Abstract
Mass drug administration is one of the public health strategies recommended by the World Health Organization for the control and elimination of seven neglected tropical diseases (NTDs). Because adequate coverage is vital to achieve program goals, periodically conducting surveys to validate reported coverage to guide NTD programs is recommended. Over the past decade, the Centers for Disease Control and Prevention (CDC) and collaborators conducted more than 30 two-stage cluster household surveys across three continents. The questionnaires gathered coverage data and information relevant to improving NTD programs including NTD-related attitudes and practices. From the 37 coverage survey estimates obtained in those surveys, 73.3% indicated an over reporting of coverage, including all three that assessed school-based distributions. It took an average of 1 week to conduct a survey. Our experiences led us to conclude that coverage surveys are useful and feasible tools to ensure NTD elimination and control goals are reached.
Introduction
Neglected tropical diseases (NTDs) impose profound health and socio-economic burdens, including impaired childhood growth and development, hindered economic prosperity, and risk of life-long morbidity, on the world's poorest populations.1–3 Seven of the NTDs—lymphatic filariasis, onchocerciasis, schistosomiasis, trachoma, and soil-transmitted helminthiasis (ascariasis, trichuriasis, and hookworm infection) not only have extensive geographic overlap but also share mass drug administration (MDA) as the main public health intervention for their control and elimination, as recommended by the World Health Organization (WHO).4 The regular distribution of single-dose chemotherapy to an entire at-risk population is founded on the idea that successive, adequate coverage MDAs will reduce the prevalence of infection that can lead in some cases to elimination. This means that achieving and maintaining adequate drug coverage during MDAs, is paramount to the success of NTD control and elimination programs. Low coverage may necessitate additional MDAs or if unnoticed, may lead to premature impact evaluations.
Drug coverage is defined as the proportion of individuals who have ingested a drug or combination of drugs.5 Most programs rely exclusively on reported drug coverage, calculated based on the number of doses distributed as recorded in drug registers during the MDA or school-based distribution. The denominator is the estimated target population, based on official census data with accounting for annual growth rates, or on a census conducted by health workers in preparation for drug distribution. Depending on the disease targeted by the MDA and the disease prevalence, the target population consists of the whole population or a specific age-eligible population such as school-aged children (SAC).4
Drug coverage can also be determined through drug coverage surveys. Surveyed drug coverage is calculated by dividing the total number of individuals reporting to have taken the drug(s) by the total number of individuals residing in the surveyed households during the MDA. Although the main purpose of drug coverage surveys is to validate reported drug coverage, these surveys also provide an opportunity to assess other questions of interest, such as sex and age-specific coverage, drug adverse events, reasons for non-compliance as well as to collect information about MDA delivery and health education strategies.
Although coverage surveys are recommended by the WHO as a necessary means of program monitoring and are recommended by drug donation programs, they often fail to be implemented because it is perceived that the cost is prohibitively high in settings with constrained financial and human resources.5 This assumption is reinforced by misconceptions that coverage surveys must be conducted annually in each district and that the collected information is not reliable. In this work, we aim to share our experiences over the past decade performing drug coverage surveys across three continents.
Methods
All the surveys described in this work were implemented by the Ministry of Health (MoH) and partners with technical assistance from the Centers for Disease Control and Prevention (CDC).
Selection of survey area.
We conducted all coverage surveys at the MDA implementation unit (IU) level, commonly a district. Exceptionally, in cases where the districts were small, several districts were combined. Because surveys are not meant to provide an annual assessment of drug coverage in each IU, only one or more IUs were selected in each country for the coverage survey. The district selection was made in collaboration with the MoH and implementing partners based on reasonable or high reported drug coverage, missing target population data, and assumed representativeness of the district. If the reported drug coverage is low, we recommend conducting supervision visits or a focus group so that the reasons for the low coverage can be identified and immediately corrected. Because the survey is meant to assist program managers in evaluating if their program needs improvement, it is important to conduct the coverage survey at the beginning of the program implementation, either after the first or second MDA round. To ensure that recall bias is minimal, we conducted the coverage survey as soon after an MDA as possible.
Sampling frame.
