Abstract
In October of 2008, an outbreak of trichinellosis occurred in northern California that sickened 30 of 38 attendees of an event at which meat from a black bear was served. Morphologic and molecular testing of muscle from the leftover portion of bear meat revealed that the bear was infected with Trichinella murrelli, a sylvatic species of Trichinella found in temperate North America. Clinical records revealed a high attack rate for this outbreak: 78% for persons consuming any bear meat and 100% for persons consuming raw or undercooked bear meat. To our knowledge, this report is the first published report of a human trichinellosis outbreak in the United States attributed to T. murrelli, and it is the second such outbreak reported worldwide.
Introduction
Trichinellosis is a parasitic zoonosis spread only by the ingestion of meat containing viable L1 larvae of Trichinella. The classic trichinellosis syndrome occurs during the parenteral phase of the acute infection, and it is characterized by periorbital edema, muscle pain and swelling, weakness, and fever. Skin rash and peripheral edema can also occur. The systemic signs and symptoms may be preceded by diarrhea, nausea, vomiting, and abdominal pain during the enteral phase of the acute infection. Less frequently, cardiac, pulmonary, and nervous system complications may occur. Death from trichinellosis is rare.1
For over one century after it was first described in the work by Owen2 in 1835, Trichinella spiralis was thought to be the only species in the genus, with a broad, non-specific host range that grew with the global spread of domestic swine. During the past several decades, however, molecular and biological research has uncovered a much more complex picture of this parasite involving domestic, sylvatic, and marine animal populations, including an updated taxonomy that currently recognizes eight distinct Trichinella species and four genotypes that have not yet been taxonomically defined.3 T. murrelli (previously Trichinella genotype T5) has been isolated from black bears, raccoons, red foxes, bobcats, coyotes, a domestic dog in the United States,4–6 and cougars in Canada,7 and it is thought to be the predominant species infecting sylvatic hosts of temperate North America.6
The first reported human trichinellosis outbreak caused by T. murrelli was one of two horsemeat-associated outbreaks that occurred in France during a 2-month period in 1985.8 The T. murrelli outbreak was epidemiologically associated with infected horsemeat imported from the United States. The parasite was not isolated from horsemeat samples obtained from consumers, butcher shops, and wholesalers during the outbreak, but Trichinella genotype T5 was isolated from a fatal case at autopsy.6,8,9 This outbreak provided the only published information on the clinical course of disease in T. murrelli-infected humans to date.
In November of 2008, the Humboldt County (California) Public Health Branch (HCPH) staff was contacted by a local physician and informed of three presumptive trichinellosis cases. All of the patients had consumed raw or undercooked black bear meat the previous month at a community gathering. An investigation conducted by local public health staff in conjunction with the California Department of Public Health (CDPH) showed a strong association between consumption of raw or undercooked bear meat and subsequent development of symptoms consistent with trichinellosis.
Methods
After receiving the reports of the three trichinellosis cases, HCPH initiated a public health investigation to identify the extent of the outbreak, determine its source, treat any additional symptomatic persons, and implement public health measures to prevent additional cases, if necessary.
Clinical and epidemiologic investigation.
A telephone questionnaire was developed to elicit information regarding consumption of bear meat, methods of food preparation, and subsequent illness, if any. Food exposure questions dealt almost exclusively with bear meat, because the reporting physician had already identified this exposure, which had been associated with past trichinellosis outbreaks in California and was the most likely source of infection. Exposure questions included the estimated amount of bear meat reportedly consumed and degree of doneness. Qualitative descriptions of the amount of meat consumed were systematically converted to an equivalent value in ounces for quantitative analysis. Clinical information and bear meat consumption history of attendees who HCPH was unable to contact were obtained from clinic and emergency room medical records.
A retrospective cohort study was conducted to identify characteristics associated with disease. A confirmed case was defined as an illness clinically compatible with trichinellosis that occurred in someone who ate bear meat served at the meal on October 27 and had serologic evidence of infection. A clinically compatible illness was defined by the presence of three or more of the following signs or symptoms: eosinophilia (defined as eosinophils > 7% of total white blood cell count or > 1,000 eosinophils/μL), muscle aches, fever, rash, or headache. Serologic evidence of infection was a positive result for Trichinella-specific antibodies using the enzyme-linked immunosorbent assay (ELISA) described below. A probable case was defined as either serologic evidence of Trichinella infection or clinically compatible illness in a person who ate bear meat served at the meal of October 27.
Analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC). The means procedure was used to calculate descriptive statistics for continuous variables. The freq procedure was used to calculate relative risk of illness in persons who consumed bear meat at varying levels of doneness and corresponding Fisher exact P values. The corr procedure was used to calculate the Pearson correlation coefficient for the amount of raw bear meat consumed and subsequent Trichinella antibody titer. A P value of < 0.05 was considered statistically significant.
Laboratory testing.
Laboratory testing of patient serum was conducted at the US Centers for Disease Control and Prevention (CDC) using an ELISA (Scimedx, Inc., Denville, NJ) that detects Trichinella-specific antibodies to excretory/secretory antigens of T. spiralis.10,11 HCPH attempted to obtain two serum specimens drawn at least 14 days apart from all event attendees because antibodies are often not detectable until after the acute-stage illness.11,12
Parasitological studies were performed from the implicated bear meat at the CDC. A squash preparation was made from a small piece of muscle that was sliced from the exposed area of the carpus and examined microscopically for Trichinella larvae. The larval concentration in the paw was determined by counting the larvae found in 1 g partially digested muscle obtained from the same area of the carpus.
Confirmation of the Trichinella to the species level was performed using a multiplex polymerase chain reaction (PCR) -based molecular typing method based on the amplification of specific regions of the internal transcribed spacer 1 (ITS1) and ITS2 and the expansion segment V region (ESV) of the ribosomal RNA gene.13
Results
Clinical and epidemiologic investigation.
Questionnaires were administered to 31 (82%) of the attendees or their parents, and medical records were used to elicit the clinical and exposure information of the 7 attendees who HCPH was unable to contact.
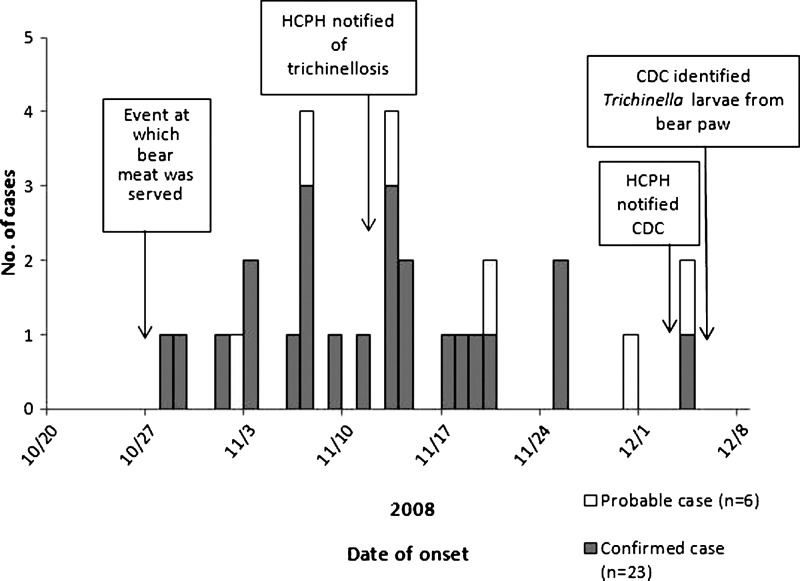
Among the 38 meal attendees, a total of 29 (76%) confirmed and probable cases of trichinellosis were identified (Figure 1). Of six probable cases, three cases had clinically compatible illness and were seronegative, two cases had only two symptoms and were seropositive, and one case had clinically compatible illness but serology was not performed. The median age of case-patients was 34 years (range = 9–61 years); 21 (72%) of 29 case-patients were male. The age and sex of the case-patients were similar to the age and sex of the meal attendees as a whole: 26 (68%) of the meal attendees were male, and the median age of attendees was 31 years (range = 1–61 years).
Figure 1.
