Abstract
Taenia solium cysticercosis infects pigs and humans. Because antiparasitic treatment for human cysticercosis has sub-optimal efficacy, alternative regimes are needed. Seven antiparasitic regimens were tested in 42 naturally infected pigs with cysticercosis, and compared with prednisone alone (n = 6) or no treatment (n = 6). The numbers of viable cysts in muscles and in the brain were examined after necropsy and were significantly decreased in pigs receiving combined albendazole plus praziquantel, albendazole alone, or oxfendazole. Pigs receiving praziquantel alone and nitazoxanide had numerous surviving cysts. Control (untreated) pigs and prednisone-treated pigs had many more viable cysts, suggesting no effect. Combined albendazole plus praziquantel, and oxfendazole, showed a strong cysticidal effect and provide suitable alternative treatments to be further explored for their use for treatment of human neurocysticercosis.
Introduction
Neurocysticercosis, infection of the central nervous system by the larval stage of Taenia solium, is a growing global public health problem.1 This disease is the main cause of secondary epilepsy in developing countries and an emerging disease in developed countries because of migration from disease-endemic zones.2,3
The life cycle of this zoonotic disease involves humans and pigs. Humans host the adult form or intestinal tapeworm, and are the only natural definitive hosts. The tapeworm is composed by a head or scolex plus immature, mature, and gravid proglotids, each containing up to 50,000 infective eggs, which are intermittently released into the environment with feces. When eggs are ingested by a pig, or accidentally by a human, the embryos are liberated, cross the intestinal wall, and are carried by the circulatory system to almost any organ or tissue, where they develop as the larval stage or cysticerci. The cycle is completed when humans ingest cysticerci in raw or poorly cooked infected pork and develop a tapeworm.4
Neurocysticercosis is a pleomorfic disease. Cysts can lodge in the brain parenchyma, ventricles, and/or subarachnoid space. The therapeutic approaches vary depending on each case and usually involves symptomatic medication (analgesics, antiepileptics), anti-inflammatory drugs, antiparasitic drugs and/or surgery.5,6 The antiparasitic drugs praziquantel (PZQ) and albendazole (ABZ) have been used in humans since 1979, and their cysticidal efficacy has been largely demonstrated in humans and pigs.7–10 After a long controversy, it is currently accepted that antiparasitic drugs eliminate viable brain parasites, and this elimination is associated with decreased seizure frequency, although cysticidal treatment may be contraindicated in some patients.7,11
In humans, ABZ is considered the drug of choice because of availability and costs and slightly higher efficacy.12 However, ABZ only kills approximately 70% of cysts and clears all living parasites in only 30% to 40% of treated patients after a course of treatment.7,13,14 On the basis of previous successful studies of pig treatment with oxfendazole (OFZ), this controlled study was developed to explore the efficacy of combined therapy (ABZ plus PZQ) versus ABZ or PZQ alone. Nitazoxanide (NZX), a broad-spectrum drug with activity against a wide variety of intestinal parasites in animals and humans15,16 was also evaluated. In addition, the performance of prednisone (PDN) was evaluated because of a possible role in altering the immune equilibrium, which could result in the death of the parasites.17
Materials And Methods
Study design and settings.
An open, randomized, controlled trial in naturally cysticercosis-infected pigs was used to compare the cysticidal efficacy of different antiparasitic treatment schemes. All procedures were performed at the San Marcos School of Veterinary Medicine. The study was approved by the Animal Ethics Committee of the School of Veterinary Medicine, Universidad Nacional de San Marcos, Lima, Peru.
Animals.
Fifty-four naturally infected pigs were bought from informal slaughterhouses (with no control from local authorities) in Huancayo and Huaraz, two highly endemic cities in the Peruvian highlands, and transported to veterinary facilities in Lima. Pig infection was grossly established by positive tongue examination (palpation of lingual nodules, implying the presence of active cysticercosis infection),18 and confirmed by serologic analysis (serum enzyme-linked immuno-electro transfer blot).19 All pigs were vaccinated against hog cholera, coded, and ear-tagged. Each treatment group was housed in a single pen.
Treatment regimens.
The pigs were randomly allocated to nine different groups of six pigs each. Each group of pigs received a individual scheme of treatment including ABZ, PZQ, combined ABZ plus PZQ, NZX, and OFZ, All groups treated with ABZ received PDN as concomitant anti-inflammatory therapy, as shown in Table 1.
Table 1.