We used the protocol described by the Global Program to Eliminate Lymphatic Filariasis (GPELF), which is based on the WHO Expanded Program on Immunization (EPI) survey methodology.6,7 We elaborated some of the details concerning household selection and expanded the questionnaire to evaluate integrated MDAs and to collect information about drug adverse events, reasons for non-compliance as well as to collect information about MDA delivery, health education strategies, and other topics important for the specific NTD program, such as the availability of water and sanitation.
Selection of villages.
Thirty clusters were systematically selected within the study area with probability proportional to the estimated population size. The first step was to create a list of all the primary sampling units (PSU), typically villages or neighborhoods. It is important that this list is mutually exclusive and exhaustive, to ensure that all villages have the opportunity to be selected. The next step was to add the population figure for each PSU. Population figures were gathered as much as possible from a single source, such as census data or another reliable source, even if these data were outdated or estimated. If population data could not be obtained for some villages, figures were estimated by asking local authorities to compare their population to villages with known population figures.
Sampling units were then selected with probability proportional to the size of its population. This is done by establishing a sampling interval, based on the total population of the survey area divided by the number of clusters. The random start point or initial cluster is chosen by selecting a random number between zero and the sampling interval, after which the sampling interval is added until the total number of desired clusters is selected.
Selection of houses.
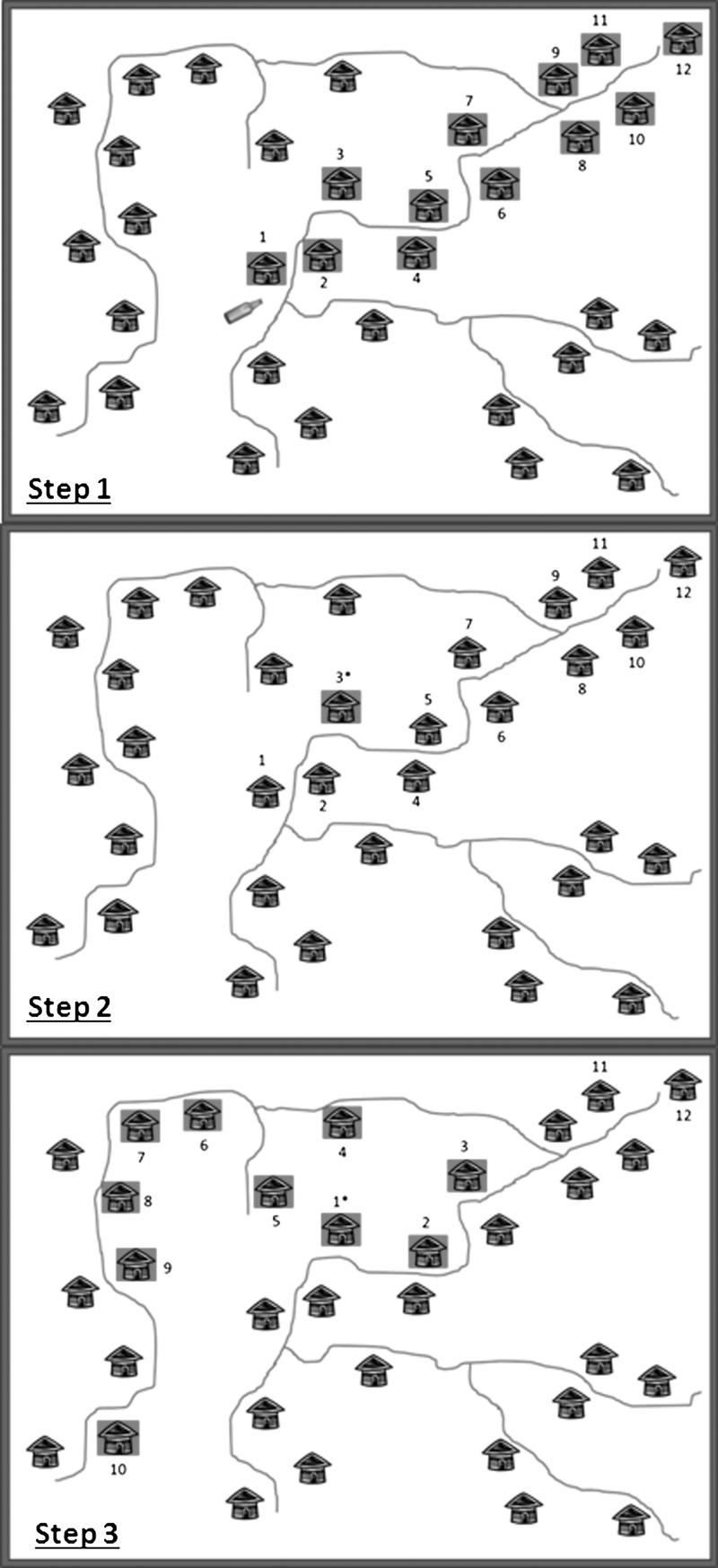
Within each of the selected clusters, 10 houses or compounds were selected using a “modified random walk” method (Figure 1).8 Initially, a central location within the cluster was identified (i.e., a central crossroad near the village leader's home) from which a random direction was selected by spinning a bottle. If the direction indicated by the bottle spin did not correspond with a road or path, the bottle was turned clockwise until a road was encountered, indicating the direction of travel. The survey team followed the routes that most closely aligned with the indicated direction until they reached the village boundary, while simultaneously enumerating all houses lining both sides of the routes. Survey teams often used the sun's position as a guide to follow the directional line to the village boundary. Uninhabited houses, public buildings, or businesses that did not also serve as residences were not included in the enumeration. A random number, between one and the total number of houses counted along the directional line, was selected usually by selecting a random number from the serial number of a bank note. This number corresponded to the first household to be interviewed. To identify the second household, the surveyors returned to the road, continued away from the center of the cluster, and turned left any time they encountered a path or road. Any time a house was encountered on the left side of the road, this house was selected. Sampling continued in this manner until 10 houses were visited. If none of the household members were present at the time of the initial visit, the team returned once to attempt to survey the household before leaving the village. If the house was still empty, the house was marked as absent, but was not replaced. In case too many houses were missed, an additional survey was planned.
