Abstract
We studied the frequency and patient risk factors for postoperative periprosthetic fractures after primary total hip replacement (THR). With a mean follow-up of 6.3 years, 305 postoperative periprosthetic fractures occurred in 14,065 primary THRs. In multivariable-adjusted Cox regression analyses, female gender (Hazard ratio [HR], 1.48;95% confidence interval [CI]:1.17–1.88), Deyo-Charlson comorbidity score of 2 (HR, 1.74 for score of 2;95% CI: 1.25–2.43) or 3 or higher (HR, 1.71;95% CI: 1.26–2.32), ASA class of 2 (HR, 1.84;95% CI: 0.90–3.76), 3 (HR, 2.45;95% CI: 1.18–5.1) or 4 or higher (HR, 2.68;95% CI: 0.70–10.28) were significantly associated with higher risk/hazard and cemented implant with lower hazard (HR, 0.68; 95% CI: 0.54–0.87) of postoperative periprosthetic fractures. Interventions targeted at optimizing comorbidity management may decrease postoperative fractures after THR.
Periprosthetic fracture is a devastating complication of total hip replacement (THR). Periprosthetic fractures in THR patients are associated with increased mortality at 30-days [1] and at 1-year [2]. In addition, periprosthetic fractures following THR are associated with significant morbidity due to accompanying pain, functional limitation and the need for additional surgery. This morbidity and mortality risk is increasingly relevant to both the patients and the surgeons, since the age-range for patients undergoing THR is expanding to include both younger and older patients and the demand for these procedures in increasing [3].
Evidence regarding age and gender as risk factors for periprosthetic femoral fractures after THR is contradictory as reported in a recent review [4]. Studies show that the risk of periprosthetic fractures after THR in females is higher [5, 6] [7, 8], similar [9, 10] or lower [11], compared to men. One large registry study that adjusted for age, gender and primary versus revision surgery, reported that age >70 years [12] and female gender [12] were risk factors for periprosthetic fractures after THR. Old age was associated with higher postoperative fractures in another small retrospective study of 16 periprosthetic fractures [13]. Most evidence comes from several small retrospective studies, most limited by small sizes of <1,000 patients and <20 fractures/series and no adjustment for important covariates or confounders in the analyses [5, 6] [7, 8] [9].
Evidence related to other important risk factors is also limited. A Swedish registry study of 1,049 periprosthetic fractures reported that certain implant types (Charnley and Exeter type implant) had a higher risk of periprosthetic factors following THR [14]; however, this study did not examine patient characteristics as risk factors. Two registry studies reported that previous history of fracture was a risk factors for periprosthetic fracture [10] [15]. These studies had small sample sizes and did not adjust for important covariates/confounders (comorbidity, body mass index etc.).
None of the previous studies have examined comorbidity as risk factor for periprosthetic fractures after THR. With an aging population, increasing comorbidity and its consequences are becoming a real challenge for the health care system. Similarly, with the exception of one underpowered case-control study of 31 periprosthetic fractures that found no association of body mass index (BMI) with risk of periprosthetic fractures in unadjusted analyses [9], no additional studies of obesity as risk factor exist to our knowledge. Consequently, the recent review of periprosthetic fractures by Franklin and Malchau does not mention these risk factors (comorbidity and BMI), likely due to lack of data [4]. The obesity epidemic has substantial impact on patient outcomes and with associated increased risk of cardiovascular disease, its impact on healthcare costs is enormous [16] [17] [18]. It is important to understand the impact of comorbidity and BMI on an important complication such as periprosthetic fracture, since the indication of THR is expanding to include a broader range of patients across strata of age, BMI and comorbidity load. Therefore, well-powered studies examining these and other patient risk factors for periprosthetic fractures following THR are needed.
Our objective was to examine patient risk factors for periprosthetic fractures, while controlling for important covariates and confounders. Using 20-year data from the AA Total Joint Registry, which captures data prospectively, we studied the frequency of postoperative periprosthetic fractures after primary THR. We examined whether key demographic (age, gender) and clinical characteristics (body mass index, comorbidity) of interest were associated with the risk of postoperative periprosthetic fractures after THR.