Antiparasitic drug schemes tested in groups of six naturally infected cysticercotic pigs
| Group | Albendazole | Praziquantel | Oxfendazole | Nitazoxanide | Prednisone |
|---|---|---|---|---|---|
| 1 | 15 mg/kg/day, 7 days | None | None | None | 0.5 mg/kg/day, 5 days |
| 2 | None | 75 mg/kg/day, 1 day | None | None | None |
| 3 | 15 mg/kg/day, 7 days | 75 mg/kg, 1 day | None | None | 0.5 mg/kg/day, 5 days |
| 4 | None | None | 30 mg/kg, days 1 and 5 | None | None |
| 5 | None | None | None | 150 mg/kg/day,* 7 days | None |
| 6 | None | None | None | None | 0.5 mg/kg/day, 5 days |
| 7 | None | None | None | None | None |
| 8 | 7.5 mg/kg/day, 7 days | 75 mg/kg, 1 day | None | None | 0.5 mg/kg/day, 5 days |
| 9 | 15 mg/kg/day, 7 days | 75 mg/kg, 1 day | None | None | 1 mg/kg/day, 5 days |
300 mg/kg on day 1, then 150 mg/kg on days 2–7.
The first three regimens were intended to simulate antiparasitic schemes given to humans and included a combination group; the fourth group received OFZ as a positive treated control group because of its proven cysticidal efficacy in cysticercosis infected pigs,20,21 given twice (days 1 and 5) to reinforce their antiparasitic effect, and the fifth group tested the cysticidal efficacy of NZX. The following two groups were non-antiparasitic treated control groups. Group 6 received PDN to rule out a hypothetical effect of steroids in altering the immune equilibrium and thus triggering parasite destruction, and group 7 did not receive any drugs. We added two more groups after the deaths of three pigs during the first week of treatment (see Results). Assuming that the cause of death was encephalitis caused by local inflammation, we implemented group 8 (½ ABZ + PZQ + PDN) by reducing the dose of ABZ by half, and group 9, duplicating the steroid dose. (ABZ + PZQ + 2 PDN). The group receiving PZQ alone did not receive concomitant steroids to avoid the consequent decrease in serum levels of PZQ and a resulting decrease in efficacy. Not using steroids could have biased our results towards increased efficacy of PZQ alone compared with the combination groups (also receiving concomitant steroids), but results showed the opposite, a lower efficacy of the PZQ alone group.
All drugs were administered orally, mixed with food, and given at a scheduled time. ABZ and NZX were administered in two doses (8:00 am and 8:00 pm); PZQ was administered in three doses of 25 mg/kg every two hours starting at 8:00 am on the same day; OFZ and PDN were administered as a single dose in the morning. All drugs were started at the same day in combined schemes.
Necropsy.
All pigs were kept at the School of Veterinary Medicine for ten weeks and then euthanized and subject to detailed necropsy to determine the numbers and stage of cysts. Animals were anesthetized by intramuscular injection using a combination of ketamine (11 mg/kg) and xylazine (2 mg/kg) and then killed by an intravenous injection of phenobarbital (9 mg/kg). Standard necropsy22 was performed immediately after euthanasia. In this procedure, psoas, legs, the tongue, and the heart were dissected, and all cysts were counted and classified by their stage of evolution.
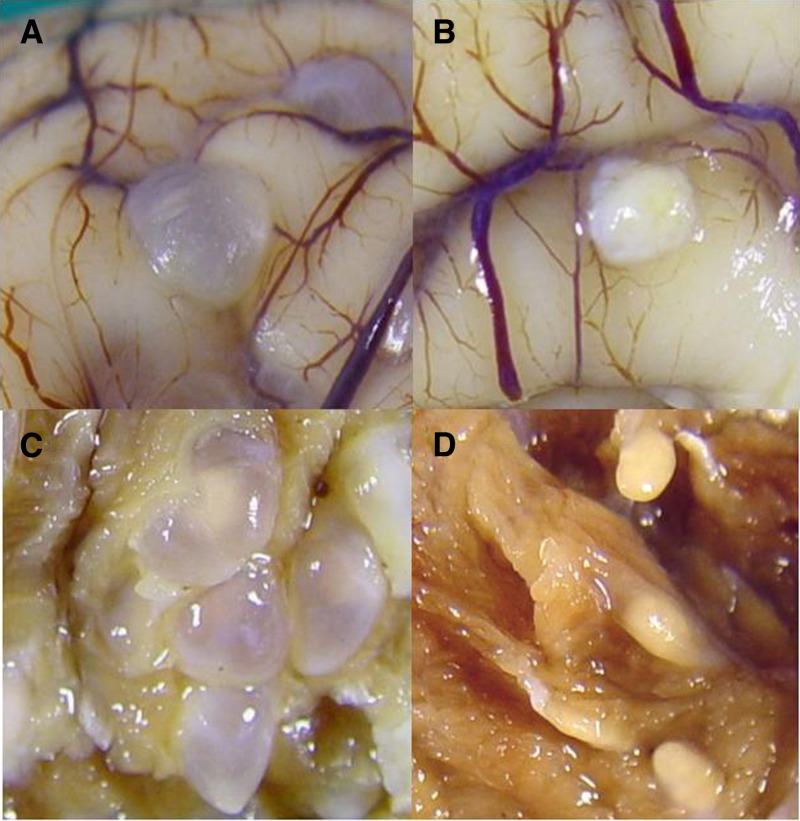
After necropsy (10 weeks after treatment), parasitic lesions were catalogued as viable, well defined, fluid-filled vesicles with clear fluid content, and non-viable lesions with a preserved cystic structure but opaque or gelatinous contents, or compact, well-defined nodular lesions, or smaller, later scar stages (Figure 1). Brains were entirely extracted and fixed in formalin and cut into 5-mm slides two weeks later for lesion counting and histologic evaluation. Pig age was calculated at necropsy by assessing eruption, development, and wearing teeth.23
Figure 1.
