Abstract
Stem or progenitor cell populations are often established in unique niche microenvironments that regulate cell fate decisions. Although niches have been shown to be critical for the normal development of several tissues, their role in the cardiovascular system is poorly understood. In this study, we characterized the cardiovascular progenitor cell (CPC) niche in developing human and mouse hearts, identifying signaling pathways and extracellular matrix (ECM) proteins that are crucial for CPC maintenance and expansion. We demonstrate that collagen IV (ColIV) and β-catenin-dependent signaling are essential for maintaining and expanding undifferentiated CPCs. Since niches are three-dimensional (3D) structures, we investigated the impact of a 3D microenvironment that mimics the in vivo niche ECM. Employing electrospinning technologies, 3D in vitro niche substrates were bioengineered to serve as culture inserts. The three-dimensionality of these structures increased mouse embryonic stem cell differentiation into CPCs when compared to 2D control cultures, which was further enhanced by incorporation of ColIV into the substrates. Inhibiting p300-dependent β-catenin signals with the small molecule IQ1 facilitated further expansion of CPCs. Our study represents an innovative approach to bioengineer cardiac niches that can serve as unique 3D in vitro systems to facilitate CPC expansion and study CPC biology.
Keywords: extracellular matrix, cardiac tissue engineering, stem cell, scaffold, heart
1. Introduction
The mammalian heart is the first organ to develop and function in the fetus. It contains three major cell lineages, cardiac myocytes (CMs), smooth muscle cells (SMCs) and endothelial cells (ECs), which are believed to derive from a common cardiovascular progenitor cell (CPC) [1-4]. This progenitor represents one of the earliest stages in mesodermal specification to the cardiovascular lineage. It can be identified in mouse embryos or embryonic stem (ES) cell derivatives by expression of the kinase insert domain protein receptor-expressing (KDR, also known as Flk1). Subsequent studies have reported additional markers, defining multipotent mouse CPCs including Islet1 (Isl1+) and NK2 transcription factor-related, locus 5 (Nkx2.5+) [1-3]. We isolated Flk1+ progenitor cells from differentiating mouse ES or induced-pluripotent stem (iPS) cells that possess the ability to differentiate into all three cell types of the cardiovascular lineage as well as hematopoietic cells [5]. A comparable CPC is also present during human cardiogenesis [6]; however, this Isl1+/Flk1+ progenitor is rapidly lost postnatally and is not detectable in the adult heart.
Endogenous Isl1+ CPCs are found in discrete clusters within the developing right ventricle, the atria and outflow tracts of both human and mouse hearts [3, 6], which are reminiscent of a stem cell niche. Niches are anatomically defined, three-dimensional (3D) microenvironments that regulate stem or progenitor cell fate through cell-cell interactions, cell-matrix contacts and localized soluble factors [7-9]. Significant progress has been made in characterizing such specific microenvironments in selected stem cell compartments, including the hematopoietic, epidermal, intestinal and neural stem cell niches; however, the characteristics of the cardiovascular niche are poorly defined [10]. A major challenge in stem cell biology is defining the components of such niches that are crucial for regulating stem/progenitor cell development and maintenance, as well as exploiting this knowledge for therapeutic potential. The interplay between stem or progenitor cells and their niche creates a dynamic system necessary for maintaining a balance between self-renewal and differentiation of the cells. Wnt/β-catenin signaling is important for the maintenance and self-renewal of stem/progenitor cells of various lineages and has also been implicated in cardiac development [11]. In the cardiovascular system, Wnt signaling through β-catenin appears to regulate both the specification and the proliferation of Isl1+ CPCs [12]. β-catenin directly binds and regulates the Isl1 promoter in cardiovascular cells [13]. Ablation of β-catenin in Isl1+ cells lead to the disruption of multiple aspects of cardiogenesis including a defective outflow tract and right ventricular development, resulting in embryonic lethality [13-14]. These data strongly suggest that β-catenin signaling plays an important role in the specification and maintenance of Isl1+ CPCs.
Extracellular matrix (ECM) modulates cell anchorage and activity of signaling molecules by direct interaction with stem cells and their progeny [7-8, 15]. The ECM of the heart is generally composed of collagens, particularly collagen types I (ColI; equals ∼85% of the cardiac collagens), III, IV (ColIV) and VI; fibronectin, elastin, glycoproteins and proteoglycans [8, 16]. These ECM components are expressed in precise temporal and spatial patterns, and are constantly remodeled during fetal cardiac development and in diseased states [17]. While fibrillar collagens like ColI play a major role in disease-related remodeling processes [18], ColIV and several glycoproteins, including laminins, are involved in cardiovascular development and have been implicated to contribute to the stem cell niche in the hematopoietic and nervous systems [19]. ColIV and laminin have also been identified to regulate cardiac stem cells in the adult heart [10, 17]; however, their role in regulating the cardiovascular niche in the developing heart remains unclear.
To determine if CPCs also reside within a niche in the developing heart, we examined fetal human and murine hearts for the presence of an anatomically distinct niche and attempted to characterize the ECM molecules and signaling pathways that mediate its interactions with CPCs. We demonstrate that the cardiovascular niche is enriched for the ECM protein ColIV, which plays a key role in controlling CPC fate. In addition, we show that inhibiting p300-dependent β-catenin signaling is critical for CPC in vitro expansion. To recapitulate the 3D cardiovascular niche microenvironment in vitro, we created unique electrospun scaffolds and demonstrate that three-dimensionality itself supports CPC expansion.
2. Materials and methods
2.1. Human and murine tissue procurement and processing
First-trimester (6-12 weeks of developmental age) human fetal hearts were discarded material obtained from elective terminations of pregnancies performed by Family Planning Associates in Los Angeles. Mouse embryos were isolated from CF1 mice at E15.5 gestational age. All heart tissues were immediately washed after harvest in sterile phosphate buffered saline (PBS, HyClone, Logan, UT), fixed in 10% buffered formalin for 12 hours, then rinsed in tap water and transferred to 70% ethanol. Fixed specimens were embedded in paraffin and cut into 5 μm sections by the UCLA Translational Pathology Core Laboratory (TPCL). Hematoxylin/eosin staining was performed by TPCL.
