Abstract
An Israeli traveler returning from Tanzania presented with a relapsing febrile illness. A diagnosis of Trypanosoma brucei rhodesiense infection was established by blood smear after nearly a month. Blood polymerase chain reaction failed to provide an early diagnosis of human African trypanososmiasis. Recognition of suggestive signs should prompt physicians to perform repeated tests before ruling out human African trypanososmiasis.
Introduction
Travelers to Africa may be at risk of acquiring human African trypanososmiasis (HAT)—one of the most important neglected tropical diseases.1 Failure to recognize HAT in its early stages may lead to severe morbidity and even mortality. We present a case of HAT in an Israeli traveler, where the diagnosis was elusive despite suggestive clinical features.
Case Report
A 31-year-old previously healthy Israeli woman traveled to Tanzania on an 11-day Safari. A week after leaving mainland Tanzania, and a day after her return to Israel she developed fever and severe headache. She was admitted to the Sheba Medical Center—a tertiary hospital in Israel. Routine laboratory tests showed mild leucopenia, thrombocytopenia, and mildly elevated transaminases (Table 1 ). Thick and thin blood smears were negative for malaria or other pathogens. Other evaluations, including chest radiography, brain computerized tomography, and a lumbar puncture, were all normal. Physical examination was unrevealing except for a small purplish painless lesion near the patient's buttock. This was considered a possible eschar, and the patient was started on doxycycline, for the purported diagnosis of African tick bite fever.
Table 1.
Main laboratory parameters during acute rhodesiense HAT case
| Parameter | 1st admission (during 1st bout of fever) | 2nd admission (after 2nd bout of fever) | 3rd admission (during 3rd bout of fever) | 4th admission (6 months after treatment) |
|---|---|---|---|---|
| Hemoglobin (gr/dL) | 12.7 | 9.5 | 8.6 | 13.2 |
| Leukocytes (X109/L) | 3.3 | 6.9 | 7.7 | 7.5 |
| Platelets (X109/L) | 93 | 342 | 286 | 297 |
| C reactive protein (mg/L) | 178 | 104 | 171 | 1.9 |
| Serum globulin (gr/L) | 3.3 | 5.8 | 5.7 | 2.6 |
| Alanine transaminase (IU/L) | 81 | 15 | 13 | 11 |
| Alkaline phosphatase (IU/L) | 262 | 73 | 64 | 64 |
| Rickettsia serology | Negative | Positive | ND | ND |
| Antinuclear antibodies | ND | Positive | Positive | Negative |
| Anticardiolipin antibodies | ND | Positive | Positive | Negative |
Shaded cells = abnormal results; ND = not done.
However, fever, headache, and vomiting continued unchanged; the cutaneous lesion grew in size; concern was raised for the possibility of HAT, and the patient recalled having been bitten by tsetse flies while on Safari in the Serengeti Reserve. A repeated malaria antigen test (BinaxNOW; Inverness Medical, Scarborough, ME) became positive, but was deemed false positive, because repeated blood smears were negative for malaria.
Blood smears were also negative for trypanosomes (although blood concentration techniques were not used). Microscopy from a biopsy from the lesion failed to show trypanosomes.
After nearly a week the patient's fever and headache resolved, the blood count and transaminase levels normalized, and the patient was discharged from the hospital. She remained asymptomatic for 10 days afterward, when fever recurred for 2 days and resolved spontaneously. Physical exam showed healing of the cutaneous lesion, and was otherwise negative. Routine laboratory tests showed only anemia and markedly increased C-reactive protein levels. Multiple additional tests were unrevealing, including blood cultures, Plasmodium, and Borrelia blood polymerase chain reaction (PCR) tests. Chest computer tomography revealed a small pleural effusion on the left. A repeated serological test showed seroconversion to spotted fever rickettsiosis (specific Rickettsia africae serology is not available in Israel), but serology also showed a markedly positive antinuclear antibody level, and antimitochondrial and anticardiolipin antibodies (Table 1). Despite additional negative blood smears (taken while the patient was afebrile), concern for possible HAT increased. A PCR test for the presence of Trypanosoma brucei DNA was performed both on the patient's blood and on DNA eluted from the biopsy specimen taken earlier: both were negative.
A week later the patient had another 3 day bout of fever. This time on physical examination, mild, tender posterior-cervical lymphadenopathy was found, in addition to an unusual, large, non-pruritic annular pink macule at the base of the neck (Figure 1), compatible with a trypanid rash. A blood smear—taken this time during rigors—revealed trypanosomes, and a diagnosis of HAT was established. A serum sample taken at this time was also positive for T. brucei DNA on PCR. This sample was tested with two specific primers (as described elsewhere2,3); it was positive for Trypanosoma brucei rhodesiense serum resistance-associated gene, and was negative for Trypanosoma brucei gambiense-specific glycoprotein, results compatible with infection with T. b. rhodesiense.