Figure 1.
Improved random walk method. Step 1: Spin a bottle in the village center to determine the direction of travel. Count all houses following a straight line until the village boundary. *In this example, 12 houses are counted. Step 2: Randomly choose a number between 1 and the total number of houses counted. This number corresponds to the first house to be surveyed. *In this example, the random number is 3, so the 3rd house is the first house to survey. Step 3: Select the subsequent houses by continuing away from the village center. Include all houses on the left side of the road. Turn left each time a road is encountered on the left. Continue until 10 houses are surveyed.
Selection of persons.
At the house or compound, the interviewers explained the purpose of the survey and obtained verbal consent from the head of the household or another adult household member. The survey team first performed a household census identifying all persons living in the household during the MDA. For the coverage survey, all household members were asked to respond to the survey questions, regardless of his/her MDA eligibility; a proxy household member was eligible to respond for household members who were either absent or unable to respond (i.e., small children). In most surveys, it was noted if a proxy provided the answer. In the case of evaluating a MDA for SAC, only household members that fell within this age category were included in the survey. If there was an additional questionnaire, either a randomly selected adult household member or the head of household was asked to respond to these questions, depending on the questionnaire.
Questionnaire.
The coverage questionnaires used closed-ended questions to collect sex, age, and drug coverage variables for each individual living in the household at the time of the MDA. In some cases, school attendance and whether or not the person answered for him/herself were also added. A template drug coverage survey questionnaire was adapted in collaboration with the MoH during the survey planning stage to ensure it was appropriate for local conditions and to account for each country's individual program needs. The questionnaire varied based on 1) MDA drug package(s); 2) specific target groups (i.e., SAC); and 3) local languages and cultural conditions.
In most surveys, an additional questionnaire was included containing structured, open and closed-ended questions about NTD knowledge, attitudes, and practices (KAP), adverse events following drug ingestion, MDA awareness and/or feedback, issues of compliance, morbidity prevalence or availability of safe water and sanitation. These questions were added to furnish NTD program managers with additional information relevant to improving drug coverage or improving public health programs in general.
Logistics
Survey team.