2.2. Immunohistochemistry
Slides were deparaffinized and subjected to antigen retrieval (95°C for 30 minutes each in 10 mM Tris, 1 mM EDTA, 0.05% Tween 20 solution, pH 9.0 followed by 10 mM citrate solution in PBS, pH 6.0). Endogenous peroxidases were quenched in a 0.9% solution of H2O2 in methanol for 20 minutes at room temperature (RT). All sections were then exposed to blocking buffer (5% horse serum, 0.05% Tween 20 in PBS) for 15 minutes. Primary antibodies were diluted in blocking buffer and applied over night at 4°C followed by incubation with biotinylated secondary antibodies for 30 minutes at RT. A complete list of all primary antibodies is provided in Supplemental Table 1. Secondary antibodies (1:500; all from Vector Labs, Burlingame, CA) were detected using the ABC system as directed (Vector Labs). For mouse primary antibodies on mouse tissues, the M.O.M. kit (BMK-2202, Vector Labs) was used as directed. Bright-field images were acquired using a Zeiss Axiovert 200 microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
2.3. Immunofluorescence staining and confocal microscopy
Unstained sections were deparaffinized and rehydrated. Antigen retrieval was performed and all slides were further processed as described before [20]. After secondary antibody incubation, all slides were exposed to DAPI solution (D8417, 5 mg/ml in PBS, Sigma Aldrich, St. Louis, MO) for 5 minutes followed by mounting using ProLong Gold antifade mounting medium (Molecular Probes, Carlsbad, CA). Fluorescence images were acquired using a confocal TCS SP2 AOBS laser-scanning microscope system (Leica Microsystems Inc., Mannheim, Germany). Images were processed with Adobe Photoshop CS4 (Adobe Systems Inc., San Jose, CA).
2.4. Mouse ES cell cultures
D3 ES cells (CRL-1934, ATCC, Manassas, VA) were maintained in an undifferentiated state on mitomycin-C-treated (M4287, Sigma), primary mouse embryonic fibroblasts (MEFs) in leukemia inhibitory factor (LIF) supplemented medium [Knockout Dulbecco's modified Eagle's medium supplemented with 15% ES cell-qualified fetal calf serum (ES-FCS), 0.1 mM β-mercaptoethanol, 2 mM glutamine, 0.1 mM nonessential amino acids (all from Invitrogen, Carlsbad, CA) and 1,000 U/ml recombinant LIF (Chemicon, Temecula, CA)] at 37°C, 5% CO2 as described before [5]. αMHC-luciferase expressing CGR8 ES cells (alpha-MHC-Luc ES cells; a kind gift of Richard T. Lee, Harvard Medical School [21]) were maintained MEF-free on 0.1% gelatin (G1890, Sigma) in LIF supplemented medium at 37°C, 5% CO2. The medium was changed on a daily base.
2.5. In vitro differentiation experiments
To remove MEFs, D3 ES cells were collected by trypsinization and plated 2× on 0.1% gelatin-coated culture dishes for 20 minutes at 37°C. All nonadherent cells were used for further experiments. ES cells were then either introduced into a dynamic suspension culture system for generating spontaneously differentiating embryoid bodies (EBs) or they were cultured on 0.1% gelatin- or extracellular matrix-coated plates and flasks as described before in detail [5]. Collagen type I- and IV-, laminin- and fibronectin-coated labware was purchased from BD Biocoat (BD Biosciences, Bedford, MA). To induce differentiation into cardiovascular progenitors, all cells were cultured in LIF-free α-minimum essential medium (Invitrogen), supplemented with 10% ES-FCS, 0.1 mM β-mercaptoethanol, 2 mM glutamine, and 0.1 mM nonessential amino acids (α-MEM). For CPC expansion, α-MEM was further supplemented with IQ1 (4 μg/ml; [22]).
2.6. Magnetic Cell Sorting and CPC Differentiation
To induce differentiation into Flk1-positive cardiovascular progenitors, undifferentiated αMHC-Luc ES cells were trypsinized and cultured in α-MEM for 4 days on ColIV-coated flasks as described before [5]. Cells were harvested and the Flk1-positive CPCs were isolated by indirect magnetic cell sorting (MACS) using a purified rat anti-mouse Flk1 antibody (550549 [1:200]; BD Pharmingen, San Diego, CA) and magnetic microbeads (Miltenyi Biotec, Auburn, CA). Flk1-positive CPCs were then exposed for 4 days to collagen type I- and IV-, laminin- and fibronectin in α-MEM.
2.7. Flow cytometry
Fluorescence-activated cell sorter (FACS) analysis was performed as described before [5]. Cells were stained using phycoerythrin (PE)-conjugated monoclonal rat anti-mouse Flk1 antibody (12-5821 [1:200]; eBioscience Inc., San Diego, CA). A PE-conjugated rat IgG2a κ isotype (12-4321 [1:200]; eBioscience) served as control. Staining with 7-aminoactinomycin D (559925; BD Pharmingen, San Diego, CA) was performed to exclude dead cells. Cells were analyzed on a BD LSR II cytometer (BD Biosciences). Data analysis was performed using FlowJo 8.6.3 software (Tree Star Inc., Ashland, OR).
2.8. Luciferase Assays
αMHC promoter activity was assessed with a luciferase assay kit (Promega Corporation, Madison, WI). Briefly, after the culture medium was removed, the cells were washed once with 1× PBS and lysed with ice-cold 300 μl of reporter lysis buffer. 200 μl of the cell lysate was then added to 50 μl of luciferase substrate solution. Bioluminescence generated was measured for 2 seconds using a Monolight 3010 luminometer (BD Pharmingen). The luminescence readings obtained were normalized to the protein content of the corresponding cell lysate determined by a Bradford assay according to the manufacturer's protocol (BioRad, Hercules, CA). Results are depicted in relative light units (RLU).