Macroscopic aspect of viable (A) and non-viable (B) cysticercotic cysts in pig brain and viable (C) and non-viable (D) cysticercotic cysts in muscle.
Cyst evagination.
Approximately 30 vesicular cysts per pig obtained from muscles (psoas and anconeal) were recovered and tested for viability by evagination procedure as follows.24 Excised cysts obtained from muscles were washed in sterile 0.15 M phosphate-buffered saline, pH 7.2, and incubated in pepsin (1%, pH 2.0, at 37 C) for 30 minutes. Cysts were washed in phosphate-buffered saline and incubated in trypsin (1:1,000, pH 8.9) plus bile salt (Sigma, St. Louis, MO) at 37°C for one hour. The visualization of scolex outside the bladder wall was considered as indicator of viability. Whenever no viable cysts were present or there were only a few, degenerating cysts were used to complete the numbers for evagination.
Histologic analysis.
Viable and non-viable cysts in brain and muscle were embedded in paraffin and stained with hematoxylin and eosin, alizarin red and Von Kossa stains to evaluate their stages of degeneration and the presence of calcium deposits.
Data analysis.
Because of logistic constraints, only six pigs were allocated to each group in evaluating differences by using non-parametric analysis. Absolute values, medians, and ranges for cyst numbers in brain and muscle are reported. Differences in viable cyst populations between each group and the corresponding untreated control group were compared by using the Mann-Whitney test, and the level of significance was defined as P < 0.05. For the groups that showed a significant difference compared with the control, the statistical power was estimated by using the Power Analysis and Sample Size software (NCS, Kaysville, UT) and a one-sided two-sample t-test. Because muscle samples and pigs had different weights, the analysis of numbers of cysts in muscles was repeated by adjusting for the calculated total muscle weight for each pig, assuming that muscles represent approximately 25% of pig weight.23 Pigs that died in the initial week after treatment were not included in the analysis. All statistical analysis was performed with a 95% significance level by using the Stata software (StataCorp LP, College Station, TX).
Results
Adverse events.
Three pigs died during antiparasitic therapy, 2 at day 4 (one receiving ABZ + PDN, and one receiving PDN alone), and one at day 7 (receiving ABZ + PZQ + PDN). Two other pigs died before the end of the trial, one at day 55 (receiving ABZ + PZQ + PDN) and one at day 65 (receiving OFZ). On the basis of this results, the pig that died at day 7 (ABZ + PZQ + PDN group) likely died of encephalitis caused by massive cyst destructions because this pig had approximately 140 brain cysts and marked inflammation at necropsy. The deaths of the other four pigs were related to adverse events, not directly related to the antiparasitic drug itself. Probable causes were acute enteritis (one case, day 4), pneumonia (2 cases, days 4 and 55), and hepatitis (one case, day 65). Male pigs in the NZX group had distended bladders, likely secondary to hypertrophic prostate glands. Estimated pig ages ranged between 8 and 54 months (mean ± SD = 30.1 ± 16.8 months).
Parasiticidal efficacy in muscle cysts.
Initial analysis evaluating cysts per kilogram (not adjusted by pig weight) showed that combined schemes using ABZ plus PZQ, as well as ABZ alone and OFZ, had statistically significant higher efficacy in destroying cysts compared with control pigs. The pigs in the NZX group had more cysts than the control group (medians = 755 versus 495), and PZQ and PDN showed less cysts than the control group (23.2%, medians = 380 versus 495 and 49.5%, medians = 250 versus 495, respectively), but without statistical significance.