Cases of trichinellosis (N = 29) associated with consumption of bear meat in Humboldt County, California in 2008.
Additionally, 37 (97%) of 38 attendees reported that they had consumed at least some bear meat, and of these 37 attendees, 29 (78%) attendees became ill. One person reported symptoms consistent with trichinellosis (muscle aches, abdominal pain, vomiting, and rash) but did not consume bear meat, and therefore, this person did not meet the case definition. The attack rates for persons consuming any raw or undercooked bear meat and persons consuming cooked meat only were 100% and 33%, respectively (Table 1) . Of the eight non-ill attendees, all were children under 8 years of age who ate only cooked bear meat and had a negative serologic test result.
Table 1.
Case status, attack rates, and relative risks among those who ate bear meat at the implicated meal in Humboldt County, California in 2008
| Bear meat exposure | Ate | Did not eat | RR | 95% CI | P value* | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Total | AR (%) | Cases | Total | AR (%) | ||||
| Any raw or undercooked | 25 | 25 | 100 | 4 | 13 | 31 | 3.25 | 1.44, 7.35 | < 0.001 |
| Cooked only | 4 | 12 | 33 | 25 | 26 | 96 | 0.34 | 0.16, 0.77 | < 0.001 |
| Any bear meat | 29 | 37 | 78 | 0 | 1 | 0 | NA | – | – |
Fisher exact two-sided test.
AR = attack rate; CI = confidence interval; RR = relative risk.
The median amount of raw or undercooked bear meat consumed by the 18 persons for whom an estimate was obtained was 1.5 oz (range = 0.50–28 oz).
The median incubation period was 17 days (range = 1–38 days). The most common signs and symptoms among confirmed and probable cases included muscle aches (100%), weakness or fatigue (88%), fever (76%), periorbital or facial edema (67%), and pruritic rash (66%) (Table 2) . One person with a past history of cardiac problems was hospitalized for overnight observation because of chest pain but was released the next day after the possibility of complications from Trichinella infection was ruled out. There were no reports of complications or deaths up to 3 months after the start of the outbreak.
Table 2.
Signs and symptoms of persons who attended the implicated meal (N = 38) in Humboldt County, California in 2008
| Sign or symptom | Confirmed (N = 23) | Probable (N = 6) | Not a case (N = 9) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Total | Percent | Number | Total | Percent | Number | Total | Percent | |
| Muscle aches | 22 | 22 | 100 | 6 | 6 | 100 | 1 | 8 | 13 |
| Elevated creatine kinase | 8 | 10 | 80 | 1 | 4 | 25 | NA | NA | NA |
| Weakness or fatigue | 19 | 20 | 95 | 3 | 5 | 60 | 0 | 8 | 0 |
| Eosinophilia | 12 | 13 | 92 | 1 | 5 | 20 | 0 | 4 | 0 |
| Fever | 20 | 23 | 87 | 2 | 6 | 33 | 4* | 8 | 50 |
| Headache | 14 | 19 | 74 | 5 | 5 | 100 | 0 | 7 | 0 |
| Pruritic rash | 17 | 23 | 74 | 2 | 6 | 33 | 1 | 9 | 0 |
| Chills | 13 | 19 | 68 | 1 | 6 | 17 | 0 | 7 | 0 |
| Periorbital/facial edema | 13 | 20 | 65 | 3 | 4 | 75 | 1† | 8 | 13 |
| Abdominal pain | 10 | 19 | 53 | 3 | 5 | 60 | 1 | 8 | 0 |
| Diarrhea | 9 | 20 | 45 | 1 | 5 | 20 | 0 | 8 | 0 |
| Vomiting | 6 | 19 | 32 | 2 | 5 | 40 | 1 | 8 | 0 |
Fever occurred in four children under 5 years of age, all of whom consumed cooked bear meat only, and none reported any other clinical signs or symptoms. No fever onset date was given, and therefore, this sign cannot be definitively linked to consumption of bear meat on October 27.
The patient was a 1-year-old child who reportedly consumed a very small amount of cooked bear meat on October 27. The child had no other signs or symptoms and was serologically negative for Trichinella infection.