Methods
Source population
The study cohort consisted of every primary THR performed at the AA medical center between 1989 and 2008. The total joint registry at the AA medical center has captured outcomes including revision, fracture and others are captured for every patient who underwent joint replacement since the beginning of THR in 1969 and TKR in 1971 [19]. We chose this 20-year time period (1989–2008) to have a more recent sample of patients with large sample of patients at risk, since the surgical techniques for hip arthroplasty have not changed dramatically in this period and this time period would allow us to have enough numbers of periprosthetic fractures to perform meaningful analyses, since periprosthetic fractures are infrequent. Another advantage of using data from this period was the availability of two additional important variables (body mass index (BMI) and American Society of Anesthesiologist (ASA) class) in institutional electronic datasets since 1989.
Predictor variables
In addition to key demographic and clinical variables, we examined diagnosis and implant fixation as potential risk factors for postoperative periprosthetic fractures following primary THR. Demographics included gender and age, as categorized in previous studies (≤60, 61–70, 71–80 and >80 years) [20–22]. Clinical variables included body mass index (BMI), American Society of Anesthesiology (ASA) and comorbidity measured with Deyo-Charlson index. BMI was categorized as <25, overweight, 25–29.9, obese and very obese, 30–39.9, or extremely obese, ≥40 as previously [20], as per WHO classification [23]. ASA score, categorized as class I-II vs. III-IV [24], a validated measure of peri-operative mortality and immediate post-operative morbidity [24, 25], was retrieved by a database managed by the Department of Anesthesiology. Deyo-Charlson index [26], a validated measure of comorbidity, consists of a weighted scale of 19 comorbidities (including cardiac, pulmonary, renal, hepatic disease, diabetes, cancer, hemiplegia, HIV etc.), expressed as a summative score [27, 28]. Deyo-Charlson is the most commonly used comorbidity index in the medical literature, which is predictive of patient morbidity and mortality in general populations. A complete list of comorbidities included in Deyo-Charlson index is shown in Appendix 1. A history of previous thromboembolic event (occurrence of deep vein thrombosis or pulmonary embolism; yes/no) or previous major cardiac event (occurrence of arrhythmia, myocardial infarction or congestive heart failure; yes/no), two common complications of THR, were also assessed as predictors. Diagnosis was categorized as osteoarthritis, rheumatoid/inflammatory arthritis, and avascular necrosis versus other. Implant fixation was categorized as cemented (includes hybrid) or uncemented implant.
Study Outcome
The main outcome of the study was the occurrence of postoperative periprosthetic fracture from the first postoperative day onwards, which is recorded for every patient post-THR. Since intraoperative fractures are likely to have different mechanism compared to postoperative fractures, we studied post-operative periprosthetic fractures only. To avoid misclassification of intraoperative fractures on the day of THR as postoperative fractures (and increase specificity) due to errors in recording of the exact time of the fracture, same day fractures were excluded.
Statistical Analyses
Summary statistics were calculated for patient characteristics as mean (standard deviation (SD)) or proportions. We performed univariate Cox regression analyses of each of the nine independent variables of interest and postoperative periprosthetic fracture after primary THR. The independent variables were gender, age, BMI, Deyo-Charlson index, ASA class, underlying diagnosis, implant fixation, previous cardiac event and previous thromboembolic disease (categorized as in predictor variables section). We included the variables significantly associated in univariate regression with p<0.05 in a backward selection multivariable-adjusted Cox regression model (model 1); this model retained only those variables that were significant with p<0.05. Sensitivity analysis was performed by including (forcing) all variables with p-value <0.20 from univariate analyses in a multivariable Cox regression analysis (model 2). Hazard ratios (HR) and 95% confidence intervals (CI) are presented. We performed additional sensitivity analyses to assess if the association of risk factors, such as demographic variables or cement status differs by the time of periprosthetic fracture, by repeating model 1 for two time frames: (1) fractures within 1 year; and (2) fractures >1 year later.
Results
The study cohort included 11,772 patients with 14,065 primary THRs. Mean age at surgery was 65 years, BMI was 29 kg/m2 and mean follow-up was 6.3 years (Table 1). Forty eight percent were men, 16% underwent bilateral THR, 9% were older than 80 years, 18% had Deyo-Charlson index of 3 or more and 39% were ASA class 3 or higher. Osteoarthritis was the underlying diagnosis in 87% and 63% had uncemented implant fixation.
