Abstract
The aim of this work was to investigate the potential usefulness of Trypanosoma cruzi lysate, recombinant protein JL7, and peptides P013, R13, JL18, JL19, and P0β as serological markers for human Chagas disease. We analyzed 228 sera from Brazilian Chagas disease patients classified into four clinical groups and 108 from non-chagasic patients. We defined the diagnostic sensitivity, specificity, and Kappa index measured by enzyme-linked immunosorbent assay (ELISA). As previously described, the highest values of diagnostic parameters were achieved for T. cruzi lysate and JL7; peptide P013 showed high specificity but low sensitivity. The other peptides resulted in lower sensitivity and specificity in our ELISA than T. cruzi lysate and JL7 protein. Antibodies against JL7 protein were mainly detected in sera from patients with severe chagasic cardiomyopathy, compared with those from the indeterminate form, whereas peptides failed to discriminate between the clinical forms of the disease.
Chagas disease, caused by the hemoflagellate Trypanosoma cruzi, is a widespread tropical disease affecting at least 8 million people primarily in Latin American countries.1 The acute phase lasts 1 or 2 months and is usually symptomless,2 however most of the infected individuals enter a life-long chronic phase with its asymptomatic and symptomatic forms. The asymptomatic, also called indeterminate form is characterized by the absence of clinical symptoms. However, ∼40% of persons with chronic T. cruzi infection develop symptoms of visceral damage, which may include cardiac lesions, digestive alterations, or both clinical manifestations (cardiac plus digestive).2
In the chronic phase, the primary method for diagnosis is the search of antibodies by serological testing, whereas the secondary diagnostic techniques are parasitological tests.1 So far, conventional serological tests include complement fixation,3,4 indirect immunofluorescence assay (IFA),5 indirect hemagglutination assay (IHA),6 direct agglutination with 2-mercaptoethanol (DA-2ME),7 and enzyme-linked immunosorbent assay (ELISA).8–10 These tests usually use crude or semi-purified parasite preparations, often derived from a stage present only in the insect and in cultures at 26°C (epimastigote) but absent in the human host. Other assays incorporate more defined parasite components, like multiple fusion proteins containing epitopes from various T. cruzi antigens.11–15 Moreover, a rapid immunochromatographic assay (Chagas Stat-Pak, Chembio Diagnostic Systems, Medford, NY) has also been developed by employing a mixture of T. cruzi recombinant antigens, presenting a different degree of performance.16–18 However, in the absence of a true gold standard, it is still necessary to carry out at least two different serological tests to establish a reliable diagnosis of Chagas disease. Indeed, the World Health Organization (WHO) consensus guidelines recommend to perform a third assay or repeat sampling to confirm or exclude the diagnosis if two serological tests are in disagreement.19
In 1991, a Workshop organized by the Ibero-American Project of Biotechnology evaluated the diagnostic potential of phage expressed T. cruzi recombinant antigens, Antigen 2, Antigen 13, SAPA, H49, A13, JL5, JL7, JL8, JL9, and RAI by phage dot array immunoassays.20 Results showed that the best recombinant antigen to be used in serodiagnosis of chronic T. cruzi infection was JL7 (also called H49 or Antigen 1), however a more efficient format than dot array was suggested.20 In addition, the serodiagnostic efficiency of the ELISA for six recombinant antigens (H49, JL7, B13, JL8, A13, and 1F8) was carried out by using sera from different geographical areas of Latin America.21 One of the goals of that work was to shed light on the usefulness of combined antigens to minimize the individual variation and promote a high sensitivity test for the routine diagnosis of Chagas disease.21