NA = information not available.
Of the 30 ill persons, 26 (87%) were treated with prescription antihelmintic drugs; the treatment regimens were mebendazole (N = 12), albendazole (N = 6), mebendazole and albendazole (N = 6), and thiabendazole (N = 2). At least three of the persons who received both mebendazole and albendazole were prescribed the two regimens because of persistent muscle pain; 13 persons were cotreated with tapering doses of prednisone.
In four (13%) cases where both onset date and resolution date were available, the median duration of illness was 23.5 days (range = 14–35 days); 22 (73%) of 30 ill persons reported continued muscle pain during the final interviews conducted in mid-December, which was 6 weeks after consumption of the bear meat.
Interviews revealed that the bear had been legally hunted a few days before the event in a mountainous region in California about 100 miles east of Humboldt County. The bear was reportedly lying down when shot and appeared to be sick; it was butchered on a table that was later used to serve food. Raw dishes were prepared with chopped meat, and cooked bear meat dishes included stir fries, lentil-based stews, and rice/meat mixtures.
Laboratory testing.
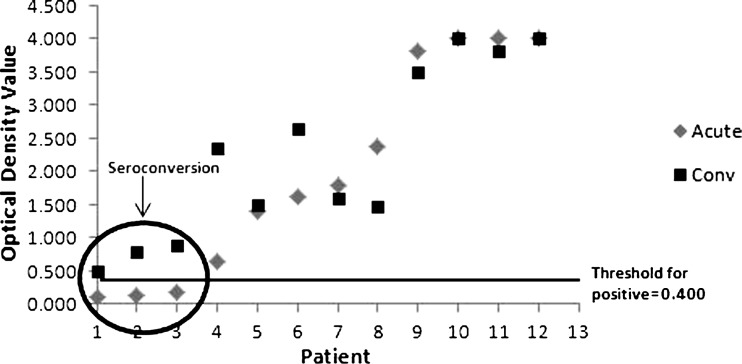
Serum for Trichinella antibody testing was obtained from 36 (95%) of 38 meal attendees. Forty-eight serum specimens from 29 ill and 7 non-ill attendees were drawn between November 25, 2008 (29 days post-exposure) and January 9, 2009 (74 days post-exposure). Of the 36 attendees who submitted serum for antibody testing, acute-phase specimens were obtained a median of 44 days (range = 29–49 days) after the date of the meal. Of the 26 ill persons submitting serum for whom an illness onset date was available, the acute-phase specimens were obtained a median of 21.5 days (range = 7–42 days) after onset of symptoms. Trichinella-specific antibodies were detected in 25 of 28 case-patients who submitted specimens for testing; 20 acute-phase specimens were positive. Serology was also performed for eight of nine persons who were not case-patients, and all were negative. Of the 12 persons who submitted both acute- and convalescent-phase specimens, all were ill; sera were drawn a median of 27 days (range = 12–41 days) apart, and seroconversion was shown in three (23%) cases (Figure 2). There was a significant positive correlation between the amount of raw or undercooked meat consumed and subsequent Trichinella antibody titer (P = 0.03). Three persons had positive serology results only after a second specimen was collected and tested. The first specimens, which were negative, were collected at 32, 36, and 36 days post-exposure. Subsequent positive results were seen in specimens collected 72, 45, and 44 days post-exposure, respectively. Of these three cases, two people reported consuming ∼0.5 oz raw or undercooked bear meat, and the third person reported consuming ∼1 oz.
Figure 2.
Change in Trichinella spp. antibody levels observed in ill persons submitting acute- and convalescent-phase serum in Humboldt County, California, 2007–2008.
Encapsulated Trichinella larvae were seen in the squash preparation prepared from the bear paw (Figure 3). Seventy-six larvae were obtained from the 1-g paw muscle sample. The recovered larvae were identified as T. murrelli by multiplex PCR.
Figure 3.

Trichinella larva encysted in muscle tissue from bear paw.