2.9. Gene expression analysis
Total RNA was extracted from cells using the RNeasy Plus Mini Kit as per manufacturer's instructions (Qiagen, Valencia, CA). First strand cDNA was generated from 2 μg of total RNA by using the Omniscript™ Reverse Transcriptase Kit (Qiagen). Semi-quantitative RT-PCR was performed as previously described [5]. The sequences of each primer set, including their annealing temperature and cycles, are published [5]. Quantitative real-time PCR was performed as previously described [23]. Primer sets specific for mouse Flk1 (QT00097020) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; QT01658692) were obtained from Qiagen (QuantiTect Primer Assay).
RNA samples from undifferentiated mouse ES cells and mouse ES cell-derived Flk1- cells and Flk1+ CPCs were analyzed at the UCLA Illumina Microarray Laboratory. Briefly, biotinylated cRNA was prepared using the Illumina RNA Amplification Kit (Ambion Inc., Austin, TX). Samples were hybridized on a Sentrix MouseRef-8 Expression BeadChip System (Illumina Inc., San Diego, CA. Scanning was performed according to the Illumina BeadStation 500 manual. Microarray raw data were analyzed using BeadStudio software version 1.5.1.3. Differential expression analysis was selected to quantify gene expression intensity values as well as to determine changes of the gene expression levels between undifferentiated ES cells (reference group) and Flk1- cells and Flk1+ CPCs. To filter out nonspecific signal intensities, local background subtraction was performed. Only genes with intensities >0.99 were selected for analysis. A differential score of 13 demonstrated that gene expression from differentiated mouse ES cells had changed significantly when compared with genes of undifferentiated ES cells.
2.10. Fabrication of electrospun substrates
To fabricate 3D cell culture inserts, a solution containing 10% gelatin (G1890, Sigma) and 10% polycaprolactone (PCL; 19561, Polysciences, Inc., Warrington, PA) in 1,1,1,3,3,3-hexafluoro-2-propanol (HFP; 105228, Sigma) was exposed to electrospinning as described before [24]. After fabrication, the 3D electrospun substrates (300–400 mm thick) were adhered to the bottom of a standard 24-well tissue culture plate with sterile Silastic Medical Adhesive, Type A (3244393, Dow Corning, Midland, MI), followed by disinfection using 70 % ethanol for 20 min followed by 3× 5 min rinses in sterile water and PBS. Some of the substrates were then coated for 1 hour at RT using collagen type IV (354233, BD Biosciences) at a concentration of 100 μg/ml (diluted in 0.05N Hydrochloric acid (H1758, Sigma)). All electrospun substrates (coated and uncoated controls) were subsequently analyzed using scanning electron microscopy (SEM) as described before [24]. Fiber diameters and pore sizes were measured on SEM images. Average fiber diameters were determined from measurements taken perpendicular to the long axis of the fibers within representative microscopic fields (20 measurements per field). The pores formed at the interstices of the fibers were measured using ImageJ software (free download available at http://rsbweb.nih.gov/ij/). For each sample, at least 5 scanning electron micrographs at 2000× magnification were used for image analysis and pore size measurements. Immunofluorescence imaging was performed as described above to test if the collagen type IV-coating was carried out successfully.
2.11. Statistical Analyses
All data are presented as mean ± standard deviation (SD). Statistical significance was assessed by Student's t test or ANOVA with Tukey's multiple comparison tests using StatView software. P-values less than 0.05 were defined as statistically significant.
3. Results
3.1. Characterization of the endogenous CPC niche
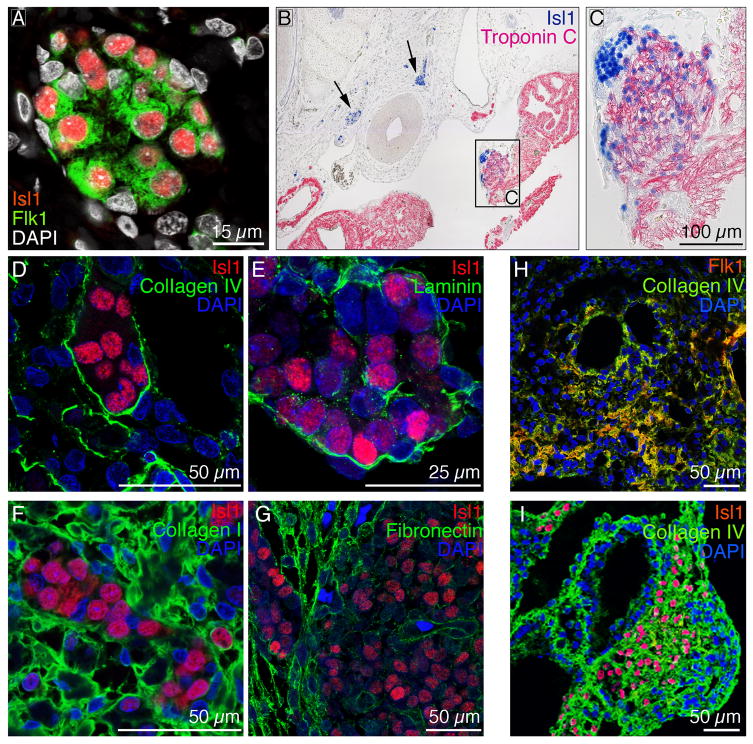
Multipotent endogenous CPCs have been identified in the fetal mouse heart based on the expression of the cardiogenic transcription factor Isl1 [2-3]. We have previously described a system to potentiate differentiation of murine ES cells to CPCs based on culturing them on ColIV. These Flk1+ progenitor cells express Isl1 as well as other known CPC markers including Nkx2.5 (Suppl. Fig. 1A) and possess the ability to differentiate into functional cardiovascular cells, including CMs, SMCs and ECs (Suppl. Fig. 2 and 3), as we previously described [5]. Immunofluorescence imaging of human first trimester (6-12 weeks of developmental age) and murine E15.5 hearts revealed that the endogenous cardiac Isl1+ cells also show strong expression of Flk1 (Fig. 1A). These Isl1+/Flk1+ CPCs are localized into discrete clusters within the right ventricular free wall, atria and outflow tracks (Fig. 1B and C). Interestingly, immunohistochemical staining demonstrated that differentiating CPCs, which migrate away from the progenitor clusters, down-regulate Isl1 while up-regulating mature cardiac markers such as troponin C (Fig. 1C). To determine if the clusters of Isl1+/Flk1+ cells occupy a distinct niche and to characterize its ECM composition, we employed immunofluorescence staining. Basement membrane (BM) proteins ColIV and laminin were found within and circumscribing the Isl1+/Flk1+ CPC clusters, reminiscent of stem/progenitor cell niches in other tissues (Fig. 1D, E). In contrast, ColI and fibronectin were predominantly found outside the CPC clusters within the myocardium (Fig. 1F, G).
