Abstract
Background and Aims
Previously, we reported that exposure of hepatitis C virus (HCV) core expressing ethanol-metabolizing cells to ethanol significantly suppresses proteasome activity which exists as 26S (20S and 19S) and as an unassociated 20S particle. The replacement of the constitutive proteasomal subunits with immunoproteasome (IPR) favors antigen processing. Here, we examined the effects of ethanol consumption by HCV core transgenic mice on proteasome activity in hepatocytic lysates and in partially purified 26S proteasome and the impact of these changes on antigen presentation.
Methods
HCV- and HCV+ core transgenic mice were fed chow diet with or without 20% (v/v) ethanol in water for 4 weeks. Following the feeding regimen, hepatocytes were isolated and examined for chymotrypsin-like proteasome activity, oxidative stress and the presentation of SIINFEKL-H2Kb complex. Additionally the constitutive proteasome and IPR were purified for further analysis and identification of proteasome-interacting proteins (PIPs).
Results
Ethanol significantly decreased proteasome activity in hepatocytes of HCV+ mice and this finding correlated with oxidative stress and dysregulated methylation reactions. In isolated 26S proteasome, ethanol suppressed proteasome activity equally in HCV+ and HCV− mice. Ethanol feeding caused proteasome instability and lowered the content of both constitutive and IPR subunits in the 20S proteasome. In addition, the level of other PIPs, PA28 and UCHL5, were also suppressed after ethanol exposure. Furthermore, in ethanol-fed mice and especially, in HCV+ mice, the presentation of SIINFEKL-H2Kb complex in hepatocytes was also decreased.
Conclusion
Proteasomal dysfunction induced by ethanol feeding and exacerbated by the presence of HCV structural proteins led to suppression of SIINFEKL-H2Kb presentation in hepatocytes.
Keywords: Hepatitis C virus, proteasome interacting proteins, methylation reactions, oxidative stress, antigen presentation
Approximately 170 million individuals worldwide have a chronic hepatitis C viral (HCV) infection. Chronic viral hepatitis is a potential risk for cirrhotic liver disease and life-threatening complications of portal hypertension and hepatocellular carcinoma (Armstrong et al., 2006). Alcohol abuse accelerates the pathogenesis of HCV infection by increasing oxidative stress, fibrosis progression and the risk of death from cirrhosis. However, the exact pathogenic pattern of alcohol-aggravating effects on HCV infection is not yet clear.
HCV persistence in hepatocytes depends on the ability of the immune system to clear the virus. Specifically, induction of the immune response and the presentation of MHC class I-peptide complexes on the target infected hepatocytes are required for elimination of these infected hepatocytes. HCV negatively affects antigen presentation by altering the function of professional antigen presenting cells (APC), which are dendritic cells and macrophages (Dolganiuc and Szabo, 2011; Rehermann, 2009). Furthermore, alcohol exacerbates the effects of HCV by inducing multiple immune dysfunctions (Siu et al., 2009; Szabo et al., 2004; Szabo et al., 2010). However, while the effects of HCV and ethanol on functioning of the effector immune cells are well-established, it is still unclear whether the combination of HCV and ethanol alters MHC class I-restricted antigen presentation on HCV+ hepatocytes. Examination of hepatocytes in this context is important because, while these cells do not prime the immune response, they serve as the targets for alcohol-modified immune interventions. Further, unlike professional APC, hepatocytes enclose replicating HCV, express high levels of HCV proteins and metabolize ethanol, which creates a prime site for the synergism between HCV and ethanol metabolism in these cells.
MHC class I-restricted antigen presentation involves several steps. The most upstream step is antigen processing, where viral proteins are cleaved to short peptides by the proteasome. Only these 8–10 aa peptides can be built into the MHC class I groove. Our long-term interest in HCV studies is focused on proteasome function.
In vivo, the proteasome exists as a 26S particle (20S catalytic core and 19S caps) and as an unassociated 20S particle that is catalytically active. The 26S proteasome degrades ubiquitylated proteins and is very sensitive to oxidative stress, which causes dissociation of the 19S caps from the 20S proteolytic core (Nishizawa-Yokoi et al., 2010). The 20S proteasome degrades only un-ubiquitylated proteins and is more resistant to oxidative stress. The 20S proteasome consists of outer α-subunits and inner β-subunits in a cylindrical-shaped particle. β-subunits catalyze protein degradation and may be constitutive or specific immunoproteasome (IPR) subunits. Replacement of constitutive β subunits with IPR is crucial for maturation and for cleavage of peptides for MHC class I-restricted antigen presentation (Groettrup et al., 1997; Heink et al., 2005; Seifert and Kruger, 2008). LMP7 subunit (β5i) possesses a unique chymotrypsin-like (Cht-L) activity, which is necessary for effective generation of antigenic peptides. The existence of typical for liver mixed (“intermediate”) proteasome with partial incorporation of immunoproteasome subunits broadens the variety of generated antigenic peptides that form a complex with MHC class I to be recognized by cytotoxic T lymphocytes (CTL) (Guillaume et al., 2010).
