Abstract
We conducted cross-sectional surveys for taeniasis and cysticercosis in humans, pigs, and dogs in four northern provinces of Laos. Human cysticercosis and taeniasis prevalence was 2.2% (95% confidence interval [CI] = 1.4–3.0%) and 8.4% (95% CI = 6.9–9.9%), respectively. Eating uncooked beef, being male, province of residence, age, and ethnicity were significant risk factors for taeniasis and only province of residence was a significant risk factor for cystiercosis. Thirty-five human tapeworms were recovered during the survey and 33 (94.3%) and 2 (5.7%) were identified as Taenia saginata and T. solium, respectively. Maximum-likelihood adjusted prevalence of T. solium and T. hydatigena in pigs was 4.2% (95% CI = 0.5–7.9%) and 55.9% (95% CI = 47.5–64.3%), respectively, and T. hydatigena taeniasis in dogs was 4.8% (95% CI = 0.0–11.3%). Taenia hydatigena and T. saginata were the most prevalent taeniids in the respective pig and human populations and together may suppress T. solium transmission.
Introduction
Taenia solium is a zoonotic tapeworm that has a life cycle involving humans as the definitive adult-stage host (taeniasis) and pigs as the intermediate larval-stage host (cysticercosis). In humans, who can also be inadvertently infected with larval-stage cysticerci after ingesting eggs, the most severe clinical manifestation of infection is neurocysticercosis when cysticerci establish in the central nervous system, causing serious neurological sequelae such as epilepsy and in severe cases, death. In Southeast Asia, the epidemiology of T. solium is complicated by the co-endemicity of other Taenia species, where three species cause taeniasis in humans (T. solium, T. saginata, and T. asiatica) and three species cause cysticercosis in pigs (T. solium, T. asiatica, and T. hydatigena).1–5
Taenia solium infection disproportionately affects the poorest communities worldwide where conditions are suitable for the completion of the tapeworm life cycle, including free-roaming pig production, inadequate sanitation, poor hygiene, and low levels of education. Such conditions exist in many rural communities in Laos. However, to date no studies have been undertaken to investigate T. solium in a multi-species context. In Laos, evidence of human cysticercosis is limited to a small study that found a seroprevalence of antibody against T. solium cysticercosis of 4.8%6 and an ill-defined case of neurocysticercosis in northern Laos.7 The only data from pigs is based on carcass inspection and indicated a prevalence of 1–2%.8
Human taeniasis is also poorly understood. Many studies have reported taeniasis prevalence without determining or reporting the species causing infection,6,9–17 and prevalence estimates range from 0% to 14%, with a high degree of spatial variation.8 Only T. saginata has been reported in southern Laos18,19 and T. solium and T. saginata have been reported in northern Laos.7
The principal objective of the present study was to investigate Taenia spp. infection in humans, pigs, and dogs in four provinces in northern Laos by 1) conducting studies to estimate the prevalence and risk of taeniasis and cysticercosis in humans, 2) identifying the Taenia species causing taeniasis in humans, 3) estimating the prevalence of cysticercosis in pigs and of T. hydatigena taeniasis in dogs, and 4) combining results of different studies to draw conclusions on the ecologic factors controlling human and pig infections.
Materials And Methods
Ethics statement.
Informed consent was obtained from all human adult participants and from the parents or legal guardians of minors (children < 15 years of age). The study protocol was reviewed and approved by the Murdoch University Human Ethics Committee (Project no. 2008/266) and the Lao Ministry of Health National Ethics Committee for Health Research (no. 239/NECHR) before commencing this study.
For the studies involving dogs and pigs, the protocols were reviewed and approved by the Murdoch University Animal Ethics Committee (Project no. R2108/07), which adheres to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The Lao Department of Livestock and Fisheries does not, at this time, have a committee to review and approve scientific research protocols involving animals.
Human survey.
Study Site.
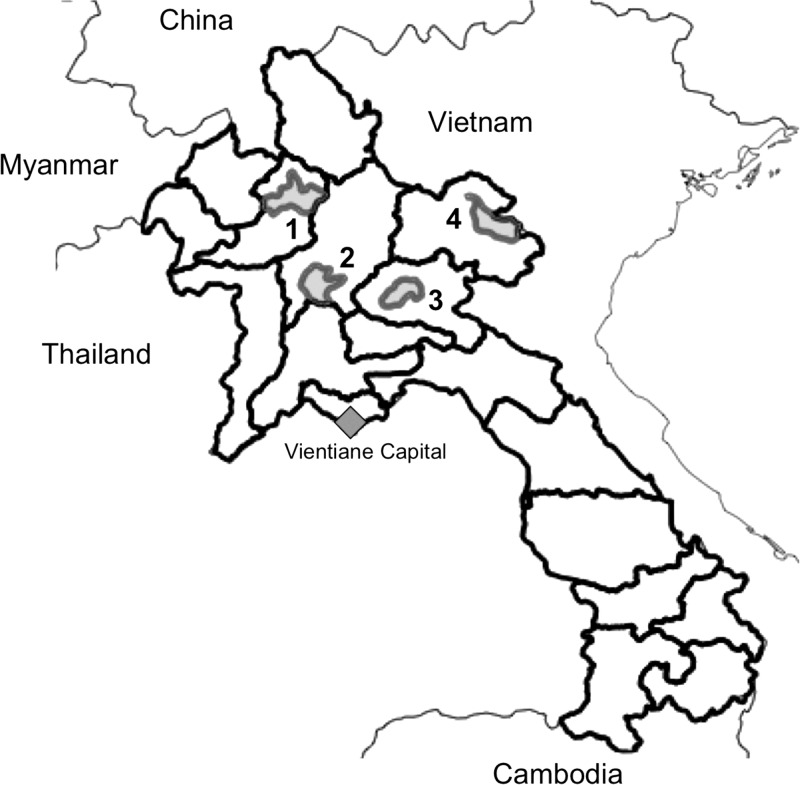
Laos is an ethnically diverse country that has 49 distinct ethnic groups classified into four ethno-linguistic families, Lao-Tai, Mon-Khmer, Hmong-Mien, and Sino-Tibetan, making up 67%, 24%, 8%, and 1% of the population, respectively.20 The study was conducted in four provinces in northern Laos: Oudomxay, Luangprabang, Huaphan, and Xiengkhuang (Figure 1), where all four ethno-linguistic families are represented. Provinces were selected in consultation with the Lao government, and the guiding principles of selection were accessibility from Vientiane and priority areas for poverty alleviation, rural development, and improving pig production.
Figure 1.
Study sites in northern Laos. 1, Xay District, Oudomxay Province; 2, Xiengngeun District, Luangprabang Province; 3, Pek District, Xiengkhuang Province; 4, Viengxay District, Huaphan Province.
Survey design.
In each province, one district was randomly selected for inclusion in this study (Figure 1). The survey was conducted in six randomly selected villages in the dry season during January–March 2009 to maximize study participation and minimize negative impacts on seasonal labor demands.The number of villages selected was constrained by the human resources available at the three levels of government administration: national, provincial, and district. Villages were selected from official listings provided by provincial government offices, and villages were excluded if a four-wheel drive vehicle could not access them.
For the sample size calculation, we conservatively estimated that 4% of households would have at least one cysticercosis or taeniasis case on the basis of prevalence data for northern Vietnam21 and prevalence estimates for Laos.22 At a precision of 10% and 95% confidence level (Equation 1) and correcting for a finite population of 150 households per village on the basis of averaged data supplied by district agriculture and forestry offices (Equation 2), 14 households were randomly selected from each village by using a random number table. The two equations used were
where N0 was the sample size required for simple random sampling, Z was the z-score for the required confidence level (1.96), P was the estimated proportion of affected households, and α was the required precision expressed as a proportion (0.1). N1 was the corrected sample size and n was the finite population size.
All household members ≥ 6 years of age were asked to participate. Village chiefs were given advance notice of the survey timing and meetings were conducted in villages the day before sampling to select households. In cases where a household refused to participate, the village chief selected a household with similar characteristics. A household questionnaire was administered to the head of each household with his or her family present to assess the house characteristics, assets owned, ownership of animals, ethnicity, education levels and literacy of the male and female heads of household, the person with greatest responsibility for preparing food, and person with the greatest responsibility for primary care. An individual questionnaire was administered to all study participants, with younger participants (< 15 years of age) interviewed in the presence of a parent or guardian who may have provided assistance in answering questions.
Data on frequency of raw meat consumption, latrine use, tapeworm segments seen in feces, and a history of taeniasis were collected. For those who consumed raw meat, we asked them to estimate the frequency of raw meat consumption: weekly, monthly, every few months, and infrequent (once or twice per year or less often). Questionnaires were administered in the Lao language and were pre-tested with persons who did not otherwise participate in the survey. In the circumstances where a person could not understand the Lao language, a household member, relative, or village chief provided verbal translation of questions to study participants with their consent.
A venous blood sample of 2–3 mL was collected and the serum fraction was stored at −20°C. Labeled plastic bags for a single fecal sample were handed out. Fecal samples were collected from the participants the next day and stored in two preservation solutions, 10% formalin and 80% ethanol, for microscopy and molecular analysis, respectively.
Persons who were Taenia egg positive or self-reported seeing fecal segments were treated with niclosamide and a purgative (bisacodyl) according to manufacturer's instructions (Vechaphant Baesaj, Visonic, Thailand) during November–December 2009. All participants were provided with detailed information on the risks associated with T. solium taeniasis and the need to safely dispose of all stools and adhere to strict hand hygiene measures. Buckets and soap were provided to all participants.
Adults were treated with 2 g of niclosamide and 15 mg of bisacodyl two hours post-treatment, children > 34 kg were treated with 1.5 g of niclosamide and 10 mg of bisacodyl, and children 11–34 kg were treated with 1 g of niclosamide and 5 mg of bisacodyl. All fecal samples were examined for scoleces and proglottids for two days after treatment. Expelled worm segments were preserved in 80% ethanol and transported back to Vientiane at ambient temperature where they were stored at 4°C until testing.