Adjusting the analysis to account for the proportion of muscle in whole pig weight (Table 2), confirmed that the three combined schemes of ABZ plus PZQ, ABZ alone, and OFZ showed almost complete efficacy for killing parasites compared with control pigs (P < 0.005, by Mann-Whitney test). Pigs in the NZX group had greater numbers of cysts than control pigs (medians = 9,433 versus 7,078), and pigs in the PDN and the PZQ groups had 23.7% (medians = 5,402 versus 7,078) and 8.0% (medians = 6,515 versus 7,078) less cysts than did the control pigs, although these differences were not statistically significant. The analysis was repeated by adjusting for the total number of cysts per animal and showed similar results.
Table 2.
Cysticidal efficacy of different antiparasitic regimens for porcine cysticercosis*
| Group description | Group | Viable cysts in muscle† | Viable cysts in brain | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median (range) | P‡ | P§ | Efficacy, % | Median (range) | P‡ | P§ | Efficacy, % | ||
| No treatment | 7 | 7,078 (2,072–11,746) | Ref. | Ref. | Reference | 25 (16–53) | Ref. | Ref. | Reference |
| PZQ alone | 2 | 6,515 (2,260–7,985) | 0.873 | 0.09 | 8.0 | 46 (6–82) | 0.631 | 0.07 | 0 |
| Prednisone | 6 | 5,401 (2,121–10,926) | 1.00 | 0.02 | 23.7 | 38 (26–54) | 0.273 | 0.02 | 0 |
| NZX | 5 | 9,433 (7,433–12,731) | 0.423 | 0.01 | 0 | 18 (8–54) | 0.631 | 0.12 | 28 |
| OFZ | 4 | 0 (0–0) | 0.003 | 0.90 | 100 | 10 (1–13) | 0.055 | 0.34 | 60 |
| ABZ + PDN | 1 | 0 (0–0) | 0.004 | 0.90 | 100 | 0 (0–4) | 0.017 | 0.39 | 100 |
| ABZ + PZQ + PDN | 3 | 0 (0–0) | 0.005 | 0.90 | 100 | 0 (0–0) | 0.009 | 0.41 | 100 |
| ½ ABZ + PZQ + PDN | 8 | 0 (0–0) | 0.002 | 0.90 | 100 | 0 (0–1) | 0.007 | 0.34 | 100 |
| ABZ + PZQ + 2 PDN | 9 | 0 (0–0) | 0.002 | 0.90 | 100 | 0 (0–1) | 0.007 | 0.41 | 100 |
Ref. = referent; PZQ = praziquantel; NZX = nitazoxanide; OFZ = oxfendazole; ABZ = albendazole; PDN = prednisone.
Numbers of cysts in muscle are adjusted by weight.
Mann-Whitney test (compared with the reference group).
Power.
Parasiticidal efficacy in brain cysts.
Evaluation of the numbers of viable cysts in brain (Table 2) showed that the cysticidal efficacy of combined ABZ + PZQ schemes was almost 100%, and most pigs had no remnant viable cysts in their brains. Albendazole had 92% efficacy with two of five pigs having small numbers of surviving brain cysts. Oxfendazole showed only 60% efficacy (10 of 25) (P < 0.05, by Mann-Whitney test). Pigs in the NZX group had 28% fewer viable brain cysts than control pigs (18 of 25, not statistically significant), and there were no differences between control pigs and pigs treated with PDN or PZQ alone in terms of cyst numbers.
Confirmation of cyst viability.