As revealed from our previous in vitro studies, ethanol metabolism suppresses proteasome function and impairs peptide processing as well as the presentation of peptide-MHC class I complex in HCV-non-expressing hepatocytes and hepatoma cells (Osna, 2009; Osna and Donohue, 2007). Furthermore, using an in vitro Huh7 HCV core+/CYP2E1+ cell line, we recently showed that proteasome activity is regulated by a dual mechanism of HCV-mediated proteasome activation via core protein-induced oxidant generation as well as by interaction between HCV core protein and the PA28–20S proteasome complex. By itself, HCV core protein elevates intracellular proteasome activity, while exposure to ethanol of core-positive cells decreases the enzyme activity (Osna et al., 2008). However, it is unclear whether this phenomenon can be reproduced in vivo in mice that express HCV structural proteins.
Here, we examined whether ethanol feeding to HCV structural protein+ mice affected proteasome catalytic activity, its composition and the presentation of peptide-MHC class I complex in hepatocytes. We hypothesized that feeding 20% ethanol in the drinking water would suppress proteasome function in HCV+ mice, dysregulate antigen processing and thereby decrease presentation of antigenic peptide-MHC class I complex on the surface of hepatocytes, the major targets for CTLs.
MATERIALS AND METHODS
Mice and ethanol feeding
We used transgenic C57Bl/6 mice with the transgene, pAlbSVPA-HCV-S, containing the structural genes (core, E1, E2 and p7) of HCV genotype 1b, under the control of the albumin promoter/enhancer. Tg mice exhibit changes in the mitochondrial redox state, which inhibits mitochondrial complex I activity, resulting in excess ROS production and depletion of mitochondrial glutathione (Korenaga et al., 2005). Female and male HCV- expressing Tg mice were bred at our VA Animal facility (Omaha, NE) by back-crossing with wild type C57Bl/6 mice, purchased from the Jackson Laboratory. Mice were treated in accordance with criteria outlined in the “Guide for the Care and Use of Laboratory Animals”. Core protein expression in mice was routinely measured by detection of HCV DNA in tail snips using PCR.
Ethanol feeding
We used four experimental groups: HCV+ mice and their HCV−littermates were each given water (control) or 20% ethanol in water (ethanol-fed) (n=5 per group). All mice were fed Purina diet ad lib (Song et al., 2002). After 5 weeks of feeding, the animals were euthanized. Hepatocytes were isolated from each animal by collagenase perfusion and then either plated on collagen-coated plates or used directly for analysis.
Expression of HCV proteins in cells was confirmed by Western blot using antibody to HCV core protein (clone 57–50, Thermo Scientific, Rockford, IL).
Oxidative stress-related parameters were examined in hepatocytes. CYP2E1 activity was measured spectrophotometrically by formation of 4-nitrocatechol (Osna et al., 2003). Pro-oxidant formation was documented by measuring MDA by thiobarbituric acid-reactive substances (TBARS) using a kit (Cayman Chemical Company, Ann Arbor, MI). Reactive oxygen species (ROS) were measured by detection with 2’7’dichlorodihydrofluorescein diacetate (DCDFA) (Otani et al., 2005). Anti-oxidant defense was quantified by measuring glutathione levels using the enzymatic recycling method (Tietze, 1969).
Cellular methylation potential was measured by determining hepatocellular SAM:SAH ratios (Kharbanda et al., 2007). The lysine methyltransferase activity was determined by quantifying methylation of lysine residues on partially purified 26S proteasome by Western Blot (WB) analysis using a specific methyl-lysine antibody (Abcam, Cambridge, MA).
Proteasome activity was measured in hepatocyte lysates as well as in 26S proteasome purified from livers of control and ethanol-fed mice. The ChT-like peptidase activity of both the 20S and 26S proteasome was detected by in vitro fluorometric assay as described (Osna et al., 2007). For 26S proteasome purification from mouse livers, we used a non-denaturating, multiple centrifugation procedure, which preserves proteasome-interacting proteins (PIPs) (Bousquet-Dubouch et al., 2009). We collected the fractions with the highest proteasome activity.
The amounts of 20S proteasome (α-subunits and constitutive β-subunits), IPR subunits, proteasome activator PA28α and other proteasome interacting proteins (PIPs) were measured in the peak fractions by WB using either Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE) or ECL-based WB (Bousquet-Dubouch et al., 2009; Osna et al., 2007).
Presentation of the peptide-MHC class I complex on HCV+ and HCV− hepatocytes derived from control- and ethanol-fed mice was determined after delivery of SIINFEKL-TE peptide to cytoplasm by Chariot TM and quantification with specific antibody of SIINFEKL-H2Kb complex expression by flow cytometry as previously described (Osna et al., 2009).
Statistical analyses
Data are expressed as mean values ± standard deviation. Comparisons among multiple groups were determined by one-way ANOVA, using a Tukey post-hoc test. For comparisons between two groups, we used Student’s t-test. A probability value of 0.05 or less was considered significant.
RESULTS
EtOH feeding of mice: ALT and triglyceride levels
Feeding with ethanol in water had no effect on ALT levels in both HCV + and HCV- mice. However, it increased triglyceride levels in the livers of HCV+ mice (Control group, 33.6±2.8 vs Ethanol group, 41.7±1.6 mg Trigs/Liver mg Trigs/Liver, p=0.034), but not in HCV- mice (Control group, 32.7±2.7 vs EtOH group, 34.7±1.9 mg Trigs/Liver, p>0.05).