Until now, an effective assay for predicting the clinical evolution of a chronic infection is lacking.15,22,23 Accordingly, in this study, we evaluated the diagnostic potential of different T. cruzi antigens, namely peptides P013, R13, JL18, JL19, and P0β by ELISA,21 and investigated the potential use of T. cruzi lysate, JL7 protein, and these peptides as markers for prognosis of the disease. Peptide R13 (EEEDDDMGFGLFD) was derived from the 13 C-terminal amino acids of TcP2β, whereas P013 (EDDDDDFGMGALF) and P0β (AESEE) were derived from the C-terminal region of TcP0 protein.24 Peptide JL18 (AYRKALPQEEEEDVGPRH) and the contiguous JL19 (VDPDFCRSTTQDAYRPVDP) were derived from T. cruzi recombinant protein JL9.24
Test sera were obtained from a careful selection of patients from the Laboratory of Chagas Disease, Hospital das Clinicas, Federal University of Goias-Goiania, Brazil. The existence of T. cruzi infection was assessed serologically by IHA, DA-2ME, IFA, and ELISA using T. cruzi Y strain (DTU Tc II) epimastigote form as antigen. Random codes were given to all samples and aliquots were sent to the Laboratorio de Biología Molecular de la Enfermedad de Chagas, INGEBI–CONICET, Buenos Aires, Argentina, for conformational testing. Moreover, 20% of sera were selected at random and recoded to use them as internal controls in different tests. At the end of the study, all codes (from Brazil) and test results (from Argentina) were sent to the manager of the Task Force on Chagas disease, Special Program for Research and Training in Tropical diseases (TDR-WHO), where codes and results were matched.
According to a conventional serological test (ELISA, IHA, IFA, DA-2ME) results, a total of 228 sera from patients with Chagas disease and 108 from patients without chagasic infection were used (Table 1). Coded sera from Chagas disease patients were classified into four groups according to clinical, electrocardiographic and radiological results, namely indeterminate form of Chagas disease (IC), digestive form of Chagas disease (DC), mild chagasic cardiomyopathy (MCC), and severe chagasic cardiomyopathy (SCC) (Table 1).
Table 1.
Clinical features of the study population*
| Serology | Disease form | No. of individuals | Age limits (years) | Mean age (years) | Sex (male/female) |
|---|---|---|---|---|---|
| Positive | IC | 58 | 12–51 | 35.3 | 25/33 |
| DC | 59 | 12–55 | 35.9 | 34/25 | |
| MCC | 57 | 24–55 | 39.2 | 29/28 | |
| SCC | 54 | 14–57 | 43.1 | 39/15 | |
| Negative | HI | 59 | 19–55 | 39.6 | 35/24 |
| nCh | 49 | 12–55 | 37.8 | 28/21 | |
| Total | 336 | 12–57 | 38.5 | 190/146 |
IC = indeterminate form of Chagas disease (normal electrocardiogram (ECG) and thoracic radiography with normal esophagus and colon x-rays); DC = digestive form of Chagas disease (megacolon and/or megaesphagus with normal ECG and thoracic radiography); MCC = mild chagasic cardiomyopathy (altered ECG, such as complete right bundle branch block, anterior left fascicular block, premature ventricular beating and alterations in ventricular repolarization with normal chest radiography and normal esophagus and colon x-rays); SCC = severe chagasic cardiomyopathy (altered ECG, high frequency of premature heart beating, ventricular auricular block, atrial fibrillation, clinical symptoms of cardiac alterations and cardiomegaly visualized by thoracic radiography); HI = healthy individual (normal ECG); nCh = no Chagas patients (other diseases).
The remaining coded sera from individuals without Chagas disease were divided into two groups (Table 1), healthy individuals with normal ECG and sero-negative for T. cruzi, however born in endemic regions, and patients with other diseases, 5 with VL (Kala-azar), 4 with muco-cutaneous leishmaniasis, 19 with autoimmune diseases (12 with Systemic Lupus Erythematosus), 16 with cardiomyopathies of non-chagasic ethiology, and 5 with another disease such as juvenile diabetes, schistosomiasis, idiopathic megaesophagus, and South American Blastomycosis. Age limits in this group were from 12 up to 55 years of age (Table 1). All sera have had no antibodies (Ab) against T. cruzi, whereas in 1 out of 5 patients with visceral leishmaniasis (VL), the IFA test was positive. Furthermore, an additional six patients with VL (1–10 years of age) were tested and two of them also resulted seropositive for IFA test, pointing to the high degree of cross-reactivity between Chagas disease and VL.