Table 1.
Demographic Features of Study Cohort as mean (standard deviation) of n (%)
| Primary THR (n=14,065) | |
|---|---|
| Mean Follow-up in years | 6.3 (4.7) |
| Male/Female | 6,819 (48.5%)/7,246 (51.5%) |
| % bilateral | 2,293 (16.3%) |
| Age at Surgery in years | 64.6 (13.7) |
| Age Category | |
| ≤60 years | 4,455 (31.7%) |
| 61–70 years | 4,252 (30.2%) |
| 71–80 years | 4,119 (29.3%) |
| >80 years | 1,239 (8.8%) |
| Body Mass Index (BMI) in kg/m2 | 29.0 (5.8) |
| BMI Category | |
| Missing | 72 (0.5%) |
| Normal < 25.0 kg/m2 | 3,429 (24.5%) |
| Overweight 25–29.9 kg/m2 | 5,334 (38.1%) |
| Obese, 30–39.9 kg/m2 | 4,589 (32.8%) |
| Morbidly Obese, III ≥ 40.0 kg/m2 | 641 (4.6%) |
| American Society of Anesthesiologists (ASA) Class | |
| 1 | 653 (4.7%) |
| 2 | 7,947 (56.7%) |
| 3 | 5,254 (37.5%) |
| 4 | 151 (1.1%) |
| Mean Deyo- Charlson Index | 1.3 (2.2) |
| Sum of comorbidities on Deyo-Charlson Index | |
| 0 | 7,520 (53.5%) |
| 1 | 2,393 (17%) |
| 2 | 1,640 (11.7%) |
| 3+ | 2,512 (17.9%) |
| Prior Cardiac Event | |
| No | 11,879 (84.5%) |
| Yes | 2,186 (15.5%) |
| Prior Thromboembolic Event | |
| No | 13,574 (96.5%) |
| Yes | 491 (3.5%) |
| Diagnosisa | |
| Osteoarthritis | 12,252 (87.1%) |
| Rheumatoid and inflammatory arthritis | 371 (2.6%) |
| Avascular necrosis | 1,021 (7.3%) |
| Other | 421 (3%) |
| Implant Fixation | |
| Uncemented | 8,796 (62.5%) |
| Cemented or hybrid* | 5,269 (37.5%) |
Rheumatoid and inflammatory arthritis category included other inflammatory arthritis such as psoriatic arthritis and ankylosing spondylitis etc.
Other diagnoses present in 421 hips included the following: Hip dysplasia, Legg-Perthe’s, disease, Slipped capital femoral epiphyses, failed previous osteotomy, failed previous arthrodeses, failed previous internal fixation, congenital dislocation of hip (CDH), pigmented villonodular synovitis, hemacrhomatosis, synovial chondromatosis among others ASA class was missing in 60 (0.4%) THRs
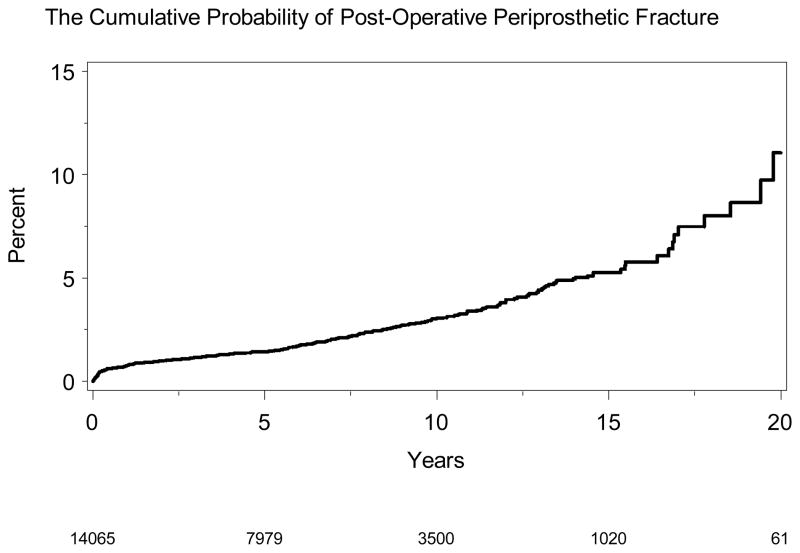
We noted 305 post-operative fractures after primary THR, which constituted our analytic dataset (Table 2). Majority of the periprosthetic fractures occurred later than 1-year after primary THR. Periprosthetic-free survival at different time-points was as follows: 1-year, 99.2% (95% CI, 99.1–99.4); 2-years, 99.0% (95% CI, 98.8–99.2); 5-years, 98.6% (95% CI, 98.4–98.8); 10-years, 97.0% (95% CI, 96.6–97.4) and 20-years, 88.9% (95% CI, 85.0–93.1). The cumulative probability of periprosthetic fracture is shown in Figure 1.