Proteasome activity: cell lysates vs purified 26S proteasome
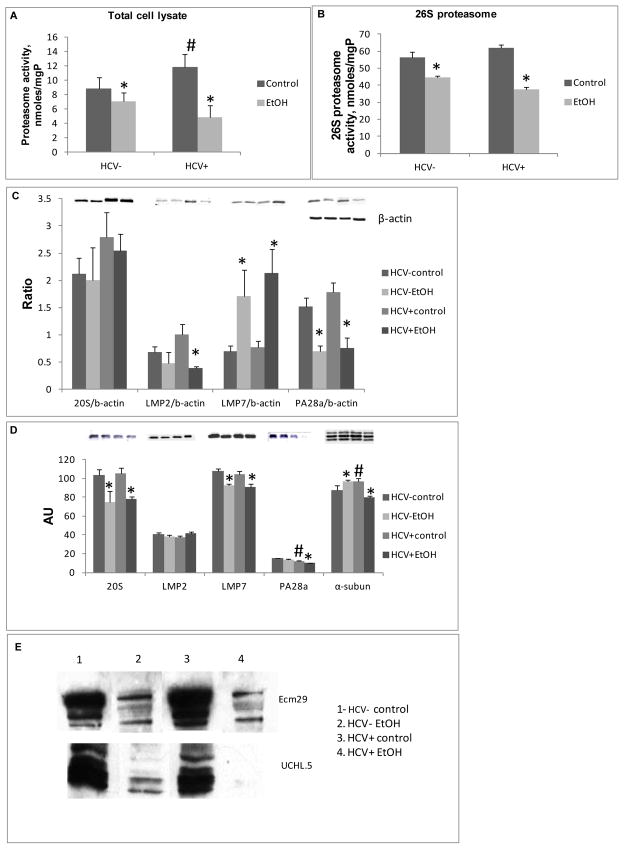
Proteasome activity was 29% higher in lysates of hepatocytes from HCV+ mice than in those from HCV−mice. However, ethanol feeding suppressed proteasome activity by 15% in hepatocytes from HCV− mice and by 69% in hepatocytes from HCV+ mice, with no difference in cell viability (Fig.1A).
Fig. 1.
Proteasome activity and expression of proteasomal proteins. (A) Cht-L proteasome activity (nmoles/mgP) in hepatocyte lysates; (B) Cht-L proteasome activity in 26S isolated proteasome from 3-5 mice/group; (C) Expression of proteasomal proteins in total cell lysates from hepatocytes detected by WB. Data (mean±StDev) from 3-5 mice/group were presented as ratio between proteasomal proteins and B-actin; (D) Expression of proteasomal proteins in isolated 26S proteasome; an equal protein load of 26S proteasome (in triplicate) was subjected to WB in lieu of internal load control since all 26S proteasomal proteins are affected by ethanol treatment; (E) 26S proteasome-associated PIPs. Ecm 29 and UCHL5, representative blot of 3 experiments with similar results on equally loaded 26S proteasome. * is p<0.05 between control and ethanol groups; # is P<0.05 between HCV+ and HCV- corresponding groups.
Since protein degradation by 26S proteasome depends on proteasome composition, we also compared the activity and composition of 26S proteasome isolated from the liver. 26S proteasome activity was lower in both HCV− and HCV+ ethanol-fed mice than in control (22% and 39%, respectively) (Fig. 1B). Using WB, we found no HCV core protein among PIPs.
Proteasome composition: hepatocyte lysates vs purified 26S proteasome
While ethanol feeding increased LMP7 and decreased PA28α in HCV+ and HCV− hepatocytes, it also decreased LMP2 expression in hepatocytes from HCV+ ethanol-fed mice (Fig. 1C). The latter observation correlated with a profound suppression in proteasome activity observed in hepatocytes of HCV+ mice after ethanol administration (Fig 1A).
In purified 26S proteasome preparations, expression of 20S proteasome (with constitutive β-subunits) and LMP7 were equally suppressed by ethanol feeding in both HCV+ and HCV− livers (Fig. 1D). Interestingly, the total proteasome α-subunit content was higher in ethanol-fed HCV− mice and lower in ethanol-fed HCV+ mice than in the corresponding control groups (Fig. 1C). PA28α content was lower in 26S proteasome particles from livers of HCV+ control mice than in those from HCV− controls and was further decreased in ethanol-fed HCV+ mice (Fig. 1C). We measured other PIPs, such as the protein, Ecm29, which regulates proteasome stability and the content of de-ubiquitylase, UCHL5, which is associated with the 19S particle and removes polyubiquitin from tagged proteins destined for degradation. Previous work showed that the contents of both Ecm29 and UCHL5 were lower in rats chronically fed ethanol intragastrically (Bousquet-Dubouch et al., 2009). Here, feeding 20% ethanol in the drinking water also decreased Ecm29 and UCHL5 content, in both HCV− and HCV+ mice (Fig. 1E) compared with controls.