Animal surveys.
Opportunistic pig surveys were conducted at three slaughter-points in Xiengkhuang and Oudomxay Provinces from May–September 2008 and at two collection points in Huaphan and Luangprabang Provinces from October 2008–January 2009. The survey team consisted of trained district and provincial agricultural and forestry government staff who visited the slaughter points approximately every two weeks. All pigs brought for slaughter on the nights the survey team visited were examined post-mortem and a blood sample was collected. The tongue and diaphragm pillar muscles were excised and examined for cysts. Pork traders prevented muscle slicing; as such, the heart, liver, mesentery, omentum, and other viscera were examined for Taenia cysts, as were all exposed muscle surfaces. Presence of cysts and data on location, age, breed, sex, and production system at last point of sale were recorded on a data collection sheet and sent to Vientiane with the blood sample and any cysts found.
Dog fecal samples were collected in the same villages as the human survey described above during January–March 2009 using a semi-structured approach. Dogs were selected if they belonged to the same household as those randomly selected for the human survey. If no dogs were present in a household, then dogs were opportunistically identified in the village. The permission of owner's was granted before sampling was undertaken. Fecal samples were collected by using manual digital extraction and preserved in two preservation solutions, 10% formalin and 80% ethanol. Demographic data and the age and sex were recorded with the sample. The samples were sent to Vientiane at ambient temperature and subsequently stored at 4°C before processing.
Laboratory analysis.
Formalin-preserved human feces was transported at room temperature to Khon Kaen University (Khon Kaen, Thailand) where samples were analyzed by the formalin-ether-concentration technique and microscopy. Formalin-preserved dog feces was transported to Murdoch University (Perth, Western Australia, Australia) at ambient temperature and examined for taeniid eggs by the saturated sodium nitrate flotation technique and microscopy.
Preserved human and pig serum samples were tested by an enzyme linked immunosorbent assay (ELISA) for Taenia metacestode circulating antigens23–25 using modifications introduced by Dorny and others.26 The optical density (OD) of the human samples were read at 490 nm with a reference at 650 nm, and the OD of the pig samples were read at 490 nm. The cut-off value was calculated as described by Dorny and others24 by using a panel of eight negative serum samples from human and pig populations in Laos. A ratio for each test was calculated by dividing the OD of the test sample by the cut-off value, and a ratio > 1 was considered positive. Samples were retested if the coefficient of variation was > 50% or if the OD of the test sample was close to the cut-off value.
DNA was isolated from proglottids expelled post-niclosamide treatment by using the DNeasy Blood and Tissue Extraction Kit (QIAGEN, Hilden, Germany). A multiplex polymerase chain reaction (PCR) for cytochrome c oxidase subunit 1 gene (cox1) was performed for species identification of T. saginata, T. solium, and T. asiatica. Primers and PCR protocols were as described27 with modifications. A PCR cocktail contained 0.4 μM of each primer (Sigma-Aldrich, St. Louis, MO, 1.25 units of GoTaq DNA polymerase in GoTaq reaction buffer supplemented with 2 mM MgCl2 (Promega, Madison, WI), and 0.2 mM of each dNTP (Promega) in a final 50-μL reaction volume. The amplification protocol consisted of 3 minutes at 94°C; followed by of 35 cycles of 30 seconds at 94°C, 30 seconds at 56°C, and 90 seconds at 72°C; plus one cycle of 5 minutes at 72°C. Subsequently, PCR-amplified products were subjected to electrophoresis on 1.5% agarose gels with a DNA ladder (Hyperladder II; Bioline, London, United Kingdom). Positive control DNA for the three Taenia species were extracted from proglottids (kindly supplied by the Institute of Tropical Medicine, Antwerp, Belgium).
Data analysis.
The questionnaire and laboratory test data were entered into a spreadsheet (Excel; Microsoft, Redmond, WA) and subsequent analysis was carried out in STATA/IC version 10 (Stata Corp LP, College Station, TX). The socioeconomic status of each household was calculated by use of principal component analysis of household assets28,29 after replacement of missing values with the mean of the respective asset for that ethnic group. All assets were dichotomous. The households were ranked into wealth quintiles according to their cumulative standardized asset scores.
Prevalence of cysticercosis seropositivity in the human and pig populations were calculated as the proportion of positive antigen ELISA results in the sampled population. Taeniasis prevalence in humans and dogs was calculated as the proportion of fecal samples with taeniid eggs in the sampled population. In addition, human taeniasis prevalence was calculated for those persons who had eggs detected and/or self-reported tapeworm segments in their feces. In the pig study, the Pearson's chi-square test and Fisher's exact test were used to explore associations between infection status (carcass inspection and antigen ELISA seropositivity) and age, breed, sex, and production system at last point of sale.
For risk factor analysis in the human study, taeniasis was defined as taeniid egg–positive and/or self-reporting segments. Univariate logistic regression without adjustment was used to test associations between infection status (cysticercosis or taeniasis) and sex, location, ethno-linguistic family, age, wealth status, defecation site, taeniasis, history of taeniasis, uncooked meat consumption habits, and literacy of selected household members. Risk factors significant or borderline significant (cut-off P ≤ 0.10) in the univariate analyses were included in a multivariate random effects logistic regression model adjusting for the effect of household clustering. The results are reported as adjusted odds ratios and 95% confidence intervals (CIs). The final analysis only considered persons with complete parasitologic and serologic data. Missing data on literacy of household members because of death, divorce, or other factors were replaced with the mean for that village and rounded to 0 or 1 (illiterate or literate).
A maximum-likelihood estimator (MLE) (Equation 3) with 95% CI (Equation 4)30 was used to calculate adjusted prevalence for dog T. hydatigena taeniasis and pig cysticercosis detected by inspection at slaughter, for T. solium, T. hydatigena and T. asiatica according to the equations
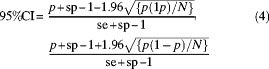 |
where p was the observed prevalence, sp was the test specificity, se was the test sensitivity, and N was the sample size. Calculations were made through 10% increments of test sensitivity assuming specificity was 100%. For dog T. hydatigena taeniasis, 100% specificity was assumed because Echinococcus spp. are not endemic to Southeast Asia. Cystic echinococcosis has been rarely reported from mainland Southeast Asia and nothing is known of its epidemiology.31 For pig cysticercosis, carcass inspection specificity has been estimated to be at or very close to 100%.26
Results
Human study.
A total of 1,582 persons in 332 households were eligible to participate in this survey. Of these persons, 1,306 (82.7%) individuals from 321 households aged 6–91 years provided blood and fecal samples, a completed questionnaire, and had valid laboratory test results. Overall, the Mon-Khmer and Lao-Tai ethnic families had the highest compliance, 88.9% and 88.6% of eligible persons, respectively. The Hmong-Mien ethnic family had the lowest compliance; only 62.5% of eligible persons provided fecal and blood samples and a completed questionnaire. Non-compliance caused by mental illness was negligible; two persons, one each from the Lao-Tai and Hmong-Mien ethnic groups, were protected by their respective families. The final survey population consisted of 553 Lao-Tai (42.3%), 523 Mon-Khmer (40.1%), and 230 (17.6%) Hmong-Mien. No Sino-Tibetan persons were recruited into this study. Differences between the compliant and non-compliant persons who were eligible to participate in the study stratified by ethnic family are shown in Table 1.
Table 1.
Demographic differences between survey participants compliant (C) and non-compliant (NC), stratified by ethnicity, Laos*
| Characteristic | Lao-Tai | Mon-Khmer | Hmong-Mien | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C, no. (%) | NC, no. (%) | P | C, no. (%) | NC, no. (%) | P | C, no. (%) | NC, no. (%) | P | |
| Sex | |||||||||
| F | 277 (89.1) | 34 (10.9) | 0.168† | 270 (91.5) | 25 (8.5) | 0.826† | 109 (67.3) | 53 (32.7) | 0.267† |
| M | 276 (92.3) | 23 (7.7) | 253 (91.0) | 25 (9.0%) | 121 (72.9) | 45 (27.1) | |||
| Province | |||||||||
| Oudomxay | 58 (96.7) | 2 (3.3) | 0.029‡ | 297 (90.8) | 30 (9.2) | 0.779† | 28 (96.6) | 1 (3.4) | < 0.001‡ |
| Luangprabang | 91 (84.3) | 17 (15.7) | 226 (91.5) | 21 (8.5) | 31 (96.9) | 1 (3.1) | |||
| Huaphan | 271 (92.5) | 22 (7.5) | 0 | 0 | 9 (17.6) | 42 (82.4) | |||
| Xiengkhuang | 133 (89.3) | 16 (10.7) | 0 | 0 | 162 (75.0) | 54 (25.0) | |||
| Household wealth status | |||||||||
| Most poor | 14 (93.3) | 1 (6.7) | 0.381† | 162 (93.1) | 12 (6.9) | 0.635‡ | 49 (51.6) | 46 (48.4) | < 0.001‡ |
| Very poor | 59 (89.4) | 7 (10.6) | 152 (88.4) | 20 (11.6) | 35 (67.3) | 17 (32.7) | |||
| Poor | 176 (91.2) | 17 (8.8) | 61 (91.0) | 6 (9.0) | 46 (78.0) | 13 (22.0) | |||
| Less poor | 178 (93.2) | 13 (6.8) | 54 (93.1) | 4 (6.9) | 56 (73.7) | 20 (26.3) | |||
| Least poor | 126 (86.9) | 19 (13.1) | 94 (91.3) | 9 (8.7) | 44 (95.7) | 2 (4.3) | |||
| Age (years) | |||||||||
| 6–10 | 69 (85.2) | 12 (14.8) | 0.035‡ | 99 (90.8) | 10 (9.2) | 0.001‡ | 49 (65.3) | 26 (34.7) | 0.203‡ |
| 11–14 | 75 (90.4) | 8 (9.6) | 70 (81.4) | 16 (18.6) | 38 (67.9) | 18 (32.1) | |||
| 15–24 | 105 (86.8) | 16 (13.2) | 102 (88.7) | 13 (11.3) | 51 (68.0) | 24 (32.0) | |||
| 25–39 | 135 (93.8) | 9 (6.2) | 109 (97.3) | 3 (2.7) | 38 (67.9) | 18 (32.1) | |||
| 40–54 | 93 (96.9) | 3 (3.1) | 98 (97.0) | 3 (3.0) | 34 (77.3) | 10 (22.7) | |||
| ≥ 55 | 76 (89.4) | 9 (10.6) | 45 (90.0) | 5 (10.0) | 20 (90.9) | 2 (9.1) | |||
C = compliant; NC = non-compliant. Missing data for non-compliant: Lao-Tai =14; Mon-Khmer = 16; Hmong-Mien = 41.
