Abstract
Rapid diagnostics tests (RDTs) detect malaria specific antigen(s) in the circulation, even when parasites are sequestered in the placenta and not visible by microscopy. However, research on their diagnostic accuracy during pregnancy is limited. Pregnant women (n = 418) were screened for malaria during routine antenatal care by using two RDTs that detect histidine-rich protein 2 (HRP2) or Plasmodium lactate dehydrogenase, and enzyme-linked immunosorbent assays with antibodies that detect dihydrofolate reductase–thymidylate synthase or heme-detoxification protein, and compared with real-time polymerase chain reaction (RT-PCR) and microscopy for evaluation of their diagnostic accuracy. Prevalence of malaria infection was high (53% by PCR). The RT-PCR and the HRP2 RDT detected most cases of malaria during pregnancy, whereas microscopy, the Plasmodium lactate dehydrogenase RDT, and enzyme-linked immunosorbent assays for dihydrofolate reductase–thymidylate synthase and heme-detoxification protein antibodies did not detect several low-density infections. Therefore, the HRP2 RDT could be a useful tool in high-transmission areas for diagnosis of malaria in asymptomatic pregnant women.
Introduction
Malaria infection is a major public health problem in sub-tropical and tropical regions throughout the world. Pregnant women, in addition to children, are at a higher risk of malaria than other adults. Infection with Plasmodium falciparum or P. vivax during pregnancy is related to adverse maternal health and poor birth outcomes.1,2 During pregnancy, malaria parasites sequester in the placenta, leading to placental changes central to the pathogenesis of placental malaria.3 In low transmission areas, malaria in pregnancy usually presents as a symptomatic, severe disease that can result in death of the mother and fetus. However, in high transmission areas, malaria infection rarely results in symptomatic disease because of acquired immunity. The main impact in these areas is malaria-related maternal anemia, low birth weight, and stillbirth.4,5
Diagnosis of malaria during pregnancy can be complicated by the absence of parasites in the peripheral blood or by parasite densities below the detection limit of microscopy caused by placental sequestration.3,6–8 Accurate detection of parasite infection in the placenta requires examination of histologic sections of fixed placental tissue, which is the gold standard for diagnosing placental malaria. Unfortunately, placental histologic analysis and microscopic examination of placental blood can only be performed after delivery. Therefore, it would be beneficial for mother and fetus to diagnose malaria in the peripheral blood earlier, followed by safe and adequate treatment. It is therefore necessary to detect the placental infection with a marker that is present in peripheral blood.
Currently available methods for the diagnosis of malaria in peripheral blood are parasite detection by microscopy, DNA or RNA detection methods such as the polymerase chain reaction (PCR), and detection of parasite antigens by rapid diagnostic tests (RDTs). The RDTs have the advantage of detecting circulating antigens, even when the parasites are sequestered in the deep circulation and not visible by microscopy. Commercially available RDTs for malaria detect one or more of the following antigens: histidine rich protein 2 (HRP2), and Plasmodium lactate dehydrogenase (pLDH) or aldolase.9,10 The RDTs are being widely deployed for diagnosis of malaria in pregnancy because of their ease of use and relatively low cost. However, their accuracy in this subpopulation has not been extensively evaluated, especially in the case of pLDH RDTs.6 Therefore, the aim of the present study was to evaluate the accuracy of an HRP2-based and a pLDH-based RDT for diagnosis of malaria in pregnancy by using peripheral blood and compare them with PCR and microscopy.
In addition, because of concerns about test stability, accuracy, species detection, antigen persistence, and antigen genetic diversity, there is still a need for improving RDT performances.9–13 Recently developed antibodies against the antigens dihydrofolate reductase– thymidylate synthase (DHFR-TS) and heme-detoxification protein (HDP) were screened for specificity against P. falciparum cultured isolate 3D7 and P. vivax samples in an enzyme-linked immunosorbent assay (ELISA).14 Two of these antibodies were evaluated for samples collected during this study for their potential use in malaria diagnosis.
Methods
Study area and population.