We first analyzed the Ab levels against T. cruzi lysate, recombinant JL7 protein, and peptides P013, R13, JL18, JL19, and P0β by ELISA. As shown in Table 2, the Ab levels against T. cruzi lysate, JL7 protein, and peptides P013, R13, and P0β were higher in sera from Chagas disease patients compared with those from non-chagasic individuals. As previously reported,12,20 T. cruzi lysate, and JL7 protein showed high sensitivity (Se) and specificity (Sp), providing the best Kappa indexes of 0.91 and 0.93, respectively, compared with the other tested antigens.
Table 2.
Sensitivity, specificity, and Kappa index of each antigen determined by ELISA*
| Antigens | Median values (AU) | Sp (%) | Se (%) | Kappa index | |
|---|---|---|---|---|---|
| ChD (N = 228) | nChD (N = 108) | ||||
| Trypanosoma cruzi | 4.31 (2.61–6.05) | 0.39 (0.09–1.34) | 89.8 | 99.6 | 0.91 |
| JL7 | 4.97 (1.11–9.99) | 0.55 (0.12–0.89) | 100 | 95.2 | 0.93 |
| P013 | 2.46 (0.35–13.64) | 0.20 (0.00–0.60) | 97.2 | 82.5 | 0.74 |
| R13 | 1.38 (0.21–10.29) | 0.21 (0.01–1.64) | 85.1 | 61.4 | 0.41 |
| JL18 | 0.83 (0.22–2.66) | 0.52 (0.11–1.59) | 78.7 | 37.3 | 0.14 |
| JL19 | 0.87 (0.20–2.44) | 0.58 (0.05–1.67) | 75.0 | 40.4 | 0.13 |
| P0β | 0.44 (0.00–7.04) | 0.23 (0.00–1.98) | 86.1 | 28.5 | 0.12 |
ELISA plates were coated with 50 ng protein/well of T. cruzi epimastigote lysate, 2 μg/well of recombinant protein JL7 or 2 μM of peptides coupled to bovine serum albumin (BSA) in 50 μL buffer carbonate. Serum samples were diluted 1/400. All samples were tested in duplicate, and sera from six healthy individuals were loaded on the same plate to determine cut-off value, as the optical density (OD) mean value plus three standard deviations. Antibody level, in arbitrary units (AU), was calculated as ratio (OD value of each serum samples/cut-off value) and, the results were expressed as median values (5th–95th percentiles).
ChD = Chagas disease; nChD = no Chagas disease; N = number of individuals; Sp = specificity; Se = sensitivity.
The peptides P013 and R13 presented Kappa values of 0.74 and 0.41, respectively (Table 2). For peptide P013, Sp and Se achieved a value of 97.2% and 82.5%, respectively; peptide R13 presented a Sp of 85.1% and a Se value of 61.4%. The remaining peptides resulted in Kappa indexes lower than 0.15 (Table 2) caused by their low Se (< 40%), although their Sp were all higher than 75%. Interestingly, peptide P013 rendered only two false positive results and none of them were sera from healthy individuals (Table 3).
Table 3.