Table 2.
Cumulative incidence of postoperative peri-prosthetic fractures
| Up to 1 year (n=102) | >1 year (n=203) | Total (≥Day 1) | |||||
|---|---|---|---|---|---|---|---|
| Day 1–30 N (% of Totala) |
Day 31–90 N (% of Totala) |
Day 91–365 N (% of Totala) |
>1–2 yrs N (% of Totala) |
>2 to 5 yrs N (% of Totala) |
>5 to 10 yrs N (% of Totala) |
>10 years N (% of Totala) |
N (% of Totala) |
| 26 (8%) | 39 (13%) | 37 (12%) | 28 (10%) | 42 (14%) | 81 (27%) | 52 (17%) | 305 (100%) |
Figure 1. Cumulative probability of postoperative periprosthetic fracture after primary THA.
X-axis shows follow-up time in years and y-axis % of postoperative periprosthetic fractures. The legend at the bottom indicates the number of THAs under observation, for example 7979 THAs were under observations at 5-years, and 3500 THAs at 10-years
Risk factors for Post-operative Periprosthetic Fractures
In univariate Cox regression analyses, we found that female gender, older age, higher Deyo-Charlson index, operative diagnosis, implant fixation and ASA class were each significantly associated with higher hazard of postoperative periprosthetic fractures after primary THR (all p-values <0.05; Table 3). BMI was not significantly associated.
Table 3.
Univariate and Multivariable-adjusted Hazard (models 1 and 2) of postoperative periprosthetic fracture following Primary Total Hip Replacement using Cox regression analysis
| Variable | Total (n=14,065) | Periprosthetic Fractures (n=305) | Univariate Hazard Ratio (95% CI) | Multivariablea model 1 Hazard Ratio (95% CI) | Multivariableb model 2 Hazard Ratio (95% CI) |
|---|---|---|---|---|---|
| Gender | p=0.002 | p=0.001 | p=0.001 | ||
| Male | 6,819 | 117 (2%) | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
| Female | 7,246 | 188 (3%) | 1.44 (1.15, 1.82) | 1.48 (1.17, 1.88) | 1.49 (1.18, 1.89) |
| Age Category (years) | p=0.02 | p=0.06 | |||
| ≤60 | 4,455 | 104 (2%) | 1.00 (REF) | 1.00 (REF) | |
| 61–70 | 4,252 | 98 (2%) | 0.88 (0.67, 1.17) | 0.91 (0.68, 1.22) | |
| 71–80 | 4,119 | 71 (2%) | 0.77 (0.57, 1.04) | 0.78 (0.55, 1.10) | |
| >80 | 1,239 | 32 (3%) | 1.45 (0.97, 2.15) | 1.37 (0.88, 2.14) | |
| Body Mass Index, kg/m2 | p=0.25 | ||||
| Normal, < 25.0 | 3,429 | 88 (3%) | 1.00 (REF) | ||
| Overweight, 25–29.9 | 5,334 | 108 (2%) | 0.84 (0.63, 1.12) | ||
| Obese, 30–39.9 | 4,589 | 94 (2%) | 1.12 (0.62, 2.01) | ||
| Morbidly Obese, ≥40.0 | 641 | 13 (2%) | 0.78 (0.59, 1.03) | ||
| Deyo-Charlson Index | p<0.001 | p<0.001 | p<0.001 | ||
| 0 | 7,520 | 136 (2%) | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
| 1 | 2,393 | 49 (2%) | 1.17 (0.84, 1.62) | 1.04 (0.74, 1.46) | 1.03 (0.73, 1.45) |
| 2 | 1,640 | 51 (3%) | 1.82 (1.32, 2.52) | 1.74 (1.25, 2.43) | 1.74 (1.24, 2.43) |
| 3+ | 2,512 | 69 (3%) | 1.85 (1.38, 2.47) | 1.71 (1.26, 2.32) | 1.69 (1.23, 2.31) |
| Operative Diagnosis | p<0.001 | p<0.001 | p<0.001 | ||