Discussion
We report the first outbreak of human trichinellosis in the United States known to be caused by the sylvatic Trichinella species T. murrelli. This outbreak exemplifies the changing epidemiology of trichinellosis in the United States from a disease caused primarily by the ingestion of contaminated domestic pork, typically infected with T. spiralis, to a disease more frequently associated with the consumption of raw or undercooked wild game meat infected with sylvatic Trichinella species.14,15
This outbreak was characterized by a high attack rate among persons consuming bear meat, which was served in a variety of ways. People who ate raw or undercooked bear meat were over three times more likely to develop a case of illness than those people who did not. All persons who consumed any of the raw or undercooked bear meat developed trichinellosis. Attack rates for other published bear meat-associated outbreaks ranged from 17% to 75% when both clinical and subclinical (i.e., the infection is asymptomatic in exposed persons with serologic evidence of infection) cases are counted.16–20 The attack rate of the French outbreak caused by T. murrelli-infected horsemeat could not be calculated, because the total number of exposed persons was not known; the rate was likely very high, because 431 confirmed and probable cases were linked to the meat from one infected horse.8
The median incubation period for the persons involved in the French outbreak was 3 weeks, which was several days longer than the median 17-day incubation period of this outbreak. It is not known whether the case-patients in this outbreak reported the date of onset of gastrointestinal or systemic symptoms; therefore, the reported incubation periods might include dates for both the enteral and parenteral stages of Trichinella infection. The wide range of incubation periods could reflect differences in the severity of infection among the case-patients, with shorter incubation periods corresponding to more severe infections.1 A high frequency of rash was observed in the current outbreak. In the French outbreaks, a high frequency of rash relative to facial edema was noted in the illnesses caused by T. murrelli compared with those illnesses caused by T. spiralis.21 High incidence of rash has also been reported in outbreaks caused by T. nativa, another sylvatic Trichinella species that is found in frigid areas of the Palearctic and Nearctic regions.22,23 More research is needed to determine whether sylvatic and domestic Trichinella species elicit different human allergic responses that would account for the perceived higher frequency of rash in outbreaks caused by the sylvatic species. The lack of asymptomatic infections in the exposed cohort could suggest that this strain of T. murrelli was moderately to highly pathogenic or that the concentration of viable larvae in the bear meat was so high that even persons who reported consuming very small portions of raw meat ingested enough infective organisms to become ill. Indeed, a large number of larvae were found in the 1-g muscle sample from the paw. Although we cannot extrapolate the larval burden found in a section of the paw to the rest of the animal, because the carpus is not proximate to any of the three main predilection muscles for Trichinella infection in bears (i.e., the tongue, masseters, and diaphragm), we can speculate that this black bear was heavily infected.24,25
The majority (73%) of persons with myalgia reported persistent muscle pain, which might have been affected by initiating antihelmintic therapy later in the course of infection. Ideally, antihelmintics should be administered just days after ingestion of the infected meat when newborn larvae are starting to migrate from the intestine to the striated muscles of the host. However, most patients are only treated after symptoms appear, and newborn larvae have already begun to invade and encyst in muscles. Nonetheless, because it is not known how long Trichinella adult females continue to produce larvae in the human intestine, antihelmintic treatment of all infected persons is recommended as late as 4–6 weeks after infection to reduce the burden of larvae migrating to and encysting in the muscles.1
All ill persons were able to receive combinations of antihelmintic drugs, steroids, and analgesics for disease treatment and symptom management, and the cohort was followed until 6 weeks after the implicated meal. At that time, over 70% of persons who reported myalgia were still experiencing muscle pain. As a result, the median duration of illness reported here is an underestimate of the true duration of illness of the cohort. Although the existence of chronic trichinellosis has been a subject of debate in the literature, myalgia and other clinical signs, symptoms, and sequelae have been reported after acute trichinellosis, and they may persist from months to years.1,26,27
Interviews with meal attendees revealed that the bear was butchered on a table that was later used to serve food. If the table on which the bear was butchered was not appropriately cleaned and disinfected before it was used to serve food, cross-contamination of other food items with pieces of larvae-containing meat could have occurred, which could explain the illness in the person who did not reportedly consume any bear meat. Unfortunately, the questionnaire did not include questions that allowed for the study of the role of potential cross-contamination of cooked bear meat or other dishes by residual raw bear meat left on the butchering table.