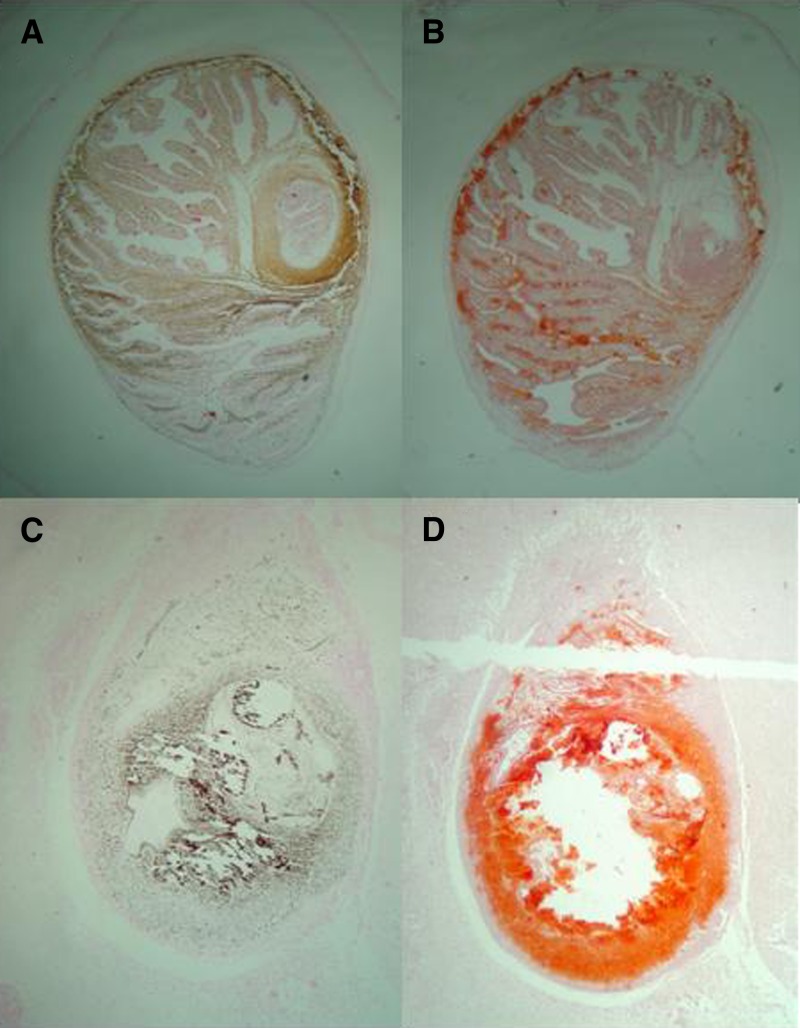
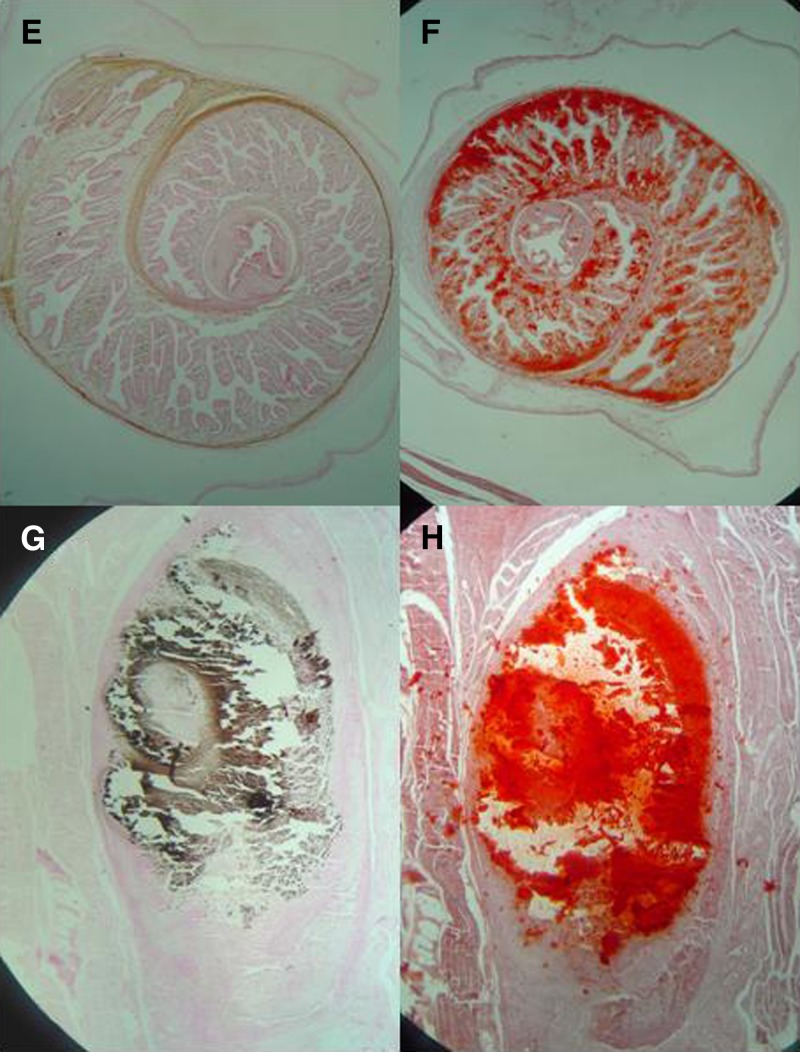
The capacity to evaginate and become an adult tapeworm was evaluated in apparently viable cysts obtained from muscles. The results confirmed the cysticidal effect of ABZ, combined schemes, and OFZ and showed the same poor effect of PDN, NZX, and PZQ alone (Supplemental Table). Histologic evaluation demonstrated the presence of calcium, which was more marked in degenerating, non-viable cysts. The lesions were macroscopically identified during necropsy and preliminary confirmed by staining with hematoxylin and eosin as viable cystic lesions and non-viable cysts (degenerating). Calcium was assessed in these lesions by using Alizarin red and Von Kossa stains. In viable cysts, calcium was restricted to the calcareous corpuscles. Calcium deposits then increased progressively according to the macroscopic degree of degeneration of the lesions (Figure 2 ).
