Abstract
Consistent with the common embryonic origin of liver and pancreas as well the similar glucose-sensing systems in hepatocytes and pancreatic β-cells, it should not be surprising that liver stem cells/hepatocytes can transdifferentiate into insulin-producing cells under high-glucose culture conditions or by genetic reprogramming. Persistent expression of the pancreatic duodenal homeobox-1 (Pdx1) transcription factor or its super-active form Pdx1-VP16 fusion protein in hepatic cells reprograms these cells into pancreatic β-cell precursors. In vitro culture at elevated glucose concentrations or in vivo exposure to a hyperglycemia are required for further differentiation and maturation of liver-derived pancreatic β-cell precursor into functional insulin-producing pancreatic β-like cells. Under appropriate conditions, multiple pancreatic transcription factors can work in concert to reprogram liver stem/adult liver cells into functional insulin-producing cells. If such autologous liver-derived insulin-producing cells can be made to escape the type 1 diabetes-associated autoimmunity, they may serve as a valuable cell source for future cell replacement therapy without the need for life-long immunosuppression.
1. Introduction
Type 1 diabetes (T1D) is a disorder characterized by the autoimmune-mediated destruction of insulin-producing β-cells in the pancreas. Insulin replacement therapy for T1D has proven to be ineffective in preventing long-term complications associated with fluctuations in blood glucose levels. Moreover, islet cell transplantation, while effective in achieving insulin-independent, persistent euglycemia, is limited by the lack of donor pancreata and the requirement of life-long immunosuppressive medication. Investigators are therefore seeking autologous stem/adult cells sources of Insulin-Producing Cells (IPCs) to restore euglycemia. Hepatic stem cells/adult hepatocytes are excellent candidates for generating β-cell surrogates, because liver and pancreas share a common bipotential precursor cell within the embryonic endoderm [1]. Hepatocytes and pancreatic β-cells also possess built-in glucose-sensing systems, allowing them to respond to changes in blood glucose concentrations. Recent studies demonstrate that conversion or transdifferentiation of hepatic stem cells [2–4], human fetal liver cells [5,6], human hepatoma cells [7] and human adult hepatocytes [8] into functional IPCs by genetic manipulation provides a promising option for cell replacement and gene therapy. Transdifferentiation/reprogramming of cells sharing a common embryonal origin can often be achieved by expressing tissue-specific/selective transcription factors (TFs) from one cell type into the other [9,10]. Due to space limitations, this review focuses on the experimental evidence of hepatic stem cell transdifferentiation into functional pancreatic IPCs, either by manipulating the external microenvironment (e.g., culture under high-glucose conditions and transplantation into diabetic mice) or by introducing key pancreatic transcription factor genes, particularly Pdx1-VP16 and Pax4.
2. Role of Pdx-1 in conversion of liver hepatocytes into pancreatic cells
Endocrine islet cell differentiation and maturation during embryonic development is a complex process controlled by a unique network of gene regulation. Numerous TFs are known to play significant roles in specifying the different pancreatic cell types [11–13]. The Pancreatic and duodenal homeobox gene-1 (Pdx-1) is the major pancreatic fate-determining TF. During embryogenesis, Pdx-1 is expressed in all progenitor cells destined to become exocrine and endocrine pancreas [14,15], and no pancreatic tissue exists in Pdx-1 knockout mice. In humans, the lack of functional Pdx-1 protein results in agenesis of the pancreas [16]. In the adult, Pdx-1 expression is restricted to β-cells and ~20% of δ-cells, where it plays a key role in insulin gene expression and maintenance of β-cell function. Ectopic expression of Pdx-1 in the mouse liver results in the hepatocytes expressing pancreatic genes and proteins with both exocrine and endocrine phenotype [17,18]. However, neither of these genes has successfully converted hepatocytes into purely endocrine pancreas [17,18]. Severe hepatitis observed in mice treated with Pdx-1 [19] was attributed to the production of pancreatic exocrine enzymes released by Pdx-1-induced pancreatic/hepatic hybrid cells [19]. Other studies demonstrate that transient expression of Pdx1-VP16, a constitutively active form of Pdx1, designed to activate target genes without the need for protein partners [20], in the liver resulted in more effective conversion of liver to pancreatic tissue than Pdx1 [21–23]. Therefore, treatment of liver hepatocytes with Pdx-1 or Pdx1-VP16 does not determine the specification of endocrine pancreas, suggesting that multiple TFs and suitable partners plus suitable soluble factors may be required for selective hepatocyte conversion into endocrine pancreas, especially insulin-producing β-like cells.
