Abstract
This study reports the first urban human case of Rocky Mountain spotted fever caused by Rickettsia rickettsii, in Costa Rica. An 8-year-old female who died at the National Children's Hospital 4 days after her admission, and an important and significant observation was the presence of an “eschar” (tache noire), which is typical in some rickettsial infections but not frequent in Rocky Mountain spotted fever cases.
Introduction
Rocky Mountain spotted fever (RMSF) is an infectious disease caused by the bacterial agent Rickettsia rickettsii. Human infections are frequently reported throughout the western hemisphere in countries such as Argentina, Brazil, Canada, Colombia, México, Panamá, and United States.1,2 Nowadays, the international recommendation of the World Health Organization (WHO) for the diagnosis of fever cases with maculopapular rashes includes the analysis for spotted group fever (SGF)-related rickettsial infection. In Costa Rica, R. rickettsii, was first identified in 19773 and several subsequent outbreaks have been reported since then, with high mortality rates (∼60%).4 Costa Rican cases5 are mostly reported in areas north to the central volcanic system, specifically in the towns of Limon, Sarapiqui, and San Carlos, as well as in Turrialba (east) and San Ramón (west).5 All cases were epidemiologically associated with the rain and tropical forests characterized by dense vegetation, and large populations of small rodents, which are highly infested with ticks, the vector of the pathogens.
The incubation period for RMSF is ∼3–12 days. A shorter incubation period is usually associated with disease severity. Only 60% of the RMSF patients remember a tick bite, because these are painless and occur at body sites that people usually overlook, like scalp, armpit, and lower legs. There are a few reports of R. rickettsii infection with observable signs at the site of the tick bite called eschars-touche noir,6 unlike the ones observed with other rickettsial species such as Rickettsia parkeri7 and Rickettsia massiliae.8 Clinical symptoms initiate with high fever usually ≥ 39.5°C, headache, generalized myalgia, chills, and digestive problems including nausea, vomiting, diarrhea, and anorexia. Because of the unspecificity of the symptoms, RMSF is usually misdiagnosed for other infectious diseases, such as leptospirosis, meningococcemia, enterocolitis, or even dengue. As the disease progresses, a generalized maculopapular rash may be observed that tends to concentrate on hands and feet, and become petequial in the more severe forms of the disease.2,9–11 Because of misdiagnosis, fatal cases are usually associated with lack or late administration of specific treatment. Here, we describe the first urban human fatal case associated with RMSF disease, in the metropolitan area of San Jose, the capital of Costa Rica, which presented a tick bite eschar on the abdominal area.
Case report
On April 2, 2010, a female, 8 years and 11 months of age, a resident of an urban area called San Rafael de Desamparados, located ∼6 km south of downtown San Jose, arrived at the main children's hospital in San Jose, Costa Rica (Hospital Nacional de Niños- [HNN]). At the time of admission, the patient reported clinical symptoms of 7 days of evolution characterized by frontal headache, fever, abdominal pain, and severe myalgia. In the last 3 days, the patient developed a maculopapular rash that started in both forearms and progressed into a generalized exanthema mainly in palms and soles. The patient reported suffering from asthma attacks that were under clinical control. The mother of the patient denied any recent visit to non-urban areas, not even to the city borders. No other members of the family or neighbor reported symptoms of the disease. The physical examination of the patient at the time of arrival to the HNN, reported that the patient was alert, conscious, with a generalized myalgia, and dehydrated. The patient had a fever of 38.5°C, a heart rate of 136 beats/minute, an oxygen saturation of 97%, the arterial pressure of 84/30 mm of Hg with a mean arterial pressure of 50 mm of Hg, conjunctival injection, and generalized maculopapular rash, however more severe in face, palms, and soles. Abundant petequia were observed in the lower limbs and mucosa, and coalescent hemorrhagic lesions were present in the inguinal area and thighs. No lymphadenopathies or signs of neurological dysfunction were reported and a negative optical return loss. Abdominal palpation was reported as soft and very painful superficially as well as deep inside. Laboratory cabinet tests at admittance were as follow: Hb: 11.5 g/dL, leukocytes: 7 040/mL with 54% polimorphonuclear, 30% bands and toxic granulation, platelets: 22,000/mL, and C-reactive protein: 248. Creatinine and ureic acid blood concentrations were normal. Upon admission into the hospital the patient developed hypotension, oliguria, hepatic dysfunction, and coagulopathy, and was immediately transferred into the intensive care unit where assisted respiration was initiated. Treatment with gamma immunoglobulin was started because of the possibility of Kawasaki disease. Electrocardiograms were relatively normal with a mild decrease in the systolic function and the coronaries had a normal appearance. On April 5, 3 days after admission into the hospital, a laparoscopic abdominal surgery was performed that showed no significant malfunctions. Upon consultation with the infectologist, Kawasaki disease was ruled out because the rashes were not characteristic of this disease and instead rickettsiosis or staphylococcemia should be considered first, but leptospirosis, ehrlichiosis, and meningococcemia were also possible. Immediately, intravenous ciprofloxacin treatment was applied at 360 mgs every 12 hours. By April 6, 4 days after hospital admission, all bacterial cultures returned negative. On the same day, a neurologist declared the patient brain dead.