Liver oxidative stress and proteasome function
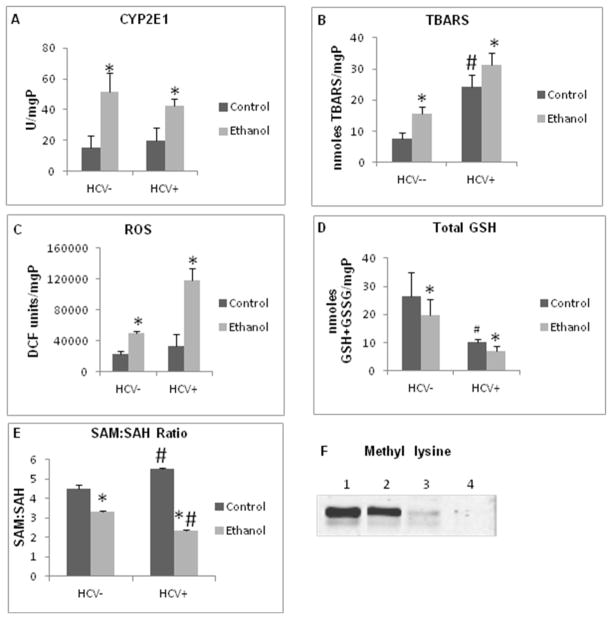
Oxidative stress significantly influences proteasome function (Bardag-Gorce et al., 2006; Kessova and Cederbaum, 2005; Osna and Donohue, 2007) and bi-phasically regulates proteasome activity depending on the level of generated oxidants (Osna et al., 2008). Here, CYP2E1 activity was equally enhanced by ethanol feeding in HCV− and HCV+ mice. MDA (measured as TBARS) was higher in HCV+ control mice than in HCV− littermates and was further increased by ethanol feeding, with significantly higher MDA levels in HCV+ ethanol-fed mice; ROS levels were also higher in ethanol-fed HCV+ than in corresponding HCV−mice (Figs. 2A- C). Total glutathione (GSH + GSSG) was 20% lower in ethanol-fed HCV−mice than in controls and this decline was greater in HCV+ ethanol-fed mice than their corresponding controls (Fig. 2D).
Fig. 2.
Oxidative stress and methylation status in mouse hepatocytes: (A) CYP2E1 activity; (B) TBARS; (C) ROS; (D) Total glutathione. All experiments were run at least from 3–5 mice in a group and are shown as mean±StDev. * is p<0.05 between control and ethanol groups; # p<0.05 between HCV+ and corresponding HCV− groups. (E) SAM:SAH ratio in hepatocytes SAM:SAH ratio was determined in hepatocytes isolated from 3 or more mice from each group and is presented as mean±StDev; (F) Methyl lysine expression in 26S proteasome. Methyl lysine is quantified by WB in 26S proteasome (a representative blot from 3 independent experiments with similar results). * is p<0.05 between control and ethanol groups; # p<0.05 between HCV+ and corresponding HCV− groups.
Methylation status in mouse livers and proteasome function
The methylation status of the 20S proteasome also affects its enzyme activity (Austin, 2009). To this end, we measured the S-adenosylmethionine (SAM): S-adenosylhomocysteine (SAH) ratio in hepatocytes as well as the content of methyl lysine in the partially-purified 26S proteasome. The intracellular SAM:SAH ratio was slightly higher in HCV+ control mice than in HCV− control mice, while ethanol feeding decreased this ratio in both HCV+ and HCV− mice, with a more prominent decline in HCV+ mice (Fig. 2E). In addition, the level of 26S proteasome-associated methyl lysines was significantly reduced in HCV+ mice (Fig. 2E-F), slightly potentiating by ethanol-feeding.
Ethanol and HCV decrease MHC class I-restricted antigen presentation on hepatocytes
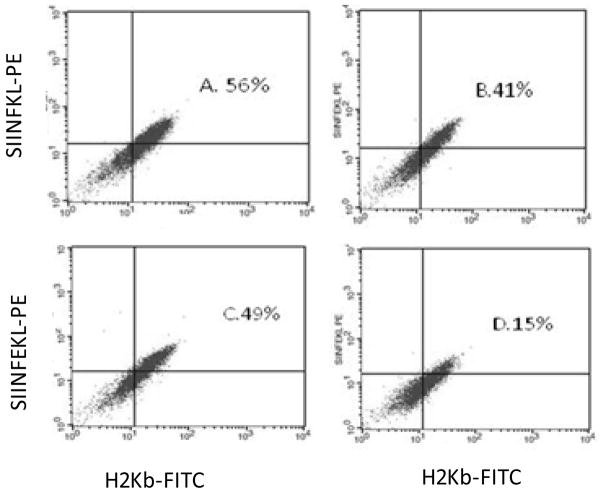
MHC class I-restricted antigen presentation is a downstream indicator of proteasome/immunoproteasome function. As shown before, the ovalbumin peptide, SIINFEKL-TE is cleaved by the proteasome, yielding SIINFEKL, which is presented on the cell surface in a complex with mouse MHC class I (H2Kb) (Osna et al., 2009; Rock et al., 2004). SIINFEKL-TE was delivered into the cytoplasm of hepatocytes obtained from control and ethanol-fed HCV + and HCV - mice and then cell surface SIINFEKL-H2Kb presentation was measured by flow cytometry utilizing specific antibody to SIINFEKL-H2Kb complex. Hepatocytes from ethanol-fed HCV− mice presented a 31% lower SIINFEKL-H2Kb compared with cells from their corresponding controls (Figs. 3A & B). However, presentation SIINFEKL-H2Kb complex on HCV+ hepatocytes was 69% suppressed by ethanol feeding and was 63% lower than in HCV− control mice (Figs .3C & D). The latter was consistent with a similar magnitude of decrease in proteasome activity.
