Abstract
Anemia is a common nutritional problem, and it has a remarkably high prevalence rate in Southeast Asia. In this study, children from 6 to 36 months were investigated to determine (1) the prevalence of anemia and (2) risk factors associated with anemia. Convenience sampling was used to select three villages in three different regions in Burma. Hemoglobin and anthropometric indicators were measured for 872 children. Logistic regression analyses were used to determine factors associated with anemia. The overall prevalence of anemia was 72.6%, with 40.0% having severe anemia. Predictors of anemia are a young age (P < 0.001), mother with anemia (P < 0.001), height-for-age Z score < −2 (P = 0.017), low family income (P < 0.001), mothers without primary education (P = 0.007), drinking unboiled water (P = 0.029), and fever in the last 3 months (P = 0.001). There is a high prevalence of anemia in children, and their nutritional status is quite poor. To control anemia, humanitarians and governments should launch comprehensive interventions.
Introduction
Anemia is one of the most common nutritional problems in many parts of the world, especially in developing countries.1 In Southeast Asia, the overall prevalence of anemia is one of the highest in the world, with 616 million people at risk.2 In children, major health consequences include impaired cognitive and physical development and increased mortality and morbidity.1,3 Low intake of iron-rich food is the main cause of anemia.4 Other causes include infectious diseases, deficiencies of micronutrients such as folate and vitamin B12,3 inherited conditions such as thalassaemia,5 and environmental pollutants such as lead.6
From the impact of civil war for over 40 years, 1 million children in Burma are reported malnourished; 9–12% have severe malnutrition.7 The national under 5 years mortality (104/1,000) is among the highest in Southeast Asia.7 Meanwhile, infectious diseases such as tuberculosis and malaria are widely spread in Burma.7 These findings indicate that children in this country may suffer from serious anemia. However, data on prevalence of anemia are very limited. Only one study from 8 years ago reported that, with a food supply but inadequate intake of micronutrients, the anemia prevalence of children in refugees of the Burma/Thailand border was 72.0%.8 There was little study of children from villages in rural areas, where children may suffer from not only micronutrient deficiencies but also a shortage of food.
In this study, three regions located at Kachin Special Region 1 (KSR1), Kachin Special Region 2 (KSR2; both located in the northeast part of Burma), and Shan State Special Region 4 (SSR4; located in the east part of Burma) were investigated. According to the report from the Burma government, the nutritional status of children under 3 years old (using underweight rate as the health indicator) is below the union level in these areas, and the Shan region has the lowest level.9 In the early 1990s, these regions received humanitarian assistance for children's immunization and malaria control, and to a certain extent, it greatly improved the health of local children. Nevertheless, children in these areas are still facing the problem of food shortage, especially lack of micronutrient supply. Humanitarian organizations intend to launch a nutritional supplement program to improve the children's health in these areas. Evaluation before the implementation is necessary for humanitarian relief planners to select appropriate interventions and budget wisely for an overall relief effort.10
The purpose of this study was to assess the nutritional status and determine the prevalence of anemia among children (aged 6–36 months) in rural areas of Burma. Interviews and field-friendly measurements were used to obtain information on anemia and anthropometry. The second objective was to examine nutritional, health-related, socioeconomic, and demographic factors and identify the factors that might be associated with anemia.
Methods
Participants.
This cross-sectional survey was conducted in Burma from January to May of 2011. Three villages were selected from rural areas using convenient sampling. Cluster sampling was used for household selection, and a total of 859 households having at least one child aged 6–36 months was screened. Malaria has been largely eliminated from these villages; only one surveyed child was reported to have had malaria in the last 3 months and was excluded from this study. Finally, 857 households with 872 children eventually participated in the study (KRS1 [N = 360], KRS2 [N = 311], and SSR4 [N = 201]). A total of 13 sibling pairs were included in the study.
Data collection.