The study was conducted in Nanoro, Boulkiemdé Province, Burkina Faso, during November 2010 –August 2011, where P. falciparum is the dominant malaria species. Malaria transmission in the study region has a peak during the rainy season (June–December). The study population comprised pregnant women > 15 years of age and at a gestational age ≥ 15 weeks visiting the health center for their routine antenatal care. Because the women were screened during their antenatal care visit, they were mostly asymptomatic and not febrile. This study was performed in conjunction with an ongoing study assessing the safety and efficacy of three artemsinin-based combination therapy (ACT) (artesunate–amodiaquine, arthemeter–lumefantrine, or artesunate–mefloquine) for treatment of malaria in pregnant women (clinicaltrials.gov: NCT00852423). Ethical approval to conduct this study in conjunction with the PREGACT-trial was obtained from the Ethical Committee of the University Hospital in Antwerp (registration no. ITG 10 30 2 732), and from the Institutional Ethics committee of Center Muraz, Burkina Faso (registration no. 019-2010/CE-CM).
Study design and sample collection.
Pregnant women attending regular antenatal care were screened at each visit with an HRP2-based RDT (SD-Bioline Malaria Antigen P.f; Standard Diagnostics, Inc., Gyeonggi-do, South Korea) for patient recruitment into the PREGACT study. The women were recruited in the PREGACT study if they had a positive result in the RDT that was confirmed by microscopy. They were subsequently allocated to an ACT treatment group and followed-up actively after treatment.
When informed consent was obtained, finger prick blood (250–500 μL from one or two finger pricks) was collected in a tube containing EDTA (microvette; Sarstedt, Nümbrecht, Germany, or capiject, Terumo Europe N.V., Leuven, Belgium). The blood sample was transferred to the laboratory where it was used to prepare thick and thin blood smears. Blood was spotted on filter paper for real-time PCR (RT-PCR) and the sample was stored at 4°C. A second RDT, Advantage Malcard Pf and PAN (J. Mitra and Co., New Delhi, India), which detects pLDH, was performed immediately or several weeks later, depending on test availability. The presence of the DHFR-TS and HDP antigens was tested by ELISA when all samples were collected. Women who were screened for this study and had a positive result in the SD-Bioline RDT received free anti-malarial treatment, either according to national treatment guidelines of Burkina Faso (chloroquine), or according to the PREGACT trial protocol (artesunate–amodiaquine, arthemeter–lumefantrine, or artesunate–mefloquine) if the women were included in the PREGACT trial.
Laboratory procedures.
Finger prick blood was applied to the RDTs (SD Bioline Malaria Antigen P.f. and Advantage Malcard) according to the procedures described by the manufacturers. The RDTs were selected on the basis of World Health Organization (WHO) evaluation; the SD-Bioline RDT was one of the top-10 performing HRP2 RDTs, and the Advantage Malcard RDT was the best performing pLDH tests for detecting PAN and Pf.9,10 Manufacturer's storage temperature specifications (4 –30°C) were maintained during transportation (on ice) and at storage (in a monitored cold room at 18°C).
Microscopy was performed according to international and good clinical practice guidelines by local expert microscopists.15,16 Briefly, thick and thin blood smears were stained with Giemsa and parasites were counted against 200 leukocytes, with parasite negative results based on screening of 100 microscopic fields at 1,000 × magnification. In case of lower parasitemia (< 10 parasites/200 leukocytes), parasites were counted against 500 leukocytes. A leukocyte count of 8,000 cells/μL was assumed to calculate the parasite density per microliter.17 Slides were examined by two readers and in case of discordant results by a third reader. Discordant results were defined as a difference between the two readers in 1) positive and negative, 2) with parasitemias > 400 parasites/μL if the higher count divided by the lower count was > 2 or 3) with parasitemias ≤ 400 parasites/μL if the higher reading density was more than one log10 higher than the lowest reading. The final result was recorded as the geometric mean of the readings.
Blood was spotted on Whatman (Maidstone, United Kingdom) 903 protein saver cards, air-dried, and stored at room temperature in sealed bags with desiccant until transport and further processing in the Netherlands. DNA was isolated from the protein saver cards according to the method of Boom and others18 and as described16 and kept at −20°C until use.
A P. falciparum-specific 18S ribosomal DNA RT-PCR was performed on a CFX96™ real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA) and a FAM-labeled Taqman probe as described.16,19,20 If there were discordant results in duplicate samples (i.e., one positive result and one negative result), the RT-PCR for that sample was repeated. Results were not considered positive until they gave a signal in at least two of the duplicate samples.