Figure 1.
(A) Immunofluorescence staining of 9 week human hearts reveals that endogenous Isl1+ CPCs (red) also express Flk1 (green). DAPI-stained cell nuclei are shown in white. (B-C) Immunohistochemistry shows that endogenous Isl1+ CPCs are localized in clusters (B, see arrows). Differentiating CPCs down-regulate Isl1 (blue) and up-regulate mature cardiac markers such as troponin C (pink) (C). (D-G) ColIV (D; green) and laminin (E; green) are tightly expressed around the CPC clusters, whereas ColI (F; green) and fibronectin (G; green) are predominantly expressed outside the niche within the myocardium. Cell nuclei are shown in blue (DAPI). (H-I) Flk1+ and Isl1+ CPCs (red) co-localized with ColIV (green) in differentiating mouse embryoid bodies. DAPI-stained nuclei are shown in blue.
3.2. ES cell-derived CPCs and ECM
It has been suggested that ECM proteins are capable of directing ES cell differentiation to mesodermal fates, including the cardiovascular lineages [5, 20, 25]. We wanted to determine if ColIV and laminin were associated with Isl1+/Flk1+ cells in differentiating EBs at time points when commitment to the cardiovascular lineage occurs. As expected, cells in differentiating EBs down-regulated pluripotency markers such as Oct4 and Nanog and up-regulated early mesodermal (brachyury), endodermal (alpha-fetoprotein) and ectodermal (nestin) markers (Suppl. Fig. 4). We found that ECM proteins ColI and IV, laminin and fibronectin were robustly expressed (Suppl. Fig. 5) and co-localized with Isl1+/Flk1+ cells within developing EBs (Fig. 1H and I; Suppl. Fig. 6).
3.3. Impact of ECM on cardiovascular fate
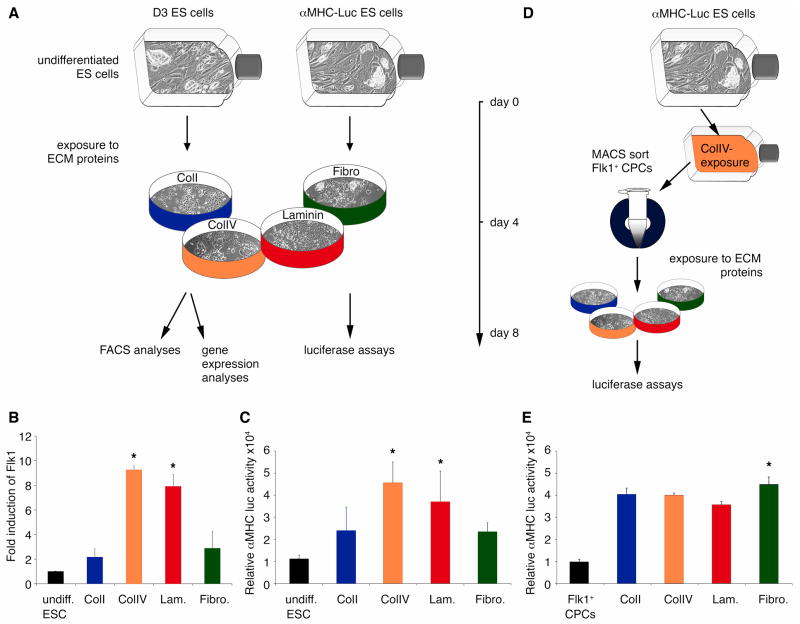
To determine the effects of the ECM proteins on cardiovascular differentiation, we cultured wildtype mouse ES cells as well as alpha-MHC-Luc ES cells on ECM protein-coated, conventional 2D culture surfaces (Fig. 2A). We found that ES cells, cultured for 4 days on the ColIV and laminin, showed significantly increased Flk1 gene expression when compared to ES cells that had been cultured on either ColI or fibronectin (Fig. 2B). FACS analyses also demonstrated that ColIV (2.13 ± 0.29%; P<0.002) significantly increased the percentage of Flk1+ candidate CPCs when compared to ColI (0.71 ± 0.09%), laminin (0.98 ± 0.05%) and fibronectin (0.8 ± 0.01%). To explore the effect of the BM ECM proteins on cardiac differentiation, we exposed alpha-MHC-Luc ES cells to ColI, ColIV, laminin and fibronectin. Similar to the results with Flk1, luciferase activity was 2-fold higher in cells cultured on ColIV and laminin (Fig. 2C). To determine if this increase in cardiac differentiation was due to ColIV and laminin increasing the number of Flk1+ CPCs versus an ability of ColIV and laminin to enhance cardiac differentiation of Flk1+ CPCs, we isolated Flk1+ cells from the alpha-MHC-Luc ES cells and then differentiated them on the various ECM substrates (ColI, ColIV, laminin or fibronectin) (Fig. 2D). Purified, alpha-MHC-Luc ES cell-derived Flk1+ CPCs cultured on ColI, ColIV, or laminin showed a similar propensity for cardiac differentiation as measured by alpha-MHC-dependent luciferase activity (Fig. 2E). Only fibronectin, an ECM protein that we found to be localized outside of the putative CPC niche, enhanced differentiation of Flk1+ CPCs into cardiac myocytes (Fig. 2E).
Figure 2.