To ensure that a coverage survey is an independent assessment of coverage, the MoH was asked to select interviewers, often health workers or students, who were not involved in the MDA implementation. Generally, we used three or four teams composed of two surveyors each. Surveyors were not required to have any particular survey skills before training, however at least one team member was required to have a basic familiarity with the survey area and to be fluent in the local language or dialect. Preferably, the second surveyor was from the national level and could perform subsequent coverage surveys throughout the country. The number of personnel devoted to a coverage survey depended on human resource availability and a desired time frame.
Training.
All surveyors and supervisors attended a training organized by the MoH and CDC. Topics addressed included the purpose of the survey, sampling methodology, ethical considerations, and questionnaire administration. Depending on the complexity of the survey tool, surveyors completed a 2- or 3-day training course, consisting of classroom training and roleplay as well as a practical field exercise to ensure that the interviewers adequately understood the sampling methodology and administered the questionnaire in a standardized fashion. On the basis of comments from the survey team, the questionnaire was adapted during the training.
Data Management and Statistical Analysis
Data entry and management were carried out using Epi Info (CDC, Atlanta, GA) and data analysis were performed using SAS v9.2 (SAS Institute Inc., Cary, NC) or SUDAAN 10.0.1 (RTI International Research Triangle Park, NC). Drug coverage for each drug or drug package was defined as the proportion of surveyed individuals who reported ingesting the drug during the MDA campaign. Point estimates and confidence intervals (CIs), with accounting for the clustering design, were calculated using SUDAAN's cluster survey program or the PROC SURVEY commands in SAS. Various strata were often created to assess sex or age-specific coverage. Because of the survey design, the probability of selection was assumed to be approximately equal across clusters and the sample was for that reason assumed to be approximately self-weighting and thus no weighting variables were needed.
For the additional questionnaire, a univariate analysis was conducted and odds ratios were calculated for risk factors associated with drug compliance.
Ethical Considerations
All the survey protocols were reviewed by the ethical committee from CDC and the respective MoH and were deemed by all to be a program evaluation activity. Before administering the questionnaire, verbal consent was obtained from the head of the household or another adult household member. As well, individual members of the household had the right to refuse to answer the survey questions or have questions answered on their behalf.
Results
Over the past decade, we have collaborated on 32 drug coverage surveys providing 37 drug coverage estimates. Surveys were carried out in eight countries throughout the Americas, Africa, and Asia (Figure 2 ). Table 1 summarizes the study findings for these surveys, including survey area, target disease(s), reported coverage, surveyed coverage, and other indicators evaluated. (Data on “other indicators” are not presented here.)
Figure 2.
Map of surveyed countries.
Table 1.
Overview of the drug coverage surveys: 2000–2011*
| Country, years | Targeted disease | Reported coverage | Surveyed coverage | Other variables |
|---|---|---|---|---|
| Haiti 2000†9 | Lymphatic filariasis | 98.7% | 71.3% (66.7–75.9%) | LF KAP, reasons for non-compliance, drug adverse events, morbidity prevalence |
| Haiti 2002‡ | Lymphatic filariasis | 86.6% | 78.5% (74.4–82.6) | LF KAP, participation in previous MDAs, reasons for non-compliance, drug adverse events |
| Haiti 2003 | ||||