For the ELISA, 37.5 μL of blood in EDTA was lysed with 75 μL of cold distilled water, mixed with an equal amount of 50 mM sodium carbonate, pH 9.6, and coated in duplicate on two ELISA plates (medium binding; Greiner Bio-One, Frickenhausen, Germany) for one hour at room temperature. The next day, wells were washed three times with phosphate-buffered saline, 0.1% Tween-20. The ELISA was then performed with α-HDP H16 (5 μg/mL) or α-DHFR-TS D20 (5 μg/mL) as described.16
Data collection and statistical analysis.
Sample size was calculated to be able to determine with 95% confidence if the sensitivity and specificity was 90% ± 5%, resulting in at least 150 positive women and 150 negative women to be recruited.21 With an expected parasite prevalence of 30% and expected study participant dropout rate of 5–10%, recruitment was continued until at least 165 (150 + 10%) women had a positive result in the HRP2 RDT. Test outcome was collected on case record forms and double-entered in an Access database (Microsoft, Redmond, WA). Calculations, including those for sensitivity, specificity, and prevalence, were performed by using STATA version 11.2; StataCorp LP, College Station, TX). The Shapiro-Wilk test was used to test for normality (W > 0.9), and not normal distributed variables such as parasite densities were log transformed for the analyses when required. The difference in mean parasite density as determined by microscopy and RT-PCR was determined by using a paired t-test. The difference in mean parasite density (determined by real-time PCR) between microscopy-positive and microscopy-negative cases was tested by using a two-sample t-test. P values < 0.05 were considered statistically significant. Kappa values were calculated to measure the level of agreement between the diagnostic tests. 95% confidence intervals (CIs) for calculated values are indicated when applicable.
Results
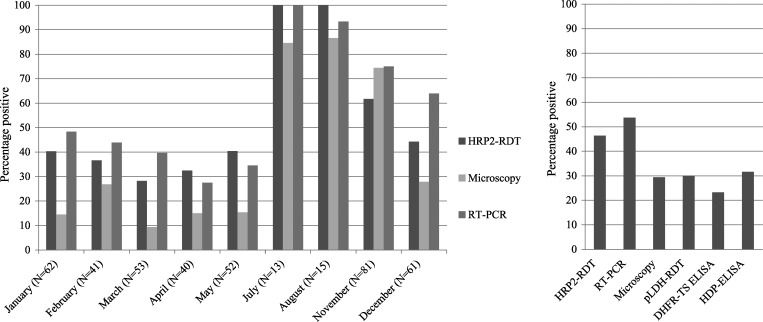
During November 2010–August 2011, 418 pregnant women were recruited and all women were routinely tested with the SD-Bioline RDT (HRP2). With this RDT, 194 (46%) of 418 (95% CI = 42–51%) women had a positive test result for P. falciparum. Overall, the HRP2 RDT and RT-PCR detected more cases than microscopy (Figure 1). Because some samples were too coagulated or there was little blood left, not all women could be tested with the pLDH RDT (n = 6) and ELISA (n = 71). The filter paper for PCR was missing for one participant, and in the first period of the study (November), no microscopy slides were prepared (n = 38). When restricting the analysis to the available microscopy results, malaria prevalence in pregnant women that were included in the study was 30% (95% CI = 25–34%) (112 of 380) by microscopy, 47% (95% CI = 42–52%) (178 of 380) by HRP2 RDT, and 53% (95% CI = 48–58%) (201 of 380) by RT-PCR. Prevalence by RT-PCR was slightly higher, 54% (95% CI = 49–59%) (224 of 417), when all recruited women are included.
Figure 1.
Percentage of women with positive results in malaria diagnostic tests for asymptomatic pregnant women in Nanoro, Burkina Faso. Left: Percentages of persons with positive results by histidine-rich protein rapid diagnostic test (HRP2 RDT), microscopy, and polymerase chain reaction (PCR) per month. Results from June are not available because women could not be included in the study during that month. Numbers of participants screened for each month is shown in parentheses. In November, for real-time–PCR (RT-PCR), n = 80 and for microscopy, n = 43. Right: Percentages of persons with positive results for all tests during the study. For HRP2 RDT, n = 418; for RT-PCR, n = 417; for microscopy, n = 380; for Plasmodium lactate dehydrogenase (pLDH) RDT, n = 412; and for enzyme-linked immunosorbent assays, n = 347.
