev Growth Differ. 2002;44:225–238. doi: 10.1046/j.1440-169x.2002.00637.x. [DOI] [PubMed] [Google Scholar]
- 36.Yoshizato K. Biochemistry and cell biology of amphibian metamorphosis with a special emphasis on the mechanism of removal of larval organs. Int Rev Cytol. 1989;119:97–149. doi: 10.1016/s0074-7696(08)60650-6. [DOI] [PubMed] [Google Scholar]
- 37.Yoshizato K. Molecular mechanism and evolutional significance of epithelial-mesenchymal interactions in the body- and tail-dependent metamorphic transformation of anuran larval skin. Int Rev Cytol. 2007;260:213–260. doi: 10.1016/S0074-7696(06)60005-3. [DOI] [PubMed] [Google Scholar]
- 38.Fu L, Ishizuya-Oka A, Buchholz DR, et al. A causative role of stromelysin-3 in extracellular matrix remodeling and epithelial apoptosis during intestinal metamorphosis in Xenopus laevis. J Biol Chem. 2005;280:27856–27865. doi: 10.1074/jbc.M413275200. [DOI] [PubMed] [Google Scholar]
- 39.Shi Y-B, Fu L, Hasebe T, et al. Regulation of extracellular matrix remodeling and cell fate determination by matrix metalloproteinase stromelysin-3 during thyroid hormone-dependent post-embryonic development. Pharmacol Ther. 2007;116:391–400. doi: 10.1016/j.pharmthera.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreiber AM, Mukhi S, Brown DD. Cell-cell interactions during remodeling of the intestine at metamorphosis in Xenopus laevis. Dev Biol. 2009;331:89–98. doi: 10.1016/j.ydbio.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su Y, Shi Y, Stolow MA, et al. Thyroid hormone induces apoptosis in primary cell cultures of tadpole intestine: cell type specificity and effects of extracellular matrix. J Cell Biol. 1997;139:1533–1543. doi: 10.1083/jcb.139.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stolow MA, Shi Y-B. Xenopus sonic hedgehog as a potential morphogen during embryogenesis and thyroid hormone-dependent metamorphosis. Nucleic Acids Res. 1995;23:2555–2562. doi: 10.1093/nar/23.13.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishizuya-Oka A, Hasebe T, Shimizu K, et al. Shh/BMP-4 signaling pathway is essential for intestinal epithelial development during Xenopus larval-to-adult remodeling. Dev Dyn. 2006;235:3240–3249. doi: 10.1002/dvdy.20969. [DOI] [PubMed] [Google Scholar]
- 44.Walker MR, Stappenbeck TS. Deciphering the ‘black box’ of the intestinal stem cell niche: taking direction from other systems. Curr Opin Gastroenterol. 2008;24:115–120. doi: 10.1097/MOG.0b013e3282f4954f. [DOI] [PubMed] [Google Scholar]
- 45.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 46.He XC, Yin T, Grindley JC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imai T, Tokunaga A, Yoshida T, et al. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol Cell Biol. 2001;21:3888–3900. doi: 10.1128/MCB.21.12.3888-3900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leedham SJ, Brittan M, McDonald SA, et al. Intestinal stem cells. J Cell Mol Med. 2005;9:11–24. doi: 10.1111/j.1582-4934.2005.tb00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amano T, Yoshizato K. Isolation of genes involved in intestinal remodeling during anuran metamorphosis. Wound Repair Regen. 1998;6:302–313. doi: 10.1046/j.1524-475x.1998.60406.x. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu K, Ishizuya-Oka A, Amano T, et al. Isolation of connective-tissue-specific genes involved in Xenopus intestinal remodeling: thyroid hormone up-regulates Tolloid/BMP-1 expression. Dev Genes Evol. 2002;212:357–364. doi: 10.1007/s00427-002-0250-3. [DOI] [PubMed] [Google Scholar]
- 51.Kress E, Samarut J, Plateroti M. Thyroid hormones and the control of cell proliferation or cell differentiation: paradox or duality? Mol Cell Endocrinol. 2009;313:36–49. doi: 10.1016/j.mce.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 52.Heimeier RA, Das B, Buchholz DR, et al. Studies on Xenopus laevis intestine reveal biological pathways underlying vertebrate gut adaptation from embryo to adult. Genome Biol. 2010;11:R55. doi: 10.1186/gb-2010-11-5-r55. [DOI] [PMC free article] [PubMed] [Google Scholar]