Abstract
We evaluated the effectiveness and safety of intralesional meglumine antimoniate (MA) in 24 not submitted to previous treatment patients with cutaneous leishmaniasis (CL) and with contraindication to systemic therapy. Each treatment consisted of one to four intralesional applications of MA at 15-day intervals. Patients' age ranged from 3 to 90 years; fourteen were females. Intralesional treatment in the absence of any relevant toxicity was successful in 20 (83.3%) patients. Three patients required additional treatment with amphotericin B and one required systemic MA. None of the patients developed mucosal lesions when followed up to 60 months. Intralesional MA is an effective and less toxic alternative treatment of patients with CL and contraindication to systemic therapy.
American tegumentary leishmaniasis (ATL) affects the skin (cutaneous leishmaniasis, CL) and/or mucous membranes, and is caused by protozoa of the genus Leishmania, transmitted through the bite of sandflies.1
Pentavalent antimonials are the first-line drugs for the treatment (TTM) of ATL,2,3 and several adverse events (AEs) are associated with them.4–6 Intralesional (IL) meglumine antimoniate (MA) was evaluated for TTM of 24 patients with CL at Evandro Chagas Clinical Research Institute (IPEC), Oswaldo Cruz Foundation (FIOCRUZ), Rio de Janeiro, Brazil, between January 1, 2000 and December 31, 2006. The study was approved by the Ethics Committee on Human Research of IPEC; all patients signed a free informed consent form. All patients had a confirmed parasitological diagnosis of CL, had not been previously submitted to TTM, and had contraindication to systemic use of MA. A scale adapted from the Aids Table for Grading the Severity of Adverse Events7 was used for the evaluation of AE and baseline clinical alterations, where G1 = mild, G2 = moderate, G3 = severe, and G4 = life-threatening. Contraindications to systemic antimonial therapy were 1) presence of baseline clinical alterations corresponding to G3; 2) presence of baseline laboratory alterations corresponding to G2; 3) presence of baseline electrocardiographic alterations corresponding to G3 or G4 (baseline adjusted QT interval [QTadj] ≥ 0.46 ms was considered G3); 4) psychiatric disorders or high probability of low compliance with systemic TTM.AE were monitored by clinical examination, electrocardiogram (EKG), complete blood count and blood biochemistry, before, during, and soon after the end of TTM.
The MA was supplied by the Brazilian Ministry of Health (Aventis Pharma, São Paulo, Brazil). Each TTM consisted of 1–4 IL applications of MA, at 15-day intervals. The IL MA was injected subcutaneously until completely infiltrating the base of the lesion. Immediate cure was defined as epithelization up to 90 days after IL TTM. Lesion progression until complete healing was monitored through absence of crusts up to 1 month after epithelization, desquamation up to 3 months, infiltration up to 6 months, and erythema up to 9–12 months, as well as the absence of mucosal lesions.8 Patients who presented lesion reactivation after TTM were retreated using the same or an alternative regimen; additional IL TTM or other medications were applied according to the presence or absence of EKG changes at the occasion of retreatment and/or therapeutic failure.
The nonparametric Mann-Whitney test was used to compare the distribution of continuous variables (lesion area, volume of infiltrated medication per lesion area, etc.) between two groups (presence or absence of AE, presence or absence of reactivation, etc.). A nonparametric Nelson-Aalen estimate was used to evaluate cumulative hazard function in months until healing. Outcomes such as loss to follow-up or change of TTM were considered censures. Survival curves were stratified according to gender and site of lesion, and compared by weighted log-rank test.
Patients' age ranged from 3 to 90 years (median = 64); most were female (N = 14). Duration of the lesions until diagnosis ranged from 15 days to 12 months (median = 3 months).
Most patients had associated chronic systemic diseases (N = 20). Baseline clinical alterations corresponding to G3 (N = 5) and/or baseline laboratory (G2) and/or electrocardiographic alterations (G3/G4) (N = 16) were also observed (Table 1). In addition, two patients refused intramuscular (IM) TTM, and one patient had mental disorders and was an alcoholic.