The fieldwork was carried out by the staff from Health Unlimited (HU) with provincial health officers coordinating the work. Blood testing and physical examinations were all performed by local health professionals. Training of examiners and interviewers was conducted at HU, Kachin, Burma, before data collection. Working arrangement, initial site survey, and preliminary questionnaire testing were completed during the initial site visit to Burma.
Questionnaires were used to collect the children's socioeconomic and demographic information; self-reported health-related status (diseases or disease-related symptoms occurring in the last 3 months) and food frequency information were also collected by interviewing the children's primary caregivers (typically mothers). According to the data from extensive consultation of local health staff, a pilot study, and previous investigation,11 frequencies in the recent month of four kinds of food typically consumed in the local area (grains, vegetables, meat, and eggs) were ranked as (1) never or less than one time per week, (2) one to six times per week, and (3) daily.
Blood samples were obtained from finger pricks of children and mothers. Hemoglobin (Hb) levels were measured during the fieldwork using Hemoscan Blood Hemoglobin Photometer (Hemoscan POCT, Chengdu BSD instrument Co LTD, Chengdu, China). The presence and severity of anemia were diagnosed using age-based Hb criteria designated by the World Health Organization (WHO),12 which defines moderate anemia as Hb < 110 g/L and severe anemia as Hb < 80 g/L for both children aged 6–36 months and female adults.
The Infant/Child Shorrboard (Infant/Child Shorrboard YSZ-1, Keda Medical Instruments Co. Ltd., Shanghai, China) was used to measure both child stature and recumbent infant length (if the child was < 85 cm). Weight was measured using a portable scale (Portable scale, Xinman Medical Instruments Co. Ltd., Shanghai, China) with infants'/children's clothing, shoes, and diapers removed (measured to the nearest 0.1 kg). Mid-upper arm circumference (MUAC) was measured at the left arm using single-slotted insertion tapes. Head circumference (HC) was also measured by a tape. The growth and development statuses of children were evaluated by weight-for-age ratio Z score (WAZ), height-for-age ratio Z score (HAZ), weight-for-height ratio Z score (WHZ), and HC Z score according to WHO's Child Growth Standards 2006.13 WAZ < −2, HAZ < −2, and WHZ < −2 are defined as underweight, stunting, and wasting, respectively. HC Z score < −2 is defined as small HC. Because of different standards of defining wasting, MUAC lower than the cut-off value of 12.5 cm or MUAC for age under the 15th percentile of the data from children in the United States was also considered as wasting.14,15
Ethics.
The study was conducted in compliance with the Declaration of Helsinki and approved by Peking University. Written consent was obtained from the primary caregivers of all participants before data collection.
Statistical analyses.
Statistical Product and Service Solutions version 15.0 was used to carry out the analysis. Descriptive statistics were completed for all the variables and presented as median or frequencies. χ2 or test for trend was completed at the 95% level to determine significance before analyzing the data (only the factors with P or trend P < 0.05 were used to develop the regression model). Logistic regression (with the method of Backwards Wald) was used to obtain odds ratios (ORs) and 95% confidence intervals (95% CIs) to analyze the associations between anemia and the potential risk factors. Non-significant variables were removed from the final model. P value < 0.05 is considered as statistically significantly different.
Results
Prevalence of anemia.