Trichinellosis is a nationally notifiable condition in the United States, and it is reportable in 48 states, New York City, and the District of Columbia.28 US outbreaks attributed to the consumption of bear meat have been reported since the mid-20th century primarily in Alaska but also in California and the Northeast.16–20,29 More recently, 50 (40%) of 126 total cases and 9 (60%) of 15 outbreaks reported to the CDC during 1997–2007 were attributed to the consumption of raw or undercooked bear meat.15,18,30 Interestingly, another black bear-associated trichinellosis outbreak occurred in October of 2008 among residents of another northern California county. All five persons who reportedly consumed raw meat from the infected bear developed trichinellosis.31 The infecting species of Trichinella was not determined, because no meat was left for testing.
Serology using the T. spiralis kit proved to be an effective method to confirm infection caused by T. murrelli in this outbreak; however, two of the probable cases had negative serologic test results after testing acute-phase specimens. Seroconversion was not shown in these two cases because follow-up specimens were not collected. Antibodies may be detectable by ELISA as early as 12–14 days after infection,32 but they are more frequently not detected until 20–35 days post-infection.11
T. murrelli is known to be widely distributed in North America, and it is an important etiologic agent of trichinellosis in several sylvatic hosts. Eating raw or undercooked bear meat is a risk factor for acquiring Trichinella infection. Therefore, thoroughly cooking wild game meat to an internal temperature of 160°F (71.1°C) before consumption helps eliminate the risk of trichinellosis. Although Trichinella-infected wild animals may not show signs of illness, the American Veterinary Medical Association advises hunters against eating or handling wild game animals that appeared to have been ill.33 This guidance is provided in the materials given to persons applying for hunting licenses and hunting safety course curricula, but these messages should be reinforced and targeted to the broader population as well.
ACKNOWLEDGMENTS
The authors thank Dr. Rodney Cade (Redwood Family Practice), Debra Judeikis (Humboldt County Public Health), and Paige Gupton (Centers for Disease Control and Prevention) for their contributions to the success of the outbreak investigation.
Footnotes
Authors' addresses: Rebecca L. Hall, Susan P. Montgomery, Patricia Wilkins, Alexandre J. da Silva, Isabel McAuliffe, Marcos de Almeida, Henry Bishop, Blaine Mathison, and Jeffrey L. Jones, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: bqu5@cdc.gov, zqu6@CDC.GOV, pwilkins@cdc.gov, abs8@cdc.gov, ibm4@cdc.gov, bnz0@cdc.gov, hsb2@cdc.gov, gqa4@cdc.gov, and jlj1@cdc.gov. Ann Lindsay, Chris Hammond, and Ron Largusa, Public Health Branch, Humboldt County Department of Health, Eureka, CA, E-mails: ann.lindsay@stanford.edu, chris.hammond@stjoe.org, and RLargusa@co.humboldt.ca.us. Benjamin Sun, Center for Infectious Diseases, California Department of Public Health, Sacramento, CA, E-mail: bgs9@cdc.gov.