Prevalence of reactive serum samples from patients of non-chagasic ethiology and healthy individuals*
| Antigens | nChD (N = 108) | HI (N = 59) | VL (N = 5) | ML (N = 4) | AD (N = 19) | Other diseases (N = 21) |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Trypanosoma cruzi | 11 (10.2) | 5 (8.5) | 1 (20) | 2 (50) | 1 (5.3) | 2 (9.5) |
| JL7 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| P013 | 2 (1.9) | 0 (0) | 0 (0) | 0 (0) | 2 (10.5) | 0 (0) |
| R13 | 16 (14) | 10 (16.9) | 0 (0) | 2 (50) | 3 (15.8) | 1 (4.8) |
| JL18 | 23 (20.2) | 16 (27.1) | 0 (0) | 1 (25) | 0 (0) | 6 (28.6) |
| JL19 | 27 (23.7) | 14 (23.7) | 0 (0) | 1 (25) | 3 (15.8) | 9 (42.9) |
| P0β | 13 (12.1) | 3 (5.1) | 0 (0) | 3 (75) | 1 (5.3) | 6 (28.6) |
nChD = no Chagas disease; HI = healthy individual; VL = visceral leishmaniasis (Kala-azar); ML = muco-cutaneous leishmaniasis; AD = autoimmune disease; Other Diseases = no chagasic cardiomyopathy, juvenile diabetes, schistosomiasis, idiopathic megaesophagus, and South American Blastomycosis; N = number of individuals.
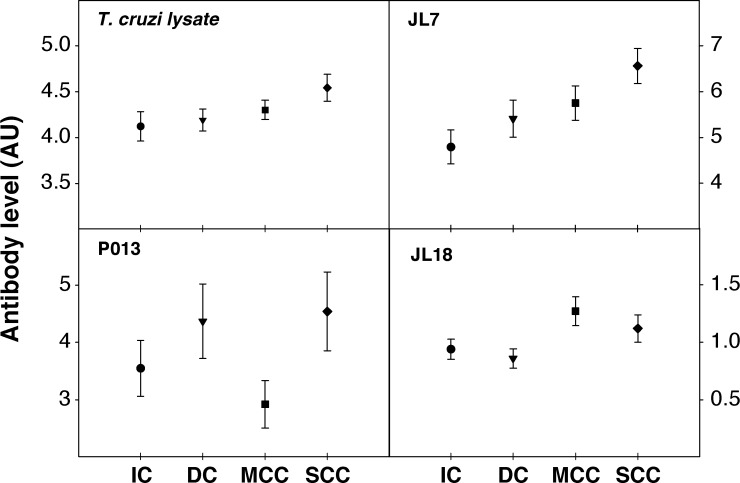
When the presence of Abs against these antigens was analyzed in sera of the patients with different clinical forms of chronic Chagas disease, we observed that the positivity of T. cruzi lysate, JL7, and peptides P013 and R13 were similar for all of them (Table 4). On the other hand, a higher prevalence of sera reactivity against peptides JL18 and JL19 was found in patients with severe or mild cardiomyopathy. Twenty-two out of 54 sera from patients with SCC (40.7%) recognized P0β peptide. Sera from patients with digestive disease showed a higher percentage of positivity with T. cruzi lysate, JL7, and peptides P013 and R13 (Table 4). We further considered whether Ab levels against the different antigens might allow us to differentiate among the clinical forms of Chagas disease. By using a non-parametric analysis for more than two groups (Kruskal-Wallis test), we observed a significant difference between the Ab levels elicited against JL7 protein (P < 0.025) and peptide JL18 (P < 0.028) by patients belonging to four clinical forms (Figure 1). No differences were observed with the other antigens (Figure 1 and data not shown). To determine which clinical form differed from each other, a Tukey test to unequal samples was carried out, comparing the mean value of Ab levels elicited against JL7 protein by patients belonging to one clinical form to the mean value obtained in the other clinical situations. Interestingly, anti-JL7 Ab concentration (by optical density [OD]) was higher in sera from patients with SCC, compared with those from IC (P < 0.019) (Figure 1 ). No analysis was performed with data from peptide JL18 because it showed poor Se and Ab levels barely above the cut-off value.
Table 4.