For chi-square test for difference between C and NC.
For Fisher's exact test for difference between C and NC.
Survey population structures stratified by ethnicity are shown in Table 2. Significant differences were observed for all characteristics with the exception of sex. Most (49.0%) Lao-Tai persons were from Huaphan Province, no Mon-Khmer persons were selected in Huaphan and Xiengkhuang Provinces, and most (70.4%) Hmong-Mien persons were from Xiengkhuang Province. The highest proportion of impoverished participants were from the Mon-Khmer ethnic family, and the highest proportion of least and less poor participants were from the Lao-Tai ethnic family. The Mon-Khmer and Hmong-Mien ethnic families had the highest proportion of participants defecating in the open and the highest proportion of persons living in a household with an illiterate female head of household (Table 1).
Table 2.
Survey population characteristics stratified by ethnicity, Laos*
| Characteristic | Total | Lao-Tai | Mon-Khmer | Hmong-Mien | χ2† | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |||
| Sex | ||||||||||
| F | 656 | 50.2 | 277 | 50.1 | 270 | 51.6 | 109 | 47.4 | 1.2 | 0.562 |
| M | 650 | 49.8 | 276 | 49.9 | 253 | 48.4 | 121 | 52.6 | ||
| Province | ||||||||||
| Oudomxay | 383 | 29.3 | 58 | 10.5 | 297 | 56.8 | 28 | 12.2 | 1,000.0 | < 0.001 |
| Luangprabang | 348 | 26.7 | 91 | 16.5 | 226 | 43.1 | 31 | 13.5 | ||
| Huaphan | 280 | 21.4 | 271 | 49.0 | 0 | 0.0 | 9 | 3.9 | ||
| Xiengkhuang | 295 | 22.6 | 133 | 24.1 | 0 | 0.0 | 162 | 70.4 | ||
| Wealth status | ||||||||||
| Most poor | 225 | 17.2 | 14 | 2.5 | 162 | 31.0 | 49 | 21.3 | 292.1 | < 0.001 |
| Very poor | 246 | 18.8 | 59 | 10.7 | 152 | 29.1 | 35 | 15.2 | ||
| Poor | 283 | 21.7 | 176 | 31.8 | 61 | 11.7 | 46 | 20.0 | ||
| Less poor | 288 | 22.1 | 178 | 32.2 | 54 | 10.3 | 56 | 24.4 | ||
| Least poor | 264 | 20.2 | 126 | 22.8 | 94 | 18.0 | 44 | 19.1 | ||
| Age (years) | ||||||||||
| 6–10 | 217 | 16.6 | 69 | 12.5 | 99 | 18.9 | 49 | 21.3 | 26.7 | 0.003 |
| 11–14 | 183 | 14.0 | 75 | 13.6 | 70 | 13.4 | 38 | 16.5 | ||
| 15–24 | 258 | 19.8 | 105 | 19.0 | 102 | 19.5 | 51 | 22.2 | ||
| 25–39 | 282 | 21.6 | 135 | 24.4 | 109 | 20.8 | 38 | 16.5 | ||
| 40–54 | 225 | 17.2 | 93 | 16.8 | 98 | 18.7 | 34 | 14.8 | ||
| ≥ 55 | 141 | 10.8 | 76 | 13.7 | 45 | 8.6 | 20 | 8.7 | ||
| Defecation site | ||||||||||
| Latrine | 861 | 65.9 | 475 | 85.9 | 275 | 52.6 | 111 | 48.3 | 171.6 | < 0.001 |
| Open | 445 | 34.1 | 78 | 14.1 | 248 | 47.4 | 119 | 51.7 | ||
| Male head of HH | ||||||||||
| Illiterate | 228 | 17.5 | 25 | 4.5 | 164 | 31.4 | 39 | 17.0 | 134.4 | < 0.001 |
| Literate | 1,078 | 82.5 | 528 | 95.5 | 359 | 68.6 | 191 | 83.0 | ||
| Female head of HH | ||||||||||
| Illiterate | 504 | 38.6 | 83 | 15.0 | 296 | 56.6 | 125 | 54.4 | 225.4 | < 0.001 |
| Literate | 802 | 61.4 | 470 | 85.0 | 227 | 43.4 | 105 | 45.6 | ||
HH = household.
Calculated across all groups and ethnicities.
The prevalence of cysticercosis and taeniasis stratified by ethnicity for population and individual variables are shown in Table 3. The prevalence of cysticercosis antigen ELISA positivity was 2.2% (95% CI = 1.4–3.0%), ranging at the village level from 0.0% to 11.3%; 14 villages had no detectable cysticercosis cases and 10 villages had at least one seropositive case. Greater than half (15 of 29) of the cases were detected in three villages in Oudomxay Province. Univariate analysis showed that only province was significantly associated with cysticercosis antigen ELISA positivity. After controlling for clustering at the household level by random effects logistic regression, only Luangprabang Province (adjusted odds ratio = 0.26, 95% CI = 0.07–0.98) was significantly associated with reduced risk of cysticercosis antigen ELISA positivity.
Table 3.
Prevalence of cysticercosis (antigen-capture ELISA) and taeniasis (egg detection plus self-reported) by population level and individual level characteristics, stratified by ethnicity, Laos*
| Characteristic | Proportion cysticercosis antigen ELISA positive (95% CI) | Proportion egg positive or self-reported taeniasis (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | LT | MK | HM | Total | LT | MK | HM | |
| Total | 2.2 (1.4–3.0) | 1.6 (0.6–2.7) | 3.3 (1.7–4.8) | 1.3 (0.0–2.8) | 8.4 (6.9–9.9) | 7.8 (5.5–10.0) | 11.7 (8.9–14.4) | 2.6 (0.5–4.7) |
| Sex | ||||||||
| F | 2.1 (1.0–3.2) | 1.1 (0.0–2.3) | 3.7 (1.4–6.0) | 0.9 (0.0–2.7) | 4.7 (3.1– 6.4) | 3.9 (1.7–6.3) | 7.0 (4.0–10.1) | 0.9 (0.0–2.7) |
| M | 2.3 (1.2–3.5) | 2.2 (0.4–3.9) | 2.7 (0.7–4.8) | 1.7 (0.0–4.0) | 12.2 (9.6–14.7) | 11.6 (7.8–15.4) | 16.6 (12.0–21.2) | 4.1 (0.5–7.7) |
| Province | ||||||||
| Oudomxay | 3.9 (2.0–5.9) | 0.0 | 5.1 (2.5–7.6) | 0.0 | 13.1 (9.7–16.4) | 12.1 (3.4–20.7) | 13.8 (9.9–17.8 | 7.1 (0.0–17.3) |
| Luangprabang | 1.1 (0.0–2.3) | 2.2 (0.0–5.3) | 0.9 (0.0–2.1) | 0.0 | 9.2 (6.1–12.2) | 9.9 (3.6–16.1) | 8.8 (5.1–12.6) | 9.7 (0.0–20.7) |
| Huaphan | 1.1 (0.0–2.3) | 1.1 (0.0–2.4) | – | 0.0 | 5.4 (2.7–8.0) | 5.5 (2.8–8.3) | – | 0.0 |
| Xiengkhuang | 2.4 (0.6–4.1) | 3.0 (0.0–5.9) | – | 1.9 (0.0–4.0) | 4.4 (2.1–6.8) | 9.0 (4.1–14.0) | – | 0.6 (0.0–1.8) |
| Wealth status | ||||||||
| Most poor | 3.1 (0.8–5.4) | 0.0 | 3.7 (0.8–6.6) | 2.0 (0.0–6.1) | 10.2 (6.2–14.2) | 0.0 | 13.6 (8.2–18.9) | 2.0 (0.0–6.1) |
| Very poor | 4.1 (1.6–6.6) | 0.0 | 5.9 (2.1–9.7) | 2.9 (0.0–8.7) | 11.0 (7.0–14.9) | 11.9 (3.4–20.4) | 13.2 (7.7–18.6) | 0.0 |
| Poor | 1.1 (0.0–2.3) | 1.1 (0.0–2.7) | 0.0 | 2.2 (0.0–6.6) | 6.7 (3.7–9.6) | 7.4 (3.5–11.3) | 9.8 (2.1–17.5) | 0.0 |
| Less poor | 2.1 (0.4–3.7) | 2.8 (0.4–5.3) | 1.9 (0.0–5.6) | 0.0 | 7.3 (4.3–10.3) | 8.4 (4.3–12.5) | 7.4 (0.2–14.6) | 3.6 (0.0–8.6) |
| Least poor | 1.1 (0.0–2.4) | 1.6 (0.0–3.8) | 1.1 (0.0–3.2) | 0.0 | 7.6 (4.4–10.8) | 6.3 (2.0–10.7) | 9.6 (3.5–15.6) | 6.8 (0.0–14.6) |
| Age (years) | ||||||||
| 6–10 | 3.2 (0.9–5.6) | 4.3 (0.0–9.3) | 4.0 (0.1–8.0) | 0.0 | 7.3 (3.9–10.9) | 2.9 (0.0–7.0) | 14.1 (7.2–21.1) | 0.0 |
| 11–14 | 1.1 (0.0–2.6) | 0.0 | 1.4 (0.0–4.3) | 2.6 (0.0–8.0) | 2.2 (0.0–4.3) | 2.7 (0.0–6.4) | 2.9 (0.0–6.9) | 0.0 |
| 15–24 | 1.2 (0.0–2.5) | 1.0 (0.0–2.8) | 2.0 (0.0–4.7) | 0.0 | 4.3 (1.8–6.7) | 2.9 (0.0–6.1) | 6.9 (1.9–11.9) | 2.0 (0.0–5.9) |