Table 1.
Frequency of associated systemic chronic diseases and frequency of baseline clinical, laboratory, and eletrocardiographic alterations*
| Frequency of associated systemic chronic diseases | Frequency of baseline clinical alterations | Frequency of baseline laboratory alterations | Frequency of baseline EKG alterations | ||||
|---|---|---|---|---|---|---|---|
| Disease | Frequency (CST) | Clinical alteration | Frequency (CST) | Laboratory alteration | Frequency (CST) | EKG alteration | Frequency (CST) |
| Hypertension | 14 (4) | High blood pressure | 5 (4) | Increased urea | 7 | Increased QTadj interval | 6 (6) |
| Heart disease | 8 | Trisomy of chromosome 21 | 1 (1) | Increased creatinine | 6 | Supraventricular tachycardia | 1 (1) |
| Dyslipidemia | 1 | Unilateral nephrectomy | 1 (1) | Increase of lipase | 1 | Bundle branch block | 3 (3) |
| Hypothyroidism | 1 | Buttocks abscesses | 1 (1) | Hyperglycemia | 4 | Axis deviation to the left | 3 (3) |
| Chronic kidney disease | 3 | Increased amylase | 4 (1) | Change in ventricular repolarization | 3 (3) | ||
| Alcoholism | 2 (2) | Increased AST | 2 | Sinus tachycardia | 1 (1) | ||
| Psychiatric disorders | 1 (1) | Increased ALT | 2 | Atrial fibrillation | 1 (1) | ||
| Diabetes mellitus | 5 | Increased alkaline phosphatase | 2 | ||||
| Anemia | 1 | ||||||
| Hypercholesterolemia | 1 | ||||||
| Thrombocytopenia | 1 | ||||||
EKG = eletrocardiographic; CST = cases that contraindicated systemic treatment; QTadj = (QT × 0.04)/sqrt(RR × 0.04); AST = aspartate transaminase; ALT = alanine transaminase.
The number of lesions per patient ranged from 1 to 6 (median = 1). Each patient received 1 to 8 applications of MA (median = 2), considering all repeated IL TTM. The average of the total volume of MA injected per lesion was ∼19.7 mL (median = 6.5 mL).
The only clinical AE observed during IL therapy was mild-to-moderate pain at the site of application. Laboratory or electrocardiographic AE occurred in 10 patients (41.7%) and were classified as G1 (anemia [N = 1], hyperglycemia [N = 1], elevated urea [N = 1], elevated alkaline phosphatase [N = 2], elevated lipase levels [N = 2]) or G2 (elevated lipase [N = 2], and elevated creatinine levels [(N = 1]). G3 AE occurred in two patients (enlargement of QTadj in EKG). No significant association (P = 0.841) was observed between total IL MA dose and presence or absence of AE.