(A) D3 ES and alpha-MHC-Luc ES cells were cultured for 4 days on ColI (blue), ColIV (orange), laminin (Lam.; red) or fibronectin (Fibro.; green). (B) Quantitative real-time PCR demonstrates a significant increase in Flk1 expression in differentiating ES cells cultured on ColIV or laminin *P<0.05 versus undifferentiated (undiff.) ES cells; ColI- and Fibro-exposed cultures. (C) Luciferase assays show significantly higher alpha-MHC-luciferase activities in ColIV- and Lam.-exposed cultures. *P<0.05 versus undiff. ES cells; ColI- and Fibro-exposed cultures. (D) alpha-MHC-Luc ES cells were cultured for 4 days on ColIV before Flk1+ CPCs were MACS-sorted. Flk1+ CPCs were then exposed for 4 days to ColI (blue) or ColIV (orange), Lam (red) or Fibro (green). (E) Luciferase assays show significantly higher alpha-MHC-dependent luciferase activity in Fibro-exposed cultures. *P<0.05 versus alpha-MHC-Luc ES cell-derived Flk1+ CPCs; ColI-, ColIV- and Fibro.-exposed cultures.
3.4. β-catenin signaling and CPC fate
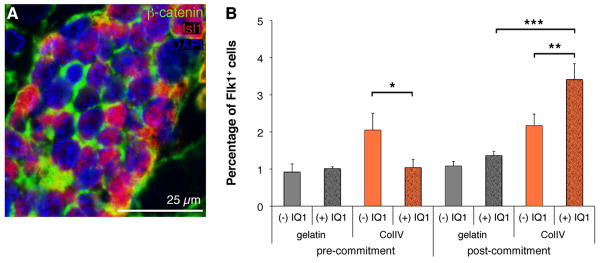
The Wnt/β-catenin pathway has been shown to play an important role in the self-renewal and subsequent differentiation of Isl1+ CPCs derived from mouse ES cells and hearts [12-13]. Consistent with these findings, endogenous Isl1+/Flk1+ CPCs in the cardiovascular niche in fetal human and mouse hearts express high levels of β-catenin (Fig. 3A). To determine if genes within the Wnt/β-catenin signaling pathway were preferentially expressed in CPCs, we analyzed global gene expression in mouse ES cell-derived Flk1+ progenitors compared to Flk1- cells using microarrays. Wnt signaling molecules, wingless-type MMTV integration site 9A (Wnt9a) and wingless-related MMTV integration site 1 (Wnt1) as well as Wnt membrane receptor frizzled homolog 4 (Fzd4) were preferentially expressed in Flk1+ cells (Table 1). B-catenin (Ctnnb1) as well SRY-box containing gene 7 (Sox7) and myelocytomatosis oncogene (c-myc), both β-catenin targets were up-regulated (Table 1). Consistent with activation of the Wnt/β-catenin pathway, negative regulators such as Wnt inhibitory factor 1 (Wif-1), adenomatosis polyposis coli (Apc), dickkopf homolog 1 (Dkk1), glycogen synthase kinase 3 β (Gsk3b) and the β-catenin degrading enzyme ubiquitin-conjugating enzyme E2B (Ube2b) were all down-regulated in Flk1+ progenitors (Table 1). Paradoxically, the Wnt/β-catenin pathway has been shown to have stage-specific effects on cardiac differentiation, both promoting and inhibiting this process [22]. Wnt/β-catenin's divergent effects may be explained by the fact that β-catenin/CBP-mediated transcription is critical for cell proliferation without differentiation, whereas a switch to β-catenin/p300-mediated transcription is critical to initiate a differentiation program with a more limited proliferative capacity [22, 26]. To explore these divergent β-catenin signaling pathways in Flk1+ CPCs, we utilized the small molecule antagonist IQ1 that selectively inhibits p300-dependent β-catenin signaling [22]. IQ1 specifically blocks the coactivator p300 from interacting with the protein β-catenin, a process that is required for the initiation of differentiation, while at the same time enhancing the interaction between the coactivator CBP and β-catenin thereby promoting the β-catenin-driven expansion of stem cells and preventing spontaneous differentiation [22, 26]. After confirming that gene expression profiles of pre-expansion Flk1+ CPCs were similar to Flk1+ CPCs that had been expanded using IQ1-supplemented medium (Suppl. Fig. 1B), we cultured ES cells on either gelatin or ColIV-coated 2D culture surfaces in the absence or presence of IQ1 introduced at two separate developmental time points (Fig. 3B). In the first group, IQ1 was added at day 0 of the culture prior to commitment to the cardiovascular lineage (pre-commitment). In group two, IQ1 was added at culture day 2, after ES cells had begun to differentiate (post-commitment). When added to undifferentiated ES cells, IQ1 had an inhibitory effect on the cardiovascular differentiation potential of ES cells cultured on ColIV as determined by the percentage of Flk1+ CPCs within the cultures (ColIV (-) IQ1: 2.05 ± 0.45% versus ColIV (+) IQ1: 1.04 ± 0.22%; P<0.0001) (Fig. 3B). In contrast, when administered after 2 days of differentiation, post-commitment to the cardiovascular lineage, IQ1 significantly increased the number of Flk1+ CPCs within the ColIV cultures (3.41 ± 0.43%; P=0.016) compared to the cultures without IQ1 supplement (2.17 ± 0.31%) (Fig. 3B). These data support a model where the increase in CPCs seen with IQ1 is related to its ability to block differentiation and promote expansion of CPCs versus promoting increased CPCs through enhancing ES differentiation to CPCs.
Figure 3.
(A) Immunofluorescence staining of an E15.5 mouse heart demonstrates high levels of expression of β-catenin (green) in Isl1+ CPCs (red). DAPI-stained nuclei are shown in blue. (B) FACS of differentiating ES cells cultured on either gelatin or ColIV with or without IQ1 (4 μg/ml). *P<0.0001 compared to ColIV pre-commitment with IQ1; **P=0.016 compared to ColIV post-commitment with IQ1; ***P<0.0001 compared to gelatin post-commitment with IQ1.
Table 1.