| 25–39 | 1.8 (0.2–3.3) | 0.7 (0.0–2.2) | 2.8 (0.0–5.9) | 2.6 (0.0–8.0) | 13.5 (9.5–17.5) | 13.3 (7.5–19.1) | 14.7 (7.9–21.4) | 10.5 (0.3–20.7) |
| 40–54 | 3.1 (0.8–5.4) | 1.1 (0.0–3.2) | 5.1 (0.7–9.5) | 2.9 (0.0–8.9) | 11.6 (7.3–15.8) | 12.9 (6.0–19.8) | 13.3 (6.4–20.1) | 2.9 (0.0–8.9) |
| ≥ 55 | 3.5 (0.5–6.6) | 3.9 (0.0–8.4) | 4.4 (0.0–10.7) | 0.0 | 10.6 (5.5–15.8) | 7.9 (1.7–14.1) | 20.0 (7.8–32.2) | 0.0 |
| Defecation site | ||||||||
| Latrine | 2.2 (1.2–3.2) | 1.9 (0.7–3.1) | 3.2 (1.2–5.4) | 0.9 (0.0–2.7) | 8.8 (6.9–10.7) | 7.6 (5.2–10.0) | 13.5 (9.4–17.5) | 2.7 (0.0–5.8) |
| Open | 2.3 (0.9–3.6) | 0.0 | 3.2 (1.0–5.4) | 1.7 (0.0–4.0) | 7.6 (5.2–10.1) | 9.0 (2.5–15.5) | 9.7 (6.0–13.4) | 2.5 (0.0–5.4) |
| Male head of HH | ||||||||
| Illiterate | 1.3 (0.0–2.8) | 0.0 | 1.8 (0.0–3.9) | 0.0 | 9.6 (5.8–13.5) | 4.0 (0.0–12.3) | 11.6 (6.6–16.5) | 5.1 (0.0–12.4) |
| Literate | 2.4 (1.5–3.3) | 1.7 (0.6–2.8) | 3.9 (1.9–5.9) | 1.6 (0.0–3.3) | 8.2 (6.5–9.8) | 8.0 (5.6–10.3) | 11.7 (8.4–15.0) | 2.1 (0.0–4.1) |
| Female head of HH | ||||||||
| Illiterate | 2.8 (1.3–4.2) | 0.0 | 3.7 (1.5–5.9) | 2.4 (0.0–5.1) | 8.7 (6.3–11.2) | 7.2 (1.5–12.9) | 11.8 (8.1–15.5) | 2.4 (0.0–5.1) |
| Literate | 1.9 (0.9–2.8) | 1.9 (0.7–3.2) | 2.6 (0.5–4.7) | 0.0 | 8.2 (6.3–10.1) | 7.9 (5.4–10.3) | 11.4 (7.3–15.6) | 2.9 (0.0–6.1) |
ELISA = enzyme-linked immunosorbent assay; CI = confidence interval; LT = Lao-Tai ethnicity; MK = Mon-Khmer ethnicity; HM = Hmong-Mien; HH = household.
The prevalence of Taenia egg positivity was 2.9% (95% CI = 2.0–3.8%), and the estimated taeniasis prevalence, egg positive plus self-reported, was 8.4% (95% CI = 6.9–9.9%), ranging at the village level from 0.0% to 6.9% and 0.0% to 17.0%, respectively. The proportion of persons reporting a history of taeniasis was 27.0% (95% CI = 24.5–29.4%). For persons with current taeniasis, 90.0% (95% CI = 84.3–95.7%) reported having a history of taeniasis compared with 21.2% (95% CI = 18.8–23.5%) for uninfected persons. Only egg positive and/or self-reporting cases were considered in the risk factor analysis. Univariate analysis showed that a history of taeniasis was strongly associated with increased risk of having a current taeniasis infection. Other factors significantly associated with taeniasis were sex, province, age, ethnicity, and consumption of raw meat. Two multivariate analyses of risk factors associated with taeniasis were carried out, including and excluding the variable history of taeniasis (Table 4). History of taeniasis was strongly correlated with age (χ2 = 121.9, P < 0.001), and inclusion in the model gave the perception that age was protective (Table 4). The exclusion of history of taeniasis from the analysis resulted in sex, province of origin, age, ethnicity, and infrequent consumption of uncooked beef being significantly associated with a current taeniasis infection (Table 4). The consumption of uncooked pork and uncooked fermented pork were not significantly associated with taeniasis after controlling for other risk factors.
Table 4.
Risk factors significantly (P < 0.050) associated with taeniasis, as determined by multiple logistic regression modeling controlling for household clustering, Laos*
| Model† | Population characteristic | Risk factor | Adjusted OR | 95% CI |
|---|---|---|---|---|
| 1 | Province | Oudomxay | Ref. | |
| Luangprabang | 0.71 | 0.40–1.29 | ||
| Huaphan Province | 0.32 | 0.12–0.84 | ||
| Xiengkhuang Province | 0.38 | 0.15–0.93 | ||
| Age (years) | 6–10 years old | Ref. | ||
| 11–14 years old | 0.17 | 0.05–0.62 | ||
| 15–24 years old | 0.24 | 0.09–0.67 | ||
| 25–39 years old | 0.42 | 0.17–1.04 | ||
| 40–54 years old | 0.29 | 0.11–0.75 | ||
| ≥ 55 years old | 0.22 | 0.08–0.60 | ||
| Ethnicity | Lao-Tai ethnicity | Ref. | ||
| Mon-Khmer ethnicity | 0.90 | 0.45–1.83 | ||
| Hmong ethnicity | 0.34 | 0.12–0.96 | ||
| Previous taeniasis | No previous taeniasis | Ref. | ||
| History of taeniasis | 32.98 | 15.63–69.56 | ||
| Raw beef consumption | Doesn't eat | Ref. | ||
| Weekly | 0.81 | 0.27–2.37 | ||
| Monthly | 1.48 | 0.71–3.09 | ||
| Every few months | 1.07 | 0.49–2.35 | ||
| Infrequent | 4.13 | 1.50–11.36 | ||
| 2 | Sex | Female | Ref. | |
| Male | 2.20 | 1.34–3.63 | ||
| Province | Oudomxay Province | Ref. | ||
| Luangprabang Province | 0.65 | 0.38–1.13 | ||
| Huaphan Province | 0.26 | 0.11–0.63 | ||
| Xienkhuang Province | 0.36 | 0.15–0.86 | ||
| Age (years) | 6–10 years old | Ref. | ||
| 11–14 years old | 0.24 | 0.07–0.75 | ||
| 15–24 years old | 0.42 | 0.17–1.00 | ||
| 25–39 years old | 1.11 | 0.53–2.34 | ||
| 40–54 years old | 0.86 | 0.39–1.90 | ||
| ≥ 55 years old | 0.82 | 0.34–1.94 | ||
| Ethnicity | Lao-Tai ethnicity | Ref. | ||
| Mon-Khmer ethnicity | 0.86 | 0.45–1.65 | ||
| Hmong ethnicity | 0.26 | 0.10–0.70 | ||
| Raw beef consumption | Doesn't eat | Ref. | ||
| Weekly | 1.68 | 0.61–4.65 | ||
| Monthly | 2.43 | 1.27–4.65 | ||
| Every few months | 1.88 | 0.91–3.87 | ||
| Infrequent | 5.99 | 2.51–14.25 |
OR = odds ratio; CI = confidence interval; Ref. = referent; Infrequent = consumes once or twice per year or less often.
Model 1 = taeniasis OR adjusted for sex, province, age, history of taeniasis, ethnicity, and frequency of raw fermented pork sausage, raw pork, and raw beef consumption; Model 2 = taeniasis OR adjusted for sex, province, age, ethnicity, and raw fermented pork sausage consumption, raw pork consumption, and raw beef consumption.
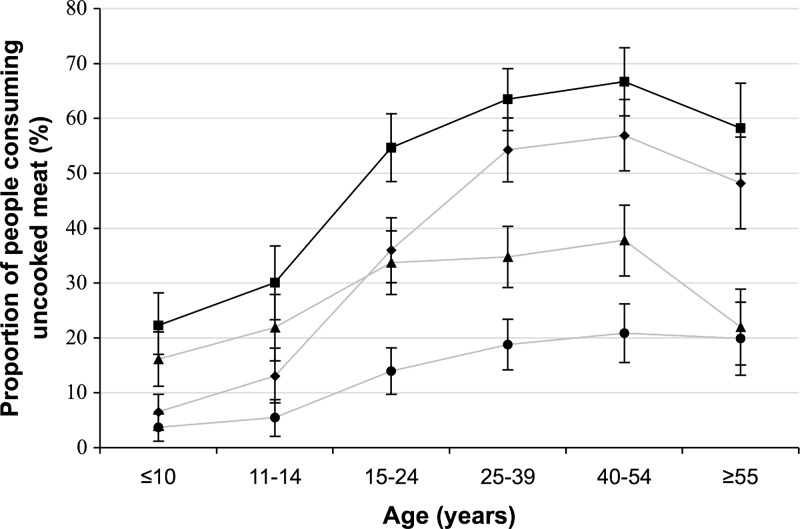
The proportion of people reporting consumption of any uncooked meat, uncooked pork, fermented pork sausage, and uncooked beef was 50.2% (95% CI = 47.5–52.9%), 13.9% (12.1–15.8%), 28.8% (26.3–31.2%), and 36.7% (34.1–39.4%), respectively. The prevalence of eating any uncooked meat increased significantly with age (χ2 = 145.8, P < 0.001), and similar results were observed for eating uncooked beef (χ2 = 214.2, P < 0.001), uncooked pork (χ2 = 48.7, P < 0.001), and fermented pork sausage (χ2 = 41.3, P < 0.001) (Figure 2). Uncooked beef consumption had the highest peak prevalence of 56.9% (95% CI = 50.4–63.4%) in the 40–54 year age group; the peak prevalence of eating uncooked pork and uncooked fermented pork sausage was 20.9% (95% CI = 15.5–26.2%) and 37.8% (95% CI = 31.3–44.2%), respectively, also in the 40–54 year age group (Figure 2).