Prevalence of reactive serum samples from patients with different clinical forms of Chagas disease*
| Antigens | ChD (N = 228) | IC (N = 58) | DC (N = 59) | MCC (N = 57) | SCC (N = 54) |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Trypanosoma cruzi | 227 (99.6) | 57 (98.2) | 59 (100) | 57 (100) | 54 (100) |
| JL7 | 217 (95.2) | 54 (93.1) | 56 (94.9) | 54 (94.7) | 53 (98.1) |
| P013 | 188 (82.5) | 49 (84.5) | 48 (81.3) | 45 (78.9) | 46 (85.2) |
| R13 | 140 (61.4) | 35 (60.3) | 36 (61.0) | 35 (61.4) | 34 (63.0) |
| JL18 | 85 (37.3) | 19 (32.8) | 14 (23.7) | 26 (45.6) | 26 (48.1) |
| JL19 | 92 (40.4) | 22 (37.9) | 16 (27.1) | 30 (52.6) | 24 (44.4) |
| P0β | 65 (28.5) | 16 (27.6) | 18 (30.5) | 9 (15.8) | 22 (40.7) |
ChD = Chagas disease; IC = indeterminate form of Chagas disease; DC = digestive form of Chagas disease; MCC = mild chagasic cardiomyopathy; SCC = severe chagasic cardiomyopathy; N = number of individuals.
Figure 1.
Performance of antigens for prognosis of Chagas disease. Enzyme-linked immunosorbent assay (ELISA) plates were coated with 50 ng protein/well of Trypanosoma cruzi epimastigote lysate, 2 μg/well of recombinant protein JL7 or 2 μM of peptides coupled to bovine serum albumin (BSA) in 50 μL buffer carbonate. Serum samples were diluted 1/400 and tested in duplicate. Sera from six healthy individuals were loaded on each plate to determine cut-off value, as the optical density (OD) mean value plus three standard deviations. Results were expressed as ratio calculated as (OD value of each serum samples/cut-off value) ±SE. IC = indeterminate form of Chagas disease; DC = digestive form of Chagas disease; MCC = mild chagasic cardiomyopathy; SCC = severe chagasic cardiomyopathy.
Our current results, together with previous reports,20,21 extend the repertoire of parasite proteins that can be used to perform an appropriate serological test framed in WHO requirements for T. cruzi infection diagnosis. Indeed, a WHO-TDR Committee declared in 2007 that searching of biomarkers to predict progression of Chagas disease is a priority research area in this field. Ongoing studies have focused on endothelin 1, tumor necrosis factor-α, B-type natriuretic peptide, angiotensin-converting enzyme, and also autoantibodies as candidates for disease prognosis.23,25–27 Among the last ones, cross-reactive Abs against β1-adrenergic and M2 muscarinic receptors have been associated with different arrhythmogenic anomalies, which may contribute to the distinct cardiac alterations observed in patients with SCC.28 Although receptor Abs are the result of molecular mimicry with parasite ribosomal P proteins,29,30 R13, P013, and P0β peptides were not good candidates as serological markers of Chagas disease in this study. Only the concentration of Abs against JL7 protein showed a significant increase (P < 0.019) in sera from patients with SCC compared with those with the asymptomatic form, suggesting that this antigen may be useful for differential prognosis, i.e., a high concentration of Abs against JL7 may be a marker for progression of disease to a severe cardiopathy. The finding of a patient in the asymptomatic form with high levels of Abs against JL7 might indicate in this particular individual a future progression to a severe form of cardiopathy. Moreover, it is noteworthy that JL7 protein was not recognized by sera from patients with cardiomyopathies of non-chagasic ethiology, not even by those from patients with visceral and muco-cutaneous leishmaniasis, which confirms that this antigen is also an excellent diagnostic reagent for Chagas disease. This study was carried out with sera collected from patients resident in geographical regions in which strains belonging to TcII prevail. Thus, this antigen needs to be validated in cohorts of patients from different geographical origin in which different parasite lineages prevail to assess its potential for prognosis of Chagas disease severity in all endemic regions.