| Verretes | Lymphatic filariasis, Soil-transmitted helminthiasis | 97.5% | 66.6% (57.1–76.0%) | LF KAP, reasons for non-compliance, drug adverse events, morbidity prevalence |
| Saut d'eau | 102.7% | 89.5% (86.6–92.3%) | ||
| Port de Paix | 83.7% | 75.3% (69.0–81.6%) | ||
| Milot (4 communes) | 79.1% | 80.5% (75.7–85.2%) | ||
| Characol | 159.7% | 87.6% (83.6–91.6%) | ||
| Nigeria 200310 | ||||
| Nasarawa and Plateau State | Lymphatic filariasis, Onchocerciasis | 84.6% | 62.7% (51.6–73.8%) | LF KAP, MDA feedback, morbidity prevalence, reason for non-compliance, school-attendance |
| Nasarawa and Plateau State | Lymphatic filariasis | 74.3% | 71.6% (65.1–78.2%) | |
| Haiti 2004§ | ||||
| Cap Haitian | Lymphatic filariasis | 63.0% | 62.7% (−)‡ | LF KAP, MDA feedback, reasons for non-compliance, drug adverse events |
| Croix des Bouquets | 50.0% | 55.0% (−)‡ | ||
| Togo 200411 | ||||
| Amou | Lymphatic filariasis | 81.4% | 73.3% (61.8–84.8%) | Detailed morbidity prevalence |
| Kozah, Binah, Doufelgou | 86.8% | 77.0% (67.0–87.0%) | ||
| Kpendjal, Tone | 81.5% | 81.0% (77.1–84.8%) | ||
| Nigeria 200512 | Lymphatic filariasis, Onchocerciasis, Malaria | −¶ | 68.0% (64.0–72.0%) | ITN ownership, ITN usage |
| Togo 2007 | Lymphatic filariasis, Onchocerciasis, Soil-transmitted helminthiasis | 84.0% | 82.1% (76.0–88.0%) | LF KAP, reasons for non-compliance, drug adverse events, provision of water and sanitation, ITN ownership, general questions |
| Schistosomiasis | 91% (89.0%–93.0%) (5–15 yrs) 94% (92.0%–96.0%) (whole population) | |||
| Bangladesh 2009 | ||||
| Munshigani | Soil-transmitted helminthiasis | 70.7% | 54.3% (44.8–63.8%) | STH KAP, school enrollment, water, sanitation and hygiene, MDA knowledge |
| Lakshmipur | 97.4% | 68.3% (60.1–76.4%) | ||
| Cambodia 200913 | Soil-transmitted helminthiasis | 97.8% | 80.9% (69.1–92.6%) | STH KAP, school enrollment, water, sanitation and hygiene, MDA knowledge, deworming intake in other settings |
| Haiti 2009∥ | ||||
| Acul du Nord/Bas-Limbe | Lymphatic filariasis, Soil-transmitted helminthiasis | 96.0% | 69.0% (62.0–77.0%) | NTD KAP, ITN coverage, morbidity prevalence, MDA feedback and cost, reasons for non-compliance, drug adverse events |
| Limbe | 94.0% | 89.0% (89.0–90.0%) | ||
| Grand Riviere du Nord | 171.0% | 77.0% (70.0–84.0%) | ||
| Croix des bouquets | 90.0% | 47.0% (42.0–50.0%) | ||
| Cabaret | 92.0% | 63.0% (50.0–70.0%) | ||
| Saint Louis du Nord | 103.0% | 77.0% (69.0–85.0%) | ||
| Bassin Bleu | 70.0% | 76.0% (71.0–81.0%) | ||
| Gros-Morne | 90.0% | 77.0% (71.0–82.0%) | ||
| Petite Riviere de l'Artibonite | 97.0% | 77.0% (70.0–84.0%) | ||
| Saint Marc | 112.2% | 78.0% (70.0–85.0%) | ||
| Malawi 2010** | Lymphatic filariasis | 80.0% | 66.8% (60.3–73.4%) | Coverage during 2 consecutive years, reasons for non-compliance |
| Mali 2011** | Lymphatic filariasis, | 87.0% | 62.0% (53.9–70.1%) | – |
| Schistosomiasis | 90.0% | 57.2% (49.1–65.4%) | – | |
| Cameroon 2011 | Lymphatic filariasis, | 80.3% | 76.9% (72.0–81.9%) | NTD KAP, morbidity prevalence, MDA feedback, reasons for non-compliance, drug adverse events |
| Trachoma, | 93.0% | 86.8% (80.9–92.7%) | ||
| Schistosomiasis | – | 39.8% (30.7–49.1%) | ||
NTD = neglected tropical diseases; MDA = mass drug administration; KAP = knowledge, attitudes, and practice; ITN = insecticide-treated nets.
Selection of the households was done by segmenting.
Clusters were randomly selected around 40 distribution posts (Direny unpublished data).
A KAP survey was administered to one household resident > 14 years of age. Reported coverage is based on the total population, whereas surveyed coverage is based on adults > 14 years of age.
Coverage data was collected at the sub-district level. Reported coverage was not available at this level.
Villages were selected by random sampling, results were weighted (Philius unpublished data).