Microarray analysis of differential gene expression of Wnt/β-catenin signaling pathway in ES cell-derived Flk1+ CPCs.
| Gene symbol | Gene name | Fold increase in expression in Flk1+ vs Flk1- cells |
|---|---|---|
| Wnt9a | wingless-type MMTV integration site 9A | 11.2 |
| Wnt1 | wingless-related MMTV integration site 1 | 2.3 |
| Ctnnb1 | catenin, beta 1 | 1.32 |
| Sox7 | SRY-box containing gene 7 | 3.04 |
| Fzd4 | Frizzled homolog 4 | 2.23 |
| c-myc | myelocytomatosis oncogene | 6.64 |
| Wif-1 | Wnt inhibitory factor 1 | 0.07 |
| Apc | adenomatosis polyposis coli | 0.08 |
| Dkk1 | dickkopf homolog 1 | 0.23 |
| Gsk3b | glycogen synthase kinase 3 beta | 0.68 |
| Ube2b | ubiquitin-conjugating enzyme E2B | 0.42 |
3.5. Recapitulation of the 3D niche microenvironment
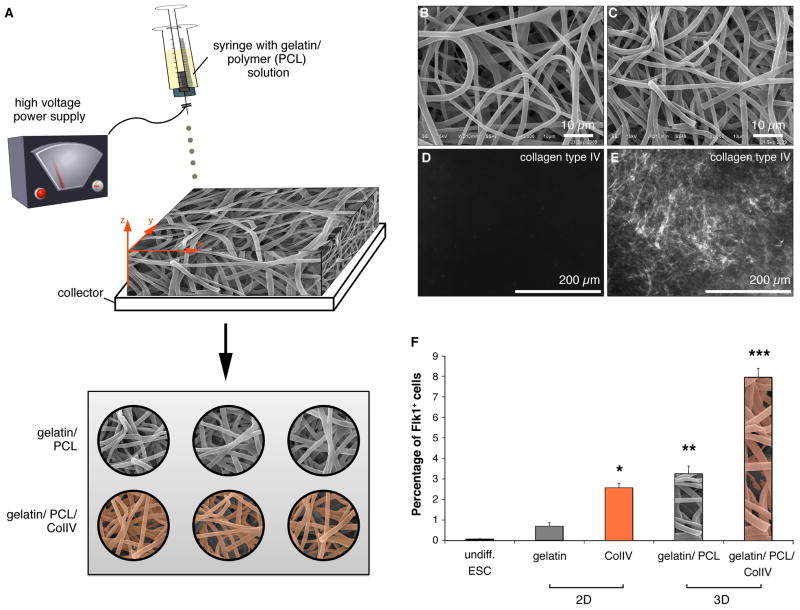
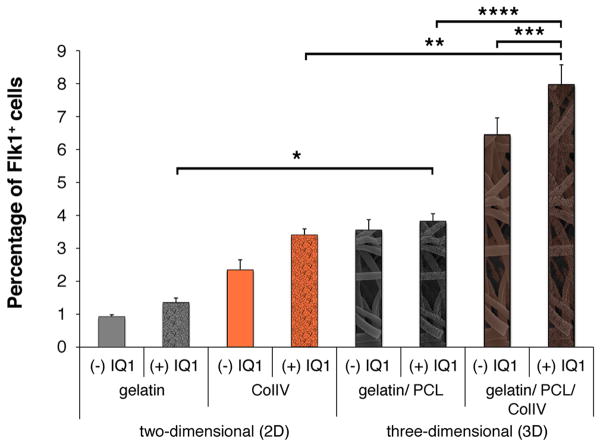
ColIV increases the differentiation potential of mouse ES cells into CPCs in vitro in two-dimensional (2D) culture systems. However, ColIV is really part of a 3D niche microenvironment in vivo, and thus 2D systems can not faithfully recapitulate these more complex cell-matrix interactions. Thus, to begin to study the effects of three-dimensionality on CPC fate we bioengineered a 3D CPC niche in vitro employing electrospinning technologies. To fabricate 3D cell culture scaffolds, 10% gelatin and 10% polycaprolactone (PCL) were mixed and electrospun to create hybrid scaffolds as described in detail previously [24]. In order to mimic not only the structural but also the biological properties of the endogenous CPC niche, some of the electrospun substrates were coated with ColIV (Fig. 4A). The ultrastructure of the resulting fibrous scaffolds, both non-coated (Fig. 4B) and ColIV-coated (Fig. 4C) are shown. The fiber size was comparable in non-coated (1.67 ± 0.45 μm) and ColIV-coated substrates (1.51 ± 0.26 μm), but the average pore size decreased after ColIV-coating (10.23 ± 2.88 μm2 and 5.96 ± 1.47 μm2) electrospun scaffolds. Immunofluorescence imaging was performed to confirm the successful coating of the electrospun material with ColIV (Fig. 4D and E). To assess the impact of three-dimensionality on CPC expansion, ES cells were cultured on either 2D coated surfaces or the 3D gelatin/PCL or 3D gelatin/PCL/ColIV electrospun cell culture inserts (Fig. 4F). Similar to our previous results, 2D ColIV increased the percentage of Flk1+ cells significantly when compared to the 2D gelatin cultures (2.57 ± 0.21% compared to 0.7 ± 0.17%; P=0.004). However, providing a 3D microenvironment further increased the fraction of Flk1-expressing CPCs (3.26 ± 0.37%; P<0.0001). Introducing ColIV to the 3D scaffold, significantly enhanced this effect (7.96 ± 0.43%; P<0.0001) (Fig. 4F).
Figure 4.
(A) Schematic of the electrospinning set-up and protocol of 2D versus 3D experiments. (B-C) Scanning electron micrographs of electrospun gelatin-PCL hybrid substrates without (B) or with (C) ColIV-coating. (D-E) Compared to the non-coated controls (D), immunofluorescence staining reveals the successful ColIV-coating of the electrospun material (E). (F) FACS analyses revealed an increased percentage of Flk1+ cells from 3D gelatin/PCL/ColIV cultures. *P=0.004 versus undiff ES cells and 2D gelatin cultures. **P<0.0001 versus undiff. ES cells and 2D gelatin and 2D ColIV cultures. ***P<0.0001 versus undiff. ES cells and gelatin, 2D ColIV and 3D gelatin/PCL cultures.