Postmortem diagnosis (autopsy).
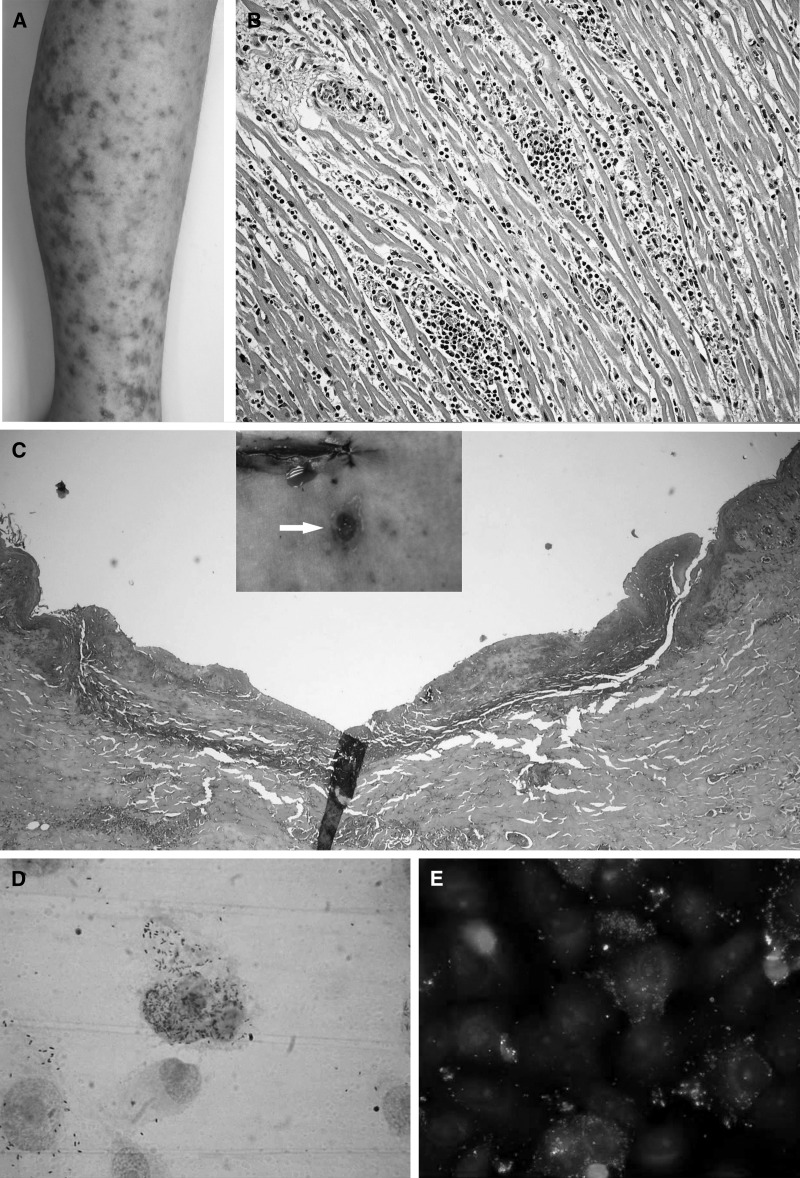
The macroscopic findings at the autopsy were as follow: a generalized maculopapular exanthema; presence of hemorrhagic and centro-necrotic papules in face, palms, and soles; numerous petequia distributed in the lower limps, and confluent purpuric lesions on the anterior and lower waist and both thighs (Figure 1A). Histologic analysis showed a leukocytoclastic vasculitis with microclots in different tissues and several organs, skin, hearth, gastrointestinal tract, bladder, brain, and skeletal muscles. The vasculitis was observed mainly in small communicating vessels, arteries, and veins. A diffuse severe myocarditis associated to the severe vasculitis (Figure 1B), a portal mononuclear hepatitis, focal interstitial nephritis, and acute tubular necroses were also noted. An important and significant observation was the presence of an “eschar” (tache noire), which is typical in some rickettsial infections, but not frequent in RMSF cases. The lesion measured 9 mm in diameter, and looked like a deep excavated necrotic ulcer, localized at the left side of the paraumbilical zone (Figure 1C). Cultures of lung fluids, cerebrospinal fluids, and blood were negative for bacteria, fungi, and mycobacteria. Samples were sent to the Center for Disease Control and Prevention (CDC, Atlanta, GA) for immunohistochemical analysis for Leptospira and Neisseria meningitidis, with negative results and positive for SFG rickettsias. Polymerase chain reaction (PCR) for liver and kidney were positive for Rickettsia sp. and negative for HSV-1 and HSV-2, enterovirus, alphavirus, flavivirus, ehrlichia, rubella, measles, and influenza A/Sw1.