Figure 2.
Histological staining for calcium in a cerebral cysts in pig. In a viable cyst, Von Kossa stain (A) and Alizarin red (B) show stained calcareous corpuscles. Degenerating cysts show a significant agglutination of calcium with both techniques: Von Kossa (C) and Alizarin red (D). A similar pattern is seen in viable cysts from muscle, in which Von Kossa (E) and Alizarin red (F) stain mainly calcareous corpuscles, and in degenerating lesions, a significant agglutination of calcium is seen with both techniques: Von Kossa (G) and Alizarin red (H).
Discussion
Cysticidal efficacy of antiparasitic treatment of human neurocysticercosis is sub-optimal. Most patients are left with viable cysts after an initial course of treatment and between one-fourth and one-third of brain cysts survive. Improved more efficacious treatments are required. In this study we compared in a naturally infected pig model a series of antiparasitic regimens. The combination of ABZ and PZQ was consistently effective in muscle and brain cysts (even if ABZ dose was decreased by half or if steroids were doubled), followed by ABZ treatment. Nitazoxanide or PZQ alone did not show satisfactory results.
Our data may have implications for human therapy. Albendazole is currently the drug of election to treat neurocysticercosis, but its efficacy to kill parasites ranges from 41.2% to 89.8% of individual cysts, with complete cure in only 30–40% of patients.25 We have shown that combined ABZ + PZQ therapy is equally or more effective and could be used under a variety of alternative schemes. This combination has been used in hydatid disease in animal models and in human disease and showed better results than monotherapy.26 The initial published experience in cysticercosis with a combined scheme of ABZ plus PZQ showed that the combination had 86.7% efficacy versus 50% for ABZ alone and 43.3% for PZQ alone.27 A later study in India in children with a single degenerating brain cysticercus found complete resolution of lesions at three months in 60% of children receiving combined ABZ + PZQ and 72% at six months, compared with 42% and 52% of children receiving ABZ alone; however, these differences were not statistically significant.28 The rationale of this double scheme is to damage the parasite in different ways to expose more antigenic proteins and improve its destruction and clearance by cellular immune responses of the host.
As previously shown, OFZ consistently killed muscle parasites. However, its efficacy seems to be lower in brain. In this study, we used two doses of OFZ. It remains to be determined whether the addition of a third drug could increase efficacy without extending treatment time beyond a week. Oxfendazole is currently marketed only for veterinary use. Given its proven action against cysts, this drug may provide an effective oral antiparasitic regimen for a single dose treatment of neurocysticercosis in humans. Our data suggests that the use of PDN alone does not result in parasite death, at least in the brain. Its beneficial effects in the treatment of human cysticercosis are thus more likely related to the modulation of the inflammatory response and associated edema.
Histologic evaluation showed that calcium is present from the early stages of degeneration and can be identified by histologic stains. Given that calcified lesions in humans play a role in epileptogenesis,29 histologic comparison of cysts from animals treated with different antiparasitic and antiinflammatory schemes can provide controlled data on this subject.
In conclusion, this study using the pig model showed that combined ABZ plus PZQ, ABZ alone, and OFZ are effective antiparasitic drugs for treatment of cysticercosis, and the combined schemes had the best antiparasitic effect in brain, with a statistically significant reduction in viable brain cysts. Treatment with PDN alone (one day), NZX, and PDN left many surviving cysts, suggesting minimal or no cysticidal effect. Combined ABZ plus PZQ may provide a more efficient antiparasitic treatment for human cysticercosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Sanchez and R. Manzanedo for veterinarian help, S. Rodriguez for test processing, and S. Santivañez for data analysis support.
Footnotes
Financial support: This study was partially supported by Fogarty International Center/National Institutes of Health (training grant no. TW001140) and the Bill and Melinda Gates Foundation (grant no. 23981). Hector H. Garcia is a Wellcome Trust Senior Research Fellow.