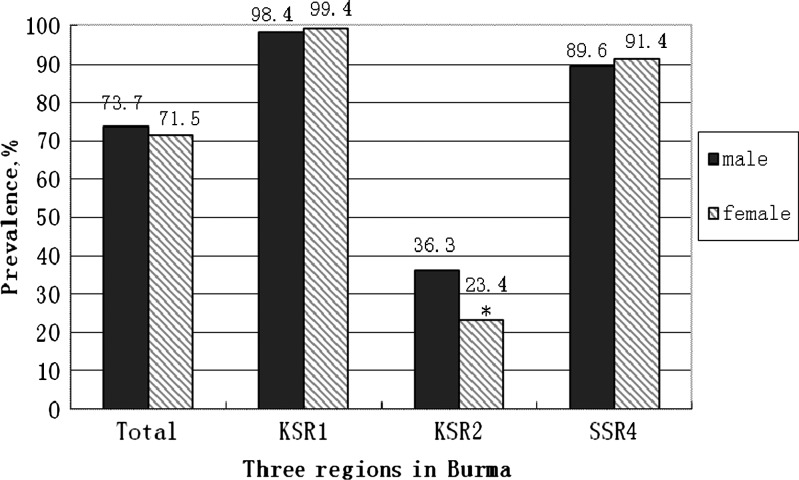
A total of 872 children aged 6–36 months participated in this study; 52.2% were male, and 41.8% were female. The mean age was 20.5 ± 9.8 months for males and 20.2 ± 9.8 months for females. The overall prevalence of anemia was 72.6%, with 40.0% having severe anemia (< 80 g/L). The prevalence of anemia by gender of three regions is shown in Figure 1. Overall, there was no gender difference for anemia. However, boys had a higher prevalence rate in KRS2 (χ2 = 6.03, P = 0.01). KSR1 had the highest anemia rate for both boys and girls. Children under 24 months were more at risk compared with older children in single-factor analysis (χ2 = 5.90, P = 0.015).
Figure 1.
The prevalence of anemia in children (6–36 months) in three regions of Burma (N = 872). *P < 0.05.
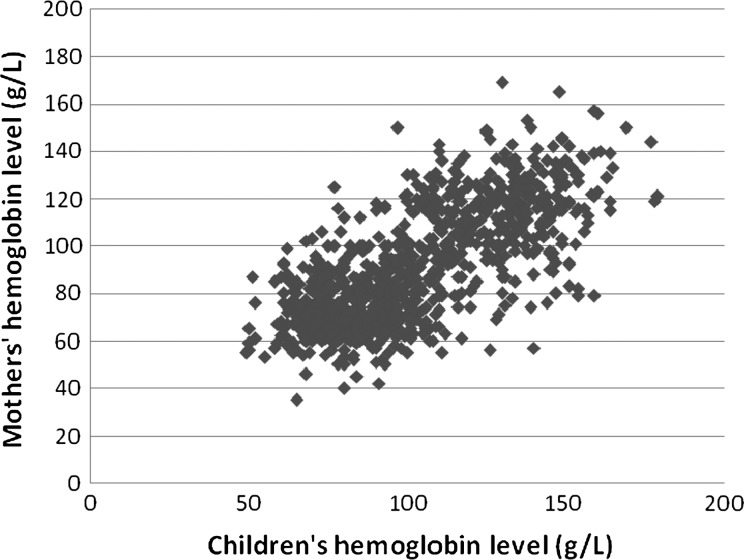
The overall anemia prevalence of children's mothers was 62.2%. The prevalence in KSR1, KSR2, and SSR4 was 97.8%, 15.6%, and 74.4%, respectively. In addition, mothers' Hb levels were significantly correlated with their children's Hb levels (r2 = 0.511, P < 0.001) (Figure 2).
Figure 2.
The correlation between children's Hb level (6–36 months) and their mothers' Hb level (N = 872, r2 = 0.511, P < 0.001).
Growth and development indicators of participants.
In this study, 39.7% of children were considered as underweight (WAZ < −2), 55.2% were stunted (HAZ < −2), and 13.2% were wasted (WHZ < −2). When considering MUAC for age, 29.2% of the children were under the 15th percentile level of US children, and 6.6% were wasted from the standard of MUAC < 12.5cm. The result of HC measurement showed that 13.5% of the children had a small HC (HC Z scores < −2). There were no gender differences for most health indicators, except for MUAC for age, which showed that there were more boys under the 15th percentile level (χ2 = 6.98, P = 0.030).
Compared with non-anemic children, anemic children were significantly more stunted, underweight, and wasted (defined by MUAC < 12.5 cm) according to single-factor analyses (χ2 = 45.08, P < 0.001; χ2 = 8.88, P = 0.003; χ2 = 6.83, P = 0.009, respectively).