| Osteoarthritis | 12,252 | 235 (2%) | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
| Rheumatoid arthritis | 371 | 15 (4%) | 1.54 (1.06, 2.23) | 1.36 (0.78, 2.36) | 1.39 (0.95, 2.03) |
| Avascular necrosis | 1,021 | 32 (3%) | 2.55 (1.66, 3.91) | 2.58 (1.67, 3.98) | 2.51 (1.61, 3.89) |
| Other | 421 | 23 (5%) | 1.76 (1.05, 2.97) | 1.42 (0.98, 2.01) | 1.37 (0.78, 2.39) |
| Cemented | p=0.01 | p=0.002 | p=0.005 | ||
| Uncemented | 8,796 | 190 (2%) | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
| Cemented | 5,269 | 115 (2%) | 0.75 (0.59, 0.94) | 0.68 (0.54, 0.87) | 0.69 (0.54, 0.89) |
| ASA class | p=0.006 | p=0.03 | p=0.04 | ||
| 1 | 653 | 8 (1%) | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
| 2 | 7,947 | 161 (2%) | 1.73 (0.85, 3.51) | 1.84 (0.9, 3.76) | 1.90 (0.92, 3.90) |
| 3 | 5,254 | 131 (2%) | 2.44 (1.19, 4.98) | 2.45 (1.18, 5.1) | 2.48 (1.18, 5.21) |
| 4 | 151 | 3 (2%) | 2.69 (0.71, 10.15) | 2.68 (0.7, 10.28) | 2.54 (0.65, 9.87) |
| Prior Cardiac event | p=0.08 | p=0.37 | |||
| No | 11,879 | 255 (2%) | 1.00 (REF) | 1.00 (REF) | |
| Yes | 2,186 | 50 (2%) | 1.31 (0.97, 1.78) | 1.16 (0.84, 1.62) | |
| Prior Thromboembolic Event | p=0.20 | ||||
| No | 13,574 | 293 (2%) | 1.00 (REF) | ||
| Yes | 491 | 12 (2%) | 1.46 (0.82, 2.59) |
p-values indicate the overall significance of the variable, for example, in model 1, ASA class was significantly associated with risk of fractures with p value of 0.03.
Multivariable model 1 was a Cox regression model that considered all significant variables from the univariate analyses with p-value <0.05 and retained only those significantly associated in the multivariable model by using a backward selection process; the c-statistic of the multivariable model was 0.64
Multivariable model 2 was a Cox regression model obtained by retaining all variables from the univariate analyses with p-value <0.20 and forcing all of them in the model; the c-statistic of the multivariable model was 0.64
ASA, American Society of Anesthesiologists; CI, confidence interval, REF, reference category; p, p-value
In multivariable-adjusted model (model 1), age was no longer significant. Women had 48% higher hazard/risk of postoperative periprosthetic fracture after primary THR compared to men (p=0.001; Table 3). A higher Deyo-Charlson index almost doubled the risk of fracture (p<0.001). Higher ASA class of 3 or 4 also doubled the risk (p=0.03). Cemented implants were associated with 30% lower risk of fractures (p=0.002; Table 3). Diagnosis was significantly associated with risk of postoperative periprosthetic fractures (p<0.001). In particular, diagnosis of avascular necrosis was associated with 160% higher hazard of periprosthetic fractures. Sensitivity analyses using a multivariable regression that retained all variables with p-value <0.20 (model 2) showed the same results as the model 1 above. There was no change in significance of the variables and minimal attenuation of hazards ratios (HR).