Figure 1.
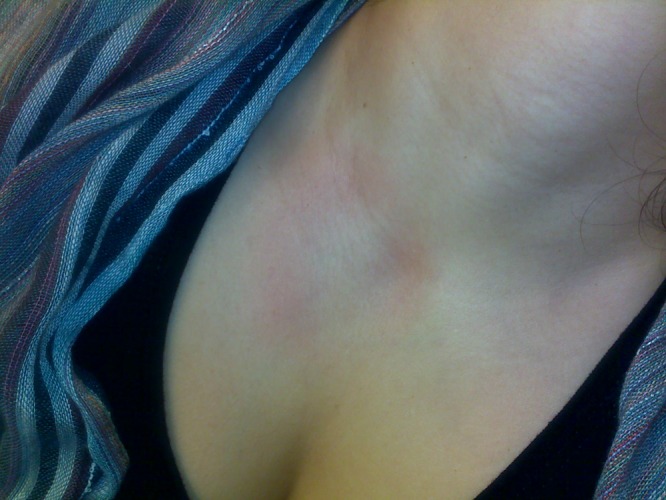
Human African trypanosomiasis—trypanid rash.
The patient was without any neurological symptoms. Lumbar puncture revealed normal cerebrospinal fluid. The patient was treated with a complete course of suramin. The first dose was followed by an evanescent, non-pruritic rash of large macules; further doses were without any adverse effects, and the patient recovered uneventfully. Six months later she was in good health; physical examination, routine laboratory tests, antinuclear antibody levels, and a repeated lumbar puncture were all normal (Table 1).
Comment
Human African trypanososmiasis is among the most important of the “neglected” tropical diseases. And although numbers of gambiense HAT are in decline,1 this trend does not affect rhodesiense HAT, where the number of reported HAT cases in travelers appears to be increasing, usually acquired in game parks in Tanzania, Kenya, or Malawi.4–6
Untreated, rhodesiense HAT is uniformly fatal, and significant morbidity and mortality may ensue even with appropriate therapy. Despite its severity and prevalence, there has been little change in its management and treatment during the last century.
Our patient may be regarded as a classical presentation of rhodesiense HAT. All the clinical features, including the initial skin lesion, the relapsing fever pattern, the posterior cervical lymphadenopathy (Winterbottom's sign), and the trypanid rash (Figure 1) would have been easily recognizable 90 years ago.7 And yet, the diagnosis proved to be elusive despite the high level of suspicion.
Trypomastigotes can sometimes be diagnosed from a biopsy of the initial chancre, however their absence—as in our case—does not rule out HAT.
Serological tests—the main screening tool for gambiense HAT—are not used in rhodesiense HAT. This in part reflects the inherent delay for seroconversion (3–4 weeks).8 In addition, occasional cases of false positive serology in travelers have been documented.9
Multiple blood smears were taken during our patient's illness, but were negative until the third bout of fever. This may have been caused by either intermittent parasitemia or low level parasitemia (below the detection level of blood smears, especially since blood concentration techniques were not used). In light of the patient's negative blood smears, we attempted to use blood PCR to diagnose HAT. However, PCR did not improve on microscopy, and was false negative between fever bouts. Blood PCR has recently been shown to be a promising test for the diagnosis (but not for follow-up) of gambiense HAT,10 but there is no data regarding its use in rhodesiense HAT. Its role should therefore be considered as investigational: a negative PCR result does not preclude HAT.
Diagnostic efforts in our case were further complicated by false positive serological reactions. Rickettsia africae infection, which was the first diagnosis considered in our patient, was theoretically confirmed by seroconversion. In addition, a false positive malaria antigen test emerged during illness, and several autoantibodies were positive as well (Table 1). The patient could easily have been misdiagnosed as a case of probable lupus erythematosus (as per American Rheumatology Association [ARA] criteria: autoantibodies, lymphopenia, serositis = pleural effusion and rash). Immunosuppressive therapy in that case could have resulted in the patient's demise from “lupus cerebritis,” when HAT would have moved to its neuroinvasive phase.
Autoantibodies are known to occur in many infections. In HAT patients, the presence of antinuclear antibodies (ANAs) has been described in the past, with up to 84% of gambiense HAT cases positive for ANAs, probably reflecting an intense, polyclonal B cell activation.11 A misdiagnosis of systemic lupus erythematosus and a delay in antitrypanosomal treatment has been described in the past in gambiense HAT cases.12 To our knowledge, this is the first documentation of ANAs in human rhodesiense HAT, however monkeys experimentally infected with T. b. rhodesiense invariably developed high titers of ANAs within a month of infection.13
Conclusions
A relapsing fever pattern in a traveler returning from a sojourn in East Africa should alert the clinician to the possibility of rhodesiense HAT. Typical physical findings may take weeks to evolve, and multiple blood smears may be required to make the diagnosis. HAT may be associated with multiple false positive infectious and autoimmune serologies, and the risk of a misdiagnosis is real. A possible association between blood smear positivity and its timing in relation to the onset of rigors should be further explored. The role of blood PCR in the diagnosis of rhodesiense HAT remains to be established.