3. Pdx1-VP16-mediated liver-stem cell transdifferentiation into IPCs
Despite the fact that ectopic expression of Pdx1 [17,18] or Pdx1-VP16 [21–23] could induce transdifferentiation of hepatocytes into pancreatic cells, the underlying molecular mechanisms remained unclear, mainly because it is difficult to extrapolate from in vivo studies to individual gene changes at the cellular and molecular levels. One approach is to establish in vitro model systems using hepatic stem cells, such as WB-F344 rat liver epithelial cells (or WB cells) derived from normal liver cells from an adult male Fisher 344 rat. WB cells represent the cultured counterpart of liver stem-like cells [24,25] and exhibit the capacity to differentiate into both mature hepatocytes and biliary epithelial cells [24–27]. To establish an in vitro system to study the molecular mechanism of the liver to pancreas transdifferentiation, we generated WB-1 cell line, a stably transfected WB cells containing genes for both CMV-Pdx1-VP16 and rat insulin-I gene promoter-driving green fluorescence protein (RIP-eGFP) [2]. WB-1 cells are also insensitive to glucose stimulation and exhibit a profile of gene expression similar to rat β-cell line INS-1 cells and rat pancreas, with the exception of the absent expression of late-stage pancreatic genes (such as Pax4, Pax6 and Isl-1) related to developing β-cells and genes (SUR1, Kir6.2, Snare 25, and IAPP) related to regulated insulin release, suggesting that WB-1 cells are precursor cells of endocrine pancreas [2].
We previously demonstrated that rat hepatic oval stem cells [28] and bone marrow-derived stem cells [29] can be induced under the high-glucose in vitro culture conditions and in vivo transplantation to trans-differentiate and mature into glucose-responsive IPCs. To determine whether long-term high-glucose culture can induce the WB-1 cells to undergo further differentiation, we continued to culture WB-1 cells in the high-glucose medium. After long-term culture in a high-glucose medium, the β-cell precursor WB-1 cells exhibited the capacity to produce and release insulin in a glucose-responsive manner [2]. Transplantation of these precursor cells into streptozotocin-induced diabetic mice promotes complete β-cell differentiation and maturation as evidenced by restoration and maintenance of nomoglycemia [2]. The post-transplanted WB-1 cells formulate glandular structures similar to insulinoma INS-1 cells with typical neuroendocrine cytology resembling native β-cells; exhibit a gene expression profile indistinguishable from functional pancreatic β-cells [2]; and express multiple β-cell-specific proteins including pancreatic TFs Pdx1, Nkx2.2, Nkx6.1, Pax4, and Isl-1 [30] as well as abundant amount of insulin. These cells show no evidence of glucagon or pancreatic exocrine protein production. We have therefore established that external condition (i.e. high-glucose culture or cell transplantation into diabetic mice) can promote liver-derived pancreatic β-cell precursors to further differentiate and mature into functional IPCs.