Figure 2.
Proportion of study population consuming uncooked meat by age category. Solid black line indicates any uncooked meat; gray line with circles indicates uncooked pork; gray line with triangles indicates uncooked fermented pork sausage; gray line with diamonds indicates uncooked beef.
Of the 110-taeniasis positive persons who were treated with niclosamide, proglottids were expelled from 35 persons and PCR showed that 33 tapeworms were T. saginata and 2 were T. solium. The T. solium worms were recovered from a seven-year-old boy from Oudomxay Province who was antigen ELISA negative and from a 34-year-old man from Xiengkhuang Province who was antigen ELISA positive. Both persons reported not eating uncooked pork or fermented pork sausage. The T. saginata worms were recovered from 27 males and six females (age range = 19–78 years) from all provinces. Thirty-two of the T. saginata cases were antigen ELISA negative, and 32 reported eating uncooked beef. Both T. solium cases were egg positive by formol ethyl acetate concentration and one was self-reported. Sixteen (48.5%) of the 33 T. saginata cases were egg positive by formol ethyl acetate concentration and 29 (87.9%) of 33 were self-reported.
Pig study.
Inspection data results from Oudomxay Province were not used in the analysis because of submission of incorrectly completed forms. A total of 590 pig carcasses, with a matching serum sample, were inspected in three provinces: 209 in Luangprabang, 190 in Huaphan, and 191 in Xiengkhuang. Data on the variables breed, age, sex, and production system at last point of sale were collected for 538, 528, 540 and 518 pigs, respectively (Table 5). Carcass inspection detected five pigs (0.8%) with cysts consistent with the morphology of T. solium; 1.0–1.5 cm fluid-filled muscle cysts with a single white scolex. All infected pigs were heavily infected (without counting) and had viable and degenerated cysts and age was significantly associated with T. solium detection (Table 5). One hundred thirty-two (22.4%) carcasses were detected with cysts consistent with the morphology of T. hydatigena; large, visceral, fluid filled cysts with a single white scolex. The prevalence of T. hydatigena detection was significantly greater in free-range pigs (Table 5). Two pigs, one each from Luangprabang and Huaphan Provinces, had a dual infection with T. solium and T. hydatigena. One pig in Huaphan Province was detected with cysts consistent with T. asiatica; small fluid-filled cysts present in the liver, spleen, and lung. This pig was a 15-month-old female obtained from a penned production system. Non-specific liver lesions consistent with parasitemia were detected in 16 (2.7%) pigs; these pigs were considered inspection negative in the absence of histopathologic test results to definitively identify Taenia.
Table 5.
Prevalence of pig cysticercosis (Taenia solium, T. hydatigena, and T. asiatica) by carcass inspection and seroprevalence of pig cysticercosis (antigen-capture ELISA) by location, sex, breed, age, and production system at last point of sale, Laos*
| Risk factor | No. (%) | Carcass inspection prevalence of pig cysticercosis (95% CI) | Cysticercosis antigen-capture ELISA positivity | ||||
|---|---|---|---|---|---|---|---|
| T. solium (95% CI) | P | T. hydatigena (95% CI) | P | Prevalence (95% CI) | P | ||
| Total | 590 | 0.8 (0.1-1.6) | 22.4 (19.0-25.7) | 68.5 (64.7–72.2) | |||
| Province (n = 590) | |||||||
| Luangprabang | 209 (35.4) | 1.4 (0.0–3.1) | 0.335† | 22.5 (16.8–28.2) | 0.173‡ | 71.3 (65.1–77.5) | 0.177‡ |
| Huaphan | 190 (32.2) | 1.1 (0.0–2.5) | 26.3 (20.0–32.6) | 70.5 (64.0–77.1) | |||
| Xiengkhuang | 191 (32.4) | 0 | 18.3 (12.7–23.8) | 63.3 (56.5–70.2) | |||
| Age (months) (n = 528) | |||||||
| ≤ 6 | 70 (13.3) | 0 | 0.010† | 27.1 (16.6–37.7) | 0.629‡ | 68.6 (57.6–79.6) | 0.389‡ |
| 7–12 | 250 (47.4) | 0 | 22.8 (17.6–28.0) | 73.2 (67.7–78.7) | |||
| 13–18 | 128 (24.2) | 1.6 (0.0–3.7) | 19.5 (12.6–26.4) | 68.0 (59.8–76.1) | |||
| ≥ 18 | 80 (15.2) | 3.8 (0.0–8.0) | 25.0 (15.4–34.6) | 63.8 (53.1–74.4) | |||
| Breed (n = 538) | |||||||
| Lao indigenous | 473 (87.9) | 1.1 (0.1–2.0) | – | 24.7 (20.8–28.6) | 0.077† | 72.1 (68.0–76.1) | 0.107‡ |
| Exotic | 37 (6.9) | 0 | 10.8 (0.6–21.0) | 56.8 (40.5–73.0) | |||
| Cross-breed | 28 (5.2) | 0 | 14.3 (1.1–27.5) | 64.3 (46.2–82.4) | |||
| Sex (n = 540) | |||||||
| F | 275 (50.9) | 0.7 (0.0–1.7) | 0.151† | 22.5 (17.6–27.5) | 0.423‡ | 69.5 (64.0–74.9) | 0.674‡ |
| M | 130 (24.1) | 2.3 (0.0–4.9) | 20.0 (13.1–26.9) | 67.7 (59.6–75.8) | |||
| Castrated male | 135 (25.0) | 0 | 26.7 (19.2–34.2) | 72.6 (65.0–80.2) | |||
| Production system at lastpoint of sale (n = 518) | |||||||
| Penned/corralled | 345 (66.6) | 1.2 (0.0–2.3) | 1.000† | 19.4 (15.2–23.6) | 0.024† | 67.2 (62.3–72.2) | 0.253† |
| Free roaming | 167 (32.2) | 0.6 (0.0–1.8) | 29.3 (22.4–36.3) | 74.3 (67.6–80.9) | |||
| Mixed | 6 (1.2) | 0 | 33.3 (0.0–74.7) | 66.7 (25.3–100.0) | |||
ELISA = enzyme-linked immunosorbent assay; CI = confidence interval; Mixed = sometimes penned and sometimes free-roaming.
By Fisher's exact test.
By Pearson's chi-square test.
Serum samples from 404 (68.5%) pigs were reactive in the antigen ELISA, and no significant association was observed for province, breed, age, sex, and production system (Table 5). All five T. solium and one T. asiatica inspection-positive pigs had serum samples reactive in the antigen ELISA. Of 132 T. hydatigena inspection-positive pigs, 129 were reactive and three were non-reactive. Fourteen of the pigs with non-specific liver lesions were serum reactive in the antigen ELISA and two were non-reactive.
Estimates of true prevalence of T. solium, T. hydatigena, T. asiatica cysticercosis in pigs and T. hydatigena taeniasis in dogs using the maximum-likelihood estimator for carcass inspection with a sensitivity ranging from 10 to 100% and assuming specificity was 100% are shown in Table 6.
Table 6.
Estimated true prevalence of pig cysticercosis (Taenia solium, T. hydatigena, and T. asiatica) and dog taeniasis (T. hydatigena) adjusted by the maximum-likelihood estimation model for increments of test sensitivity, assuming specificity of 100%, Laos*
| Test sensitivity (%) | Estimated true prevalence of pig cysticercosis, % (95% CI) | Estimated true prevalence of T. hydatigena taeniasis in village dogs, % (95% CI) | ||
|---|---|---|---|---|
| T. solium | T. hydatigena | T. asiatica | ||
| 100 | 0.8 (0.1–1.6) | 22.4 (19.0–25.7) | 0.2 (0.0–0.5) | 1.9 (0.0–4.5) |
| 90 | 0.9 (0.1–1.8) | 24.9 (21.1–28.6) | 0.2 (0.0–0.6) | 2.1 (0.0–5.0) |
| 80 | 1.1 (0.1–2.0) | 28.0 (23.8–32.2) | 0.2 (0.0–0.6) | 2.4 (0.0–5.6) |
| 70 | 1.2 (0.2–2.3) | 32.0 (27.2–36.8) | 0.2 (0.0–0.7) | 2.7 (0.0–6.4) |
| 60 | 1.4 (0.2–2.6) | 37.3 (31.7–43.0) | 0.3 (0.0–0.8) | 3.2 (0.0–7.5) |
| 50 | 1.7 (0.2–3.2) | 44.7 (38.0–51.5)† | 0.3 (0.0–1.0) | 3.8 (0.0–9.0) |
| 40 | 2.1 (0.3–4.0) | 55.9 (47.5–64.3)† | 0.4 (0.0–1.3) | 4.8 (0.0–11.3)† |
| 30 | 2.8 (0.4–5.3)† | 74.6 (63.4–85.8) | 0.6 (0.0–1.7)† | 6.3 (0.0–15.1)† |
| 20 | 4.2 (0.5–7.9)† | – | 0.8 (0.0–2.5)† | 9.5 (0.0–22.6) |
| 10 | 8.5 (1.1–15.9) | – | 1.7 (0.0–5.0) | 19.0 (0.0–45.2) |
CI = confidence interval; – = > 100% prevalence calculated.