Figure 1.
(A) Maculopapular rash with areas of central necrosis violacea. (B) Lymphocytic myocarditis with vasculitis in small vessels. (C) Skin section of the lesion (Tache noire), showing necrosis and leukocytoclastic. (D) Gimenez staining of VERO E 6 cells infected with isolated Rickettsia. (E) Immunofluorescense of VERO E6 cells infected with isolated R. rickettsii.
Confirmatory (laboratory) Rickettsia rickettsii diagnosis.
Further analysis of samples recovered at the autopsy (e.g., brain, liver, spleen, lungs, blood, and skin), was performed at the Laboratory of Virology, Facultad de Microbiología, Universidad de Costa Rica.
In brief, PCR using SFG Rickettsia-specific primers, previously designed for the detection of the citrate synthase gene (gltA) of R. rickettsii,12 were performed and all the tissue samples tested resulted positive. Worthwhile to mention is the fact that the skin sample was taken from a site near the eschar (not from the eschar itself), and this sample showed the strongest PCR band. This would strongly suggest that the eschar and the disease were a consequence of the tick bite. Furthermore, macerated tissues were inoculated into VERO E6 and primary chick embryo cell monolayers, and incubated at 37°C in 5% CO2 for 7 days. Rickettsia replication in the cell cultures was detected by Giménez staining and later confirmed by immunofluorescence using specific R. rickettsii antisera,13 as shown in Figure 1D and E. Male guinea pigs were inoculated intraperitoneally with cell lysates of positive infected cell cultures (1 × 106 and 1 × 105 cells, respectively). The animals were monitored daily for the appearance of symptoms and weight loss. Infected animals died 4–5 days after inoculation, presenting severe orquitis, 10–25% weight loss, and significant temperature rise, all consistent with virulent R. rickettsii infections. Internal tissues of affected animals were processed by PCR to detect the ompA gene of SFG rickettsiae.14 Amplified PCR products were sequenced using the Genetic Analyzer 3130 (Applied Biosystems/HITACHI, CA, USA), edited and analyzed by BLAST (basic local alignment search tool). The amplified segments were also sent to the laboratory of Dr. Marcelo Labruna at the School of Veterinary Medicine, Universidad de Sao Paulo, Brazil, for corroboration of the results. All tissue samples tested were positive by PCR for Rickettsia-specific fragments: gltA and ompA and the sequences of amplified segments showed a 100% homology with R. rickettsii. Both sequences were analyzed in Brazil and Costa Rica. Rickettsia rickettsii was identified as the responsible agent for the fatal outcome.
Initially, the clinical history did not reveal enough data to guide the physicians to the proper diagnosis and some details may have been overlooked. After the patient's death, the personnel of the pathology service at the HNN interviewed her mother, who referred that the family had moved a few weeks before to a new home and that the girl complained of an insect bite on the abdominal area, where the eschar was later found. She also mentioned that they had a pet dog, but the dog was not at their home any more.
For another research project, some of the neighboring dogs were bled for serological analysis and the dog belonging to the patient (it was at another house) was seropositive for R. rickettsii antibodies by indirect immunofluorescence (results will be published separately).
Conclusions.
Although rickettsial diseases have been described previously in Costa Rica in several occasions; this is the first report of a human fatal case presenting an eschar, and the first report of an infection acquired in a residential area of the capital city, San José.