4. Effectiveness of Pdx1 versus Pdx1-VP16 in liver-to-pancreas transdifferentiation
Forced expression of the bmaster control geneQ Pdx1 [17,18] or its modified form Pdx1-VP16 [2,21–23] reprograms liver cells to take a different, but related, differentiation pathway toward a pancreatic fate. Controversy arose as to whether expression of Pdx1 alone is sufficient to convert the liver cells into pancreatic beta-cells [17,19]. Transient adenovirus-mediated expression of Pdx1 alone in mouse liver activates pancreatic endocrine and exocrine genes [17,18], the latter reportedly resulting in severe hepatitis [19]. Our recently published study [2] showed that Pdx1-VP16 can selectively trans-differentiate rat hepatic stem-like WB cells into IPCs without any evidence of exocrine differentiation. These studies raise an important and clinically relevant question: do Pdx1 and Pdx1-VP16 have similar potency in mediating liver-to-pancreas transdifferentiation? Comparisons can be ambiguous, when adenovirus vectors harboring a transcription factor cDNA have differing extents and duration of gene expression. In view of the remarkable capacity of lentiviral vector (LV) for delivering and integrating transgene into both dividing and non-dividing cells, we transduced rat hepatic stem-like WB cells with LV-Pdx1 or LV-Pdx1-VP16, and generated single-cell-derived cell lines that stably express either Pdx1 or Pdx1-VP16 [3]. With these cell lines, we studied: the repertoire of both short-term and long-term expression of Pdx1- or Pdx1-VP16-induced pancreatic gene expression; and their capacity to serve as beta-cell surrogates in restoring euglycemia in streptozotocin-treated diabetic mice. We found that cell lines expressing either Pdx1 or Pdx1-VP16 long-term exhibited similar profiles for expression of genes related to pancreatic β-cell development and function; and similar capacity of reversal of hyperglycemia in diabetic mice [3]. However, short-term expression of Pdx1 or Pdx1-VP16 demonstrated that Pdx1-VP16 is much more efficient in initiating liver-to-pancreas transdifferentiation [3], this finding confirming the recent observations that adenovirus-mediated transient expression of Pdx1-VP16 in mouse liver is more effective than Pdx1 [22,23]. In summary, long-term, persistent expression of either Pdx1 or Pdx1-VP16 is similarly effective in converting hepatic stem cells into pancreatic endocrine precursor cells that, upon transplantation into diabetic mice, can become functional IPCs and restore euglycemia.
5. Impact of Pax4 on Pdx1-VP16-mediated liver-to-endocrine pancreas transdifferentiation
Although Pdx1-VP16 expression induces hepatic stem cell transdifferentiation into pancreatic precursor cells, these incompletely reprogrammed cells fail to become glucose-sensitive IPCs in the absence of late-stage pancreatic TFs [2]. Pax4 is known to promote late-stage β-cell differentiation and maturation [31], and it is positioned at the most upstream among the silent pancreatic TFs in WB-1 cells. Introduction of Pax4 gene by lentiviral vector into WB-1 cells thus creating WB-1A cell line results in the activation of the late-stage TFs (Pax6, Isl-1, and MafA) and β-cell function-related genes (SUR1, Kir6.2, Snare 25, and IAPP); and generation of a gene expression profile similar to that of functional rat insulinoma INS-1 cells and post-transplanted functional Pdx1-VP16-expressing WB-1 cells [2]. The newly generated WB-1A cells produce abundant insulin, exhibit glucose-responsive insulin release in vitro and a rapid reversal of hyperglycemia after transplantation into streptozotocin-induced diabetic mice [4]. Removal of transplanted WB-1A cells resulted in a rebound hyperglycemia, confirming that they were responsible for the observed normoglycemia. Therefore, simultaneous expression of Pdx1-VP16 and Pax4 reprograms liver stem cells into functional, mature β-like cells exhibiting glucose-responsive insulin release [4].
6. Summary of liver stem cell transdifferentiation
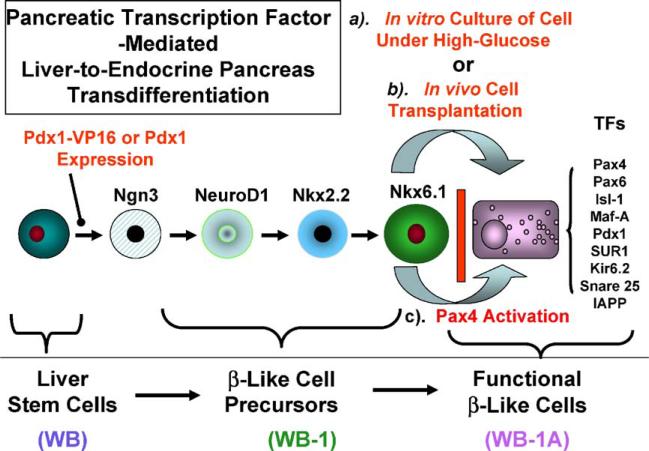
Based on our published findings [2–4], the molecular events for Pdx1-VP16-induced transdifferentiation of hepatic stem-like WB cell into functional insulin-producing β-like cells can be summarized in Fig. 1. Pdx1-VP16 expression converts the hepatic stem-like WB cells into pancreatic precursor cells characterized by stage-specific TF expression, in the absence of further differentiation, but these precursor cells cannot respond to glucose challenge in vitro. Generation of glucose-responsive, liver-derived β-cell-like IPCs from these precursor cells appears to require: (a) a long-term culture in high-glucose medium [2], (b) transplantation of the cells into diabetic mice [2], or (c) introduction of Pax4 into the Pdx1-VP16-expressing cells [4].