The Advantage Malcard RDT (pLDH) and both ELISAs detected fewer cases than RT-PCR and the HRP2 RDT (Figure 1). The two RDTs had a good agreement with each other and with microscopy (Table 1). All infections detected by microscopy, RT-PCR, and HRP2 RDT were P. falciparum. However, the pLDH RDT showed positive results only for the PAN line and not P. falciparum in three cases, but they were all detected by the P. falciparum-specific RT-PCR and had low parasite densities, i.e., 26, 28, and < 4 parasites/μL, respectively.
Table 1.
Percentage of agreement and kappa value ± SE for level of agreement between diagnostic tests for malaria for asymptomatic pregnant women in Nanoro, Burkina Faso*
| Test | Microscopy | HRP2 RDT | pLDH RDT | RT-PCR | DHFR-TS ELISA |
|---|---|---|---|---|---|
| HRP2 RDT | 80.5% (0.60 ± 0.05) | ||||
| pLDH RDT | 85.7% (0.66 ± 0.05) | 83.0% (0.65 ± 0.05) | |||
| RT-PCR | 75.0% (0.51 ± 0.05) | 87.0% (0.74 ± 0.05) | 74.9% (0.51 ± 0.04) | ||
| DHFR-TS ELISA | 66.1% (0.19 ± 0.06) | 62.0% (0.22 ± 0.05) | 66.2% (0.19 ± 0.05) | 58.7% (0.20 ± 0.05) | |
| HDP ELISA | 67.7% (0.24 ± 0.06) | 62.0% (0.22 ± 0.05) | 66.2% (0.21 ± 0.05) | 57.8% (0.18 ± 0.05) | 74.6% (0.40 ± 0.05) |
P < 0.05 for all comparisons. A kappa value < 0.20 is considered a poor agreement, 0.21– 0.40 fair agreement, 0.41– 0.60 moderate agreement, 0.61– 0.80 good agreement, and 0.81–1.00 very good agreement. HRP2 = histidine-rich protein 2; RDT = rapid diagnostic test; pLDH = Plasmodium lactate dehydrogenase; RT-PCR = real time–polymerase chain reaction; DHFR-TS = dihydrofolate reductase–thymidylate synthase; ELISA = enzyme-linked immunosorbent assay; HDP = heme-detoxification protein.
Accuracy with microscopy as reference test.
When microscopy was used as the reference test, the RT-PCR had the highest sensitivity, followed by the HRP2 RDT (Table 2). The pLDH RDT had the highest specificity (95% CI = 86–93%). The other tests had significantly lower specificities (Table 2). The sensitivity for both ELISAs was low but the specificity was higher. However, this specificity was not sufficient to justify use of the ELISAs as diagnostic tests (Table 2).
Table 2.
Diagnostic test accuracy with microscopy as reference test for detection of malaria in asymptomatic pregnant women in Nanoro, Burkina Faso*
| Test | No. | Sensitivity | 95% CI | Specificity | 95% CI |
|---|---|---|---|---|---|
| RT-PCR | 380 | 97.3% (109/112) | 92–99% | 65.7% (176/268) | 60–71% |
| HRP2 RDT | 380 | 96.4% (108/112) | 91–99% | 73.9% (198/268) | 68–79% |
| pLDH RDT | 378 | 75.9% (85/112) | 67–84% | 89.8% (239/266) | 86–93% |
| DHFR-TS ELISA | 316 | 41.2% (40/97) | 31–52% | 77.2% (169/219) | 71–83% |
| HDP ELISA | 316 | 47.4% (46/97) | 37–58% | 76.7% (168/219) | 71–82% |
CI = confidence interval; RT-PCR = real-time–polymerase chain reaction; HRP2 = histidine-rich protein 2; RDT = rapid diagnostic test; pLDH = Plasmodium lactate dehydrogenase; DHFR-TS = dihydrofolate reductase–thymidylate synthase; ELISA = enzyme-linked immunosorbent assay; HDP = heme-detoxification protein.
Accuracy with PCR as reference test.