Woodhall unpublished data.
Data unknown.
Eight of the 32 survey estimates for which we had comparable reported coverage results provided by the NTD program (25%) fell in the 95% CI of the surveyed coverage, thus validating the reported coverage. In 23 cases (71%) the surveys indicated that the reported coverage over-reported the MDA coverage. Only in one survey (3%) the reported coverage estimate was lower than the surveyed estimate. In several settings in Haiti the reported coverage exceeded 100% (Saut d'eau, 2003; Characol, 2003; Grand Riviere du Nord, 2009; Saint Louis du Nord, 2009; and Saint Marc, 2009), however this was not supported by the coverage survey. Our findings show that in the case of school distribution (Cambodia, Bangladesh, and Cameroon-Praziquantel), there was an important discrepancy between reported and surveyed coverage.
In all but two surveys, we were able to use census data for the proportional to the estimated population size sampling. In Haiti 2004, village population lists were compiled with information from local key-informants and in Haiti 2009 surveys, villages were selected randomly and results were weighted. In several surveys, one or more selected clusters were inaccessible for various reasons (Nigeria 2003, Bangladesh 2009, Cameroon 2011), but clusters were never replaced. The number of individuals surveyed in the drug coverage survey ranged from 637 and 2,921 and the refusal rate in all surveys was < 0.2%. The gender ratio (female/male) was between 0.96 and 1.17.
Several surveys assessed drug coverage over successive MDAs. In addition, MDA delivery was assessed by asking feedback regarding the organization of MDAs in settings, such as Haiti, where distribution posts were used to distribute the drugs. The health education campaigns were evaluated by questions addressing MDA awareness and KAP surrounding NTDs. In most surveys, information about drug adverse events and reasons for MDA non-compliance were asked. To evaluate other aspects of the program, questions were added on water and sanitation, insecticide-treated bed nets ownership and morbidity prevalence.
Most of the surveys took less than a week of field work with each team typically completing two clusters each day. The total time frame for completing a coverage survey depended on number of survey teams, proximity of adjacent clusters, as well as mode and ease of transportation. The cost depended on the availability of cars, distance from the capital, and rates of per diem but was in most cases around $5000.
Discussion
As programs for the control and elimination of neglected tropical diseases scale up, the role of monitoring and evaluation of NTD programs is becoming increasingly important. The two main indicators for NTD control and elimination programs are drug coverage as a performance indicator for program implementation and disease prevalence as an impact indicator. The success of MDA to disrupt the transmission of NTDs and consequently reach the elimination and control goals is dependent on achieving adequate drug coverage. To assess drug coverage, there is a need for a survey tool that requires minimum financial and human resources but that can independently evaluate the quality of reported coverage data. The importance of this was demonstrated by the fact that 74% of the survey estimates indicated that reported coverage was overestimated. Because the drug coverage survey used the MDA implementation unit as the survey area, the results can assist program managers to address the inaccurate coverage reports in the survey area and potentially other districts. Especially in settings with low school attendance levels, population-based surveys are particularly important for programs that target SAC through school-based interventions. Our findings show that in the case of school distribution (Cambodia, Bangladesh), there was an important discrepancy between reported and surveyed coverage. Drug coverage surveys also provide NTD program managers with additional pertinent information such as reasons for non-compliance, drug adverse events, feedback on MDA delivery strategies, and evaluation of health education messaging.
For the previously stated reasons, we are convinced that the financial and human resources needed to conduct these surveys are a worthwhile investment. Additionally, coverage surveys may be cost-saving if the results lead to improved MDA delivery strategies and better targeting of non-compliant groups, and therefore reduce the number of treatment rounds required to interrupt transmission. It is also important to note that coverage surveys do not need to be performed in every IU after every MDA round and have been demonstrated to be easily integrated with other variables of interest or even other public health programs.