Fig. 3.
SIINFEKL-H2Kb presentation on/in hepatocytes. SIINFEKL-H2Kb presentation was measured by flow cytometry with antibody to SIINFEKL-H2Kb, 3 experiments (a representative experiment is shown). (A) HCV− control; (B) HCV− ethanol; (C) HCV+ control; (D) HCV+ ethanol.
DISCUSSION
For MHC class I folding, proteins and long peptides are degraded to short peptides by the proteasome. Here, we studied the in vivo effects of ethanol alone, HCV structural proteins and the combination of HCV proteins and ethanol exposure on the activity and subunit composition of the isolated 26S proteasome particle.
Remarkably, while proteasome activity was higher in crude hepatocyte lysates of control HCV+ mice than HCV− mice, proteasome activation was not observed in the 26S preparation isolated from HCV+ control mouse livers. Because we found no detectable core protein among the various 26S PIPs and because HCV core protein increases proteasome activity (Osna et al., 2008), this could explain why isolated 26S proteasome was equally suppressed by ethanol feeding in HCV+ and HCV− mice. Enhanced proteasome suppression was seen in crude hepatocyte lysates of ethanol-fed HCV+ core protein expressing mice. These results also suggest that the cross-talk between HCV core protein and proteasome in ethanol-exposed liver cells is necessary for severe impairment of enzyme function.
The total Cht-L proteasome activity in hepatocytes reflects the activity of the 20S proteasome as both a free particle and as part of the 26S holoenzyme. Total proteasome activity may decrease by reduced expression of constitutive β-subunits and IPRs, or due to reduced access by substrate proteins to the 20S proteolytic core. Access to the 20S catalytic core in the 26S proteasome is regulated by 19S particles (hinge and lid) and by the 20S proteasome inducer, PA28. Here, ethanol feeding to mice indeed reduced the expression of LMP7, the major IPR subunit with Cht-L activity, in both HCV+ and HCV− mice. However, the total LMP7 subunit was not decreased in hepatocytes from ethanol-fed HCV − mice. This indicates that ethanol alone selectively reduces the replacement (or de-novo synthesis) of constitutive proteasome subunits with IPR in the 20S proteolytic core of the 26S proteasome or it decreases 26S proteasome stability.
Next, we analyzed the expression of PIPs that associate with the 26S proteasome, concentrating on PIPs important for proteasome stability and normal proteasome function such as PA28α, Ecm29 and UCHL5, which were previously shown to be altered by intragastric ethanol feeding (Bardag-Gorce, 2010; Bousquet-Dubouch et al., 2009). Consistent with previous findings, we discovered that ethanol feeding decreased the levels of Ecm29 and UCHL5. Because Ecm29 is a protein that regulates 26S proteasome stability and proteasome coupling to intracellular secretory compartments engaged in quality control (Kleijnen et al., 2007), the reduction in 20S proteasome subunit content within the 26S proteasome was likely a consequence of proteasome instability. This result also revealed that Ecm29 did not co-purify with the 26S proteasome, indicating that its binding to the 26S enzyme was disrupted by ethanol or ethanol metabolism. Furthermore, the amount of 19S-particle-associated deubiquitylase, UCHL5 also declined in ethanol-fed mice. Thus, ethanol feeding alone destabilizes the 26S proteasome, and which depleties of the 20S particle.
PA28 α is an activator of the 20S (and hybrid 26S/20S) proteasome. It binds to the α-subunits on the 20S core enzyme and enhances accessibility of substrate proteins to the interior of the proteolytic core. The intrahepatic supply of PA28α was reduced in all ethanol fed mice, resulting in suppressed proteasome activity. In addition, the content of PA28α associated with the 26S proteasome was decreased in control HCV+ mice and further reduced in these mice after ethanol feeding. Also, the total amount of α-subunits of proteasome, which are regulated by PA28α to enhance substrate access to the 20S catalytic core, was decreased only in 26S proteasome preparations from HCV+ ethanol-fed mice. These results indicate that this larger form of the proteasome is functionally the most defective among those tested here. Thus, we speculate that suppression of proteasome function in ethanol-fed HCV+ mice may be partially attributed to ethanol-elicited alterations in the accessibility of antigenic substrates to the proteasome catalytic core due to reduced PA28α expression.