When PCR was used as the reference test, the highest sensitivity was found with the HRP2 RDT, followed by the pLDH RDT, microscopy, and the ELISAs. Sensitivity of the ELISAs was < 50% (Table 3). Conversely, microscopy had the highest specificity, followed by pLDH RDT and HRP2 RDT. The ELISAs had the lowest specificity (approximately 78%) (Table 3).
Table 3.
Diagnostic test accuracy with RT-PCR as reference test for detection of malaria in asymptomatic pregnant women in Nanoro, Burkina Faso*
| Test | No. | Sensitivity | 95% CI | Specificity | 95% CI |
|---|---|---|---|---|---|
| Microscopy | 380 | 54.2% (109/201) | 47–61% | 98.3% (176/179) | 95–100% |
| HRP2 RDT | 417 | 81.3% (182/224) | 76–86% | 93.8% (181/193) | 89–97% |
| pLDH RDT | 411 | 54.8% (120/219) | 48–62% | 97.9% (188/192) | 95–99% |
| DHFR-TS ELISA | 346 | 37.5% (69/184) | 31–45% | 82.7% (134/162) | 76–88% |
| HDP ELISA | 346 | 40.2% (74/184) | 33–48% | 77.8% (126/162) | 71–84% |
CI = confidence interval; RT-PCR = real-time–polymerase chain reaction; HRP2 = histidine-rich protein 2; RDT = rapid diagnostic test; pLDH = Plasmodium lactate dehydrogenase; DHFR-TS = dihydrofolate reductase–thymidylate synthase; ELISA = enzyme-linked immunosorbent assay; HDP = heme-detoxification protein.
Effect of parasite density on diagnostic test accuracy.
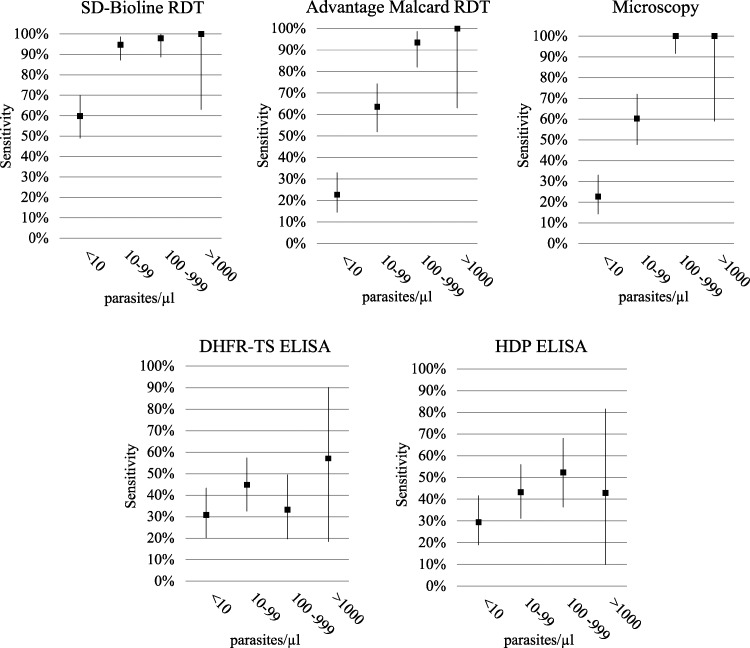
Parasite densities detected by microscopy and RT-PCR were statistically different (P < 0.00001). Parasite density ranged from 30 to 64,471 parasites/μL by microscopy and from ≤ 4 to 4066 parasites/μL by RT-PCR. Among women positive by RT-PCR, 75% (169 of 224) had a parasite density < 100 parasites/μL, with a low geometric mean parasite density of 15.7 (95% CI = 11.5 –21.3). The sensitivity of pLDH RDT and microscopy depended on parasite density (Figure 2). Mean parasite density of women with a negative blood slide but a positive RT-PCR result (sub-microscopic infections, n = 92) was significantly lower (2.9 parasites/μL; 95% CI = 2.2–3.9) than those having positive results for both tests (62.1 parasites/μL, 95% CI = 42.0 –91.9) (P < 0.00001).
Figure 2.
Sensitivity versus parasite density for malaria diagnostic tests for asymptomatic pregnant women in Nanoro, Burkina Faso. Sensitivities (y-axis) of the different diagnostic tests compared with real-time-PCR (RT-PCR) as a reference test stratified by parasite density in parasite/microliter (x-axis) that was determined by RT-PCR.