Footnotes
Financial support: This work was supported by grants from the World Health Organization/Special Program for Research and Training in Tropical Diseases; the Argentinean National Council for Science and Technology (CONICET), the University of Buenos Aires, and the National Agency of Scientific and Technological Promotion (FONCYT BID 1201/OC-AR, PICT 05-06802, PICT 01–14389 and BID 1728/ OC-AR PICT 2439).
Authors' addresses: Silvia A. Longhi, Silvia B. Brandariz, Sonia O. Lafon, Leticia L. Niborski, and Alejandro G. Schijman, INGEBI – CONICET, Buenos Aires, E-mails: longhi@dna.uba.ar, brandariz.silvia@gmail.com, sonialafon@mincyt.gov.ar, niborski.leticia@gmail.com, and schijman@dna.uba.ar or aleschijman@gmail.com. Alejandro O. Luquetti, Instituto de Patología Tropical e Saúde Pública—Universidad Federal de Goiás, Goiania, Brazil, E-mail: aluquetti@gmail.com. Karina A. Gómez, Vuelta de Obligado 2490, Buenos Aires 1428, E-mail: drkagomez@gmail.com.
References
- 1.PAHO-WHO . 2006. Estimación cuantitativa de la enfermedad de Chagas en las Américas. OPS/HDM/CD/425-06. [Google Scholar]
- 2.World Health Organization . Control of Chagas disease: Second Report of the WHO Expert Committee. Geneva: WHO; 2002. WHO Technical report series 905. [Google Scholar]
- 3.Muniz J, Freitas G. Contribuição para o diagnóstico da doença de Chagas pelas reações de imunidade. II - Isolamento de polissacarídeos de Schizotrypanum cruzi e de outros tripanosomídeos, seu comportamento nas reações de precipitação, de fixação do complemento e de hipersensibilidade. Os “Tests” de floculação (sublimado e formol-gel) Rev Bras Biol. 1944;4:421–438. [Google Scholar]
- 4.Cerisola JA, Rosenbaum MB. Complement-fixation reaction in the diagnosis of Chagas' disease. Prensa Med Argent. 1958;45:1454–1463. [PubMed] [Google Scholar]
- 5.Alvarez M, Cerisola JA, Rohweder RW. Test de inmunofluorescencia para el diagnóstico de la enfermedad de Chagas. Bol Chil Parasitol. 1968;23:4–8. [PubMed] [Google Scholar]
- 6.Cerisola JA, Fatala Chaben M, Lazzari JO. Hemagglutination test for the diagnosis of Chagas's disease. Prensa Med Argent. 1962;49:1761–1767. [PubMed] [Google Scholar]
- 7.Vattuone NH, Yanovsky JF. Trypanosoma cruzi: agglutination activity of enzyme-treated epimastigotes. Exp Parasitol. 1971;30:349–355. doi: 10.1016/0014-4894(71)90098-1. [DOI] [PubMed] [Google Scholar]
- 8.Voller A, Draper C, Bidwell DE, Bartlett A. A microplate enzyme-linked immunosorbent assay (ELISA) for Chagas disease. Lancet. 1975;1:426–429. doi: 10.1016/s0140-6736(75)91492-0. [DOI] [PubMed] [Google Scholar]
- 9.Cura EN, Ruiz AM, Velazquez E, Malagrino N, Orn A, Segura EL. Estandarización de un kit de confirmación (FATALAKIT) para el inmunodiagnóstico de la infección por el Trypanosoma cruzi. Medicina (B Aires) 1993;53:82. [Google Scholar]
- 10.Caballero Z, Sousa O, Marques W, Saez-Alquezar A, Umezawa S. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determinate cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin Vaccine Immunol. 2007;14:1045–1049. doi: 10.1128/CVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da Silveira JF, Umezawa ES, Luquetti AO. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol. 2001;17:286–291. doi: 10.1016/s1471-4922(01)01897-9. [DOI] [PubMed] [Google Scholar]