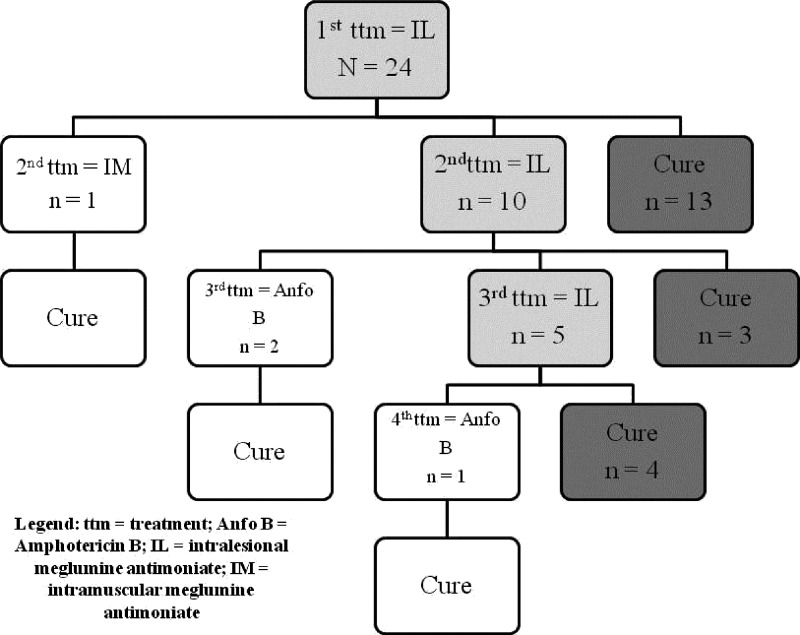
Figure 1 shows patients evolution from the first IL TTM up to healing. Four patients who needed other regimens, after IL therapy, until complete healing, required a greater volume of IL medication when compared with patients who healed exclusively with IL therapy (P = 0.006; median = 65.50 mL versus median = 12.25 mL, respectively). However, there was no significant difference (P = 0.310) in the total number of lesions between patients cured exclusively with IL MA and patients who demanded additional regimens. Considering all IL TTM, 20 patients (83.3%) achieved complete healing. Among those four patients who did not achieve lesion healing, two presented local injuries (frequent traumas or burn); all healed their lesions after the secondary TTM (three with amphotericin B and one with systemic MA, the last being previously contra-indicated caused by buttocks abscesses).
Figure 1.
Patients follow-up, since the first intralesional treatment.
No significant differences in lesion area (P = 0.185), volume of infiltrated medication per lesion area (P = 0.965), or total volume of medication infiltrated in the first TTM (P = 0.864) were observed between the group presenting reactivation (N = 11), and the group who cured after one IL TTM (N = 13).
Stratified analysis of the probability of lesion healing according to gender revealed no significant differences between survival curves (P = 0.250).
Lower limb lesions healed faster (median = 1.31 months) than lesions located at other sites (median = 14.65 months) (P = 0.037). One patient had no description of lesion site. These results were in disagreement with those of Schubach and others9 in IL TTM, and Marsden and others10 in IM TTM. Among eight patients with lesions in lower limbs, seven (87.5%) healed exclusively with IL TTM; and five (62.5%) did not reactivate the lesions. From 15 patients with lesions in other sites, 13 (86.7%) healed only with IL TTM; 53.3% demanded additional TTM. The percentage of cure was similar between patients with lower limb lesions and those with other sites lesions. Ampuero11 demonstrated that lesions take longer to heal in the lower limbs, but with a better cure rate and rarer reactivation when compared with lesions in other sites.
Despite 41.7% had AE, these were mild and self-limited. Effectiveness and safety of this TTM has been previously demonstrated in patients not necessarily with contra-indications to the systemic use of MA.12
Effectiveness of IL TTM was similar to that reported for higher IM doses of MA,13–15 with less AE.13–15 We recently reported a 44-patient series with older individuals who underwent low-dose intermittent IM MA,16 with worse effectiveness and more AE.
The number of MA infiltrations and the injection volume per patient were lower than those of standard IM TTM, a fact rendering IL TTM more tolerable and economically attractive.17–20 Lack of difference between development of AE and total volume of medication suggests that systemic absorption after IL infiltration is not sufficient to produce severe systemic AE.
Reactivation after trauma has been reported after conventional TTM of CL21 and is explained, in part, by persistence of the parasite in CL scars12,21–23; there are also reports of patients who developed CL at sites of frequent traumas years after being infected.24
Three patients who needed amphotericin B have shown clinical resistance after 2–3 IL MA TTM. The fourth patient, who did not cure with IL therapy after resolution of buttocks abscesses that temporarily contraindicated systemic MA, received a low IM dose of MA, reaching cure, a finding that is in contrast to the reports in literature of resistance with the use of suboptimal antimonial doses.20
We observed that the patients who did not evolved into cure exclusively with IL TTM received a larger volume of MA in IL TTM, probably caused by a greater number of required injections of MA, trying to obtain the cure only with IL TTM. The higher volume of medication could not be explained by a larger number of lesions per patient. Similarly, reactivation could not be explained by lesion area, suggesting that other factors in the parasite-host relationship may predispose to reactivation.