Authors' addresses: Armando E. Gonzalez and Mercy G. Ramirez, School of Veterinary Medicine, Avenida Circunvalacion 2800, San Borja, Lima, Peru, E-mails: agonzale@jhsph.edu and mercyvet@gmail.com. Javier A. Bustos, Juan A. Jimenez, Mary L. Rodriguez, and Hector H. Garcia, Department of Microbiology, School of Sciences and Center for Global Health-Tumbes, Universidad Peruana Cayetano Heredia, Lima, Peru, H. Delgado 430, SMP, Lima, Peru, and Instituto Peruano de Parasitología Clínica y Experimental, Lima, Peru, E-mails: javier@peruresearch.org, jajch@hotmail.com, mary_luz_rodriguez@hotmail.com, and hgarcia@jhsph.edu. Robert H. Gilman, Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, E-mail: rgilman@jhsph.edu.
References
- 1.Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4:653–661. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 2.Schantz PM, Wilkins PP, Tsang VC. Immigrants, imaging and immunoblots: the emergence of neurocysticercosis as a significant public health problem. In: Scheld WM, Craig WA, Hughes JM, editors. Emerging Infections. Vol. 2. Washington, DC: American Society for Microbiology Press; 1998. pp. 213–241. [Google Scholar]
- 3.Wallin MT, Kurtzke JF. Neurocysticercosis in the United States: review of an important emerging infection. Neurology. 2004;63:1559–1564. doi: 10.1212/01.wnl.0000142979.98182.ff. [DOI] [PubMed] [Google Scholar]
- 4.Yoshino K. Studies on the post-embryonal development of Taenia solium: III. On the development of cysticercus cellulosae within the definitive intermediate host. J Med Assoc Formosa. 1933;32:166–169. [Google Scholar]
- 5.Nash TE, Neva FA. Recent advances in the diagnosis and treatment of cerebral cysticercosis. N Engl J Med. 1984;311:1492–1496. doi: 10.1056/NEJM198412063112307. [DOI] [PubMed] [Google Scholar]
- 6.Garcia HH, Del Brutto OH. Taenia solium cysticercosis. Infect Dis Clin North Am. 2000;14:97–119. doi: 10.1016/s0891-5520(05)70220-8. [DOI] [PubMed] [Google Scholar]
- 7.Del Brutto OH, Roos KL, Coffey CS, Garcia HH. Meta-analysis: cysticidal drugs for neurocysticercosis: albendazole and praziquantel. Ann Intern Med. 2006;145:43–51. doi: 10.7326/0003-4819-145-1-200607040-00009. [DOI] [PubMed] [Google Scholar]
- 8.Sinha S, Sharma BS. Neurocysticercosis: a review of current status and management. J Clin Neurosci. 2009;16:867–876. doi: 10.1016/j.jocn.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Flisser A, Gonzalez D, Shkurovich M, Madrazo I, Correa D, Rodriguez-Carbajal J, Cohen S, Rodriguez-del-Rosal E, Collado M, Fernandez B. Praziquantel treatment of porcine brain and muscle Taenia solium cysticercosis. 1. Radiological, physiological and histopathological studies. Parasitol Res. 1990;76:263–269. doi: 10.1007/BF00930823. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez AE, Garcia HH, Gilman RH, Lopez MT, Gavidia C, McDonald J, Pilcher JB, Tsang VC. Treatment of porcine cysticercosis with albendazole. Am J Trop Med Hyg. 1995;53:571–574. doi: 10.4269/ajtmh.1995.53.571. [DOI] [PubMed] [Google Scholar]
- 11.Garcia HH, Pretell EJ, Gilman RH, Martinez SM, Moulton LH, Del Brutto OH, Herrera G, Evans CA, Gonzalez AE. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350:249–258. doi: 10.1056/NEJMoa031294. [DOI] [PubMed] [Google Scholar]
- 12.Sotelo J, del Brutto OH, Penagos P, Escobedo F, Torres B, Rodriguez-Carbajal J, Rubio-Donnadieu F. Comparison of therapeutic regimen of anticysticercal drugs for parenchymal brain cysticercosis. J Neurol. 1990;237:69–72. doi: 10.1007/BF00314663. [DOI] [PubMed] [Google Scholar]
- 13.Botero D, Uribe CS, Sanchez JL, Alzate T, Velasquez G, Ocampo NE, Villa LA. Short course albendazole treatment for neurocysticercosis in Columbia. Trans R Soc Trop Med Hyg. 1993;87:576–577. doi: 10.1016/0035-9203(93)90095-8. [DOI] [PubMed] [Google Scholar]