- 12.Silva ED, Pereira VR, Gomes JA, Lorena VM, Cançado JR, Ferreira AG, Krieger MA, Goldenberg S, Correa-Oliveira R, Gomes YM. Use of the EIE-recombinant-Chagas-Biomanguinhos kit to monitor cure of human Chagas' disease. J Clin Lab Anal. 2002;16:132–136. doi: 10.1002/jcla.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umezawa ES, Luquetti AO, Levitus G, Ponce C, Ponce E, Henriquez D, Revollo S, Espinoza B, Sousa O, Khan B, da Silveira JF. Serodiagnosis of chronic and acute Chagas' disease with Trypanosoma cruzi recombinant proteins: results of a collaborative study in six Latin American countries. J Clin Microbiol. 2004;42:449–452. doi: 10.1128/JCM.42.1.449-452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CD, Cheng KY, Jiang LX, Salbilla VA, Haller AS, Yem AW, Bryant JD, Kirchhoff LV, Leiby DA, Schochetman G, Shah DO. Evaluation of a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening. Transfusion. 2006;46:1737–1744. doi: 10.1111/j.1537-2995.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- 15.Cooley G, Etheridge RD, Boehlke C, Bundy B, Weatherly DB, Minning T, Haney M, Postan M, Laucella S, Tarleton RL. High throughput selection of effective serodiagnostics for Trypanosoma cruzi infection. PLOS Neg Trop Dis. 2008;2:1–12. doi: 10.1371/journal.pntd.0000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luquetti AO, Ponce C, Ponce E, Esfandiari J, Schijman A, Revollo S, Anez N, Zingales B, Rangel-Aldao R, Gonzalez A, Levin MJ, Umezawa ES, Franco da Silveira J. Chagas' disease diagnosis: a multicentre evaluation of Chagas Stat-Pack, a rapid immunochromatographic assay with recombinant proteins of Trypanosoma cruzi. Diagn Microbiol Infect Dis. 2003;46:265–271. doi: 10.1016/s0732-8893(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 17.Ponce C, Ponce E, Vinelli E, Montoya A, de Aguilar V, Gonzalez A, Zingales B, Rangel-Aldao R, Levin MJ, Estfandiari J, Umezawa ES, Luquetti AO, da Silveira JF. Validation of a rapid and reliable test for diagnosis of Chagas' disease by detection of Trypanosoma cruzi-specific antibodies in blood of donors and patients in Central America. J Clin Microbiol. 2005;43:5065–5068. doi: 10.1128/JCM.43.10.5065-5068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sosa-Estani S, Gamboa-León MR, Del Cid-Lemus J, Althabe F, Alger J, Almendares O, Cafferata ML, Chippaux JP, Dumonteil E, Gibbons L, Padilla-Raygoza N, Schneider D, Belizan JM, Buekens P. Working Group Use of a rapid test on umbilical cord blood to screen for Trypanosoma cruzi infection in pregnant women in Argentina, Bolivia, Honduras and Mexico. Am J Trop Med Hyg. 2008;79:755–759. [PubMed] [Google Scholar]
- 19.World Health Organization . Report of the Scientific Working Group on Chagas Disease. Buenos Aires, Argentina, 17–20 April 2005. Geneva: World Health Organization; http://apps.who.int/tdr/publications/tdr-research-publications/reporte-enfermedad-chagas/pdf/swg_chagas.pdf Available at: [Google Scholar]
- 20.Levin MJ, Franco da Silveira J, Frasch AC, Camargo ME, Lafon S, Degrave WM, Rangel-Aldao R. Recombinant Trypanosoma cruzi antigens and Chagas' disease diagnosis: analysis of a workshop. FEMS Microbiol Immunol. 1991;89:11–20. doi: 10.1111/j.1574-6968.1991.tb04965.x. [DOI] [PubMed] [Google Scholar]