Sensitivity analyses were performed to examine if the association of factors differed by time, by reanalyzing data split into fractures up to 1 year and those at >1 year. Gender was significantly associated with the higher risk of periprosthetic fractures within 1 year with HR, 2.61 [95% CI, 1.68, 4.05] but not at >1 year with HR of 1.13 (95% CI, 0.85, 1.50). Deyo-Charlson index was significantly associated at both within 1 year and >1 year. Cemented implant was only significantly associated with lower risk of periprosthetic fractures at >1 year with HR of 0.63 (95% CI, 0.47–0.85) and not associated at follow-up to 1-year, HR 1.04 (95% CI, 0.67–1.60). Diagnosis was associated significantly at >1 year, but not up to 1 year.
Discussion
In this study, we reported the frequency and predictors of postoperative periprosthetic fracture in 11,772 patients with 14,065 primary THRs from our medical center. We found that female gender, Deyo-Charlson index of 3 or higher and ASA class of 3 or 4 were each independently associated with 1.5–2.5 times higher risk of periprosthetic fractures after primary THR. Obesity was not associated with risk of periprosthetic factors. Cemented implants were associated with 30% lower risk of fractures. Several findings deserve further discussion.
We found that higher comorbidity as assessed by Deyo-Charlson index of 2 or 3 or higher were each independently associated with twice the risk of periprosthetic fracture after primary THR, compared to patient with Deyo-Charlson index of 0. To our knowledge, none of the previous studies in primary THR have reported this association, which may partially be due to lack of use of validated comorbidity measures such as Deyo-Charlson index in previous studies. This finding adds to the literature. This finding has practical application since Deyo-Charlson index is easy calculable at preoperative evaluation, and patients with high comorbidity can be made aware of higher risk of periprosthetic fractures. It is important to note that the overall prevalence of periprosthetic fracture is low, so the absolute risk is still low even in patients with higher comorbidity.
An important negative finding of our study was the lack of association of BMI and periprosthetic fracture. A small case-control study of 31 fractures from the Finnish register also failed to find any association between BMI and periprosthetic fractures [9]; however, the lack of association in this previous study could have been due to lack of power (n=31). Our study included >14,000 THRs and >300 periprosthetic fractures, with analyses that controlled for important covariates and confounding factors and confirmed this finding. Due to a large sample size, our study had adequate power and therefore we are confident that this is not a false negative finding. This is an important finding, implying that obesity should not be viewed as a risk factor for periprosthetic factures after THR.
An ASA class of 3 or higher was independently associated with higher risk of periprosthetic fracture, a novel finding from our study. We are not aware of any THR studies that have reported this association in multivariable-adjusted analyses. It is well-known that ASA class correlates well with perioperative mortality. ASA is assessed preoperatively in all patients undergoing replacement and therefore it could be factored in assessing the risk of periprosthetic fracture. Higher ASA and higher Deyo-Charlson index indicate more frailty, which may be partially responsible for higher risk of periprosthetic fracture. Although, these measures have some correlation, we have found that measures are not collinear (correlation coefficient ranges 0.30–0.35), i.e., it is statistically valid to use both ASA class and Deyo-Charlson comorbidity index in the analyses, since both provide unique information.
In our study, female gender was independently associated with 50% higher risk of periprosthetic fracture in multivariable-adjusted models that controlled for all significant factors. This may be due to higher prevalence of osteoporosis in women and differences in bone structure. Our findings confirm findings of higher risk of periprosthetic fractures in women in previous retrospective case-control studies [5, 6] [7, 8]. Our study used prospectively collected data in a much large cohort (3–10 times larger) and adjusted for several important variables. One small case-control study of 31 periprosthetic fractures from the Finnish registers found no gender association [9], where as one study from Scottish registry reported lower risk in females [12].
Cement fixation was a significant risk factor for periprosthetic factor, with lower risk of periprosthetic fracture in those with a cemented implant. This may be a consequence of the effort to obtain a sufficient press-fit to gain initial stem stability [29]. Previously, implant type has been reported to be significantly associated with risk of periprosthetic fractures [14]. The increased risk of fracture with uncemented implants was evident at 1 year or later follow-up after primary THA.