Human cases of RMSF are usually related to tropical areas, where the vector is able to complete its life cycle; however, a few cases have been associated with urban environments. For example, a fatal human case was reported in Rio de Janeiro, Brazil, where a 48-year-old male acquired the infection from his brother who was a carriage-horse driver. In this case R. rickettsii was diagnosed by PCR alerting the health authorities in Rio de Janeiro of the presence of the pathogen, which was not reported in the area for more than three decades. Furthermore, Freitas and others and Lamas and others15,16 reported that 9.33% of the horses in the area were seropositive for SFG. Although the pathogenicity for horses is unknown, they are currently used in urban and marginal areas of Brazil as sentinels of the disease.
In the state of Sonora, Mexico, from 2003 to 2010 more than 600 cases of rickettsial infection have been diagnosed, most of them as a result of R. rickettsii infection of the pediatric population, which were highly lethal. During 2009, the lethality in the same area was 43% of a total of 21 registered cases, especially caused by misdiagnosis of the disease and the consequent lack or delayed application of specific treatment. An aggravating factor is that most people have dogs in their houses in Sonora, where the main rickettsial vector is the brown tick of dogs (Rhipicephalus sanguineus).17
In recent years other species of ticks and fleas,2,18,19 and some new species of Rickettsia have been associated with disease in humans in the Americas.2,20,21 Although the pathogenic mechanisms for most of them is still unknown, the health personnel should be aware of potential rickettsial infections in urban areas where rodents are present. This case illustrates and emphasizes the importance of a good initial clinical description, and the need to correlate it with all the epidemiological information available from the patient, to obtain the most accurate diagnosis in a timely manner. This case also illustrates that zoonotic diseases are not restricted to rural, selvatic areas. Therefore, it is also necessary to take into account the possibility that this and other zoonotic diseases could infect patients even if they came from an urban area. This report urges medical professionals to assist these cases promptly and with proper treatment to avoid unnecessary lethality.
ACKNOWLEDGMENTS
We thank Francisco Vega and Carlos Vargas (University of Costa Rica) for providing excellent support and technical assistance. The authors are also grateful to Marcelo Labruna and his staff (Departamento de Medicina Veterinaria Preventiva e Salude Animal, Universidade de Sao Paulo, SP, and Brazil) for his generous help in confirming Rickettsia identity. We also thank Adriana Troyo and Andres Moreira for providing support in the collection of serum samples from dogs and David Loria for his kind assistance in preparing the manuscript.
Footnotes
Financial support: This research was supported in part by grants from Netropica (grant no.9-N-2008), the Universidad de Costa Rica (project no. 803-A8-127), and by the Faculty of Microbiology, UCR, project ED-541.
Authors' addresses: Ana Patricia Argüello and Patricia Rivera, Hospital Nacional de Niños – Pathology San Jose, San Jose, Costa Rica, E-mails: anapatricia03@yahoo.com.mx and priveram@hnn.sa.cr. Laya Hun and Lizeth Taylor, Universidad de Costa Rica - Centro de Investigación en Enfermedades Tropicales, Departamento de Microbiología e Inmunología, Facultad de Microbiología San Jose, San Jose, Costa Rica, E-mails: ruchlia.hun@ucr.ac.cr and mayra.taylor@ucr.ac.cr.