The following are the possible mechanisms involved in ethanol- and HCV-induced proteasome dysfunction. As revealed from our previous cell culture study, HCV core protein regulates 20S proteasome activity by synergizing with ethanol to induce oxidative stress (Osna et al., 2008). Here, we confirmed an in vivo association between proteasome activity and the level of oxidative stress in hepatocytes of HCV− and HCV+ mice fed water or 20% ethanol. The highest degree of oxidative stress (based on elevated TBARS, ROS and glutathione depletion) was in hepatocytes from HCV+ ethanol-fed mice and was accompanied by the lowest proteasome activity, corroborating the suppressive effect of higher levels of ethanol-induced oxidative stress in compromised proteasome function (Osna et al., 2004; Osna et al., 2008). As seen in hepatocytes of HCV− ethanol-fed mice, lower-levels of oxidative stress only slightly reduced proteasome activity. Furthermore, in hepatocytes of HCV+ control mice, mild oxidative stress actually enhanced proteasome activity, which is consistent with our previous report of dual regulation of proteasome function by differential levels of oxidants(Osna et al., 2008). Because the outer α-subunits of the proteasome are the most susceptible to oxidative modifications (Ferrington and Kapphahn, 2004) and oxidatively modified proteins are the preferred substrates of the proteasome (Curry-McCoy et al., 2009), we cannot exclude the possibility that some of outer α-subunit proteasome modifications were removed in the process of proteasome-regulated “self-cleaning”. The latter may have caused the reduction in α-subunits seen here in HCV+ ethanol-fed mice While more profound inhibition of proteasome by chronic ethanol exposure is seen after intragastric feeding or feeding with Lieber deCarli ethanol diet to HCV− rodents (Bardag-Gorce et al., 2006; Donohue et al., 2007), feeding mice 20% ethanol in water clearly demonstrates that HCV proteins potentiate the effect of ethanol-induced suppression of proteasome and constitute a “second hit” in a mouse model of alcohol-elicited liver injury (Tsukamoto et al., 2009).
We recently showed that, in addition to oxidative stress, methylation potential in the liver (i.e. SAM:SAH ratio) also regulates proteasome activity (Osna et al., 2010) . Here, the SAM:SAH ratio in hepatocytes decreased to 3.3 after ethanol feeding to HCV− mice compared with a ratio of 4.5 in control hepatocytes. This ratio was further decreased to 2.4 in ethanol-fed HCV+ mice and was largely due to the elevation of SAH (not shown) and was consistent with previously published data in a rat model of alcohol liver injury (Kharbanda et al., 2005). Interestingly, we observed an increase in SAM levels in control HCV+ mice, (not shown), yet a reduction in the methylation of lysine residues on 26S proteasome, one of the identified SAM:SAH- regulated targets on proteasome was seen (Gomes et al., 2009). These data support the notion that HCV structural proteins may decrease methylation of lysine residues in vivo. Since proteasome activity is regulated by multiple factors, the stimulating effect of low oxidative stress on proteasome in HCV+ control hepatocytes seems to “override” the suppressive effect from the reduction in proteasomal methyl lysines, leading to a net increase in the proteasome activity. Nevertheless, the combination of high oxidative stress and impaired methylation reactions observed after ethanol feeding of HCV+ mice significantly blocked proteasome function.
To examine the link between proteasome activity in hepatocytes and peptide processing as the most upstream event in MHC class I restricted antigen presentation, we measured the surface expression of SIINFEKL-H2Kb, a cleaved form of intracellularly delivered SIINFEKL-TE. The expression of SIINFEKL-H2Kb in HCV−ethanol-fed mice was lower than in controls; it was also low in HCV+ control mice and further suppressed by ethanol. These findings indicate the similarity in the patterns of proteasome activation and MHC class I-restricted peptide presentation on hepatocytes suggests that current antiviral therapies that isolated from HCV+ and HCV- mice fed control or ethanol diets. Thus, exposure of uninfected hepatocytes to ethanol or HCV slightly lowers proteasome activity and subsequent presentation of peptide-MHC class I complex on the cell surface, while the combination of HCV and ethanol considerably decreases the display of this complex on hepatocytes. This means that .in HCV-expressing hepatocytes exposed to ethanol, the ability of proteasome to generate antigenic peptides for recognition by cytotoxic T-lymphocytes, in the context of MHC class I, is very limited, Such a condition would likely s provoke viral persistence in liver cells. . The latter partially explains why HCV-infection is greater in heavy drinkers than in non-drinkers and enhance antigen processing are thwarted because the processing machinery has been damaged.
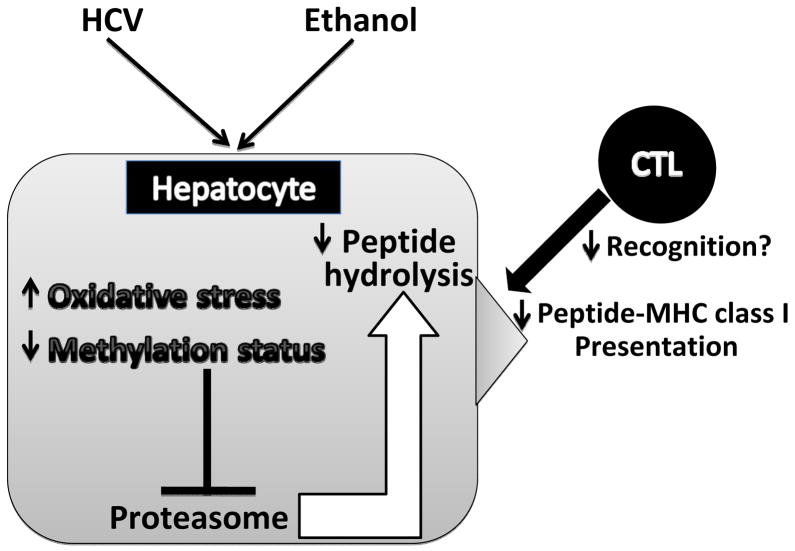
In conclusion, this study clarifies the mechanisms of ethanol and HCV-induced dysfunction of proteasome and subsequent antigen presentation in hepatocytes. Feeding 20% ethanol in water to HCV− mice slightly suppressed proteasome activity with significant potentiation of this effect in HCV+ mice. Ethanol-elicited reduction in purified 26S proteasome function is caused by miscommunication between 26S proteasome and PIPs, which destabilizes the 26S proteasome. This is followed by the reduction in the levels of 20S proteasome containing both constitutive and IPR subunits. Both HCV and ethanol feeding contribute to regulation of in vivo/ex vivo liver proteasome activity via induction of oxidative stress and impaired methylation reactions. These changes in proteasome composition/activity likely suppress processing of peptides for antigen presentation by hepatocytes, and substantially diminishes the ability of CTLs to recognize and eliminate these HCV+ ethanol-exposed hepatocytes. Our working hypothesis is presented in Fig. 4.