Socioeconomic and demographic characteristics and self-reported health-related status of children with and without anemia.
The median family annual capita income from three regions was 120.0 (interquartile range [IQR] = 50.0–214.3) US dollars; KSR1, KSR2, and SSR4 were 100.0 (IQR = 43.4–171.4), 207.3 (IQR = 100.0–300.0), and 60.0 (IQR = 30.0–100.0) US dollars, respectively. Children from families with lower income were more susceptive to anemia in single-factor analysis. Mothers' education was inversely associated with the anemia prevalence of their children. Children drinking spring and river water and unboiled water were at risk for anemia. Complementary feeding for children older than 8 months was also associated with anemia (Table 1).
Table 1.
The association of sociodemographic characters and self-reported health status with anemia for children (6–36 months) in single-factor analysis
| Variables | Children with anemia, N (%) | Children without anemia, N (%) | P value |
|---|---|---|---|
| Family annual capita income (US dollar) | 93.8 (IQR = 42.9–180.0)* | 200.0 (IQR = 115.6–272.7)* | |
| N | 633 | 239 | < 0.001† |
| > 150 | 336 (53.1) | 55 (23.0) | |
| 100–150 | 112 (17.7) | 32 (13.4) | |
| < 100 | 185 (29.2) | 152 (63.6) | |
| Education of mothers, N | 629 | 230 | < 0.001† |
| No primary education | 364 (57.9) | 41 (17.8) | |
| Primary school education | 124 (19.7) | 74 (32.2) | |
| Secondary school education or above | 141 (22.4) | 115 (50.0) | |
| Source of water, N | 544 | 219 | < 0.001 |
| Tap water | 187 (34.4) | 87 (39.7) | |
| Well water | 69 (12.7) | 86 (39.3) | |
| Spring water | 260 (47.8) | 37 (16.9) | |
| River water | 28 (5.1) | 9 (4.1) | |
| Water was boiled, N | 630 | 228 | < 0.001 |
| Yes | 288 (45.7) | 170 (74.6) | |
| No | 342 (54.3) | 58 (25.4) | |
| Intake dietary supplement, N | 633 | 239 | < 0.001 |
| Yes | 15 (2.4) | 21 (8.8) | |
| No | 618 (97.6) | 218 (91.2) | |
| Age of complementary feeding (months) N | 633 | 239 | 0.005† |
| 0–5 | 219 (34.6) | 99 (41.4) | |
| 6–8 | 323 (51.0) | 123 (51.5) | |
| > 8 | 91 (14.4) | 17 (7.1) | |
| Diarrhea in 3 months before, N | 633 | 239 | 0.070 |
| Yes | 190 (30.0) | 87 (36.4) | |
| No | 433 (70.0) | 152 (63.6) | |
| Fever in 3 months before, N | 633 | 239 | < 0.001 |
| Yes | 199 (31.4) | 23 (9.6) | |
| No | 434 (68.6) | 216 (90.4) |
Presented as median (interquartile range [IQR]).
P values presented as P values for trend using the χ2 test for trend; other P values were the P value for χ2.
In this study, 4.0% of the children (N = 35) took dietary supplements (multivitamins or vitamin A), and they were all from KSR2. Taking dietary supplements was shown to have a protective effect against anemia in the single-factor analysis (P < 0.001) (Table 1).
The health status of the children was reported by the primary care providers. Approximately 65.5% of the children had experienced an illness in the past 3 months. Reported illnesses included diarrhea (31.7%) and fever (25.7%). Only one child had been diagnosed with malaria. For diarrhea incidence, there was no difference between children with and without anemia, whereas fever cases were reported more often in anemic children (Table 1).
Food intake frequency of children with and without anemia.