- 14.Carpio A, Kelvin EA, Bagiella E, Leslie D, Leon P, Andrews H, Hauser WA. Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2008;79:1050–1055. doi: 10.1136/jnnp.2008.144899. [DOI] [PubMed] [Google Scholar]
- 15.Gilles HM, Hoffman PS. Treatment of intestinal parasitic infections: a review of nitazoxanide. Trends Parasitol. 2002;18:95–97. doi: 10.1016/s1471-4922(01)02205-x. [DOI] [PubMed] [Google Scholar]
- 16.Rossignol JF, Maisonneuve H. Nitazoxanide in the treatment of Taenia saginata and Hymenolepis nana infections. Am J Trop Med Hyg. 1984;33:511–512. doi: 10.4269/ajtmh.1984.33.511. [DOI] [PubMed] [Google Scholar]
- 17.Carpio A, Santillan F, Leon P, Flores C, Hauser WA. Is the course of neurocysticercosis modified by treatment with antihelminthic agents? Arch Intern Med. 1995;155:1982–1988. [PubMed] [Google Scholar]
- 18.Gonzalez AE, Cama V, Gilman RH, Tsang VC, Pilcher JB, Chavera A, Castro M, Montenegro T, Verastegui M, Miranda E, Bazalar H. Prevalence and comparison of serologic assays, necropsy, and tongue examination for the diagnosis of porcine cysticercosis in Peru. Am J Trop Med Hyg. 1990;43:194–199. doi: 10.4269/ajtmh.1990.43.194. [DOI] [PubMed] [Google Scholar]
- 19.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 20.Gonzales AE, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, Bernal T, Falcon N, Romero M, Lopez-Urbina MT. Effective, single-dose treatment or porcine cysticercosis with oxfendazole. Am J Trop Med Hyg. 1996;54:391–394. doi: 10.4269/ajtmh.1996.54.391. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez AE, Falcon N, Gavidia C, Garcia HH, Tsang VC, Bernal T, Romero M, Gilman RH. Time-response curve of oxfendazole in the treatment of swine cysticercosis. Am J Trop Med Hyg. 1998;59:832–836. doi: 10.4269/ajtmh.1998.59.832. [DOI] [PubMed] [Google Scholar]
- 22.Straw BJ, Meuten DJ. Physical examination. In: Leman AD, Straw BE, Mengeling WL, D'Allaire S, Taylor DJ, editors. Diseases of Swine. Seventh edition. Ames, IA: Iowa State University Press; 1992. pp. 793–807. [Google Scholar]
- 23.Flores Menendez JA, Agraz García AA. Enciclopedia Tecnica del Ganado Porcino. Cria, Explotación, Enfermedades e Industrialización. Mexico City: Primera Edicion. Ediciones Ciencia y Técnica S.A; 1987. [Google Scholar]
- 24.Cañedo L, Laclette JP, Morales E. Evagination of the metacestode of Taenia solium. In: Flisser A, Willms K, Laclette JP, Larralde C, Ridaura C, Beltran F, editors. Cysticercosis: Present State of Knowledge and Perspectives. New York: Academic Press; 1982. pp. 363–374. [Google Scholar]
- 25.Garcia HH. Antiparasitic drugs in neurocysticercosis: albendazole or praziquantel? Expert Rev Anti Infect Ther. 2008;6:295–298. doi: 10.1586/14787210.6.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamshidi M, Mohraz M, Zangeneh M, Jamshidi A. The effect of combination therapy with albendazole and praziquantel on hydatid cyst treatment. Parasitol Res. 2008;103:195–199. doi: 10.1007/s00436-008-0954-z. [DOI] [PubMed] [Google Scholar]
- 27.Guo DM, Xie SP, Jia JP. Therapeutic efficacy of praziquantel, albendazole and a combination of the two drugs in cysticercosis. Chinese Journal of Parasitology and Parasitic Diseases. 2003;21:187–188. [PubMed] [Google Scholar]
- 28.Kaur S, Singhi P, Singhi S, Khandelwal N. Combination therapy with albendazole and praziquantel versus albendazole alone in children with seizures and single lesion neurocysticercosis: a randomized, placebo-controlled double blind trial. Pediatr Infect Dis J. 2009;28:403–406. doi: 10.1097/INF.0b013e31819073aa. [DOI] [PubMed] [Google Scholar]
- 29.Nash TE, Pretell EJ, Lescano AG, Bustos JA, Gilman RH, Gonzalez AE, Garcia HH. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol. 2008;7:1099–1105. doi: 10.1016/S1474-4422(08)70243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.