Discussion
The RDTs are being increasingly implemented for the diagnosis of malaria during pregnancy, but research on their diagnostic accuracy in this sub-population is limited.6 In the present study, six diagnostic tests, comprising microscopy, two RDTs (SD-Bioline RDT and Advantage Malcard RDT), RT-PCR, and two ELISAs (detecting HDP or DHFR-TS), were evaluated for routine testing of malaria infections among pregnant women attending antenatal clinics. The Advantage Malcard RDT (pLDH) and microscopy were unable to detect a large proportion of the low-density infections that were detected by RT-PCR and the SD-Bioline RDT in asymptomatic pregnant women. As expected, the sensitivity of all tests varied with parasite density, with a higher sensitivity at high parasite density. This effect was greatest with microscopy and the pLDH RDT.
The specificity of PCR and both RDTs were low when microscopy was used as the reference test, probably because microscopy cannot detect placental sequestered parasites. The PCR and RDT detect circulating antigen or nucleic acids, which might be adequate for identifying women with placenta malaria.6 Three microscopy-positive women with parasite densities of 93, 277, and 484 parasites/μL were negative by RT-PCR. Possible explanations for this discrepancy could be degradation of target DNA or presence of an amplification-inhibiting factor.
The difference in RDT performance is most likely caused by the type of antigen detected by the RDT. However, this finding does not indicate that tests from one manufacturer are superior to those from another. This finding is reflected by the HRP2-detecting test from J. Mitra and Co. (Advantage P.f. Malaria Card), which performed similarly to the SD-Bioline Malaria Antigen P.f. in WHO evaluation of RDTs.9,10 This difference in test performance between pLDH-based and HRP2-based tests was also observed in a previously conducted meta-analysis.6
When compared with accuracy of microscopy, accuracy of the RT-PCR and RDTs was similar to the summary values reported in a previous meta-analysis, although the specificity in this study was slightly lower.6 The sensitivity of the pLDH RDTs in the meta-analysis was lower than that of the Advantage Malcard RDT in this study. In WHO evaluation of RDTs, the performance characteristics of the Advantage Malcard RDT were superior to those of other pLDH RDTs.6,9,10 The pLDH RDT and the ELISAs were performed on stored blood samples, which might have had a negative effect on the accuracy of these tests because of degradation of antigen. However, no significant difference in accuracy of the pLDH RDT performed on stored blood samples (n = 246) and fresh blood samples (n = 172) was found.
Both ELISAs, when compared with PCR and microscopy, did not have sufficient accuracy for diagnosis of malaria during pregnancy. Antibodies for these ELISAs were selected on the basis of antibody affinity, detection limit, and sufficient differences in results for positive (with P. falciparum culture 3D7) and negative (whole blood) samples.14 Other antibodies are available and if more sensitive tests detecting DHFR-TS and HDP could be developed, they might be good options for malaria diagnosis, but not in the form of the assays used in this study.
This study has shown that a remarkably high proportion of pregnant women in the study area had asymptomatic malaria infection detectable by HRP2-based RDT and PCR, despite the implementation of intermittent preventive treatment (IPTp) in Burkina Faso and the study area. It is remarkable that in July and August all women in the studied population were parasite positive by RT-PCR and the HRP2 RDT. Women could not be recruited during June 1–July 10, 2011, because of unavailability of research staff, and therefore the percentage of infected pregnant women in that period could not be determined. Although it has been demonstrated that HRP2 persists considerably after parasite clearance,16 most women were also positive by RT-PCR, which indicated that they had active infections or had cleared their infection recently. Unfortunately, information on prior malaria episodes and treatment history (including last dose of IPTp-SP) was unavailable. If these positive results indicate active infection in persons who are not treated, these malaria infections may cause severe maternal anemia, a risk factor for maternal mortality and for intrauterine growth retardation and low birth weight.4
There were many sub-microscopic infections (detected by RT-PCR), and a large proportion (64%, 59 of 92) of these infections was also detected by the HRP2 RDT. In the absence of a gold standard for malaria infection during pregnancy, it was difficult to make conclusions on the accuracy of these tests and to conclude whether the HRP2 RDT and RT-PCR were detecting more cases or whether they are detecting false-positive cases. However, sub-microscopic infections detected by PCR have been associated with maternal anemia, low birth weight, and premature delivery.22 This finding indicates that although malaria control measures such as IPTp are implemented, routinely screening pregnant women for malaria during their antenatal care visits might be necessary to further eliminate infection and its related adverse effects. In areas with low malaria transmission or high sulfadoxine-pyrimethamine resistance, screening and treatment of women during antenatal care is already being conducted, but this strategy requires an easy-to-use test with sufficient accuracy.23,24 However, the complexity of RT-PCR does not make it suitable for large-scale implementation. Therefore, the RDT based on HPR2 would be the most practical and economic alternative.