The cumulative frequency of postoperative periprosthetic fractures was 2.1% in our 20-year study with a mean follow-up of 6.3 years. It is similar to cumulative incidences of 2.5% at 11 years in 1,442 primary cemented THAs [30] and 2.3% in 6458 primary THAs for non-trauma indications in a 17-year study [31], reported in two other studies. The annual incidence of periprosthetic hip fracture of 0.1% after primary THR from the Swedish National register [10], is not dissimilar to our finding of cumulative incidence of 2.1% over a 20-year period. A rising prevalence of periprosthetic has been suspected with expanding indications for replacement. Our finding of 2.1% cumulative prevalence of periprosthetic fractures after primary THR of patients from 1989–2008 compared to 0.6% in 17,579 primary THR at our institution from 1969–1990 [32] indicates that the incidence of periprosthetic fracture may be rising. More carefully planned time-series studies accounting for important confounders are needed to test this hypothesis.
Our study has several limitations. Our estimates may be somewhat conservative due to loss to follow-up of patients, who might seek health care elsewhere for their fractures; however, this loss to follow-up is minimized by systematic intensive follow-up by dedicated total joint registry staff, who contact patients not returning for follow-up visits by performing phone interviews and sending letters and surveys to patients assessing for major complications such as fractures. It remains to be seen if these results can be replicated in other large registries that have information on the variables of interest. Our study may have residual confounding despite our ability to control for several important patient-level and comorbidity characteristics. We did not analyze the type of according to the Vancouver classification, since that was not the focus of this paper. Our study has several strengths, including a large sample size, prospective data collection by dedicated registry staff, ability to control for BMI and comorbidity, use of multivariable-adjusted estimates and robust results that did not change with sensitivity analyses.
In summary, in this 20-year study of prospectively collected data, we found that several important patient factors were associated with higher risk of postoperative periprosthetic fractures. Female gender, higher comorbidity, worse ASA class and cementless implants were associated with a higher risk of periprosthetic fractures. Obesity was not a risk factor for periprosthetic fractures. Interventions targeting and optimizing comorbidity management in patients with primary THR may reduce the risk of periprosthetic fractures. Future studies need to examine the mechanism by which these risk factors increase the risk of periprosthetic fractures. A better understanding of underlying reasons can pave the path for interventions to reduce the risk of periprosthetic fractures.
Acknowledgments
We thank Youlonda Lochler for assistance in identifying the study cohort.
“The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parvizi J, Ereth MH, Lewallen DG. Thirty-day mortality following hip arthroplasty for acute fracture. J Bone Joint Surg Am. 86-A(9):1983–2004. doi: 10.2106/00004623-200409000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya T, Chang D, Meigs JB, Estok DM, 2nd, Malchau H. Mortality after periprosthetic fracture of the femur. J Bone Joint Surg Am. 2007;89(12):2658. doi: 10.2106/JBJS.F.01538. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 4.Franklin J, Malchau H. Risk factors for periprosthetic femoral fracture. Injury. 2007;38(6):655. doi: 10.1016/j.injury.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996;(327):238. doi: 10.1097/00003086-199606000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Bethea JS, 3rd, DeAndrade JR, Fleming LL, Lindenbaum SD, Welch RB. Proximal femoral fractures following total hip arthroplasty. Clin Orthop Relat Res. 1982;(170):95. [PubMed] [Google Scholar]
- 7.Johansson JE, McBroom R, Barrington TW, Hunter GA. Fracture of the ipsilateral femur in patients wih total hip replacement. J Bone Joint Surg Am. 1981;63(9):1435. [PubMed] [Google Scholar]
- 8.Whittaker RP, Sotos LN, Ralston EL. Fractures of the femur about femoral endoprostheses. J Trauma. 1974;14(8):675. doi: 10.1097/00005373-197408000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Sarvilinna R, Huhtala HS, Sovelius RT, Halonen PJ, Nevalainen JK, Pajamaki KJ. Factors predisposing to periprosthetic fracture after hip arthroplasty: a case (n = 31)-control study. Acta Orthop Scand. 2004;75(1):16. doi: 10.1080/00016470410001708030. [DOI] [PubMed] [Google Scholar]