Biologically plausible estimates of the true prevalence of respective Taenia species.
Dog study.
Fecal samples were collected from 105 dogs from 21 villages; 32 (30.5%), 30 (28.6%), 11 (10.5%), and 32 (30.5%) from Oudomxay, Luangprabang, Huaphan and Xiengkhuang Provinces, respectively (Table 1). All dogs were raised in an unrestrained manner, the median age was 12 months (range = 2–108 months), and 63 (60%) were female and 42 (40%) were male. Two dogs (1.9%; 95% CI = 0.0–4.6) were Taenia egg positive; one a 12-month-old male dog from village 5 in Xiengkhuang Province and the other a 3-year-old male dog from village 15 in Luangprabang Province. Estimates of true prevalence in the plausible range of diagnostic sensitivity ranged from 4.8% to 6.3% (Table 6).
Discussion
This study investigated T. solium in the context of multiple Taenia species interacting in a host assemblage that includes humans, pigs, dogs, and bovines. We have documented the sympatric occurrence of four Taenia species in northern Laos where conditions suit T. solium transmission and hyperendmicity. We observed a substantial proportion of the population practicing open defecation, uncooked pork consumption was relatively common, and pig production systems were rudimentary. However, we observed a low prevalence of human cysticercosis in the survey population. The following discussion draws on the human and animal studies to hypothesize that T. hydatigena hyperendemicity in pigs and a high prevalence of persons in Laos eating uncooked beef were the strongest factors controlling Taenia ecology in this country.
This study had a number of important limitations and most notable was the relatively small sample size. There was a lack of statistical power to detect significant risk factors associated with cysticercosis even though most cases occurred in three Mon-Khmer villages in Oudomxay Province. Second, we sought to recruit all eligible household members ≥ 6 years of age and compliance varied for the different ethnic groups. Most low compliance in the Hmong-Mien ethnic family was evident in Huaphan Province and could be explained by an aversion to venipuncture and embarrassment in giving a fecal sample, both closely linked to cultural beliefs and customs. This limitation could be corrected in future studies by using a fingerprick and blood spot sampling method, the use of trained Hmong-Mien women to administer the surveys, and strengthening the consultation process. This study limitation possibly led to an underestimation of cysticercosis prevalence in this ethnic group because poor household members were most likely to have refused participation and the three cysticercosis cases were identified in the three poorest quintiles. However, taeniasis was more common in the wealthier quintiles of the Hmong-Mien ethnic group, and our data may have represented an overestimate. Families also tended to exclude household members who were mentally ill or frail, which might have had a limited effect on the estimate of taeniasis and cysticercosis prevalence within the survey population. Because the number of refusals due to frailty or mental illness was small, this effect was assumed to be negligible.
We required an accurate estimate of current human cysticercosis infection and used a circulating antigen ELISA rather than an antibody ELISA, which tends to overestimate prevalence in disease-endemic areas.21,32,33 One preliminary study in Vietnam using computed tomography and cutaneous nodule biopsy as a gold standard found a high sensitivity (94.4%) and specificity (100%) of detecting active human cysticercosis with the antigen ELISA,34 indicating the results in our study were reliable. Human cysticercosis was relatively rare in northern Laos at the community level (2.2%), but there was strong evidence of a focal distribution because just over half of the seropositive cases came from three villages in Oudomxay Province. In Asia, a focal distribution of human cysticercosis has also been observed in northern Vietnam,21 Indonesian Papua,35 and China,36 our results provide further evidence that cases tend to cluster in geographically restricted localities. The relatively high prevalence of cysticercosis in young children was unexpected and corresponded with a relatively high prevalence of taeniasis in the same age group, although the seven-year old boy with T. solium taeniasis was cysticercosis antigen ELISA negative. The specific exposures leading to increased prevalence in this age group warrants further investigation.
We observed a high taeniasis prevalence (8.4%) with spatial variation on the basis of self-reporting and detection of Taenia eggs in a single fecal sample, and these results were comparable with those of other studies in southern and central Laos.6,8–17,19 Somers and others21 estimated that self-reporting in Vietnam grossly overestimates true prevalence, but in our study almost half (17 of 35) of treated persons who expelled proglottids were initially detected by self-reporting alone. This finding was consistent with results of Flisser and others,37 who reported improved detection of tapeworm carriers by using self-reporting methods. Somers and others21 also found Taenia egg prevalence almost identical to copro-antigen prevalence, which was inconsistent with studies conducted elsewhere.38,39 Our results show conclusively that Taenia egg detection was a gross underestimate of true prevalence, and we obtained 18 additional tapeworm specimens after treating egg-negative persons who self-reported infection. Unfortunately, no scoleces were recovered after treatment, and proglottids were not recovered from more than half (20 of 38) of the egg-positive cases. These results are comparable with other studies using niclosamide and magnesium sulfate40 but poor in comparison to the use of pre- and post-niclosamide purging with electrolyte-polyethyleneglycol salt.41 The later purging salt was not suitable for use in the field in Laos. Thirty-three (94.3%) of the tapeworm specimens were identified by PCR as T. saginata, and because the efficacy of niclosamide in treating T. solium and T. saginata are assumed to be similar,42 the data indicates that T. saginata was the dominant species infecting persons in Laos.
The strongest risk factor for having a current taeniasis infection was a history of taeniasis. Ninety percent of persons with taeniasis reported a history of taeniasis, although this finding could refer to a single infection with sporadic shedding of segments. Despite this potential bias, results indicated a high prevalence of taeniasis re-infection, which was not surprising considering the high prevalence of eating uncooked meat, particularly beef. Infrequent consumption of beef, which is typically prepared as laap (a traditional meat salad prepared with lime juice, mint, chili, and pounded rice) was strongly associated with taeniasis in both multivariate models and indicates a possible link with raw meat consumption at festivals. More detailed investigations will be required to understand specific cultural practices and food preparation. We did not undertake studies of cysticercosis in cattle and buffalo in this study. However, a recent slaughterhouse-based study in five northern provinces detected cysticercosis antigen in 52% of cattle and 21% of buffalo.43 The evidence therefore suggests that taeniasis re-infection was predominantly caused by eating uncooked beef and infection with T. saginata. The two T. solium cases were not associated with knowingly eating uncooked pork and may have arisen simply from inadvertent undercooking.
The animal studies provide important insights into the ecology of Taenia in Laos and mainland Southeast Asia in general. Evidence suggests that T. hydatigena was the dominant species infecting pigs in Laos, and a strong infection pressure was exerted by a relatively large, unmanaged dog population (Canis lupus familiaris). No other definitive hosts for T. hydatigena are found in Laos. Under a strong infection pressure, immunity in the intermediate host can be acquired within two weeks of exposure to eggs and embryos entering muscles after one week will not survive,44,45 indicating that there is a short window of opportunity for infection throughout the life of the intermediate host. Immune-mediated Taenia competition in the intermediate host has been well documented for the ovine tapeworms T. ovis and T. hydatigena, in which T. ovis cyst development can be inhibited by pre-exposure to T. hydatigena eggs.45 There are no plausible reasons why these same immune-mediated competitive interactions do not occur in pigs, indicating that T. solium cyst development would be inhibited by ingestion of T. hydatigena eggs. The evidence presented here indicates that pigs may be exposed to T. hydatigena eggs at a young age through coprophagia, feed, water, and/or soil contamination and provide pigs with protective immunity because of genus-conserved immunogenic antigens. However, controlled studies will be required to elucidate the immune-mediated interactions in pigs, if they exist, and determine the impact on transmission dynamics.
The pig and dog studies were limited by the diagnostic protocols used. We used egg detection for T. hydatigena taeniasis in dogs instead of copro-antigen ELISA because we were unable to source suitable diagnostic reagents during the course of this study. No published data provide a reliable estimate of the diagnostic sensitivity and specificity of T. hydatigena egg detection in dog feces. Because proglottids are predominantly shed without defecation,46 we believe that sensitivity would be much lower than the estimated 62.5% observed for T. solium taeniasis.38,47 Our prevalence estimates of T. hydatigena were in the order of 5–6% of village dogs and this corresponded to prevalence in pigs of 50–60%.
In the pig study, the antigen ELISA was not able to differentiate the three Taenia species,33,48 and inspection data could only account for one-third of the serologically positive animals. The sensitivity of detecting T. solium cysts at slaughter can be variable and estimates range from 20% to 60%,26,49,50 but specificity has been estimated to be 100%.26 Bayesian approaches have been applied to model true prevalence of T. solium cysticercosis in the face of imperfect tests.26 However, in this present study, T. hydatigena and T. asiatica co-endemicity excluded the antigen ELISA results and the Bayesian model could not be applied. Instead of a Bayesian approach, we used a standard maximum-likelihood estimator to calculate true prevalence, which was only valid if specificity and sensitivity were known.30 We made the assumption that inspection was 100% specific and calculated true prevalence through a range of sensitivities of detecting cysts at slaughter, meaning prevalence was estimated from one degree of freedom in the observed data at each increment of sensitivity. Because inspection was constrained by traders restricting muscle slicing, we assumed the sensitivity of detecting T. solium cysts was at the lower end of the range. No data were available that enabled us to estimate the sensitivity of detecting T. hydatigena in pigs. Because T. hydatigena metacestodes mature in the fat of the mesentery and omentum and the size can vary from 1 to 7 cm,50 these cysts could be easily missed, particularly in indigenous breed pigs in Laos that typically have a high fat content.51 The adjusted inspection prevalence data for T. solium, T. hydatigena, and T. asiatica accounted for most serologically positive animals.