In conclusion, microscopic examination of peripheral blood of pregnant women and the Advantage Malcard RDT (pLDH) failed to detect a large proportion of low-density infections that were detected by RT-PCR and the HRP2 RDT, which is similar to that of previous reports.6 However, more research is needed to determine if PCR and HRP2 RDTs are detecting low-density infections, rather than recently cleared infections. For that purpose, results from histologic examination of the placenta should be compared with those obtained by RDTs and PCR conducted at delivery.
ACKNOWLEDGMENTS
We thank Anneke Taal and Yao Mnimou for collecting blood samples, performing the RDTs, and preparing slides and PCR spots; Janneke Zoeten for performing the RT-PCR; the clinical and laboratory staff at the Clinical Research Unit of Nanoro for making contributions to this study; and all patients for participating in the study.
Footnotes
Financial support: The PREGACT-trial in Burkina Faso was supported by the European-Developing Countries Clinical Trials Programme, the Belgian Cooperation, and Sanofi S. A. (Paris, France). Johanna H. Kattenberg is supported by the Foundation for Innovative New Diagnostics in a collaborative effort for improvement of malaria diagnosis.
Authors' addresses: Johanna H. Kattenberg, Inge A. J. Versteeg, Henk D. F. H. Schallig, and Petra F. Mens, Meibergdreef 39, 1105 AZ Amsterdam, The Netherlands, E-mails: E.Kattenberg@KIT.nl, I.Versteeg@KIT.nl, H.Schallig@KIT.nl, and P.Mens@KIT.nl. Christian M. Tahita and Halidou Tinto, Institut de Recherche en Sciences de la Santé 01 BP 545 Bobo-Dioulasso, Burkina Faso, E-mails: marctahita@yahoo.fr and tintohalidou@yahoo.fr. Maminata Traoré/Coulibaly, Department of Mephatra/Pharmacy, Institut de Recherche en Sciences de la Santé–Direction Régionale de l'Ouest, BP 545 Bobo-Dioulasso, Burkina Faso. Umberto D'Alessandro, Medical Research Council, Banjul, The Gambia, E-mail: traore_maminata@yahoo.fr.
References
- 1.Hartman TK, Rogerson SJ, Fischer PR. The impact of maternal malaria on newborns. Ann Trop Paediatr. 2010;30:271–282. doi: 10.1179/146532810X12858955921032. [DOI] [PubMed] [Google Scholar]
- 2.Nosten F, McGready R, Simpson JA, Thwai KL, Balkan S, Cho T, Hkirijaroen L, Looareesuwan S, White NJ. Effects of Plasmodium vivax malaria in pregnancy. Lancet. 1999;354:546–549. doi: 10.1016/s0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- 3.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 4.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 5.Nosten F, Rogerson SJ, Beeson JG, McGready R, Mutabingwa TK, Brabin B. Malaria in pregnancy and the endemicity spectrum: what can we learn? Trends Parasitol. 2004;20:425–432. doi: 10.1016/j.pt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Kattenberg JH, Ochodo EA, Boer KR, Schallig HD, Mens PF, Leeflang MM. Systematic review and meta-analysis: rapid diagnostic tests versus placental histology, microscopy and PCR for malaria in pregnant women. Malar J. 2011;10:321. doi: 10.1186/1475-2875-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–4679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 8.Uneke CJ. Diagnosis of Plasmodium falciparum malaria in pregnancy in sub-Saharan Africa: the challenges and public health implications. Parasitol Res. 2008;102:333–342. doi: 10.1007/s00436-007-0782-6. [DOI] [PubMed] [Google Scholar]
- 9.WHO . Malaria Rapid Diagnostic Test Performance—Results of WHO Product Testing of Malaria RDTs: Round 1. Geneva: World Health Organization; 2008. [Google Scholar]