In the 6–12 months group, 63.7% of children never had meat, and 64.9% of children never had eggs; only 4.1% of the children were able to have meat every day, and 5.9% of children had eggs every day. In the 13–36 months group, 30.7% and 28.8% of children never had meat and eggs, respectively, whereas 5.6% and 6.1% of the children had meat and eggs every day, respectively. However, none of the intake frequencies of the four food groups (grains, vegetables, meat, and eggs) were shown to be related to anemia.
Logistic regression analysis and anemia-related factors.
Only the data from 829 subjects (including 13 sibling pairs) were eligible for logistic regression analysis, and 43 subjects were not involved because of missing values. Factors found to be associated with anemia in this population included a young age (< 24 months), mother having anemia, stunting status, family annual capita income < 100 US dollars, lower maternal education level, drinking unboiled water, and fever in the last 3 months (Table 2). The same risk factors were found from the analysis when the 13 sibling pairs were removed (results not shown here).
Table 2.
Logistic regression for growth and development indicators and socioeconomic and demographic predictors of anemia in children aged 6–36 months (N = 829)
| Indicator variable | B | Wald | OR | 95% CI | P value |
|---|---|---|---|---|---|
| < 24 months old | 0.92 | 16.36 | 2.50 | 1.60–3.89 | < 0.001 |
| Mother has anemia | 2.47 | 118.84 | 11.84 | 7.59–18.47 | < 0.001 |
| HAZ < −2 Z score | 0.51 | 5.69 | 1.66 | 1.10–2.52 | 0.017 |
| Family annual capita income < 100 US dollars | 0.54 | 18.53 | 1.72 | 1.34–2.20 | < 0.001 |
| Mothers without primary education | 0.60 | 7.15 | 1.82 | 1.17–2.82 | 0.007 |
| Drinking unboiled water | 0.50 | 4.74 | 1.64 | 1.05–2.57 | 0.029 |
| Fever in 3 months before | 1.00 | 11.59 | 2.72 | 1.53–4.83 | 0.001 |
Anemia is hemoglobin < 110 g/L. HAZ = height-for-age Z score.
Discussion
Prevalence of Anemia.
Anemia is a major public health problem for children in Southeast Asia.16 WHO has reported that anemia contributes to 324,000 deaths and 12,500,000 disability adjusted life-years (DALYs) in this area, and these numbers are the world's highest.17 The current study has supported and extended previous findings by showing that anemia is an important public health issue in children from Burma.
In this study, anemia was clearly prevalent among children of rural areas. The overall prevalence in this population was 72.6%, which is consistent with the reported rate (72%) of refugee children 5–59 months on the Thailand/Burma border8; however, this number is much higher than the mean prevalence (49%) in children of Southeast Asia.16 It is worth noting that the anemia prevalence was unacceptably high (exceeded 90%) in KSR1 and SSR4; similar numbers were found in some parts of Asia in the 1980s.18 In terms of family income and standards of living, KSR1 and SSR4 were more disadvantaged compared with KRS2. Moreover, 35 children from KRS2 accepted vitamin supplements (multivitamins or vitamin A) provided by the United Nations International Children's Emergency Fund (UNICEF), which might have resulted in the lower prevalence of anemia in that region. The prevalence in non-refugee children without humanitarian aid may be expected to be higher.
Severe anemia is also a major concern in children. In the study, we found that 40% of the children had severe anemia. This rate is much higher than the rate of 10.4% in the study with the refugee children.8
Growth and development indicators of children.
To maximally include children who are at risk, three different standards of defining wasting were used in this study; a child meeting any one standard was considered as wasted. According to our result, with the most conservative estimates, 6.6% of the children were wasted when using MUAC < 12.5 cm. In this study, we also found a high prevalence of stunting, low weight, and small head circumference in the children. The results reflect an alarming rate of malnutrition among Burma's children. In addition, stunting was shown to be associated with anemia. Previous studies reported that anemia could lead to cognitive impairment, lack of physical capacity, and even life-threatening consequences 3 However, the causality between malnutrition and anemia in this study could not be observed.
Risk factors associated with anemia.