It is evident that future Taenia research in Southeast Asia will require the use of more robust diagnostic protocols for the animal and human studies. The gold standard for cysticercosis in pigs is dissection. However, dissection, which involves taking 0.5-cm slices through half a carcass,52 is expensive and time-consuming, and not conducive to large a geographically diverse studies. There is a genuine need for a robust, cheap, sensitive, specific, validated, and readily available test that can differentiate Taenia species in pigs.1,49 Similarly, improved tests for sensitive and specific detection of human T. solium taeniasis cases are required, and these tests are currently being developed and validated,53 but validation in multiple populations around the world should be a priority.
We have documented the occurrence of four Taenia species in Laos. The study indicated a low prevalence of T. solium cysticercosis and taeniasis in the human population and a focal distribution in northern Laos. The evidence also suggests that natural parasite competition and local food customs have an influence on T. solium transmission, presenting real opportunities for control and possible elimination. With a concerted effort to identify, treat, and follow-to-cure T. solium tapeworm carriers, thereby reducing the infection pressure on pigs, continued exposure of pigs to T. hydatigena eggs may assist in further reducing T. solium transmission. With time, and continued improvements to sanitation and pig husbandry in Laos, we might expect significant reductions in human cysticercosis prevalence.
ACKNOWLEDGMENTS
We thank the study participants in northern Laos for providing valuable time; the national, provincial, and district staff of the Ministry of Agriculture and Forestry and the Ministry of Health for their support and valued contributions to this study, Lapinh Phithacthep, Vilaywan Soukvilay, Vilayphet Viravong (National Animal Health Centre); and Virasack Som, Thongchan Sisouk, and Khouanta Douangmala (National Centre for Laboratory and Epidemiology) for providing logistic, technical, and laboratory support. We also wish to thank Dr Sue Lee from the Mahidol-Oxford Tropical Medicine Research Unit for her valuable contribution to data analysis.
Footnotes
Financial support: This study was supported by the Australian Centre for International Agricultural Research (project no. AH2006/161). James V. Conlan was supported by a Murdoch University Research Studentship award.
Authors' addresses: James V. Conlan, Aileen Elliot, Stanley Fenwick, and R. C. Andrew Thompson, School of Veterinary and Biomedical Sciences, Murdoch University, Murdoch 6150, Western Australia, Australia, E-mails: james.conlan@foodstandards.gov.au, a.elliot@murdoch.edu.au, stanley_fenwick@dai.com, and a.thompson@murdoch.edu.au. Khamphouth Vongxay, Department of Livestock and Fisheries, Ministry of Agriculture and Forestry, Vientiane, Laos, E-mail: kamputvongxay@yahoo.com. Boualam Khamlome, Department of Hygiene and Prevention, Ministry of Health, Vientiane, Laos, E-mail: drboualam2004@yahoo.com.au. Pierre Dorny, Institute of Tropical Medicine, Department of Animal Health, Nationalestraat 155, 2000 Antwerp, Belgium, E-mail: pdorny@itg.be. Banchob Sripa, Tropical Disease Research Laboratory, Division of Experimental Pathology, Department of Pathology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand, E-mail: banchob@kku.ac.th. Stuart D. Blacksell, Mahidol-Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Ratchathewi District, Bangkok, Thailand, E-mail: stuart@tropmedres.ac.
References
- 1.Conlan JV, Vongxay K, Fenwick S, Blacksell SD, Thompson RC. Does interspecific competition have a moderating effect on Taenia solium transmission dynamics in Southeast Asia? Trends Parasitol. 2009;25:398–403. doi: 10.1016/j.pt.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Willingham AL III, De NV, Doanh NQ, le Cong D, Dung TV, Dorny P, Cam PD, Dalsgaard A. Current status of cysticercosis in Vietnam. Southeast Asian J Trop Med Public Health. 2003;34((Suppl 1)):35–50. [PubMed] [Google Scholar]
- 3.Anantaphruti MT, Yamasaki H, Nakao M, Waikagul J, Watthanakulpanich D, Nuamtanong S, Maipanich W, Pubampen S, Sanguankiat S, Muennoo C, Nakaya K, Sato MO, Sako Y, Okamoto M, Ito A. Sympatric occurrence of Taenia solium, T. saginata, and T. asiatica, Thailand. Emerg Infect Dis. 2007;13:1413–1416. doi: 10.3201/eid1309.061148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somers R, Dorny P, Geysen D, Nguyen LA, Thach DC, Vercruysse J, Nguyen VK. Human tapeworms in North Vietnam. Trans R Soc Trop Med Hyg. 2007;101:275–277. doi: 10.1016/j.trstmh.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Willingham AL III, Wu HW, Conlan J, Satrija F. Combating Taenia solium cysticercosis in Southeast Asia: an opportunity for improving human health and livestock production. Adv Parasitol. 2010;72C:235–266. doi: 10.1016/S0065-308X(10)72009-1. [DOI] [PubMed] [Google Scholar]
- 6.Tran DS, Odermatt P, Le Oanh T, Huc P, Phoumindr N, Ito A, Druet-Cabanac M, Preux PM, Strobel M. Risk factors for epilepsy in rural Lao PDR: a case-control study. Southeast Asian J Trop Med Public Health. 2007;38:537–542. [PubMed] [Google Scholar]
- 7.Eom KS, Jeon HK, Rim HJ. Geographical distribution of Taenia asiatica and related species. Korean J Parasitol. 2009;47((Suppl)):S115–S124. doi: 10.3347/kjp.2009.47.S.S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conlan J, Khounsy S, Inthavong P, Fenwick S, Blacksell S, Thompson RC. A review of taeniasis and cysticercosis in the Lao People's Democratic Republic. Parasitol Int. 2008;57:252–255. doi: 10.1016/j.parint.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Rim HJ, Chai JY, Min DY, Cho SY, Eom KS, Hong SJ, Sohn WM, Yong TS, Deodato G, Standgaard H, Phommasack B, Yun CH, Hoang EH. Prevalence of intestinal parasite infections on a national scale among primary schoolchildren in Laos. Parasitol Res. 2003;91:267–272. doi: 10.1007/s00436-003-0963-x. [DOI] [PubMed] [Google Scholar]
- 10.Sayasone S, Odermatt P, Phoumindr N, Vongsaravane X, Sensombath V, Phetsouvanh R, Choulamany X, Strobel M. Epidemiology of Opisthorchis viverrini in a rural district of southern Lao PDR. Trans R Soc Trop Med Hyg. 2007;101:40–47. doi: 10.1016/j.trstmh.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Giboda M, Viengsay M, Bouaphan S, Ditrich O. Épidémiologie des parasitoses intestinales au Laos. Bull Soc Pathol Exot. 1991;84:184–193. [PubMed] [Google Scholar]
- 12.Giboda M, Ditrich O, Scholz T, Viengsay T, Bouaphanh S. Current status of food-borne parasitic zoonoses in Laos. Southeast Asian J Trop Med Public Health. 1991;22((Suppl)):56–61. [PubMed] [Google Scholar]
- 13.Chai JY, Hongvanthong B. A small-scale survey of intestinal helminthic infections among the residents near Pakse, Laos. Korean J Parasitol. 1998;36:55–58. doi: 10.3347/kjp.1998.36.1.55. [DOI] [PubMed] [Google Scholar]
- 14.Sithithaworn P, Sukavat K, Vannachone B, Sophonphong K, Ben-Embarek P, Petney T, Andrews R. Epidemiology of food-borne trematodes and other parasite infections in a fishing community on the Nam Ngum Reservoir, Lao PDR. Southeast Asian J Trop Med Public Health. 2006;37:1083–1090. [PubMed] [Google Scholar]
- 15.Pholsena K, Sayaseng B, Hongvanthong B, Vanisaveth V. The prevalence of helminth infection in Ban Nanin, Laos. Southeast Asian J Trop Med Public Health. 1991;22:137–138. [PubMed] [Google Scholar]
- 16.Vannachone B, Kobayashi J, Nambanya S, Manivong K, Inthakone S, Sato Y. An epidemiological survey on intestinal parasite infection in Khammouane Province, Lao PDR, with special reference to Strongyloides infection. Southeast Asian J Trop Med Public Health. 1998;29:717–722. [PubMed] [Google Scholar]
- 17.Erlanger TE, Sayasone S, Krieger GR, Kaul S, Sananikhom P, Tanner M, Odermatt P, Utzinger J. Baseline health situation of communities affected by the Nam Theun 2 hydroelectric project in central Lao PDR and indicators for monitoring. Int J Environ Health Res. 2008;18:223–242. doi: 10.1080/09603120701757815. [DOI] [PubMed] [Google Scholar]
- 18.Chai JY, Han ET, Shin EH, Sohn WM, Yong TS, Eom KS, Min DY, Um JY, Park MS, Hoang EH, Phommasack B, Insisiengmay B, Lee SH, Rim HJ. High prevalence of Haplorchis taichui, Phaneropsolus molenkampi, and other helminth infections among people in Khammouane Province, Lao PDR. Korean J Parasitol. 2009;47:243–247. doi: 10.3347/kjp.2009.47.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayasone S, Vonghajack Y, Vanmany M, Rasphone O, Tesana S, Utzinger J, Akkhavong K, Odermatt P. Diversity of human intestinal helminthiasis in Lao PDR. Trans R Soc Trop Med Hyg. 2009;103:247–254. doi: 10.1016/j.trstmh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Anonymous . Population and Housing Census 2005. Vientiane, Laos: Steering Committee for Census of Population and Housing, Department of Statistics, Ministry of Planning and Investment; 2006. [Google Scholar]