To assess if recapitulation of all aspects of the CPC niche in vitro would have an additive effect on CPC expansion, we combined the inhibitor of p300-dependent β-catenin signaling with three-dimensionality. We exposed undifferentiated ES cells to either the 2D or 3D culture systems and introduced IQ1 or vehicle to the cell culture medium after 2 days of culture. FACS analysis for Flk1 expression revealed that culture on 3D ColIV-coated electrospun scaffolds and inhibition of β-catenin signaling with IQ1, were additive (Fig. 5). The percentage of Flk1+ CPCs in 3D ColIV-coated/ IQ1-treated cultures (7.97 ± 0.6%; P<0.0001) was significantly greater then either 3D (3.55 ± 0.32%) or IQ1 treatment (1.35 ± 0.14% (gelatin); 2.34 ± 0.31% (ColIV)) alone.
Figure 5.
FACS analysis of differentiating ES cells that had been cultured on either gelatin- or ColIV-coated cell culture surfaces (2D), or gelatin/ PCL or gelatin/ PCL/ ColIV electrospun scaffolds (3D), with or without the presence of IQ1 post-commitment to the cardiovascular lineage. *P<0.0001 compared to gelatin with IQ1; **P<0.0001 compared to ColIV with IQ1; ***P<0.0001 compared to gelatin/ PCL/ ColIV without IQ1; ****P<0.0001 compared to gelatin/ PCL with IQ1.
4. Discussion
It has been speculated that, similar to satellite cells in adult skeletal muscle, cardiac stem cells in the adult heart are quiescent cells, surrounded by BM proteins including ColIV and laminin, and give rise to cardiovascular cells through asymmetric division when needed for cardiac repair [10, 17]. However, these adult cardiac stem cells differ widely from the cardiac progenitor cell populations described in the fetal heart and whether a stem or progenitor cell niche existed in the developing heart was unknown. This study represents the first characterization of the CPC in vivo niche in both fetal human and mouse hearts. We found that endogenous, multipotent Isl1+/Flk1+ CPCs reside within niche clusters in the right ventricular free wall, the atria and outflow tracks that were tightly circumscribed by the BM ECM proteins ColIV and laminin. This association of Isl1+/Flk1+ CPCs with ColIV and laminin was also seen in differentiating mouse ES cell-derived EBs. The ability of ColIV and laminin to enhance CPC differentiation from mouse ES cells and support their expansion is consistent with our previous studies [5, 20]. We also demonstrate that ECM proteins such as ColI and fibronectin surround these CPC niches within the fetal myocardium and may selectively promote cardiovascular differentiation of CPCs. Consistent with this model, Isl1-positive CPCs that migrated away from the niche, down-regulated Isl1 while committing to a CM phenotype, indicating the uniqueness of this in vivo niche microenvironment to maintain CPCs in their undifferentiated state. Interestingly, fibronectin specifically was capable of enhancing the differentiation of mouse ES cell-derived Flk1+ CPCs into CMs and has previously been shown to support differentiation of vascular cells in vitro [5, 25, 27]. The molecular basis for the selective effect of these ECM proteins on CPC fate is speculative, but likely is mediated by their interactions with CPC-expressed integrins.
Several specific factors that regulate cell fate decisions and thus control normal embryonic development have been discovered and include members of the bone morphogenetic protein (BMP), hedgehog, fibroblast growth factor (FGF) and Wnt families along with small molecules such as retinoic acid [15, 26]. Within this group, Wnt proteins are particularly important as they control numerous functions during development such as embryonic induction, generation of cell polarity and the specification of cell fate [26, 28]. Wnt signaling has also been implicated to be important for the maintenance and self-renewal of ES cells as well as stem/progenitor cells of various lineages, including the cardiovascular lineage [11]. Microarray analysis of CPC gene expression suggested that Wnt signaling through β-catenin was activated in CPCs and endogenous Isl1+/Flk1+ CPCs within the fetal cardiovascular niche expressed high levels of β-catenin. Wnt/β-catenin signaling has been reported to have a stage dependent, paradoxical effect on cardiac differentiation [22], but until recently, there has been no clear rationale for the dichotomous behavior of Wnt/β-catenin signaling in promoting self-renewal and proliferation in some cells while enhancing differentiation in others [26]. Recent studies have shown that β-catenin/CBP-mediated transcription is critical for stem/progenitor maintenance and proliferation without differentiation, whereas a switch to β-catenin/p300-mediated transcription is critical to initiate differentiation with a more limited proliferative capacity [22, 26]. To define the role of β-catenin signaling in CPC development, we utilized the small signaling molecule IQ1 that selectively inhibits p300-dependent β-catenin signaling [22]. We found that IQ1 had an inhibitory effect on CPC differentiation when introduced into cultures of undifferentiated mouse ES cells prior to commitment to the cardiovascular lineage, as suggested in the original reports [22]. When administered post-commitment to the cardiovascular lineage, IQ1 significantly increased Flk1+ CPC numbers, which is likely a result of expansion and prevention of differentiation of the existing pool of CPCs.
All stem and progenitor cell in vivo niches are complex 3D microenvironments but the importance of this structure to its function is unknown. However, awareness of the potential biological differences between 3D versus 2D environment has prompted investigators to bioengineer structures that mimic the 3D properties of natural ECM [8, 29-32]. To examine the importance of three-dimensionality on CPC fate, we created 3D CPC niche-like scaffolds with electrospinning [24]. When cultured in a 3D microenvironment, the percentage of Flk1+ progenitors increased significantly compared to the 2D cultures. By incorporating ColIV, a component of the endogenous CPC niche in vivo, we were able to increase the number of CPCs further. Although this work demonstrates that three-dimensionality is sufficient in increasing numbers of Flk1+ progenitors compared to conventional 2D in vitro culture systems, understanding of the mechanisms underlying the effect that three-dimensionality has on cell fate decisions will require further studies. Enhanced cell–cell and cell–matrix interactions leading to improved cell signaling in 3D cultures may play an important role [33]. Endogenous CPCs do not grow as independent units. Instead, they are surrounded in all dimensions by ECM within an isolated niche environment that provides unique cell–cell interactions, cell–ECM interactions, and exposure to a variety of soluble factors. These interactions within the niche have been shown to be critical for regulating stem cell self-renewal, proliferation, survival and differentiation in other tissues [34]. Cell–matrix interactions are greatly impaired on in vitro tissue culture plates, where cells are forced to adjust to an artificially flat and rigid surface; 3D matrices provide a closer approximation to the in vivo environment. Finally, we have demonstrated that incorporating aspects of both the physical 3D niche microenvironment and signaling pathways using IQ1 have an additive effect on CPC expansion. This suggests that further understanding of the CPC niche could facilitate additional optimization of synthetic niches in vitro to enhance CPC expansion, which will be a necessary prerequisite if CPCs are to be expanded to sufficient numbers to be useful in regenerative therapies.