ACKNOWLEDGMENTS
We thank Daphna Gutman, from the Microbiology laboratory, at the Sheba Medical Center, Tel Hashomer, for her help in the microscopical diagnosis of this case.
Footnotes
Authors' addresses: Eyal Meltzer, Eyal Leshem, Yechezkel Sidi, Shmuel Steinlauf, and Eli Schwartz, The Center for Geographic Medicine and Department of Medicine C, Sheba Medical Center, Tel Hashomer, Israel; and The Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel, E-mails: meltzere@zahav.net.il, leshem@gmail.com, ysidi@sheba.health.gov.il, Shmuel.Stienlauf@sheba.health.gov.il, and elischwa@post.tau.ac.il. Shulamit Michaeli, The Mina and Everard Goodman Faculty of Life Sciences, Bar Ilan University, Ramat Gan, Israel, E-mail: michaes@mail.biu.ac.il.
References
- 1.WHO . Human African trypanosomiasis: number of new cases drops to historically low level in 50 years. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Radwanska M, Chamekh M, Vanhamme L, Claes F, Magez S, Magnus E, de Baetselier P, Buscher P, Pays E. The serum resistance-associated gene as a diagnostic tool for the detection of Trypanosoma brucei rhodesiense. Am J Trop Med Hyg. 2002;67:684–690. doi: 10.4269/ajtmh.2002.67.684. [DOI] [PubMed] [Google Scholar]
- 3.Radwanska M, Claes F, Magez S, Magnus E, Perez-Morga D, Pays E, Buscher P. Novel primer sequences for polymerase chain reaction-based detection of Trypanosoma brucei gambiense. Am J Trop Med Hyg. 2002;67:289–295. doi: 10.4269/ajtmh.2002.67.289. [DOI] [PubMed] [Google Scholar]
- 4.Migchelsen SJ, Büscher P, Hoepelman AI, Schallig HD, Adams ER. Human African trypanosomiasis: a review of non-endemic cases in the past 20 years. Int J Infect Dis. 2011;15:e517–e524. doi: 10.1016/j.ijid.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Gautret P, Clerinx J, Caumes E, Simon F, Jensenius M, Loutan L, Schlagenhauf P, Castelli F, Freedman D, Miller A, Bronner U, Parola P. Imported human African trypanosomiasis in Europe, 2005–2009. Euro Surveill. 2009;14:1–3. [PubMed] [Google Scholar]
- 6.Jelinek T, Bisoffi Z, Bonazzi L, van Thiel P, Bronner U, de Frey A, Gundersen SG, McWhinney P, Ripamonti D. Cluster of African trypanosomiasis in travelers to Tanzanian national parks. Emerg Infect Dis. 2002;8:634–635. doi: 10.3201/eid0806.010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manson P. Tropical Diseases: A Manual of the Diseases of Warm Climates. London: Cassel & Co; 1918. [Google Scholar]
- 8.Chappuis F, Loutan L, Simarro P, Lejon V, Buscher P. Options for field diagnosis of human African trypanosomiasis. Clin Microbiol Rev. 2005;18:133–146. doi: 10.1128/CMR.18.1.133-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum J, Beck BR, Brun R, Hatz C. Clinical and serologic responses to human “apathogenic” trypanosomes. Trans R Soc Trop Med Hyg. 2005;99:795–797. doi: 10.1016/j.trstmh.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Deborggraeve S, Lejon V, Ekangu RA, Mumba Ngoyi D, Pati Pyana P, Ilunga M, Mulunda JP, Büscher P. Diagnostic accuracy of PCR in gambiense sleeping sickness diagnosis, staging and post-treatment follow-up: a 2-year longitudinal study. PLoS Negl Trop Dis. 2011;5:e972. doi: 10.1371/journal.pntd.0000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazyumba G, Berney M, Brighouse G, Cruchaud A, Lambert PH. Expression of the B cell repertoire and autoantibodies in human African trypanosomiasis. Clin Exp Immunol. 1986;65:10–18. [PMC free article] [PubMed] [Google Scholar]
- 12.Kirrstetter M, Lerin-Lozano C, Heintz H, Manegold C, Gross WL, Lamprecht P. Trypanosomiasis in a woman from Cameroon mimicking systemic lupus erythematosus. Dtsch Med Wochenschr. 2004;129:1315–1317. doi: 10.1055/s-2004-826866. [DOI] [PubMed] [Google Scholar]
- 13.Lindsley HB, Kysela S, Steinberg AD. Nucleic acid antibodies in African trypanosomiasis: studies in Rhesus monkeys and man. J Immunol. 1974;113:1921–1927. [PubMed] [Google Scholar]