- 21.Somers R, Dorny P, Nguyen VK, Dang TC, Goddeeris B, Craig PS, Vercruysse J. Taenia solium taeniasis and cysticercosis in three communities in North Vietnam. Trop Med Int Health. 2006;11:65–72. doi: 10.1111/j.1365-3156.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- 22.Pawlowski Z. Taenia solium: basic biology and transmission. In: Singh G, Prabhakar S, editors. Taenia solium Cysticercosis: from Basic to Clinical Science. Wallingford, United Kingdom: CABI Publishing Co.; 2002. pp. 1–23. [Google Scholar]
- 23.Brandt JR, Geerts S, De Deken R, Kumar V, Ceulemans F, Brijs L, Falla N. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol. 1992;22:471–477. doi: 10.1016/0020-7519(92)90148-e. [DOI] [PubMed] [Google Scholar]
- 24.Dorny P, Vercammen F, Brandt J, Vansteenkiste W, Berkvens D, Geerts S. Sero-epidemiological study of Taenia saginata cysticercosis in Belgian cattle. Vet Parasitol. 2000;88:43–49. doi: 10.1016/s0304-4017(99)00196-x. [DOI] [PubMed] [Google Scholar]
- 25.Dorny P, Phiri I, Gabriel S, Speybroeck N, Vercruysse J. A sero-epidemiological study of bovine cysticercosis in Zambia. Vet Parasitol. 2002;104:211–215. doi: 10.1016/s0304-4017(01)00634-3. [DOI] [PubMed] [Google Scholar]
- 26.Dorny P, Phiri IK, Vercruysse J, Gabriel S, Willingham AL, 3rd, Brandt J, Victor B, Speybroeck N, Berkvens D. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int J Parasitol. 2004;34:569–576. doi: 10.1016/j.ijpara.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki H, Allan JC, Sato MO, Nakao M, Sako Y, Nakaya K, Qiu D, Mamuti W, Craig PS, Ito A. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol. 2004;42:548–553. doi: 10.1128/JCM.42.2.548-553.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinmann P, Zhou XN, Li YL, Li HJ, Chen SR, Yang Z, Fan W, Jia TW, Li LH, Vounatsou P, Utzinger J. Helminth infections and risk factor analysis among residents in Eryuan county, Yunnan province, China. Acta Trop. 2007;104:38–51. doi: 10.1016/j.actatropica.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Raso G, Utzinger J, Silué KD, Ouattara M, Yapi A, Toty A, Matthys B, Vounatsou P, Tanner M, N'Goran EK. Disparities in parasitic infections, perceived ill health and access to health care among poorer and less poor schoolchildren of rural Côte d'Ivoire. Trop Med Int Health. 2005;10:42–57. doi: 10.1111/j.1365-3156.2004.01352.x. [DOI] [PubMed] [Google Scholar]
- 30.Rahme E. Estimating the prevalence of a rare disease: adjusted maximum likelihood. Statistician. 1998;47:149–158. [Google Scholar]
- 31.McManus DP. Echinococcosis with particular reference to Southeast Asia. Adv Parasitol. 2010;72:267–303. doi: 10.1016/S0065-308X(10)72010-8. [DOI] [PubMed] [Google Scholar]
- 32.Garcia HH, Gonzalez AE, Gilman RH, Palacios LG, Jimenez I, Rodriguez S, Verastegui M, Wilkins P, Tsang VC. Short report: transient antibody response in Taenia solium infection in field conditions—a major contributor to high seroprevalence. Am J Trop Med Hyg. 2001;65:31–32. doi: 10.4269/ajtmh.2001.65.31. [DOI] [PubMed] [Google Scholar]
- 33.Dorny P, Brandt J, Geerts S. Immunodiagnostic approaches for detecting Taenia solium. Trends Parasitol. 2004;20:259–260. doi: 10.1016/j.pt.2004.04.001. author reply 260–261. [DOI] [PubMed] [Google Scholar]
- 34.Erhart A, Dorny P, Van De N, Vien HV, Thach DC, Toan ND, le Cong D, Geerts S, Speybroeck N, Berkvens D, Brandt J. Taenia solium cysticercosis in a village in northern Vietnam: seroprevalence study using an ELISA for detecting circulating antigen. Trans R Soc Trop Med Hyg. 2002;96:270–272. doi: 10.1016/s0035-9203(02)90095-7. [DOI] [PubMed] [Google Scholar]
- 35.Salim L, Ang A, Handali S, Tsang VC. Seroepidemiologic survey of cysticercosis-taeniasis in four central highland districts of Papua, Indonesia. Am J Trop Med Hyg. 2009;80:384–388. [PubMed] [Google Scholar]
- 36.Chen YD, Xu LQ, Zhou XN. Distribution and disease burden of cysticercosis in China. Southeast Asian J Trop Med Public Health. 2004;35:231–239. [Google Scholar]
- 37.Flisser A, Vazquez-Mendoza A, Martinez-Ocana J, Gomez-Colin E, Leyva RS, Medina-Santillan R. Short report: evaluation of a self-detection tool for tapeworm carriers for use in public health. Am J Trop Med Hyg. 2005;72:510–512. [PubMed] [Google Scholar]
- 38.Allan JC, Velasquez-Tohom M, Torres-Alvarez R, Yurrita P, Garcia-Noval J. Field trial of the coproantigen-based diagnosis of Taenia solium taeniasis by enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1996;54:352–356. doi: 10.4269/ajtmh.1996.54.352. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Noval J, Allan JC, Fletes C, Moreno E, DeMata F, Torres-Alvarez R, Soto de Alfaro H, Yurrita P, Higueros-Morales H, Mencos F, Craig PS. Epidemiology of Taenia solium taeniasis and cysticercosis in two rural Guatemalan communities. Am J Trop Med Hyg. 1996;55:282–289. doi: 10.4269/ajtmh.1996.55.282. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez AL, Gomez O, Allebeck P, Cosenza H, Ljungstrom L. Epidemiological study of Taenia solium infections in a rural village in Honduras. Ann Trop Med Parasitol. 1997;91:163–171. doi: 10.1080/00034983.1997.11813126. [DOI] [PubMed] [Google Scholar]
- 41.Jeri C, Gilman RH, Lescano AG, Mayta H, Ramirez ME, Gonzalez AE, Nazerali R, Garcia HH. Species identification after treatment for human taeniasis. Lancet. 2004;363:949–950. doi: 10.1016/S0140-6736(04)15791-7. [DOI] [PubMed] [Google Scholar]
- 42.Flisser A. Where are the tapeworms? Parasitol Int. 2006;55((Suppl)):S117–S120. doi: 10.1016/j.parint.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Vongxay K, Conlan JV, Khuonsy S, Dorny P, Fenwick S, Thompson RCA, Blacksell SD. Seroprevalence of major bovine-associated zoonotic infectious diseases in the Lao People's Democratic Republic. Vector Borne Zoonotic Dis. 2012 doi: 10.1089/vbz.2011.0850. doi:10.1089/vbz.2011.0850. [DOI] [PubMed] [Google Scholar]
- 44.Gemmell MA. A critical approach to the concepts of control and eradication of echinococcosis/hydatidosis and taeniasis/cysticercosis. Int J Parasitol. 1987;17:465–472. doi: 10.1016/0020-7519(87)90122-6. [DOI] [PubMed] [Google Scholar]
- 45.Gemmell MA, Lawson JR, Roberts MG. Population dynamics in echinococcosis and cysticercosis: evaluation of the biological parameters of Taenia hydatigena and T. ovis and comparison with those of Echinococcus granulosus. Parasitology. 1987;94:161–180. doi: 10.1017/s0031182000053543. [DOI] [PubMed] [Google Scholar]
- 46.Deplazes P, Gottstein B, Stingelin Y, Eckert J. Detection of Taenia hydatigena copro-antigens by ELISA in dogs. Vet Parasitol. 1990;36:91–103. doi: 10.1016/0304-4017(90)90097-u. [DOI] [PubMed] [Google Scholar]
- 47.Allan JC, Wilkins PP, Tsang VC, Craig PS. Immunodiagnostic tools for taeniasis. Acta Trop. 2003;87:87–93. doi: 10.1016/s0001-706x(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 48.Deckers N, Dorny P. Immunodiagnosis of Taenia solium taeniosis/cysticercosis. Trends Parasitol. 2010;26:137–144. doi: 10.1016/j.pt.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Sciutto E, Martinez JJ, Villalobos NM, Hernandez M, Jose MV, Beltran C, Rodarte F, Flores I, Bobadilla JR, Fragoso G, Parkhouse ME, Harrison LJ, de Aluja AS. Limitations of current diagnostic procedures for the diagnosis of Taenia solium cysticercosis in rural pigs. Vet Parasitol. 1998;79:299–313. doi: 10.1016/s0304-4017(98)00180-0. [DOI] [PubMed] [Google Scholar]
- 50.OIE Cysticercosis. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2010. http://www.oie.int/eng/normes/mmanual/2008/pdf/2.09.05_CYSTICERCOSIS.pdf Office International des Epizooties. Available at.
- 51.Phengsavanh P, Ogle B, Stur W, Frankow-Lindberg BE, Lindberg JE. Feeding and performance of pigs in smallholder production systems in northern Lao PDR. Trop Anim Health Prod. 2010;42:1627–1623. doi: 10.1007/s11250-010-9612-4. [DOI] [PubMed] [Google Scholar]
- 52.Boa ME, Kassuku AA, Willingham AL, III, Keyyu JD, Phiri IK, Nansen P. Distribution and density of cysticerci of Taenia solium by muscle groups and organs in naturally infected local finished pigs in Tanzania. Vet Parasitol. 2002;106:155–164. doi: 10.1016/s0304-4017(02)00037-7. [DOI] [PubMed] [Google Scholar]
- 53.Handali S, Klarman M, Gaspard AN, Dong XF, Laborde R, Noh J, Lee YM, Rodriguez S, Gonzalez AE, Garcia HH, Gilman RH, Tsang VC, Wilkins PP. Development and evaluation of a magnetic immunochromatographic test to detect Taenia solium, which causes taeniasis and neurocysticercosis in humans. Clin Vaccine Immunol. 2010;17:631–637. doi: 10.1128/CVI.00511-09. [DOI] [PMC free article] [PubMed] [Google Scholar]