Fig. 4.
Proteasome-dependent regulation of MHC class I-restricted antigen presentation on hepatocytes by HCV and ethanol (working hypothesis): HCV and ethanol induce oxidative stress and impaired methylation status in hepatocytes. This affects the proteasome composition and consequently inhibits proteasome activity and peptide processing, which ultimately suppress the display of peptide-MHC class I complexes on hepatocytes, the targets for CTLs.
Acknowledgments
The research reported here was supported by grants from Department of Veterans Affairs National Merit Review grant (KKK) and NIH/NIAAA grants R21AA017296 (KKK), R21 AA017232 (NAO). We thank Dr. Geoffrey Thiele and his laboratory for the purification of SIINFEKL-H2Kb antibody and Dr. Carol Casey for the help with liver perfusions.
References
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- Austin L, Kharbanda K, Beard M, Osna N. Ethanol affects expression of receptors for HCV viral entry in liver cells. Hepatology. 2009;50(5 suppl):1155A–1156A. [Google Scholar]
- Bardag-Gorce F. Effects of ethanol on the proteasome interacting proteins. World J Gastroenterol. 2010;16:1349–1357. doi: 10.3748/wjg.v16.i11.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, French BA, Nan L, Song H, Nguyen SK, Yong H, Dede J, French SW. CYP2E1 induced by ethanol causes oxidative stress, proteasome inhibition and cytokeratin aggresome (Mallory body-like) formation. Exp Mol Pathol. 2006;81:191–201. doi: 10.1016/j.yexmp.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Bousquet-Dubouch MP, Nguen S, Bouyssie D, Burlet-Schiltz O, French SW, Monsarrat B, Bardag-Gorce F. Chronic ethanol feeding affects proteasome-interacting proteins. Proteomics. 2009;9:3609–3622. doi: 10.1002/pmic.200800959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry-McCoy TV, Osna NA, Donohue TM., Jr Modulation of lysozyme function and degradation after nitration with peroxynitrite. Biochim Biophys Acta. 2009;1790:778–786. doi: 10.1016/j.bbagen.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolganiuc A, Szabo G. Dendritic cells in hepatitis C infection: can they (help) win the battle? J Gastroenterol. 2011;46:432–447. doi: 10.1007/s00535-011-0377-y. [DOI] [PubMed] [Google Scholar]
- Donohue TM, Jr, Curry-McCoy TV, Todero SL, White RL, Kharbanda KK, Nanji AA, Osna NA. L-Buthionine (S,R) sulfoximine depletes hepatic glutathione but protects against ethanol-induced liver injury. Alcohol Clin Exp Res. 2007;31:1053–1060. doi: 10.1111/j.1530-0277.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004;578:217–223. doi: 10.1016/j.febslet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Gomes AV, Young GW, Wang Y, Zong C, Eghbali M, Drews O, Lu H, Stefani E, Ping P. Contrasting proteome biology and functional heterogeneity of the 20 S proteasome complexes in mammalian tissues. Mol Cell Proteomics. 2009;8:302–315. doi: 10.1074/mcp.M800058-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groettrup M, Standera S, Stohwasser R, Kloetzel PM. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc Natl Acad Sci U S A. 1997;94:8970–8975. doi: 10.1073/pnas.94.17.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume B, Chapiro J, Stroobant V, Colau D, Van Holle B, Parvizi G, Bousquet-Dubouch MP, Theate I, Parmentier N, Van den Eynde BJ. Two abundant proteasome subtypes that uniquely process some antigens presented by HLA class I molecules. Proc Natl Acad Sci U S A. 2010;107:18599–18604. doi: 10.1073/pnas.1009778107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heink S, Ludwig D, Kloetzel PM, Kruger E. IFN-gamma-induced immune adaptation of the proteasome system is an accelerated and transient response. Proc Natl Acad Sci U S A. 2005;102:9241–9246. doi: 10.1073/pnas.0501711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessova IG, Cederbaum AI. The effect of CYP2E1-dependent oxidant stress on activity of proteasomes in HepG2 cells. J Pharmacol Exp Ther. 2005;315:304–312. doi: 10.1124/jpet.105.088047. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, Mailliard ME, Baldwin CR, Beckenhauer HC, Sorrell MF, Tuma DJ. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J Hepatol. 2007;46:314–321. doi: 10.1016/j.jhep.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, Rogers DD, 2nd, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, Sorrell MF, Tuma DJ. A comparison of the effects of betaine and S-adenosylmethionine on ethanol-induced changes in methionine metabolism and steatosis in rat hepatocytes. J Nutr. 2005;135:519–524. doi: 10.1093/jn/135.3.519. [DOI] [PubMed] [Google Scholar]