In the study, children < 24 months were more likely to be anemic compared with older children, which is consistent with the literature information that anemia is common among children around the time of the growth spurt, especially between the ages of 6 and 24 months.8,19 During that period, children's physical development is rapid, and the blood volume is largely expanded, whereas the iron storage from the maternal source has usually been depleted; diet becomes a vital source for iron as a result.3 If exogenous iron is deficient, anemia occurs easily.
Additionally, maternal conditions can be a contributing factor. In this study, maternal Hb level was associated with anemia in the children. One explanation is that all family members shared the same socioeconomic environment (for example, a low family income). Also, through breastfeeding, severe maternal anemia (caused by iron deficiency) can greatly influence the iron status of their children.3,20 Children at 6–36 months are even more likely to have anemia when they are additionally in a shortage of iron-rich food. Mothers with primary education or higher were less likely to have anemic children. This finding is consistent with some but not all of the previous studies.8,19 These findings indicate that promoting maternal health and educating mothers on anemia-related information may help with controlling anemia in children.
Interestingly, in this study, we found that drinking unboiled water put children at risk for anemia. Although we did not collect the data on parasite infection, based on the previous study, we can infer that drinking unboiled water can easily cause a parasite infection that could be the cause of intestinal bleeding-induced anemia, especially when knowing that the main water source was spring or river water in these areas.2,20 From previous studies, we know that infectious diseases were a common cause of anemia, and anemic children were more susceptive to infectious diseases.7 Consistent with these findings, having fever in the past 3 months was found to be related to anemia in this study. Although malaria, to some extent, was controlled in this area, critical concerns and immediate treatment are needed, because the incidence of infectious diseases, such as parasitic infection, pneumonia, and tuberculosis, is still high.
Micronutrient deficiency is considered as the main cause of anemia.3 In public health terms, iron deficiency is by far the first cause of nutritional anemia worldwide.3 However, food intake frequency is said to be associated with anemia, which seemed to be unwarranted in this study. This finding was mainly because all the iron-rich foods were shown to have very low daily intakes in both anemic and non-anemic children. A similar lack of association was also found in other studies, especially in varying environments where many other factors other than diet exposure can increase the risk of getting anemia.11,21
Anemia, especially iron-deficiency anemia, usually can be prevented at a low cost, and the benefit/cost ratio of implementing preventive programs is recognized as one of the highest in the realm of public health.3 Based on the results in this study, multiple contributing factors can coexist in an individual or population that affect the severity. Several primary issues need to be addressed to help reduce the high prevalence in this population: (1) strengthen infrastructures and health institutions by, for example, drinking tap or well water; (2) control infectious diseases; (3) provide anemia-related health education and focus on local women; and (4) provide dietary supplements rich in bioavailable iron, folic acid, and vitamin B12. An appropriate combination of interventions may be the ideal way to reach comprehensive anemia control in this region.
Limitations.
This study was a cross-sectional study conducted in three different regions of Burma. Selection of the design is inevitable. However, the sampling process may have several limitations. On one side, because of the war, we could not use a stratified random sampling for subjects; some villages in the three regions were at war and were not accessible. The anemia prevalence of children in those villages was expected to be higher. On the other side, some recruited children were from one family and shared the same socioeconomic environment; therefore, the association between anemia and some risk factors may have been overestimated. As a result, the anemia prevalence in this survey could not truly represent the level of all Burma children. However, considering the sample size, the small number of siblings (13 pairs) cannot greatly skew the results, and the results are still able to reflect a general level of anemia in the region and the poor nutritional status of the children. Also, the risk factors for anemia were consistent from the regression analyses with or without the 13 sibling pairs.