In this series, none of the patients developed mucosal lesions over a follow-up period of up to 60 months (median = 14 months) after the last TTM. Marsden25 suggested that even low doses of MA for the TTM of CL may prevent the occurrence of mucosal lesions in the future. Furthermore, Zajtchuk and others26 did not observe significant differences in the development of mucosal lesions, within a period of 4.6 years, between low-dose (10 mg Sb5+) and high-dose (20 mg Sb5+) TTM with MA.
The present results suggest that IL MA was effective and safe for the TTM of patients with CL and contraindication to systemic therapy. Controlled studies with IL and standard and alternative TTM with systemic MA are currently being conducted to confirm this hypothesis.
Footnotes
Financial support: This study was supported by the Rio de Janeiro Municipal Health Secretariat (RJ/FIOCRUZ accord), FIOCRUZ, FAPERJ, CNPq, and PAPES4/FIOCRUZ.
Authors' addresses: Érica de Camargo Ferreira e Vasconcellos, Maria Inês Fernandes Pimentel, Armando de Oliveira Schubach, Mariza de Matos Salgueiro, João Soares Moreira, Maria de Fátima Madeira, Cibele Baptista, and Cláudia Maria Valete-Rosalino, Fundação Oswaldo Cruz – Laboratório de Vigilância em Leishmanioses Rio de Janeiro, Rio de Janeiro, Brazil, E-mails: erica.vasconcellos@ipec.fiocruz.br, maria.pimentel@ipec.fiocruz.br, armando.schubach@ipec.fiocruz.br, mariza.salgueiro@ipec.fiocruz.br, joao.moreira@ipec.fiocruz.br, fatima.madeira@ipec.fiocruz.br, cibele.baptista@ipec.fiocruz.br, and claudia.valete@ipec.fiocruz.br. Raquel de Vasconcellos Carvalhaes de Oliveira, FIOCRUZ – Laboratório de Epidemiologia Rio de Janeiro, Rio de Janeiro, Brazil, E-mail: raquel.vasconcellos@ipec.fiocruz.br. Rilza Beatriz Azeredo-Coutinho, Hospital Federal de Bonsucesso – Dermatologia Rio de Janeiro, Rio de Janeiro, Brazil, E-mail: coutinhob@ymail.com. Fátima da Conceição Silva, Fundação Oswaldo Cruz – IOC Rio de Janeiro, Rio de Janeiro, Brazil, E-mail: fconcei@ioc.fiocruz.br.
References
- 1.Ministério da Saúde . In: Manual de Vigilância da Leishmaniose Tegumentar Americana. Saúde SdVe, editor. Editora MS; 2010. p. 78. [Google Scholar]
- 2.Goodwin LG. Pentostan (sodium stibogluconate); a 50-year personal reminiscence. Trans R Soc Trop Med Hyg. 1995;89:339–341. doi: 10.1016/0035-9203(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 3.Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 4.Andersen EMC-SM, Llanos-Cuentos A, Luz-Cjuno M, Echevarria J, Miranda-Verastegui C, Colina O, Berman JD. Comparison of meglumine antimoniate and pentamidine for perureian cutaneous leishmaniasis. J Trop Med Hyg. 2005;72:133–137. [PubMed] [Google Scholar]
- 5.Aronson NE, Wortmann GW, Johnson SC, Jackson JE, Gasser RA, Jr, Magill AJ, Endy TP, Coyne PE, Grogl M, Benson PM, Beard JS, Tally JD, Gambel JM, Kreutzer RD, Oster CN. Safety and efficacy of intravenous sodium stibogluconate in the treatment of leishmaniasis: recent U.S. military experience. Clin Infect Dis. 1998;27:1457–1464. doi: 10.1086/515027. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro AL, Drummond JB, Volpini AC, Andrade AC, Passos VM. Electrocardiographic changes during low-dose, short-term therapy of cutaneous leishmaniasis with the pentavalent antimonial meglumine. Braz J Med Biol Res. 1999;32:297–301. doi: 10.1590/s0100-879x1999000300008. [DOI] [PubMed] [Google Scholar]