References
- 1.Dupouy-Camet J, Kociecka W, Bruschi F, Bolas-Fernandez F, Pozio E. Opinion on the diagnosis and treatment of human trichinellosis. Expert Opin Pharmacother. 2002;3:1117–1130. doi: 10.1517/14656566.3.8.1117. [DOI] [PubMed] [Google Scholar]
- 2.Owen R. Description of a microscopic entozoon infesting the muscles of the human body. Trans Zool Soc London. 1835;1:315–324. [Google Scholar]
- 3.Pozio E, Hoberg E, La Rosa G, Zarlenga DS. Molecular taxonomy, phylogeny, and biogeography of nematodes belonging to the Trichinella genus. Infect Genet Evol. 2009;90:606–616. doi: 10.1016/j.meegid.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Dubey JP, Hill DE, Zarlenga DS. A Trichinella murrelli infection in a domestic dog in the United States. Vet Parasitol. 2006;137:374–378. doi: 10.1016/j.vetpar.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Hill DE, Samuel MD, Nolden CA, Sundar N, Zarlenga DS, Dubey JP. Trichinella murrelli in scavenging mammals from south-central Wisconsin, USA. J Wildl Dis. 2008;44:629–635. doi: 10.7589/0090-3558-44.3.629. [DOI] [PubMed] [Google Scholar]
- 6.Pozio E, La Rosa G. Trichinella murrelli n. sp: etiological agent of sylvatic trichinellosis in temperate areas of North America. J Parasitol. 2000;86:134–139. doi: 10.1645/0022-3395(2000)086[0134:TMNSEA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Gajadhar AA, Forbes LB. A 10-year wildlife survey of 15 species of Canadian carnivores identifies new hosts or geographic locations for Trichinella genotypes T2, T4, T5, and T6. Vet Parasitol. 2010;168:78–83. doi: 10.1016/j.vetpar.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Ancelle T, Dupouy-Camet J, Bougnoux ME, Fourestie V, Petit H, Mougeot G, Nozais JP, Lapierre J. Two outbreaks of trichinosis caused by horsemeat in France in 1985. Am J Epidemiol. 1988;127:1302–1311. doi: 10.1093/oxfordjournals.aje.a114923. [DOI] [PubMed] [Google Scholar]
- 9.Dupouy-Camet J, Robert F, Guillou JP, Vallet C, Perret C, Soule C. Identification of Trichinella isolates with random amplified polymorphic DNA markers. Parasitol Res. 1994;80:358–360. doi: 10.1007/BF02351881. [DOI] [PubMed] [Google Scholar]
- 10.van Knapen F, Franchimont JH, Verdonk AR, Stumpf J, Undeutsch K. Detection of specific immunoglobulins (IgG, IgM, IgA, IgE) and total IgE levels in human trichinosis by means of the enzyme-linked immunosorbent assay (ELISA) Am J Trop Med Hyg. 1982;31:973–976. doi: 10.4269/ajtmh.1982.31.973. [DOI] [PubMed] [Google Scholar]
- 11.Wilson M, Schantz P, Nutman T. Molecular and immunological approaches to the diagnosis of parasitic infection. In: Detrick B, Hamilton RG, Folds JD, editors. Manual of Molecular and Clinical Laboratory Immunology. Washington, DC: American Society for Microbiology; 2006. pp. 557–568. [Google Scholar]
- 12.Yera H, Andiva S, Perret C, Limonne D, Boireau P, Dupouy-Camet J. Development and evaluation of a Western blot kit for diagnosis of human trichinellosis. Clin Diagn Lab Immunol. 2003;10:793–796. doi: 10.1128/CDLI.10.5.793-796.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarlenga DS, Chute MB, Martin A, Kapel CM. A multiplex PCR for unequivocal differentiation of all encapsulated and non-encapsulated genotypes of Trichinella. Int J Parasitol. 1999;29:1859–1867. doi: 10.1016/s0020-7519(99)00107-1. [DOI] [PubMed] [Google Scholar]
- 14.Gottstein B, Pozio E, Nockler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev. 2009;22:127–145. doi: 10.1128/CMR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy SL, Lopez AS, Schantz PM. Trichinellosis surveillance—United States, 1997–2001. MMWR Surveill Summ. 2003;52:1–8. [PubMed] [Google Scholar]
- 16.Clark PS, Brownsberger KM, Saslow AR, Kagan IG, Noble GR, Maynard JE. Bear meat trichinosis. Epidemiologic, serologic, and clinical observations from two Alaskan outbreaks. Ann Intern Med. 1972;76:951–956. doi: 10.7326/0003-4819-76-6-951. [DOI] [PubMed] [Google Scholar]