- 10.Lindahl H, Garellick G, Regner H, Herberts P, Malchau H. Three hundred and twenty-one periprosthetic femoral fractures. J Bone Joint Surg Am. 2006;88(6):1215. doi: 10.2106/JBJS.E.00457. [DOI] [PubMed] [Google Scholar]
- 11.Lindahl H, Eisler T, Aea Oden. Risk factors associated with the late periprosthetic femoral fractures. In: LIndahl H, editor. The periprosthetic femur fracture: a study from the Swedisj National Hip Arthroplasty Register. Department of Orthopaedics, Sahlgenska Academy; Goteberg: Goteberg University; 2006. [Google Scholar]
- 12.Meek RM, Norwood T, Smith R, Brenkel IJ, Howie CR. The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br. 2011;93(1):96. doi: 10.1302/0301-620X.93B1.25087. [DOI] [PubMed] [Google Scholar]
- 13.Wu CC, Au MK, Wu SS, Lin LC. Risk factors for postoperative femoral fracture in cementless hip arthroplasty. J Formos Med Assoc. 1999;98(3):190. [PubMed] [Google Scholar]
- 14.Lindahl H, Malchau H, Herberts P, Garellick G. Periprosthetic femoral fractures classification and demographics of 1049 periprosthetic femoral fractures from the Swedish National Hip Arthroplasty Register. J Arthroplasty. 2005;20(7):857. doi: 10.1016/j.arth.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Gjertsen JE, Lie SA, Fevang JM, Havelin LI, Engesaeter LB, Vinje T, Furnes O. Total hip replacement after femoral neck fractures in elderly patients: results of 8,577 fractures reported to the Norwegian Arthroplasty Register. Acta Orthop. 2007;78(4):491. doi: 10.1080/17453670710014130. [DOI] [PubMed] [Google Scholar]
- 16.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 17.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298(17):2020. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 18.Long DA, Reed R, Lehman G. The cost of lifestyle health risks: obesity. J Occup Environ Med. 2006;48(3):244. doi: 10.1097/01.jom.0000201568.73562.a2. [DOI] [PubMed] [Google Scholar]
- 19.Berry DJ, Kessler M, Morrey BF. Maintaining a hip registry for 25 years. Mayo Clinic experience. Clin Orthop Relat Res. 1997;(344):61. doi: 10.1097/00003086-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Singh JA, Gabriel SE, Lewallen DG. Higher Body Mass Index Is Not Associated With Worse Pain Outcomes After Primary or Revision Total Knee Arthroplasty. J Arthroplasty. 2010 doi: 10.1016/j.arth.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh JA, Lewallen D. Age, gender, obesity, and depression are associated with patient-related pain and function outcome after revision total hip arthroplasty. Clin Rheumatol. 2009;28(12):1419. doi: 10.1007/s10067-009-1267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh JA, Gabriel S, Lewallen D. The impact of gender, age, and preoperative pain severity on pain after TKA. Clin Orthop Relat Res. 2008;466(11):2717. doi: 10.1007/s11999-008-0399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Obesity; preventing and managing the global epidemic. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 24.Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA. 1961;178:261. doi: 10.1001/jama.1961.03040420001001. [DOI] [PubMed] [Google Scholar]
- 25.Weaver F, Hynes D, Hopkinson W, Wixson R, Khuri S, Daley J, Henderson WG. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18(6):693. doi: 10.1016/s0883-5403(03)00259-6. [DOI] [PubMed] [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Sax FL, MacKenzie CR, Braham RL, Fields SD, Douglas RG., Jr Morbidity during hospitalization: can we predict it? J Chronic Dis. 1987;40(7):705. doi: 10.1016/0021-9681(87)90107-x. [DOI] [PubMed] [Google Scholar]
- 29.Lindahl H. Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury. 2007;38(6):651. doi: 10.1016/j.injury.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 30.Lowenhielm G, Hansson LI, Karrholm J. Fracture of the lower extremity after total hip replacement. Arch Orthop Trauma Surg. 1989;108(3):141. doi: 10.1007/BF00934256. [DOI] [PubMed] [Google Scholar]
- 31.Cook RE, Jenkins PJ, Walmsley PJ, Patton JT, Robinson CM. Risk factors for periprosthetic fractures of the hip: a survivorship analysis. Clin Orthop Relat Res. 2008;466(7):1652. doi: 10.1007/s11999-008-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewallen DG, Berry DJ. Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect. 1998;47:243. [PubMed] [Google Scholar]