- 10.WHO . Malaria Rapid Diagnostic Test Performance—Results of WHO Product Testing of Malaria RDTs: Round 2. Geneva: World Health Organization; 2009. [Google Scholar]
- 11.Bell D, Wongsrichanalai C, Barnwell JW. Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat Rev Microbiol. 2006;4:682–695. doi: 10.1038/nrmicro1474. [DOI] [PubMed] [Google Scholar]
- 12.Baker J, McCarthy J, Gatton M, Kyle DE, Belizario V, Luchavez J, Bell D, Cheng Q. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PFHRP2-based diagnostic tests. J Infect Dis. 2005;192:870–877. doi: 10.1086/432010. [DOI] [PubMed] [Google Scholar]
- 13.Perkins MD, Bell DR. Working without a blindfold: the critical role of diagnostics in malaria control. Malar J. 2008;7((Suppl 1)):S5. doi: 10.1186/1475-2875-7-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kattenberg JH, Versteeg I, Migchelsen SJ, González IJ, Perkins MD, Mens PF, Schallig HD. New developments in malaria diagnostics: monoclonal antibodies against Plasmodium dihydrofolate reductase–thymidylate synthase, heme detoxification protein and glutamate rich protein. MAbs. 2012;4:120–126. doi: 10.4161/mabs.4.1.18529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Basic Malaria Microscopy. Part I: Learner's Guide. 2nd edition. Geneva: World Health Organization; 2010. [Google Scholar]
- 16.Kattenberg JH, Tahita CM, Versteeg I, Tinto H, Traoré/Coulibaly M, Schallig HD, Mens PF. Antigen persistence of rapid diagnostic tests in pregnant women in Nanoro, Burkina Faso and the implications for the diagnosis of malaria in pregnancy. Trop Med Int Health. 2012;17:550–557. doi: 10.1111/j.1365-3156.2012.02975.x. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood BM, Armstrong JR. Comparison of two simple methods for determining malaria parasite density. Trans R Soc Trop Med Hyg. 1991;85:186–188. doi: 10.1016/0035-9203(91)90015-q. [DOI] [PubMed] [Google Scholar]
- 18.Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermsen CC, Telgt DS, Linders EH, van de Locht LA, Eling WM, Mensink EJ, Sauerwein RW. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol Biochem Parasitol. 2001;118:247–251. doi: 10.1016/s0166-6851(01)00379-6. [DOI] [PubMed] [Google Scholar]
- 20.Wang CW, Hermsen CC, Sauerwein RW, Arnot DE, Theander TG, Lavstsen T. The Plasmodium falciparum var gene transcription strategy at the onset of blood stage infection in a human volunteer. Parasitol Int. 2009;58:478–480. doi: 10.1016/j.parint.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, Poole F, Smith PG, Sriram N, Wongsrichanalai C, Linke R, O'Brien R, Perkins M, Cunningham J, Matsoso P, Nathanson CM, Olliaro P, Peeling RW, Ramsay A. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol. 2006;4((Suppl)):S20–S32. doi: 10.1038/nrmicro1570. [DOI] [PubMed] [Google Scholar]
- 22.Arango E, Maestre A, Carmona-Fonseca J. Effecto de la infección submicroscópica o poloclonal de Plasmodium falciparum sobre la madre y el producto de la gestación. Revisión sistemàtica. Rev Bras Epidemiol. 2010;13:373–386. doi: 10.1590/s1415-790x2010000300002. [DOI] [PubMed] [Google Scholar]
- 23.McGready R, Davison BB, Stepniewska K, Cho T, Shee H, Brockman A, Udomsangpetch R, Looareesuwan S, White NJ, Meshnick SR, Nosten F. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. Am J Trop Med Hyg. 2004;70:398–407. [PubMed] [Google Scholar]
- 24.Tagbor H, Bruce J, Agbo M, Greenwood B, Chandramohan D. Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: a randomised controlled non-inferiority trial. PLoS ONE. 2010;5:e14425. doi: 10.1371/journal.pone.0014425. [DOI] [PMC free article] [PubMed] [Google Scholar]