Fig. 1.
Model of liver-to-endocrine pancreas transdifferentiation. Expression of a modified form of Pdx1-Vp16 in hepatic stem (WB) cells activates multiple downstream TF genes associated with β-cell development and convert the WB cells into β-cell precursors. However, Pdx1-Vp16 failed to activate the following late-stage pancreatic transcription factors: Pax-4, Pax-6, Isl-1, and MafA, as well as the β-cell function-related genes SUR1, Kir6.2, SNAP25, and IAPP. Functional β-like cells can be achieved by: (a) culturing them under long-term high-glucose medium, (b) transplanting cells into diabetic (hyperglycemic) mice, and (c) activating Pax4 gene.
7. Future directions
The ultimate goal of studies on the liver-to-endocrine pancreas transdifferentiation is to translate rodent data into safe and reliable strategies for generating autologous primary human hepatocyte (PHH)-derived IPCs for β-cell replacement therapies for curing Type 1 diabetes. Such efforts should be pursued by an interdisclipinary team of clinicians and basic scientists that will establish best-practice treatment protocols. Major obstacles include the limits on the PHH expandability and the lifespan of the IPCs derived from adult hepatocytes. Transduction of the patient PHHs from liver biopsies with human telomerase should improve their proliferative capacity. These PHHs can be used to derive IPCs that must be rigorously tested for their ability to restore and sustain normoglycemia in immunocompromised mice and evaluated for any potential tumorigenesis. Another intriguing issue is whether the liver-derived autologous IPCs will survive or escape the autoimmune attack from the T1D patients’ own immune cells.
Take-home messages.
Liver and pancreas share a common progenitor cell during embryogenesis, and hepatocytes and pancreatic β-cells have similar built-in glucose-sensing systems.
Liver stem cells can be induced to transdifferentiate into insulin-producing cells under high-glucose culture conditions.
Persistent expression of Pdx1 or Pdx1-VP16 in hepatic stem cells results in liver-to-endocrine pancreas transdifferentiation, giving rise to pancreatic β-cell precursors.
In vitro high-glucose culture and other soluble factors or in vivo exposure to a hyperglycemic microenvironment are needed for further differentiation of liver-derived pancreatic β-cell precursor and production of functional pancreatic β-cell-like insulin-producing cells.
Multiple pancreatic transcription factors working in concert under an appropriate microenvironment are needed to reprogram liver stem/adult cells into functional insulin-producing cells. Liver-derived insulin-producing cells may serve as cell source for future cell therapy for treatment of diabetes.
Footnotes
This work was supported by grants NIH DK064054 and DK0718311.