Being restricted by the cost and local working conditions, we did not test indicators like serum ferritin, transferrin saturation, and erythrocyte protoporphyrin. Consequently, we could not identify the types of anemia as iron deficiency, thalassemia, or sickle cell anemia. Thalassemia was also reported to have a high prevalence in Burma.22 Different types of anemia are not managed in the same manner; anemia caused by genetic factors needs to be attended differently and intervened slowly compared with iron-deficiency anemia.3 This study was focused on exploring the controllable factors associated with anemia. We hypothesize that, in these areas, the majority of anemia is caused by micronutrient deficiency along with the other risk factors.22
One issue that we studied in the investigation was the information on recent illnesses. Diarrhea and fever occurrences in the past 3 months were obtained by asking the primary caregivers to recall such incidences. Therefore, the reliability might be compromised; however, in this situation where there is a lack of regular visits to a doctor and no medical records, this method might be the only feasible method for obtaining subjects' medical history. In this study, we also did not collect the stool samples, and therefore, we cannot identify the parasitic infection. According to the recent report, the prevalence of helminths infection was as high as 70% in Burmese pregnant women on the Thailand/Burma border.23 Drinking unboiled water is related to parasitic infection.20 In the investigation, another limitation is that we used only a brief food frequency to estimate the status of food intake; as a result, the status of micronutrient intake or total caloric intake of the subjects was not accessed. Brief food frequency is usually used for nutrition surveys in children when weighed dietary records are unavailable.11 This method reflected the meat and eggs shortage in the children, which indicated a deficiency of iron.
Conclusion.
The findings of this study pointed out a high prevalence of anemia and poor nutritional status in children in rural areas of Burma. The results showed that a young age, stunting status, mother without primary education experience, low family income, drinking unboiled water, and having had a fever in the recent 3 months may be associated with anemia. Additional studies are needed to identify causes of anemia to plan proper interventions.
ACKNOWLEDGMENTS
This study was a collaboration of Peking University and Health Unlimited, and it was funded by Health Unlimited. We would like to thank all the Health Unlimited staffs and local health staffs who were involved in the field work in a tough working condition. We also thank the provincial health officers for invaluable assistance with all the provincial aspects of the survey. Also, we appreciate the time devoted by and good cooperation from all the participants.
Footnotes
Authors' addresses: Ai Zhao, Yumei Zhang, Titi Yang, Zhaoyan Liu, and Yanli Lv, Department of Nutrition and Food Hygiene, School of Public Health, Peking University Health Science Center, Beijing, China, E-mails: xiaochaai@163.com, zhangyumei111@gmail.com, lesliedidi@hotmail.com, hsaza@126.com, and 267240084@qq.com. Ying Peng and Jiayin Li, Health Unlimited Organization, Kunming, Yunnan, China, E-mails: hpapengy@gmail.com and jiayinli83@gmail.com. Peiyu Wang, Department of Social Medicine and Health Education, School of Public Health, Peking University Health Science Center, Beijing, China, E-mail: wpeiyupku@gmail.com.
References
- 1.WHO Iron Deficiency Anaemia: Assessment, Prevention and Control. A1. Guide for Programme Managers. 2001. http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/index.htm Available at: Accessed July 15, 2011.
- 2.Latham M. Human Nutrition in the Developing World. Food and Agricultural. 1997. http://www.fao.org/DOCREP/W0073e/w0073e00.htm Available at. Accessed July 15, 2011.
- 3.Kotecha PV. Nutritional anemia in young children with focus on Asia and India. Indian J Community Med. 2011;36:8–16. doi: 10.4103/0970-0218.80786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López MA, Martos FC. Iron availability: an updated review. Int J Food Sci Nutr. 2004;55:597–606. doi: 10.1080/09637480500085820. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization and Centers for Disease Control and Prevention Assessing the Iron Status of Populations: Report of a Joint World 2. 2005. http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107/en/index.htm Available at. Accessed July 20, 2011.