5. Conclusion
In this study we have characterized the CPC niche in developing human and mouse hearts. We identified crucial niche ECM proteins and demonstrated their biological relevance for controlling CPC fate. We further demonstrated that three-dimensionality is not only important for CPC expansion in vitro, but that in combination with specific inhibition of certain β-catenin signals can enhance 3D engineered ECM protein-based in vitro culture systems. While pluripotent stem cells such as ES and iPS cells can be extensively expanded in culture [35], the ability to expand tissue-specific stem or progenitor cells ex vivo is extremely limited. Hence, the development of advanced in vitro culture systems that exhibit features of natural niche microenvironments, such as the one presented here, are critically needed both for studying the role of the niche in CPC biology but also the advancement of the field of regenerative medicine.
Supplementary Material
Acknowledgments
This work was supported by the NIH (5T32HL007895-10 to K.S-L.; P01-HL080111, R01 HL70748, and R01-HL094941 to W.R.M.), CIRM (RB1-01354), Laubisch and Cardiovascular Development Funds (to W.R.M.), Fraunhofer-Gesellschaft (Attract 692263 to K.S.-L.), Ruth Kirschstein National Research Service Award (GM007185 to B.V.H; T32HL69766 to J.M.G.) and the Alberta Heritage Foundation for Medical Research Fellowship (AHFMR to A.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–32. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–50. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Schenke-Layland K, Rhodes KE, Angelis E, Butylkova Y, Heydarkhan-Hagvall S, Gekas C, et al. Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem Cells. 2008;26:1537–46. doi: 10.1634/stemcells.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–7. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 8.Votteler M, Kluger P, Walles W, Schenke-Layland K. Stem Cell Microenvironments - Unveiling the Secret of How Stem Cell Fate is Defined. Macromol Biosci. 2010;10:1302–15. doi: 10.1002/mabi.201000102. [DOI] [PubMed] [Google Scholar]
- 9.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 10.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–98. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- 12.Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–79. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, et al. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:9313–8. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, et al. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–7. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 16.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 17.Leri A, Kajstura J, Anversa P, Frishman WH. Myocardial regeneration and stem cell repair. Curr Probl Cardiol. 2008;33:91–153. doi: 10.1016/j.cpcardiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Feo JA, Sluijter JP, de Kleijn DP, Pasterkamp G. Modulation of collagen turnover in cardiovascular disease. Curr Pharm Des. 2005;11:2501–14. doi: 10.2174/1381612054367544. [DOI] [PubMed] [Google Scholar]
- 19.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–64. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 20.Schenke-Layland K, Angelis E, Rhodes KE, Heydarkhan-Hagvall S, Mikkola HK, Maclellan WR. Collagen IV induces trophoectoderm differentiation of mouse embryonic stem cells. Stem Cells. 2007;25:1529–38. doi: 10.1634/stemcells.2006-0729. [DOI] [PubMed] [Google Scholar]
- 21.Hakuno D, Takahashi T, Lammerding J, Lee RT. Focal adhesion kinase signaling regulates cardiogenesis of embryonic stem cells. J Biol Chem. 2005;280:39534–44. doi: 10.1074/jbc.M505575200. [DOI] [PubMed] [Google Scholar]
- 22.Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 2007;104:5668–73. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenke-Layland K, Xie J, Magnusson M, Angelis E, Li X, Wu K, et al. Lymphocytic infiltration leads to degradation of lacrimal gland extracellular matrix structures in NOD mice exhibiting a Sjogren's syndrome-like exocrinopathy. Exp Eye Res. 2010;90:223–37. doi: 10.1016/j.exer.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenke-Layland K, Rofail F, Heydarkhan S, Gluck JM, Ingle NP, Angelis E, et al. The use of three-dimensional nanostructures to instruct cells to produce extracellular matrix for regenerative medicine strategies. Biomaterials. 2009;30:4665–75. doi: 10.1016/j.biomaterials.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–6. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 26.Teo JL, Kahn M. The Wnt signaling pathway in cellular proliferation and differentiation: A tale of two coactivators. Adv Drug Deliv Rev. 2010;62:1149–55. doi: 10.1016/j.addr.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 27.McCloskey KE, Stice SL, Nerem RM. In vitro derivation and expansion of endothelial cells from embryonic stem cells. Methods Mol Biol. 2006;330:287–301. doi: 10.1385/1-59745-036-7:287. [DOI] [PubMed] [Google Scholar]
- 28.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 29.Badylak SF, Nerem RM. Progress in tissue engineering and regenerative medicine. Proc Natl Acad Sci U S A. 2010;107:3285–6. doi: 10.1073/pnas.1000256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lund AW, Stegemann JP, Plopper GE. Inhibition of ERK promotes collagen gel compaction and fibrillogenesis to amplify the osteogenesis of human mesenchymal stem cells in three-dimensional collagen I culture. Stem Cells Dev. 2009;18:331–41. doi: 10.1089/scd.2008.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabbany SY, James D, Rafii S. New dimensions in vascular engineering: opportunities for cancer biology. Tissue Eng Part A. 2010;16:2157–9. doi: 10.1089/ten.tea.2010.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tate CC, Shear DA, Tate MC, Archer DR, Stein DG, LaPlaca MC. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. J Tissue Eng Regen Med. 2009;3:208–17. doi: 10.1002/term.154. [DOI] [PubMed] [Google Scholar]
- 33.Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29:690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Krause DS. Regulation of hematopoietic stem cell fate. Oncogene. 2002;21:3262–9. doi: 10.1038/sj.onc.1205316. [DOI] [PubMed] [Google Scholar]
- 35.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–23. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.