- Kleijnen MF, Roelofs J, Park S, Hathaway NA, Glickman M, King RW, Finley D. Stability of the proteasome can be regulated allosterically through engagement of its proteolytic active sites. Nat Struct Mol Biol. 2007;14:1180–1188. doi: 10.1038/nsmb1335. [DOI] [PubMed] [Google Scholar]
- Korenaga M, Okuda M, Otani K, Wang T, Li Y, Weinman SA. Mitochondrial dysfunction in hepatitis C. J Clin Gastroenterol. 2005;39(4 Suppl 2):S162–166. doi: 10.1097/01.mcg.0000155517.02468.46. [DOI] [PubMed] [Google Scholar]
- Nishizawa-Yokoi A, Tainaka H, Yoshida E, Tamoi M, Yabuta Y, Shigeoka S. The 26S proteasome function and Hsp90 activity involved in the regulation of HsfA2 expression in response to oxidative stress. Plant Cell Physiol. 2010;51:486–496. doi: 10.1093/pcp/pcq015. [DOI] [PubMed] [Google Scholar]
- Osna NA. Hepatitis C virus and ethanol alter antigen presentation in liver cells. World J Gastroenterol. 2009;15:1201–1208. doi: 10.3748/wjg.15.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna NA, Clemens DL, Donohue TM., Jr Interferon gamma enhances proteasome activity in recombinant Hep G2 cells that express cytochrome P4502E1: modulation by ethanol. Biochem Pharmacol. 2003;66:697–710. doi: 10.1016/s0006-2952(03)00252-1. [DOI] [PubMed] [Google Scholar]
- Osna NA, Donohue TM., Jr Implication of altered proteasome function in alcoholic liver injury. World J Gastroenterol. 2007;13:4931–4937. doi: 10.3748/wjg.v13.i37.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna NA, Haorah J, Krutik VM, Donohue TM., Jr Peroxynitrite alters the catalytic activity of rodent liver proteasome in vitro and in vivo. Hepatology. 2004;40:574–582. doi: 10.1002/hep.20352. [DOI] [PubMed] [Google Scholar]
- Osna NA, White RL, Donohue TM, Jr, Beard MR, Tuma DJ, Kharbanda KK. Impaired methylation as a novel mechanism for proteasome suppression in liver cells. Biochem Biophys Res Commun. 2010;391:1291–1296. doi: 10.1016/j.bbrc.2009.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna NA, White RL, Krutik VM, Wang T, Weinman SA, Donohue TM., Jr Proteasome activation by hepatitis C core protein is reversed by ethanol-induced oxidative stress. Gastroenterology. 2008;134:2144–2152. doi: 10.1053/j.gastro.2008.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna NA, White RL, Thiele GM, Donohue TM., Jr Ethanol metabolism alters major histocompatibility complex class I-restricted antigen presentation in liver cells. Hepatology. 2009;49:1308–1315. doi: 10.1002/hep.22787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna NA, White RL, Todero S, McVicker BL, Thiele GM, Clemens DL, Tuma DJ, Donohue TM., Jr Ethanol-induced oxidative stress suppresses generation of peptides for antigen presentation by hepatoma cells. Hepatology. 2007;45:53–61. doi: 10.1002/hep.21442. [DOI] [PubMed] [Google Scholar]
- Otani K, Korenaga M, Beard MR, Li K, Qian T, Showalter LA, Singh AK, Wang T, Weinman SA. Hepatitis C virus core protein, cytochrome P450 2E1, and alcohol produce combined mitochondrial injury and cytotoxicity in hepatoma cells. Gastroenterology. 2005;128:96–107. doi: 10.1053/j.gastro.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, York IA, Goldberg AL. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat Immunol. 2004;5:670–677. doi: 10.1038/ni1089. [DOI] [PubMed] [Google Scholar]
- Seifert U, Kruger E. Remodelling of the ubiquitin-proteasome system in response to interferons. Biochem Soc Trans. 2008;36(Pt 5):879–884. doi: 10.1042/BST0360879. [DOI] [PubMed] [Google Scholar]
- Siu L, Foont J, Wands JR. Hepatitis C virus and alcohol. Semin Liver Dis. 2009;29:188–199. doi: 10.1055/s-0029-1214374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72:1109–1116. [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Mandrekar P, White B. Inhibition of antigen-presenting cell functions by alcohol: implications for hepatitis C virus infection. Alcohol. 2004;33:241–249. doi: 10.1016/j.alcohol.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Szabo G, Wands JR, Eken A, Osna NA, Weinman SA, Machida K, Joe Wang H. Alcohol and hepatitis C virus--interactions in immune dysfunctions and liver damage. Alcohol Clin Exp Res. 2010;34:1675–1686. doi: 10.1111/j.1530-0277.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Machida K, Dynnyk A, Mkrtchyan H. “Second hit” models of alcoholic liver disease. Semin Liver Dis. 2009;29:178–187. doi: 10.1055/s-0029-1214373. [DOI] [PMC free article] [PubMed] [Google Scholar]