- 7.DAIDS Division of AIDS table for grading the severity of adult and pediatric adverse events. DAIDS Regulatory Support Center; 2004. [Google Scholar]
- 8.Romero GA, Mendonça S, Schubach A, Noronha E, Matos M, Dietze R. O desafio para a realização de ensaios clínicos em leishmaniose tegumentar. Revista da Sociedade Brasileira de Medicina Tropical. 2006;39((III Suppl)):100–103. [Google Scholar]
- 9.Schubach AO, Marzochi KBF, Moreira JS, Schubach TMP, Araújo ML, Francesconi-do-Vale AC, Passos SRL, Marzochi MC. Retrospective study of 151 patients with cutaneous leishmaniasis treated with meglumine antimoniate. Rev Soc Bras Med Trop. 2005;38:213–217. doi: 10.1590/s0037-86822005000300001. [DOI] [PubMed] [Google Scholar]
- 10.Marsden P, Llanos Cuentas E, Lago E, Cuba C, Barreto A, Costa J, Jones T. Human mucocutaneous leishmaniasis in Tres Bracos, Bahia - Brazil. An area of Leishmania braziliensis braziliensis transmission. III. Mucosal disease presentation and initial evolution. Rev Soc Bras Med Trop. 1984;17:179–186. [Google Scholar]
- 11.Ampuero J. Efficacy and safety of low-dose pentavalent antimonial for treatment of cutaneous leishmaniasis by Leishmania (Viannia) braziliensis in Bahia, Brazil: a randomized clinical trial. Brasília - DF: Universidade de Brasília; 2009. p. 260. Thesis, Núcleo de Medicina Tropical. [Google Scholar]
- 12.Schubach A, Marzochi MC, Cuzzi-Maya T, Oliveira AV, Araújo ML, Oliveira AL, Pacheco RS, Momen H, Conceição-Silva F, Coutinho SG, Marzochi KB. Cutaneous scars in American tegumentary leishmaniasis patients: a site of Leishmania (Viannia) braziliensis persistence and viability eleven years after antimonial therapy and clinical cure. Am J Trop Med Hyg. 1998;58:824–827. doi: 10.4269/ajtmh.1998.58.824. [DOI] [PubMed] [Google Scholar]
- 13.de Paula CDSJ, Cardoso DR, Sampaio RN. A comparative study between the efficacy of pentamidine isothionate given in three doses for one week and N-methil-glucamine in a dose of 20mgSbV/day for 20 days to treat cutaneous leishmaniasis. Rev Soc Bras Med Trop. 2003;36:365–371. [PubMed] [Google Scholar]
- 14.Saldanha AC, Romero GA, Guerra C, Merchan-Hamann E, Macedo VO. Comparative study between sodium stibogluconate BP 88 and meglumine antimoniate in cutaneous leishmaniasis treatment. II. Biochemical and cardiac toxicity. Rev Soc Bras Med Trop. 2000;33:383–388. doi: 10.1590/s0037-86822000000400009. [DOI] [PubMed] [Google Scholar]
- 15.Soto J, Buffet P, Grogl M, Berman J. Successful treatment of Colombian cutaneous leishmaniasis with four injections of pentamidine. Am J Trop Med Hyg. 1994;50:107–111. doi: 10.4269/ajtmh.1994.50.107. [DOI] [PubMed] [Google Scholar]