The coverage survey protocol recommended by the GPELF to assess drug coverage is adapted from the WHO EPI survey methodology.6,7 This protocol was originally developed to provide vaccination coverage estimates, but this survey methodology has been widely adopted and adapted by public health programs.14,15 We further adapted the coverage survey protocol to improve the original EPI method while maintaining a balance of feasibility and epidemiologic rigor. This included using low tech data sampling techniques (i.e., modified random walk) and data management solutions (i.e., paper surveys and open source data management software) to ensure that our methods could be easily and independently conducted by local personnel with limited resources. Although we often used SAS or SUDAAN as our data analysis software package, coverage calculations can be performed using open source analysis software such as Epi Info. Additionally, both the Malawian and Haitian MoH subsequently implemented the protocol without external technical assistance, demonstrating the feasibility of performing those coverage surveys.
Although these surveys have been widely implemented for the past several decades, they also face several important challenges. As previously mentioned, there are several drawbacks of the sampling methodology, mainly concerning the representativeness of sample caused by the use of non-probability sampling when selecting households to be included.7,14 Compact segment sampling, PSU-level census, or census maps provide a cleaner alternative to the random walk method by allowing for the selection of a probability sample of households.14 Although we promote the use of probability sampling methods for second-stage sampling in areas with sufficient resources and skills required to undertake such a sampling frame, we would preclude the use of such surveys in the resource constrained settings where most of the MDAs are ongoing. Therefore, our surveys applied a “modified random walk” method to preserve the low cost and relatively simplicity of the EPI method, but also showed epidemiologic improvement when compared with the “next-nearest-household random walk method.”7 For the purpose of validating reported coverage and detecting gross programmatic inadequacies, lower levels of precision and unknown probabilities of selection associated with non-probability sampling methods such as the “modified random walk” are tolerable. Additionally, evidence suggests that the EPI random walk method can give accurate and precise results.15 Notably, simulations comparing non-probability sampling frames with a simple random sampling frame have shown that non-probability schemes, such as “5th nearest household” and “quadrant sampling,” can perform approximately as well as probability sampling.16 Additionally, seeing as adequate coverage thresholds are not precisely determined, we would argue that rigorous survey methodologies demanding a lot of financial and human resources determine drug coverage are not justified from a programmatic standpoint.
Critics of the original EPI cluster design suggest that the EPI method violates the assumption of negligible non-response in the study population, and introduces participant bias through replacement of non-respondent households.14 Our survey design selects households from within the PSU with non-replacement, to avoid introducing participant bias by replacing non-respondent households. Additionally, same-day call backs were conducted to decrease the likelihood of household non-response. Further, follow-up surveys were planned in the event of non-negligible non-respondent households, to assess participation bias; however, these were not necessary because of high levels of participation for each of the surveys.
Another concern raised about relying on surveys to assess coverage, is the ability of participants to recall drug consumption and respond correctly to the survey questions. However, a 2009 study examining recall bias in Togo concluded that more than 80% of respondents were able to accurately recall whether they had taken three different medications up to 1 year after an integrated MDA campaign (Budge, unpublished data). Because drug distribution days are rare and memorable events, recall bias does not contribute considerably and answers from participants are reliable.
Overall, in our experience, coverage surveys are a feasible and vital component of a comprehensive NTD program monitoring and evaluation system. By using surveyed coverage estimates in conjunction with reported coverage, NTD managers can better determine the true population coverage and take appropriate action to maintain or improve not only MDA coverage but NTD programs in general. Coverage survey benefits are multifaceted such that they can provide program oversight, assess program deficiencies, and ensure timely remedial action.
ACKNOWLEDGMENTS
We thank the Ministry of Health and the NTD program managers from Haiti, Plateau, and Nasarawa State (Nigeria), Togo, Bangladesh, Cambodia, Mali, Malawi, and Cameroun. We thank our partners (Carter Center, IMA World Health, University of Notre Dame and HKI) and CDC staff, fellows and EIS officers who assisted us conducting the survey. We thank all the surveyors and data management persons in the surveyed countries and Abdel Direny, Frank Richard, and Yao Sodahlon who assisted us organizing and conducting multiple surveys.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Financial support: We thank all the donors, including Bill and Melinda Gates Foundation, RTI/USAID, CDC, Children without Worms, GSK, ITI and MDP for financing the surveys.
Authors' addresses: Caitlin Worrell and Els Mathieu, Centers for Disease Control and Prevention - Division of Parasitic Diseases, Atlanta, GA, E-mails: uvz2@cdc.gov and emm7@cdc.gov.