- 6.Choi JW, Kim SK. Relationships of lead, copper, zinc, and cadmium levels versus hematopoiesis and iron parameters in healthy adolescents. Ann Clin Lab Sci. 2005;35:428–434. [PubMed] [Google Scholar]
- 7.Chelala C. Burma: a country's health in crisis. Lancet. 1998;352:556. doi: 10.1016/S0140-6736(05)79276-X. [DOI] [PubMed] [Google Scholar]
- 8.Kemmer TM, Bovill ME, Kongsomboon W, Hansch ST, Geisler KL, Cheney C, Shell-Duncan BK, Drewnowski A. Iron deficiency is unacceptably high in refugee children from Burma. J Nutr. 2003;133:4143–4149. doi: 10.1093/jn/133.12.4143. [DOI] [PubMed] [Google Scholar]
- 9.Department of Health Planning and Department of Health Annual Public Health Statistics Report (2007). The Government of the Union of Burma Ministry of Health. 2007. http://www.ibiblio.org/obl/show.php?cat=1967&lo=d&sl=1 Available at. Accessed July 20, 2011.
- 10.Hansch SH. Health: How Many People Die of Starvation in Humanitarian Emergencies. Center for Policy Analysis and Research on Refugee Issues Refugee Policy Group, 30; 1995. [Google Scholar]
- 11.Mikki N, Abdul-Rahim HF, Stigum H, Holmboe-Ottesen G. Anaemia prevalence and associated sociodemographic and dietary factors among Palestinian adolescents in the West Bank. East Mediterr Health J. 2011;17:208–217. [PubMed] [Google Scholar]
- 12.United Nations Children's Fund Preventing iron deficiency in women and children: background and consensus on key technical issues and resources for advocacy, planning and implementing national programmes. Proceedings of the UNICEF/UNU/WHO/MI Technical Workshop, International Nutrition Foundation (INF), Micronutrient Initiative (MI); UNICEF, New York, NY. 1998. October 7–9. [Google Scholar]
- 13.WHO WHO Multicentre Growth Reference Study Group (2006) WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. 2006. http://www.who.int/childgrowth/standards/technical_report/en/ Available at. Accessed June 15, 2011.
- 14.Medecins Sans Frontieres . Nutrition Guidelines. 1st Ed. Paris, France: Medecins Sans Frontieres; 1995. [Google Scholar]
- 15.Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34:2540–2545. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- 16.DeMaeyer E, Adiels-Tegman M. The prevalence of anemia in the world. World Health Stat Q. 1995;38:302–316. [PubMed] [Google Scholar]
- 17.Stoltzfus RJ, Mullany L, Black RE. Iron Deficiency Anaemia. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. 2005. http://www.who.int/publications/cra/chapters/volume1/0163-0210.pdf Available at. Accessed July 15, 2011.
- 18.Hercberg S, Rouaud C. Nutritional anaemia. Child Trop. 1981;133:1–36. [PubMed] [Google Scholar]
- 19.Provan D. Mechanisms and management of iron deficiency anemia. Br J Haematol. 1999;105:19–26. [PubMed] [Google Scholar]
- 20.Cifuentes E. The epidemiology of enteric infections in agricultural communities exposed to wastewater irrigation: perspectives for risk control. Int J Environ Health Res. 1998;3:203–213. [Google Scholar]
- 21.Halileh S, Gordon NH. Determinants of anemia in pre-school children in the occupied Palestinian territory. J Trop Pediatr. 2006;52:12–18. doi: 10.1093/tropej/fmi045. [DOI] [PubMed] [Google Scholar]
- 22.Yip R. Nutrition and Health in Developing Countries. Totowa, NJ: Humana Press; 1994. Iron deficiency and anemia; pp. 327–342. [Google Scholar]
- 23.Boel M, Carrara IV, Rijken M, Proux S, Nacher M, Pimanpanarak M, Paw MK, Moo O, Gay H, Bailey W, Singhasivanon P, White NJ, Nosten F, McGready R. Complex interactions between soil-Transmitted helminths and malaria in pregnant women on the Thai-Burmese border. PLoS Negl Trop Dis. 2010;4:e887. doi: 10.1371/journal.pntd.0000887. [DOI] [PMC free article] [PubMed] [Google Scholar]