References
- 1.Toledo RS, Tamekuni K, Filho MF, Haydu VB, Barbieri AR, Hiltel AC, Pacheco RC, Labruna MB, Dumler JS, Vidotto O. Infection by spotted fever rickettsiae in people, dogs, horses and ticks in Londrina, Parana State, Brazil. Zoonoses Public Health. 2011;58:416–423. doi: 10.1111/j.1863-2378.2010.01382.x. [DOI] [PubMed] [Google Scholar]
- 2.Parola P, Labruna MB, Raoult D. Tick-borne rickettsioses in America: unanswered questions and emerging diseases. Curr Infect Dis Rep. 2009;11:40–50. doi: 10.1007/s11908-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 3.Fuentes LG. 1st case of Rocky Mountain fever in Costa Rico, Central America. Rev Latinoam Microbiol. 1979;21:167–172. [PubMed] [Google Scholar]
- 4.Fuentes L, Calderón A, Hun L. Isolation and identification of Rickettsia rickettsii from the rabbit tick (Haemaphysalis leporispalustris) in the Atlantic zone of Costa Rica. Am J Trop Med Hyg. 1985;34:564–567. doi: 10.4269/ajtmh.1985.34.564. [DOI] [PubMed] [Google Scholar]
- 5.Hun L, Cortes X, Taylor L. Molecular characterization of Rickettsia rickettsii isolated from human clinical samples and from the rabbit tick Haemaphysalis leporispalustris collected at different geographic zones in Costa Rica. Am J Trop Med Hyg. 2009;79:899–902. [PubMed] [Google Scholar]
- 6.Walker D, Ismail N. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nature Rev. 2008;6:375–386. doi: 10.1038/nrmicro1866. [DOI] [PubMed] [Google Scholar]
- 7.Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SL, Tamminga CL, Ohl CA. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United State. Clin Infect Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- 8.García-García JC, Portillo A, Núñez MJ, Santibáñez S, Castro B, Oteo JA. Case report: a patient from Argentina infected with Rickettsia massiliae. Am J Trop Med Hyg. 2010;82:691–692. doi: 10.4269/ajtmh.2010.09-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masters EJ, Olson GS, Weiner SJ, Paddock CD. Rocky Mountain spotted fever: a clinician's dilemma. Arch Intern Med. 2003;163:769–774. doi: 10.1001/archinte.163.7.769. [DOI] [PubMed] [Google Scholar]
- 10.Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724–732. doi: 10.1016/S1473-3099(07)70261-X. [DOI] [PubMed] [Google Scholar]
- 11.Lacz NL, Schwartz RA, Kapila R. Rocky Mountain spotted fever. J Eur Acad Dermatol Venereol. 2006;20:411–417. doi: 10.1111/j.1468-3083.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 12.Labruna MB, Whitworth T, Horta MC, Bouyer DH, Mcbride JW, Pinter A, Popov V, Gennari SM, Walker DH. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol. 2004;42:90–98. doi: 10.1128/JCM.42.1.90-98.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horta MC, Labruna MB, Sangioni LA, Vianna MC, Gennari SM, Galvão MA, Mafra CL, Vidotto O, Schumaker TT, Walker DH. Prevalence of antibodies to spotted fever group rickettsiae in humans and domestic animals in a Brazilian spotted fever-endemic area in the state of São Paulo, Brazil: serologic evidence for infection by Rickettsia rickettsii and another spotted fever group Rickettsia. Am J Trop Med Hyg. 2004;71:93–97. [PubMed] [Google Scholar]
- 14.Roux V, Fournier PE, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol. 1996;34:2058–2065. doi: 10.1128/jcm.34.9.2058-2065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freitas MC, Grycajuk M, Molento MB, Bonacin J, Labruna MB, Pacheco Rde C, Moraes-Filho J, Deconto I, Biondo AW. Brazilian spotted fever in cart horses in a non-endemic area in southern Brazil. Rev Bras Parasitol Vet. 2010;19:130–131. doi: 10.4322/rbpv.01902012. [DOI] [PubMed] [Google Scholar]
- 16.Lamas C, Favacho A, Rozental T, Bóia MN, Kirsten AH, Guterres A, Barreira J, de Lemos ER. Characterization of Rickettsia rickettsii in a case of fatal Brazilian spotted fever in the city of Rio de Janeiro, Brazil. Braz J Infect Dis. 2008;12:149–151. doi: 10.1590/s1413-86702008000200010. [DOI] [PubMed] [Google Scholar]
- 17.Álvarez-Hernández G. Rocky Mountain spotted fever: a forgotten epidemic. Salud Publica Mex. 2010;52:1–3. doi: 10.1590/s0036-36342010000100002. [DOI] [PubMed] [Google Scholar]
- 18.Labruna M. Ecology of Rickettsia in South America. Ann N Y Acad Sci. 2009;1166:156–166. doi: 10.1111/j.1749-6632.2009.04516.x. [DOI] [PubMed] [Google Scholar]
- 19.Parola P, Labruna MB, Raoult D. Tick-borne rickettsioses in America: unanswered questions and emerging diseases. Curr Infect Dis Rep. 2009;11:40–50. doi: 10.1007/s11908-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 20.Sangioni LA, Horta MC, Vianna MC, Gennari SM, Soares RM, Galvão MA, Schumaker TT, Ferreira F, Vidotto O, Labruna MB. Rickettsial infections in animals and Brazilian spotted fever endemicity. Emerg Infect Dis. 2005;11:265–270. doi: 10.3201/eid1102.040656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paddock DC, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Okechukwu E, Blass MA, Tamminga CL, Ohl CA, McLellan SL, Goddard G, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremeeva ME. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]