- 21.Umezawa ES, Bastos SF, Camargo ME, Yamaguchi LM, Santos MR, Gonzalez A, Zingales B, Levin MJ, Sousa O, Rangel-Aldao R, Franco da Silveira J. Evaluation of recombinant antigens for serodiagnosis of Chagas' disease in South and Central America. J Clin Microbiol. 1999;37:1554–1560. doi: 10.1128/jcm.37.5.1554-1560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes YM, Lorena VM, Luquetti AO. Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem Inst Oswaldo Cruz. 2009;104((Suppl I)):115–121. doi: 10.1590/s0074-02762009000900017. [DOI] [PubMed] [Google Scholar]
- 23.Foti L, Fonseca B de P, Nascimento LD, Marques C de F, da Silva ED, Duarte CA, Probst CM, Goldenberg S, Pinto AG, Krieger MA. Viability study of a multiplex diagnostic platform for Chagas disease. Mem Inst Oswaldo Cruz. 2009;104((1 Suppl)):136–141. doi: 10.1590/s0074-02762009000900019. [DOI] [PubMed] [Google Scholar]
- 24.Levin MJ, Mesri E, Benarous R, Levitus G, Schijman A, Levy-Yeyati P, Chiale PA, Ruiz AM, Kahn A, Rosembaum MB, Torres HN, Segura EL. Identification of major Trypanosoma cruzi antigenic determinants in Chronic Chagas's Heart Disease. Am J Trop Med Hyg. 1989;41:530–538. doi: 10.4269/ajtmh.1989.41.530. [DOI] [PubMed] [Google Scholar]
- 25.Tanowitz HB, Huang H, Jelicks LA, Chandra M, Loredo ML, Weiss LM, Factor SM, Shtutin V, Mukherjee S, Kitsis RN, Christ GJ, Wittner M, Shirani J, Kisanuki YY, Yanagisawa M. Role of endothelin 1 in the pathogenesis of chronic chagasic heart disease. Infect Immun. 2005;73:2496–2503. doi: 10.1128/IAI.73.4.2496-2503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talvani A, Rocha MO, Barcelos LS, Gomes YM, Ribeiro AL, Teixeira MM. Elevated concentrations of CCL2 and tumor necrosis factor-α in chagasic cardiomyopathy. Clin Infect Dis. 2004;38:943–950. doi: 10.1086/381892. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Moreira Mda C, Heringer-Walther S, Ebermann L, Schultheiss HP, Wessel N, Siems WE, Walther T. Plasma ACE2 activity is an independent prognostic marker in Chagas' disease and equally potent as BNP. J Card Fail. 2010;16:157–163. doi: 10.1016/j.cardfail.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Mahler E, Hoebeke J, Levin MJ. Structural and functional complexity of the humoral response against the Trypanosoma cruzi ribosomal P2 beta protein in patients with chronic Chagas' heart disease. Clin Exp Immunol. 2004;136:527–534. doi: 10.1111/j.1365-2249.2004.02480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elies R, Ferrari I, Wallukat G, Lebesgue D, Chiale P, Elizari M, Rosenbaum M, Hoebeke J, Levin MJ. Structural and functional analysis of the B cell epitopes recognized by anti-receptor autoantibodies in patients with Chagas' disease. J Immunol. 1996;157:4203–4211. [PubMed] [Google Scholar]
- 30.Kaplan D, Ferrari I, Bergami PL, Mahler E, Levitus G, Chiale P, Hoebeke J, Van Regenmortel MH, Levin MJ. Antibodies to ribosomal P proteins of Trypanosoma cruzi in Chagas disease possess functional autoreactivity with heart tissue and differ from anti-P autoantibodies in lupus. Proc Natl Acad Sci USA. 1997;94:10301–10306. doi: 10.1073/pnas.94.19.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]