References
- 1.Ramaiah KD, Radhamani MP, John KR, Evans DB, Guyatt H, Joseph A, Datta M, Vanamail P. The impact of lymphatic filariasis on labour inputs in southern India: results of a multi-site study. Ann Trop Med Parasitol. 2000;94:353–364. doi: 10.1080/00034983.2000.11813550. [DOI] [PubMed] [Google Scholar]
- 2.Stoltzfus RJ, Kvalsvig JD, Chwaya HM, Montresor A, Albonico M, Tielsch JM, Savioli L, Pollitt E. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. BMJ. 2001;323:1389–1393. doi: 10.1136/bmj.323.7326.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Preventive Chemotherapy in Human Helminthiasis. Geneva: WHO; 2006. [Google Scholar]
- 5.WHO . Preventative Chemotherapy in Human Helminthiasis Coordinated use of Antihelminthic Drugs in Control Interventions a Manual for Health Professionals and Programme Managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 6.World Health Organization . Monitoring and Epidemiological Assessment of the Programme to Eliminate Lymphatic Filariasis at Implementation Unit Level. 2005. (WHO/CDS/CPE/CEE/20005.50) [Google Scholar]
- 7.WHO . Training for Mid-Level Managers: The EPI Coverage Survey. 1991. WHO/IVB/08.07. WHO/EPI/MLM/91.10. [Google Scholar]
- 8.UNICEF . Monitoring Progress towards the Goals of the World Summit for Children. UNICEF; 1995. [Google Scholar]
- 9.Mathieu E, Deming M, Lammie PJ, McLaughlin SI, Beach MJ, Deodat DJ, Addiss DG. Comparison of methods for estimating drug coverage for filariasis elimination, Leogane Commune, Haiti. Trans R Soc Trop Med Hyg. 2003;97:501–505. doi: 10.1016/s0035-9203(03)80006-8. [DOI] [PubMed] [Google Scholar]
- 10.Richards FO, Eigege A, Miri ES, Kal A, Umaru J, Pam D, Rakers LJ, Sambo Y, Danboyi J, Ibrahim B, Adelamo SE, Ogah G, Goshit D, Oyenekan OK, Mathieu E, Withers PC, Saka YA, Jiya J, Hopkins DR. Epidemiological and entomological evaluations after six years or more of mass drug administration for lymphatic filariasis elimination in Nigeria. PLoS Negl Trop Dis. 2011;5:e1346. doi: 10.1371/journal.pntd.0001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathieu E, Amann J, Eigege A, Richards F, Sodahlon Y. Collecting baseline information for national morbidity alleviation programs: different methods to estimate lymphatic filariasis morbidity prevalence. Am J Trop Med Hyg. 2008;78:153–158. [PubMed] [Google Scholar]
- 12.Blackburn BG, Eigege A, Gotau H, Gerlong G, Miri E, Hawley WA, Mathieu E, Richards F. Successful integration of insecticide-treated bed net distribution with mass drug administration in Central Nigeria. Am J Trop Med Hyg. 2006;75:650–655. [PubMed] [Google Scholar]
- 13.Chesnaye N, Sinuon M, Socheat D, Koporc K, Mathieu E. Treatment coverage survey after a school-based mass distribution of mebendazole: Kampot Province, Cambodia. Acta Trop. 2011;118:21–26. doi: 10.1016/j.actatropica.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Brogan D, Flagg EW, Deming M, Waldman R. Increasing the accuracy of the Expanded Programme on Immunization's cluster survey design. Ann Epidemiol. 1994;4:302–311. doi: 10.1016/1047-2797(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 15.Milligan P, Njie A, Bennett S. Comparison of two cluster sampling methods for health surveys in developing countries. Int J Epidemiol. 2004;33:469–476. doi: 10.1093/ije/dyh096. [DOI] [PubMed] [Google Scholar]
- 16.Bennett S, Radalowicz A, Vella V, Tomkins A. A computer simulation of household sampling schemes for health surveys in developing countries. Int J Epidemiol. 1994;23:1282–1291. doi: 10.1093/ije/23.6.1282. [DOI] [PubMed] [Google Scholar]