- 16.de Camargo Ferreira EV, de Oliveira Schubach A, Valete-Rosalino CM, de Souza Coutinho R, Conceicao-Silva F, de Matos Salgueiro M, Rosandiski Lyra M, Soares Moreira J, Azeredo-Coutinho RB, Fernandes Pimentel MI, Roberto Mortari S, de Fatima Madeira M, Pereira Quintella L, Baptista C, de Almeida Marzochi MC. American tegumentary leishmaniasis in older adults: 44 cases treated with an intermittent low-dose antimonial schedule in Rio de Janeiro, Brazil. J Am Geriatr Soc. 2010;58:614–616. doi: 10.1111/j.1532-5415.2010.02747.x. [DOI] [PubMed] [Google Scholar]
- 17.Claros P, Wienberg P, Gonzalez MA, Claros A, Claveria MA, Lopez P. Intralesional treatment of cutaneous leishmaniasis: a report of two cases. Acta Otorrinolaringol Esp. 1996;47:67–70. [PubMed] [Google Scholar]
- 18.Harms G, Chehade AK, Douba M, Roepke M, Mouakeh A, Rosenkaimer F, Bienzle U. A randomized trial comparing a pentavalent antimonial drug and recombinant interferon-gamma in the local treatment of cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1991;85:214–216. doi: 10.1016/0035-9203(91)90026-u. [DOI] [PubMed] [Google Scholar]
- 19.Sharquie KEA-TK, Chu AC. Intralesional therapy of cutaneous leishmaniasis with sodium stibogluconate antimony. Br J Dermatol. 1988;119:53–57. doi: 10.1111/j.1365-2133.1988.tb07100.x. [DOI] [PubMed] [Google Scholar]
- 20.Tallab TM, Bahamdam KA, Mirdad S, Johargi H, Mourad MM, Ibrahim K, el Sherbini AH, Karkashan E, Khare AK, Jamal A. Cutaneous leishmaniasis: schedules for intralesional treatment with sodium stibogluconate. Int J Dermatol. 1996;35:594–597. doi: 10.1111/j.1365-4362.1996.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 21.Schubach A, Haddad F, Oliveira-Neto MP, Degrave W, Pirmez C, Grimaldi G, Jr, Fernandes O. Detection of Leishmania DNA by polymerase chain reaction in scars of treated human patients. J Infect Dis. 1998;178:911–914. doi: 10.1086/515355. [DOI] [PubMed] [Google Scholar]
- 22.Mendonça MG, De Brito ME, Rodrigues EH, Bandeira V, Jardim ML, Abath FG. Persistence of Leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure? J Infect Dis. 2004;189:1018–1023. doi: 10.1086/382135. [DOI] [PubMed] [Google Scholar]
- 23.Schubach A, Cuzzi-Maya T, Oliveira AV, Sartori A, de Oliveira-Neto MP, Mattos MS, Araujo ML, Souza WJ, Haddad F, Perez Mde A, Pacheco RS, Momen H, Coutinho SG, de Almeida Marzochi MC, Marzochi KB, da Costa SC. Leishmanial antigens in the diagnosis of active lesions and ancient scars of American tegumentary leishmaniasis patients. Mem Inst Oswaldo Cruz. 2001;96:987–996. doi: 10.1590/s0074-02762001000700018. [DOI] [PubMed] [Google Scholar]
- 24.Wortmann GW, Miller RS, Blazes D, Oster CN. Cutaneous leishmaniasis following local trauma: a clinical pearl. Clin Infect Dis. 2000;31:199–201. doi: 10.1086/313924. [DOI] [PubMed] [Google Scholar]
- 25.Marsden PD, Tada MS, Barreto AC, Cuba CC. Spontaneous healing of Leishmania braziliensis braziliensis skin ulcers. Trans R Soc Trop Med Hyg. 1984;78:561–562. doi: 10.1016/0035-9203(84)90087-7. [DOI] [PubMed] [Google Scholar]
- 26.Zajtchuk JT, Netto EM, Grogl M, Neafie RC, Hessel CR, de Magalhaes AV, Marsden PD. Mucosal leishmaniasis in Brazil. Laryngoscope. 1989;99:925–939. doi: 10.1288/00005537-198909000-00006. [DOI] [PubMed] [Google Scholar]