- 17.Maynard JE, Pauls FP. Trichinosis in Alaska. A review and report of two outbreaks due to bear meat with observations on serodiagnosis and skin testing. Am J Hyg. 1962;76:252–261. [PubMed] [Google Scholar]
- 18.Nelson M, Wright TL, Pierce A, Krogwold RA. A common-source outbreak of trichinosis from consumption of bear meat. J Environ Health. 2003;65:16–19. 24. [PubMed] [Google Scholar]
- 19.Roselle HA, Schwartz DT, Geer FC. Trichnosis from New England Bear Meat: report of an Epidemic. N Engl J Med. 1965;272:304–305. doi: 10.1056/NEJM196502112720607. [DOI] [PubMed] [Google Scholar]
- 20.Wand M, Lyman D. Trichinosis from bear meat: clinical and laboratory features. JAMA. 1972;220:245–246. [PubMed] [Google Scholar]
- 21.Dupouy-Camet J, Paugam A, De Pinieux G, Lavarde V, Vieillefond A. Trichinella murrelli: pathological features in human muscles at different delays after infection. Parasite. 2001;8:S176–S179. doi: 10.1051/parasite/200108s2176. [DOI] [PubMed] [Google Scholar]
- 22.MacLean JD, Poirier L, Gyorkos TW, Proulx JF, Bourgeault J, Corriveau A, Illisituk S, Staudt M. Epidemiologic and serologic definition of primary and secondary trichinosis in the Arctic. J Infect Dis. 1992;165:908–912. doi: 10.1093/infdis/165.5.908. [DOI] [PubMed] [Google Scholar]
- 23.Schellenberg RS, Tan BJ, Irvine JD, Stockdale DR, Gajadhar AA, Serhir B, Botha J, Armstrong CA, Woods SA, Blondeau JM, McNab TL. An outbreak of trichinellosis due to consumption of bear meat infected with Trichinella nativa, in 2 northern Saskatchewan communities. J Infect Dis. 2003;188:835–843. doi: 10.1086/378094. [DOI] [PubMed] [Google Scholar]
- 24.Gamble HR, Bessonov AS, Cuperlovic K, Gajadhar AA, van Knapen F, Noeckler K, Schenone H, Zhu X. International Commission on trichinellosis: recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet Parasitol. 2000;93:393–408. doi: 10.1016/s0304-4017(00)00354-x. [DOI] [PubMed] [Google Scholar]
- 25.Kapel CM. Host diversity and biological characteristics of the Trichinella genotypes and their effect on transmission. Vet Parasitol. 2000;93:263–278. doi: 10.1016/s0304-4017(00)00345-9. [DOI] [PubMed] [Google Scholar]
- 26.Fröscher W, Gullotta F, Saathoff M, Tackmann W. Chronic trichinosis. Clinical, bioptic, serological and electromyographic observations. Eur Neurol. 1988;28:221–226. doi: 10.1159/000116271. [DOI] [PubMed] [Google Scholar]
- 27.Harms G, Binz P, Feldmeier H, Zwingenberger K, Schleehauf D, Dewes W, Kress-Hermesdorf I, Klindworth C, Bienzle U. Trichinosis: a prospective controlled study of patients ten years after acute infection. Clin Infect Dis. 1993;17:637–643. doi: 10.1093/clinids/17.4.637. [DOI] [PubMed] [Google Scholar]
- 28.Council of State and Territorial Epidemiologists State Reportable Conditions Assessment. 2010. http://www.cste.org/dnn/ProgramsandActivities/PublicHealthInformatics/StateReportableConditionsQueryResults/tabid/261/Default.aspx Available at. Accessed May 8, 2012.
- 29.Harbottle JE, English DK, Schultz MG. Trichinosis in bears in northeastern United States. HSMHA Health Rep. 1971;86:473–476. [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy ED, Hall RL, Montgomery SP, Pyburn DG, Jones JL. Trichinellosis surveillance—United States, 2002–2007. MMWR Surveill Summ. 2009;58:1–7. [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention Summary of notifiable diseases: United States, 2009. MMWR Morb Mortal Wkly Rep. 2011;58:17–18. [PubMed] [Google Scholar]
- 32.Capó V, Despommier DD. Clinical aspects of infection with Trichinella spp. Clin Microbiol Rev. 1996;9:47–54. doi: 10.1128/cmr.9.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Veterinary Medical Association Disease Precautions for Hunters. 2010. http://www.avma.org/public_health/zoonotic_risks/hunters_precautions.pdf Available at. Accessed May 8, 2012.