References
- 1.Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–81. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 2.Cao LZ, Tang DQ, Horb ME, Li SW, Yang LJ. High glucose is necessary for complete maturation of pdx-1-vp16-expressing hepatic cells into functional insulin-producing cells. Diabetes. 2004;53:3168–78. doi: 10.2337/diabetes.53.12.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang DQ, Lu S, Sun YP, Rodrigue E, Chou W, Yang C, et al. Reprogramming liver-stem WB cells into functional insulin-producing cells by persistent expression of Pdx1- and Pdx1-VP16 mediated by lentiviral vectors. Lab Invest. 2006;83:83–93. doi: 10.1038/labinvest.3700368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang DQ, Cao LZ, Chou W, Lu S, Farag C, Atkinson M, et al. Pdx1-VP16- and Pax4-Mediated Liver-to-Endocrine Pancreas Transdifferentiation. J Biol Chem. doi: 10.1038/labinvest.3700434. submitted for publication. [DOI] [PubMed] [Google Scholar]
- 5.Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, et al. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci U S A. 2003;100:7253–8. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zalzman M, Anker-Kitai L, Efrat S. Differentiation of human liver-derived, insulin-producing cells toward the beta-cell phenotype. Diabetes. 2005;54:2568–75. doi: 10.2337/diabetes.54.9.2568. [DOI] [PubMed] [Google Scholar]
- 7.Li WC, Horb ME, Tosh D, Slack JM. In vitro transdifferentiation of hepatoma cells into functional pancreatic cells. Mech Dev. 2005;122:835–47. doi: 10.1016/j.mod.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E, et al. Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A. 2005;102:7964–9. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen CN, Horb ME, Slack JM, Tosh D. Transdifferentiation of pancreas to liver. Mech Dev. 2003;120:107–16. doi: 10.1016/s0925-4773(02)00337-4. [DOI] [PubMed] [Google Scholar]
- 10.Slack JM, Tosh D. Transdifferentiation and metaplasia-switching cell types. Curr Opin Genet Dev. 2001;11:581–6. doi: 10.1016/s0959-437x(00)00236-7. [DOI] [PubMed] [Google Scholar]
- 11.Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:111–27. doi: 10.1101/gad.859401. [DOI] [PubMed] [Google Scholar]
- 12.Dohrmann C, Gruss P, Lemaire L. Pax genes and the differentiation of hormone-producing endocrine cells in the pancreas. Mech Dev. 2000;92:47–54. doi: 10.1016/s0925-4773(99)00324-x. [DOI] [PubMed] [Google Scholar]
- 13.Edlund H. Transcribing pancreas. Diabetes. 1998;47:1817–23. doi: 10.2337/diabetes.47.12.1817. [DOI] [PubMed] [Google Scholar]
- 14.Soria B. In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001;68:205–19. doi: 10.1046/j.1432-0436.2001.680408.x. [DOI] [PubMed] [Google Scholar]
- 15.Hui H, Perfetti R. Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur J Endocrinol. 2002;146:129–41. doi: 10.1530/eje.0.1460129. [DOI] [PubMed] [Google Scholar]
- 16.Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–10. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 17.Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–72. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 18.Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, et al. Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem. 2003;278:31950–7. doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- 19.Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 20.Sadowski I, Ma J, Triezenberg S, Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988;335:563–4. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- 21.Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105–15. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- 22.Imai J, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Uno K, et al. Constitutively active PDX1 induced efficient insulin production in adult murine liver. Biochem Biophys Res Commun. 2005;326:402–9. doi: 10.1016/j.bbrc.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 23.Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, et al. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54:1009–22. doi: 10.2337/diabetes.54.4.1009. [DOI] [PubMed] [Google Scholar]
- 24.Grisham JW, Coleman WB, Smith GJ. Isolation, culture, and transplantation of rat hepatocytic precursor (stem-like) cells. Proc Soc Exp Biol Med. 1993;204:270–9. doi: 10.3181/00379727-204-43663. [DOI] [PubMed] [Google Scholar]
- 25.Couchie D, Holic N, Chobert MN, Corlu A, Laperche Y. In vitro differentiation of WB-F344 rat liver epithelial cells into the biliary lineage. Differentiation. 2002;69:209–15. doi: 10.1046/j.1432-0436.2002.690414.x. [DOI] [PubMed] [Google Scholar]
- 26.Coleman WB, Wennerberg AE, Smith GJ, Grisham JW. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol. 1993;142:1373–82. [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman WB, McCullough KD, Esch GL, Faris RA, Hixson DC, Smith GJ, et al. Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am J Pathol. 1997;151:353–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, et al. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci U S A. 2002;99:8078–83. doi: 10.1073/pnas.122210699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721–32. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang LJ, Crawford AC. Morphologic characterization of Explanted Liver-Derived Insulin-Producing β-like Cells.. United States and Canadian Academy of Pathology Annual meeting; Atlanta, Georgia. February 11–17, 2006; (Abstract) [Google Scholar]
- 31.Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mech Dev. 2003;120:65–80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]